Current concepts of protective ventilation during general anaesthesia
DOI: https://doi.org/10.4414/smw.2015.14211
Ary
Serpa Neto, Marcus J
Schultz, Arthur S.
Slutsky
Summary
Mechanical ventilation with high tidal volumes (VT) has been common practice in operating theatres because this strategy recruits collapsed lung tissue and improves ventilation-perfusion mismatch, thus decreasing the need for high inspired oxygen concentrations. Positive end-expiratory pressure (PEEP) was not used routinely because it was thought to impair cardiovascular function. Over the past two decades there have been advances in our understanding of the causes and importance of ventilation-induced lung injury based on studies in animals with healthy lungs, and trials in critically ill patients with and without acute respiratory distress syndrome. Recent data from randomised controlled trials in patients receiving ventilation during general anaesthesia for surgery have demonstrated that lung-protective strategies (use of low VT, use of PEEP if indicated, and avoidance of excessive oxygen concentrations) are also of importance during intraoperative ventilation.
Introduction
More than 230 million surgical procedures are undertaken worldwide each year [1]. Complications after surgery are an important cause of mortality and morbidity; approximately 4% of patients who develop a postoperative complication die before hospital discharge, and those who survive often have reduced functional status [2, 3]. Development of postoperative pulmonary complications (PPCs) has a stronger impact on outcome of these patients; one in every five patients who develop one or more PPCs dies within 30 days of surgery, and the occurrence of PPCs is strongly associated with longer postoperative stay in hospital [2–4].
Advances in the understanding of the potential harmful effects of mechanical ventilation, resulting in so-called ventilation-induced lung injury (VILI) have led to the development of lung-protective strategies in patients with the acute respiratory distress syndrome (ARDS) [5]. Recently there have been a number of trials that suggest that certain lung-protective strategies could also benefit patients without ARDS, including those receiving intraoperative ventilation during general anaesthesia for surgery. These strategies include measures that prevent alveolar over-distension, decrease repeated alveolar opening and closing with each breath cycle, and prevent oxygen toxicity.
The scope of this narrative review is to summarise the rationale for lung-protective intraoperative ventilation strategies focusing on the potential benefits of tidal volume (VT) reduction, use of positive end-expiratory pressure (PEEP) and avoidance of excessive oxygen concentrations (FiO2).
Tidal volume
Historical rationale for tidal volume settings in the operating room
For many years, anaesthesiologists applied ventilation strategies with high VT because this strategy re-opens lung regions that collapse with each breath at end-expiration, i.e. minimises atelectasis. This was seen as beneficial because it reduced ventilation-perfusion mismatch, thereby requiring lower FiO2 [6]. While high tidal volumes were increasingly considered to be harmful in critically ill patients, in particular those with ARDS [6], use of high VTs during intraoperative ventilation was considered to be safe because of the relatively short duration (hours) of ventilation, compared with critically ill patients who could be ventilated for days to weeks.
Evidence for harm from high tidal volumes in animal models of ventilation
In the last few decades, animal research has convincingly demonstrated that ventilation with high VT can induce VILI (fig. 1B) [7]. Animal studies frequently used a multiple-hit approach in which lung injury was first triggered by a preceding insult (e.g., systemic inflammation or sepsis, pneumonia or aspiration) and then amplified by the harmful effects of large VT [8, 9]. However, several animal studies demonstrated that ventilation with high VT alone – without a preceding hit – could induce VILI [10]. This suggested that intraoperative ventilation strategies that use high tidal volumes might be harmful. Of particular note, the animal models almost always used relatively short periods of ventilation (≤12 hours), more closely simulating the clinical scenario of the operating room.
Evidence for harm from high tidal volumes in patients with ARDS
The harmful effects of ventilation with high VT in patients with ARDS were not confirmed until the landmark ARDS Network trial in 2000, which demonstrated the beneficial effect of ventilation with low VT (6 ml/kg predicted body weight, PBW) compared with conventional VT (12 ml/kg PBW) [5]. Ventilation with low VT resulted in decreased mortality and increased number of ventilator-free days [5]. Some clinicians and investigators were relatively slow to accept these findings, but subsequent trials and a meta-analysis convincingly confirmed the mortality reduction [11]. Currently, lung-protective ventilation with low VTs is considered standard of care for ARDS [11, 12].
Increasing evidence for harm from high tidal volumes in patients without ARDS
The finding that ventilation with low VTs benefits patients with ARDS evoked interest in lung-protective ventilation in patients without lung injury. One clinical trial published in 2010 found increased lung injury in a group ventilated with “high” VT (10 ml/kg PBW) compared with “low” VT (6 ml/kg predicted PBW) [13]. These findings were confirmed in a series of meta-analyses [7, 14–16] that also suggested that ventilation strategies with low VT could hasten liberation from the ventilator. Despite the growing evidence for potential harm from high VT in critically ill patients without ARDS, lung-protective ventilation is still not considered standard of care for critically ill patients who need ventilatory support but have healthy lungs. Nevertheless, a substantial reduction in VT appears to have occurred in recent years [7, 14–18].
Increasing evidence for harm from high tidal volumes during intraoperative ventilation
Several small clinical trials of intraoperative ventilation suggested that VT reduction could improve pulmonary mechanics and oxygenation [19, 20], reduce local production of inflammatory mediators [21], and shorten duration of postoperative ventilation [22].As well, one meta-analysis suggested that intraoperative ventilation strategies that use low VT could reduce the incidence of postoperative pulmonary complications [22].
Recently, three randomised controlled trials further increased the evidence for harm from intraoperative ventilation with high VT [23–25]. An Italian single-centre randomised controlled trial showed that VT reduction from 9 to 7 ml/kg PBW during abdominal surgery was associated with better postoperative pulmonary function [23]. A French multicentre randomised controlled trial showed that there was a >60% reduction in postoperative pulmonary complications in patients undergoing abdominal surgery when a ventilation strategy using a VT of 6 ml/kg PBW was compared with a VT of 12 ml/kg PBW [24]. A Chinese randomised controlled trial in patients undergoing spinal fusion showed a very impressive benefit from reducing VT from 10–12 ml/kg PBW to 6 ml/kg PBW [25]. Of note, in all three trials, lung-protective ventilation consisted of a bundle of measures: low VT
with higher levels of PEEP and recruitment manoeuvres; as such, it was impossible to conclude which protective measure caused most benefit. A recent individual patient data meta-analysis, including data from these three randomised controlled trials, suggested that benefit from lung-protection was best explained from VT reductions, and not from higher levels of PEEP [26].
Present choices in tidal volume size in the operation room
One recently published observational study on intraoperative ventilation settings in a German university hospital [17], and one large report on intraoperative ventilation practices in a large number of American university hospitals, demonstrated increased use of low VT during intraoperative ventilation: VT nearly halved, to 7‒8 ml/kg PBW [18]. One could pose the question as to whether this reduction in VT can be considered “enough”. It has been suggested that the “normal” VT in mammals is ≈6.3 ml/kg PBW [27], and it is possible, but certainly not proven, that a further reduction of VT during intraoperative ventilation would be better.
Positive end-expiratory pressure
Historical choices for PEEP settings in the operation room
Induction of anaesthesia, especially with the use of high FiO2, causes atelectasis, which increases ventilation-perfusion mismatch [28]. Ventilation strategies that use low VT could further induce alveolar instability with cyclic opening and closing of alveoli with each breath (fig. 1A and C) [29]. PEEP may open those lung regions that collapse following induction of anaesthesia, and could maintain the alveoli open during the entire breathing cycle [28]. However, adverse effects such as cardiac compromise, mandating volume expansion and perhaps even vasoactive drugs [30], could outweigh these beneficial effects. Consequently many anaesthesiologists had reservations about using PEEP.
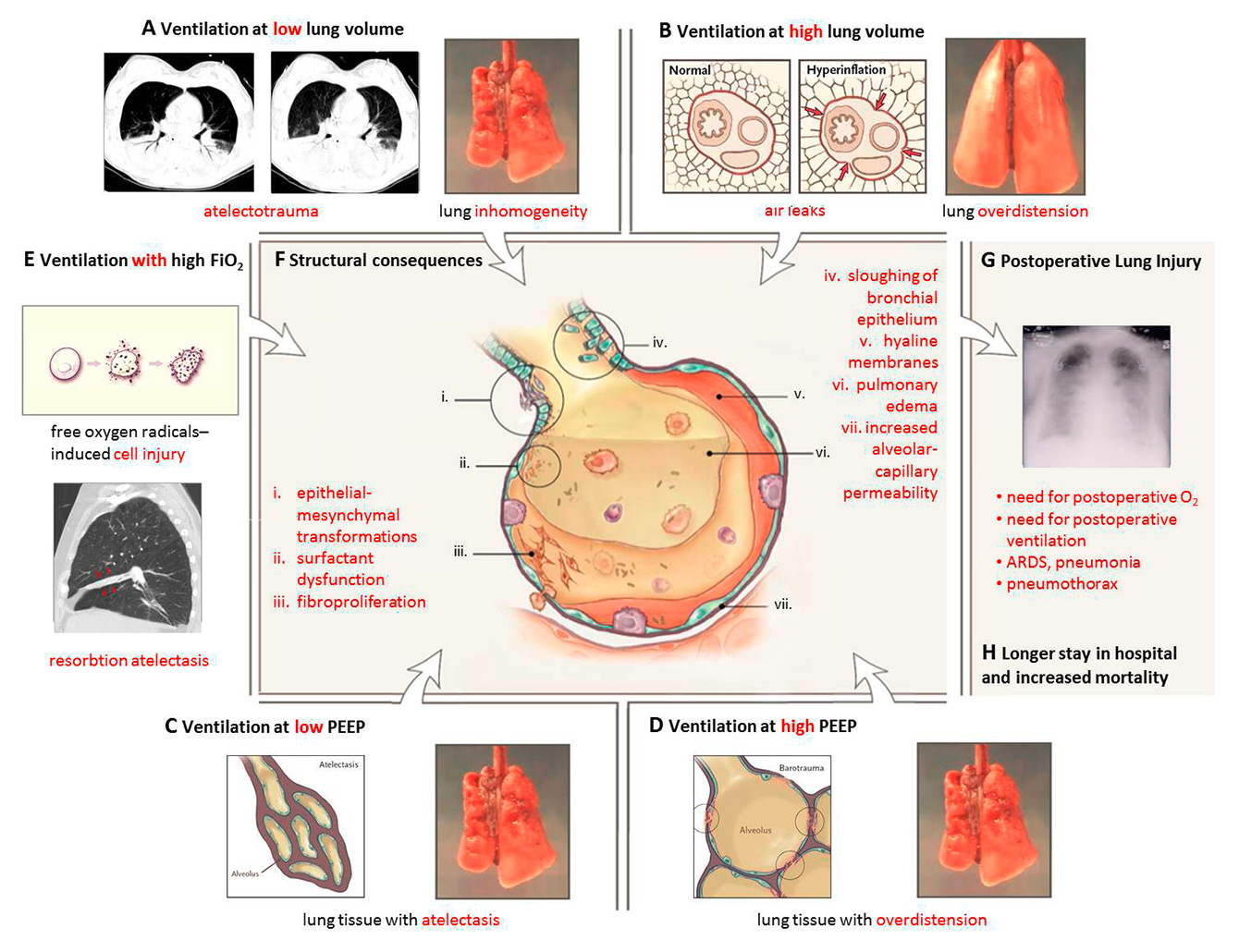
Figure 1
Postoperative pulmonary complications caused by forces generated by ventilation at low and high lung volumes, low and high PEEP and high FiO2 (Adapted from: Slutsky AS, Ranieri VM. Ventilator-Induced Lung Injury. N Engl J Med. 2013;369(22):2126–36. Copyright ©, Massachusetts Medical Society. Reprinted with permission.).
Evidence for benefit from high PEEP levels in animal models of ventilation
Atelectasis appears in a vast majority of larger mammals that receive general anaesthesia, with collapse of up to 15 to 20% of the dependent lung, worsened by the use of high FiO2and the use of muscle relaxation [31, 32]. Numerous preclinical studies have shown that ventilation strategies that used recruitment manoeuvres and PEEP could improve lung aeration, and thus improve oxygenation [33, 34]. However, PEEP could be detrimental by causing overdistention of nondependent lung regions (fig. 1D) [35].
Evidence for benefit from high PEEP levels in patients with ARDS
Independently, three well-performed randomised controlled trials in ARDS patients failed to show definitive benefit from ventilation strategies that used higher PEEP levels [36–38]; however, a meta-analyses of these trials suggested benefit from higher PEEP in ARDS patients who had lower PaO2/FiO2 ratios [39] Patients with moderate or severe ARDS who received ventilation with higher levels of PEEP had a lower mortality, and needed rescue therapies, such as inhaled nitric oxide, prone ventilation, extracorporeal membrane oxygenation, less frequently than patients who were ventilated with low levels of PEEP [39, 40] Patients with mild ARDS had no benefit from higher PEEP levels.
PEEP levels in critically ill patients without ARDS
There are only two small randomised controlled trials in patients without ARDS in which different levels of PEEP were compared. In one study, use of PEEP from 5 to 8 cm H2O compared with zero PEEP resulted in a lower incidence of ventilator-associated pneumonia and a lower risk of hypoxaemia [41]. However, there were no differences in outcomes (incidence of ARDS or hospital mortality) [41]. In the other trial, the early application of 8 cm H2O of PEEP in patients at high-risk for ARDS had no effect on the occurrence of ARDS or other associated complications [42]. In contrast, in the randomised controlled trial mentioned above comparing ventilation with a low VT (6 ml/kg PBW) to high VT (10 ml/kg PBW) in patients without ARDS [13], an independent association between use of higher levels of PEEP and the development of lung injury was noticed. Although there are no well-accepted recommendations on whether PEEP should be used in critically ill patients without ARDS, observational studies show increased use of PEEP in these patients [43–45].
PEEP during intraoperative ventilation
Atelectasis appears in the vast majority of patients during general anaesthesia for surgery and can persist for several days during the postoperative period. This is associated with an increased risk of postoperative infection and pulmonary complications [31]. The three randomised controlled trials (discussed above) of intraoperative protective ventilation compared bundles of lung-protection: low VT
withhigh levels of PEEP and recruitment manoeuvres, and high VT
without PEEP and recruitment manoeuvres [23–25]. As mentioned above, it was difficult, if not impossible, to conclude from these trials what led to the benefit of the lung-protective strategy: lower VT
orincreased PEEP levels, orrecruitment manoeuvres orall of the above. Of note, one large recent observational study suggested that use of low levels of PEEP during intraoperative ventilation with low VTs might be associated with increased risk of mortality [46]. One recent randomised controlled trial addressed the question of higher vs lower PEEP levels during intraoperative ventilation with low VTs [30]. In that trial, patients with a high risk for postoperative pulmonary complications, who were undergoing abdominal surgery, were randomised to intraoperative low VT (8 ml/kg PBW) ventilation with no PEEP and no recruitment manoeuvres, or PEEP of 12 cm H2O with recruitment manoeuvres. The 12 cm H2O PEEP level was based on physiological studies showing that a PEEP of 10 to 12 cm H2O was necessary to increase end-expiratory lung volume [47], to reduce atelectasis formation during surgery [48], and to improve compliance and oxygenation after surgery [49]. This trial showed no benefit from the higher PEEP strategy: occurrence of postoperative pulmonary complications was not affected. However, use of a higher level of PEEP was associated with intraoperative hypotension and need for vasoactive drugs. A recent individual patient data meta-analysis including data from several randomised controlled trials and observational trials of protective ventilation in the operating room suggested that high PEEP levels do not prevent postoperative pulmonary complications when low VTs are used [26].
Present choices in PEEP settings in the operation room
About 10 years ago, the recommendation was to use a ‘sufficient’ level of PEEP ‘but at least 5 cm H2O’ to minimize atelectasis and maintain oxygenation [50]; more recently it was suggested that PEEP during intraoperative ventilation should be set at 6 to 8 cm H2O [28]. However, the best level of PEEP during intraoperative ventilation remains highly uncertain. It could very well be that a minimum PEEP of 2 cm H2O is sufficient in most patients, and that a further increase should be individualized, e.g., based on perioperative levels of oxygenation. It is also uncertain whether ventilation strategies that use higher levels of PEEP are beneficial in obese patients, or patients undergoing laparoscopic abdominal surgery, during which insufflation of gas in the abdominal cavity could induce more atelectasis.
Inspired fraction of oxygen
Historical choices in oxygen fractions settings in the operation room
Since PEEP was not widely used in the operating room, oxygenation was improved by using high inspired FiO2 (despite the fact that this could induce reabsorption atelectasis) and/or high VT (fig. 1E) [51]. It was also claimed that patients undergoing surgery could benefit from higher FiO2 [52], as randomised controlled trials showed less postoperative nausea and vomiting, and a reduced risk of surgical site infection, in patients who were ventilated with FiO2as high as 80% [52, 53].
High oxygen fractions in animal models of ventilation
High FiO2may induce pulmonary injury that could at least in part be induced by increased oxidative stress via increased levels of reactive oxygen-derived free radicals, with an influx of inflammatory cells, increased permeability and endothelial cell injury [54]. In experimental models of lung injury, coexisting lung inflammation increases susceptibility to oxygen toxicity [55]. High FiO2 induce the production of large amounts of reactive oxygen species that can overwhelm natural antioxidant defences and injure cellular structures [56]. Alterations in the permeability induced by lung injury increases the alveolar-capillary oxygen gradient increasing the risk of oxygen toxicity [57].
Potential harm from high oxygen fractions in critically ill patients who need ventilatory support
It has been suggested that normoxia should be the target in patients with ARDS, as this might prevent neurocognitive dysfunction in those who survive [54]. However, there is some evidence that both ventilation with high FiO2and high levels of blood oxygenation are associated with increased mortality in critically ill patients [57, 58], an effect that appeared to be independent of other factors such as disease severity. Similar associations were found in patients following resuscitation from cardiac arrest [59], patients with ischaemic stroke [60], and traumatic brain injury [61]. Two recent systematic reviews and meta-analyses found that arterial hyperoxia was associated with worse hospital outcome in various subsets of critically ill patients [61–63].
Evidence for harm from high oxygen fraction during intraoperative ventilation
At present, there are no sufficiently powered studies that have investigated the effects of higher FiO2on the occurrence of postoperative pulmonary complications.
Present choices in inspired fraction of oxygen in the operation room
The above mentioned recently published observational studies on intraoperative ventilation settings in one university hospital in Germany [17] and in a large number of university hospitals in the USA [18] show increased use of higher FiO2during intraoperative ventilation: for example, FiO2doubled to 80% in neurosurgery patients [17]. One could pose the question as to whether this increased use of higher FiO2 is safe, given some evidence for harm in nonsurgical patients.
Consequences and future directions for research
The harmful effects of high VT mechanical ventilation in patients under short-term ventilation during general anaesthesia for surgery are now recognised (fig. 1G) [64, 65]. Recent findings suggest that higher levels of PEEP (10‒12 cm H2O) do not protect against postoperative pulmonary complications, and may even cause harm, at least in nonobese patients. And finally, we are uncertain as to whether higher FiO2is beneficial or harmful in surgical patients.
Several clinical trials addressing intraoperative ventilation are currently ongoing. The PROVE Network investigators initiated an international multicentre randomised controlled trial in obese patients at high risk for postoperative pulmonary complications during abdominal surgery. The Protective Ventilation With Higher Versus Lower PEEP During General Anesthesia for Surgery in Obese Patients (PROBESE) trial will compare higher and lower levels of PEEP during low VT ventilation [66].
It is uncertain whether intraoperative ventilation with higher levels of PEEP is protective during general anaesthesia for surgical procedures other than abdominal surgery, such as thoracic surgery. A large randomised controlled trial comparing protective with conventional ventilation (VT of 5 ml/kg PBW plus PEEP vs VT of 10 ml/kg PBW without PEEP) in surgery for lung cancer is ongoing [67]. The PROVE Network investigators are also planning a trial in patients receiving one-lung ventilation for thoracic surgery (Protective Ventilation With Higher Versus Lower PEEP During General Anesthesia for Thorax Surgery, PROTHOR) [68], and they are planning a trial in patients undergoing laparoscopic abdominal surgery. These two randomised controlled trials will compare low VT ventilation with different levels of PEEP. What are presently lacking are well-performed randomised controlled trials of different levels of FiO2during intraoperative ventilation.
In conclusion, during intraoperative ventilation, VT should be kept low, perhaps 6 to 8 ml/kg PBW, but maybe even lower. It is less certain whether surgery patients benefit from levels of PEEP >2 cm H2O; it could be beneficial in individual patients, but it comes at a price of more haemodynamic compromise. Finally, there is a paucity of clinical data addressing the oxygen fraction to utilise during surgery.
References
1 Pearse RM, Moreno RP, Bauer P, et al. Mortality after surgery in Europe: a 7 day cohort study. Lancet. 2012;380:1059–65.
2 Canet J, Gallart L, Gomar C, et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology. 2010;113:1338–50.
3 Mazo V, Sabaté S, Canet J, et al. Prospective external validation of a predictive score for postoperative pulmonary complications. Anesthesiology. 2014;121:219–31.
4 Serpa Neto A, Hemmes SN, Barbas CS, et al. Incidence of mortality and morbidity related to postoperative lung injury in patients who have undergone abdominal or thoracic surgery: a systematic review and meta-analysis. Lancet Respir Med. 2014;2:1007–15.
5 ARDS Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–8.
6 Bendixen HH, Hedley-Whyte J, Laver MB. Impaired oxygenation in surgical patients during general anesthesia with controlled ventilation. A concept of atelectasis. N Engl J Med. 1963;269:991–6.
7 Serpa Neto A, Nagtzaam L, Schultz MJ. Ventilation with lower tidal volumes for critically ill patients without the acute respiratory distress syndrome: a systematic translational review and meta-analysis. Curr Opin Crit Care. 2014; 20:25–32.
8 Slutsky AS, Tremblay LN. Multiple system organ failure. Is mechanical ventilation a contributing factor? Am J Respir Crit Care Med. 1998;157:1721–5.
9 Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest. 1997;99:944–52.
10 Webb HH, Tierney DF. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection by positive end-expiratory pressure. Am Rev Respir Dis. 1974;110:556–65.
11 Putensen C, Theuerkauf N, Zinserling J, Wrigge H, Pelosi P. Meta-analysis: ventilation strategies and outcomes of the acute respiratory distress syndrome and acute lung injury. Ann Intern Med. 2009;151:566–76.
12 Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637.
13 Determann RM, Royakkers A, Wolthuis EK, et al. Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: a preventive randomized controlled trial. Crit Care. 2010;14:R1.
14 Serpa Neto A, Cardoso SO, Manetta JA, et al. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA. 2012;308:1651–9.
15 Serpa Neto A, Simonis FD, Schultz MJ. How to ventilate patients without acute respiratory distress syndrome? Curr Opin Crit Care. 2015;21:65–73.
16 Serpa Neto A, Simonis FD, Barbas CS, et al. Association between tidal volume size, duration of ventilation, and sedation needs in patients without acute respiratory distress syndrome: an individual patient data meta-analysis. Intensive Care Med. 2014;40:950–7.
17 Treschan TA, Schaefer MS, Subasi L, Kaisers W, Schultz MJ, Beiderlinden M. Evolution of ventilator settings during general anaesthesia for neurosurgery: An observational study in a German centre over 15 years. Eur J Anaesthesiol. 2015;[in press].
18 Wanderer JP, Ehrenfeld JM, Epstein RH, et al. Temporal trends and current practice patterns for intraoperative ventilation at U.S. academic medical centers: a retrospective study. BMC Anesthesiol. 2015;15:40.
19 Chaney MA, Nikolov MP, Blakeman BP, Bakhos M. Protective Ventilation Attenuates Postoperative Pulmonary Dysfunction in Patients Undergoing Cardiopulmonary Bypass. J Cardiothorac Vasc Anesth. 2000;14:514–8.
20 Michelet P, D’Journo XB, Roch A, Doddoli C, Marin V, Papazian L, et al. Protective ventilation influences systemic inflammation after esophagectomy: a randomized controlled study. Anesthesiology. 2006;105:911–9.
21 Zupancich E, Paparella D, Turani F, Munch C, Rossi A, Massaccesi S, et al. Mechanical ventilation affects inflammatory mediators in patients undergoing cardiopulmonary bypass for cardiac surgery: A randomized clinical trial. J Thorac Cardiovasc Surg. 2005;130:378–83.
22 Hemmes SN, Serpa Neto A, Schultz MJ. Intraoperative ventilatory strategies to prevent postoperative pulmonary complications: a meta-analysis. Curr Opin Anaesthesiol. 2013;26:126–33.
23 Severgnini P, Selmo G, Lanza C, et al. Protective mechanical ventilation during general anesthesia for open abdominal surgery improves postoperative pulmonary function. Anesthesiology. 2013;118:1307–21.
24 Futier E, Constantin JM, Paugam-Burtz C, et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med. 2013;369:428–37.
25 Ge Y, Yuan L, Jiang X, Wang X, Xu R, Ma W. Effect of lung protection mechanical ventilation on respiratory function in the elderly undergoing spinal fusion. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2013;38:81–5.
26 Serpa Neto A, Hemmes SNT, Barbas CSV, et al. Protective Versus Conventional Ventilation for Surgery: A systematic review and individual patient data meta-analysis. Anesthesiology. 2015; [in press].
27 Tenney SM, Remmers JE. Comparative quantitative morphology of the mammalian lung: Diffusing area. Nature. 1963;197:54–6.
28 Dreyfuss D, Saumon G. Ventilator-induced Lung Injury – Lessons from experimental studies. Am J Respir Crit Care Med. 1998;157:294–323.
29 Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369:2126–36.
30 PROVE Network Investigators for the Clinical Trial Network of the European Society of Anaesthesiology, Hemmes SN, Gama de Abreu M, Pelosi P, Schultz MJ. High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): a multicentre randomised controlled trial. Lancet. 2014;384:495–503.
31 Hedenstierna G, Edmark L. Mechanisms of atelectasis in the perioperative period. Best Pract Res Clin Anaesthesiol. 2010;24:157–69.
32 Borges JB, Hedenstierna G, Bergman JS, Amato MB, Avenel J, Montmerle-Borgdorff S. First-time imaging of effects of inspired oxygen concentration on regional lung volumes and breathing pattern during hypergravity. Eur J Appl Physiol. 2015;115:353–63.
33 Hanson A, Göthberg S, Nilsson K, Hedenstierna G. Recruitment and PEEP level influences long-time aeration in saline-lavaged piglets: an experimental model. Paediatr Anaesth. 2012;22:1072–9.
34 Blomqvist H, Wickerts CJ, Berg B, Frostell C, Jolin A, Hedenstierna G. Does PEEP facilitate the resolution of extravascular lung water after experimental hydrostatic pulmonary oedema? Eur Respir J. 1991;4:1053–9.
35 Bellardine Black CL, Hoffman AM, Tsai LW, et al. Relationship between dynamic respiratory mechanics and disease heterogeneity in sheep lavage injury. Crit Care Med. 2007;35:870–8.
36 Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–36.
37 Mercat A, Richard JC, Vielle B, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299:646–55.
38 Meade MO, Cook DJ, Guyatt GH, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299:637–45
39 Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 2010;303:865–73.
40 Suzumura EA, Figueiró M, Normilio-Silva K, et al. Effects of alveolar recruitment maneuvers on clinical outcomes in patients with acute respiratory distress syndrome: a systematic review and meta-analysis. Intensive Care Med. 2014;40:1227–40.
41 Manzano F, Fernández-Mondéjar E, Colmenero M, et al. Positive-end expiratory pressure reduces incidence of ventilator-associated pneumonia in nonhypoxemic patients. Crit Care Med. 2008;36:2225–31.
42 Pepe PE, Hudson LD, Carrico CJ. Early application of positive end-expiratory pressure in patients at risk for the adult respiratory-distress syndrome. N Engl J Med. 1984;311:281–6.
43 Esteban A, Anzueto A, Frutos F, et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA. 2002;287:345–55.
44 Esteban A, Ferguson ND, Meade MO, et al. Evolution of mechanical ventilation in response to clinical research. Am J Respir Crit Care Med. 2008;177:170–7.
45 Esteban A, Frutos-Vivar F, Muriel A, et al. Evolution of mortality over time in patients receiving mechanical ventilation. Am J Respir Crit Care Med. 2013;188:220–30.
46 Levin MA, McCormick PJ, Lin HM, Hosseinian L, Fischer GW. Low intraoperative tidal volume ventilation with minimal PEEP is associated with increased mortality. Br J Anaesth. 2014;113:97–108.
47 Futier E, Constantin JM, Petit A, et al. Positive end-expiratory pressure improves end-expiratory lung volume but not oxygenation after induction of anaesthesia. Eur J Anaesthesiol. 2010;27:508–13.
48 Neumann P, Rothen HU, Berglund JE, Valtysson J, Magnusson A, Hedenstierna G. Positive end-expiratory pressure prevents atelectasis during general anaesthesia even in the presence of a high inspired oxygen concentration. Acta Anaesthesiol Scand. 1999;43:295–301.
49 Maisch S, Reissmann H, Fuellekrug B, et al. Compliance and dead space fraction indicate an optimal level of positive end-expiratory pressure after recruitment in anesthetized patients. Anesth Analg. 2008;106:175–81.
50 Schultz MJ, Haitsma JJ, Slutsky AS, Gajic O. What tidal volumes should be used in patients without acute lung injury? Anesthesiology. 2007;106:1226–31.
51 de Graaff AE, Dongelmans DA, Binnekade JM, de Jonge E. Clinicians’ response to hyperoxia in ventilated patients in a Dutch ICU depends on the level of FiO2. Intensive Care Med. 2011;37:46–51.
52 Hovaguimian F, Lysakowski C, Elia N, Tramèr MR. Effect of intraoperative high inspired oxygen fraction on surgical site infection, postoperative nausea and vomiting, and pulmonary function: systematic review and meta-analysis of randomized controlled trials. Anesthesiology. 2013;119:303–16.
53 Togioka B, Galvagno S, Sumida S, Murphy J, Ouanes JP, Wu C. The role of perioperative high inspired oxygen therapy in reducing surgical site infection: a meta-analysis. Anesth Analg. 2012;114:334–42.
54 Bhandari V, Elias JA: Cytokines in tolerance to hyperoxia-induced injury in the developing and adult lung. Free Radic Biol Med. 2006;41:4–18.
55 Aggarwal NR, Brower RG. Targeting normoxemia in acute respiratory distress syndrome may cause worse short-term outcomes because of oxygen toxicity. Ann Am Thorac Soc 2014;11:1449–53.
56 Kallet RH, Matthay MA. Hyperoxic acute lung injury. Respir Care 2013;58:123–40.
57 de Jonge E, Peelen L, Keijzers PJ, et al. Association between administered oxygen, arterial partial oxygen pressure and mortality in mechanically ventilated intensive care unit patients. Crit Care. 2008;12:R156.
58 Altemeier WA, Sinclair SE. Hyperoxia in the intensive care unit: why more is not always better. Curr Opin Crit Care. 2007;13:73–8.
59 Kilgannon JH, Jones AE, Shapiro NI, et al. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303:2165–71.
60 Cornet AD, Kooter AJ, Peters MJ, Smulders YM. Supplemental oxygen therapy in medical emergencies: more harm than benefit? Arch Intern Med. 2012;172:289–90.
61 Davis DP, Meade W, Sise MJ, et al. Both hypoxemia and extreme hyperoxemia may be detrimental in patients with severe traumatic brain injury. J Neurotrauma. 2009;26:2217–23.
62 Damiani E, Adrario E, Girardis M, et al. Arterial hyperoxia and mortality in critically ill patients: a systematic review and meta-analysis. Crit Care. 2014;18:711.
63 Helmerhorst HJ, Roos-Blom MJ, van Westerloo DJ, de Jonge E. Association Between Arterial Hyperoxia and Outcome in Subsets of Critical Illness: A Systematic Review, Metaanalysis, and Meta-Regression of Cohort Studies. Crit Care Med. 2015;43:1508–19.
64 Staehr-Rye AK, Eikermann M. Eliminate postoperative respiratory complications: preoperative screening opens the door to clinical pathways that individualise perioperative treatment. Eur J Anaesthesiol. 2015;32:455–7.
65 Canet J, Sabaté S, Mazo Valentín, et al. Development and validation of a score to predict postoperative respiratory failure in a multicentre European cohort: A prospective, observational study. Eur J Anaesthesiol. 2015;32:458–70.
66 Technische Universität Dresden. Protective Ventilation With Higher Versus Lower PEEP During General Anesthesia for Surgery in Obese Patients (PROBESE). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000- [cited 2015 Apr 17]. Available from: http://clinicaltrials.gov/show/NCT02148692 NLM Identifier: NCT02148692.
67 Assistance Publique – Hôpitaux de Paris. Pulmonary Surgery and Protective Mechanical Ventilation (VPP). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000- [cited 2015 Apr 17]. Available from: http://clinicaltrials.gov/show/NCT00805077 NLM Identifier: NCT00805077.
68 Schultz MJ (2015, April 17). Protective ventilation during thoracic surgery (PROTHORAX). Retrieved from: https://sites.google.com/site/proveneteu/provenet-studies
