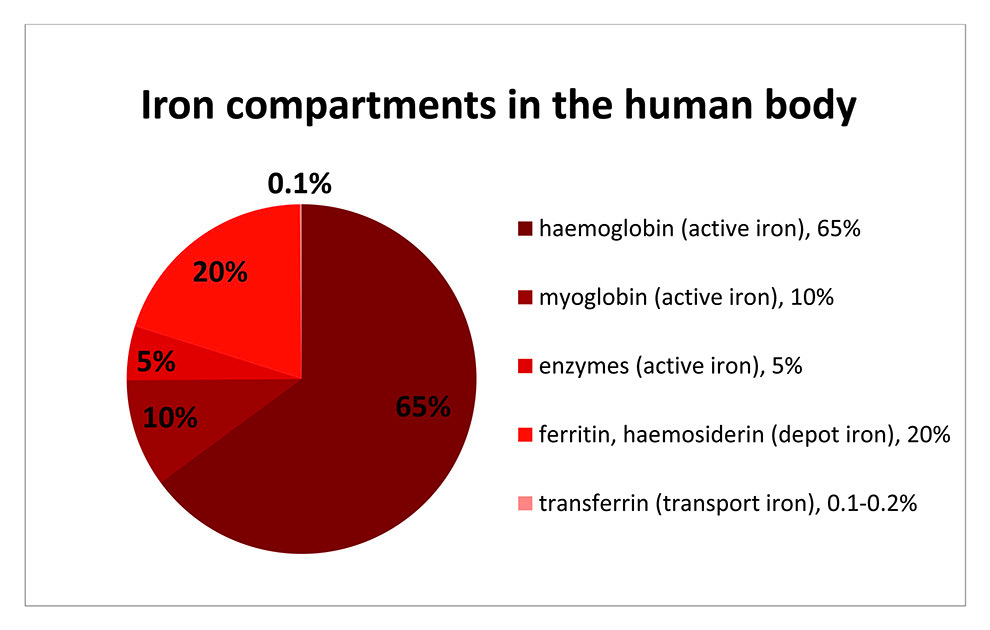
Figure 1
Iron compartments.
DOI: https://doi.org/10.4414/smw.2015.14196
Consensus statement of the Swiss Society of Sports Medicine
Iron deficiency among athletes, in males and more often in females, is a commonly encountered condition for the sports medicine physician. Iron deficiency is one of the most common deficits globally with a clear predominance in adolescence and in menstruating females [1]. Data from a general Swiss population show frequencies for iron deficiency for menstruating females of 22.7%, for male military recruits of 7.2% and for iron deficiency anaemia of 2.2% (females) and 0.1% (males) [2, 3]. In sports the rate of iron deficiency is distinctly higher, up to 52% in female adolescent athletes, and occurs more often in endurance sports and in disciplines with a high prevalence of eating disorders [4–6]. These abnormal findings need a careful clinical look as iron deficiency affects many organ systems of the body and not just oxygen transport, especially in sports [7].
On the other hand, Switzerland has recently experienced some kind of “iron hype” for various reasons. Not all of them seem to be rational [8, 9]. The problem is enhanced by the finding of an earlier study among Swiss top athletes, showing that iron supplements were consumed to some extent uncritically and in excess [10, 11].
Sports medicine physicians are often in charge of male and female athletes at their peak performance. In this role, they are aware of the importance of adequately diagnosing and treating iron deficiency in all its aspects, but at the same time to prevent iron overload. This article is a consensus statement of the Swiss Society of Sports Medicine and provides an overview and practical guidelines for the diagnosis and treatment of iron deficiency in sports and should help clinicians in decision making.
Iron is a transition metal and has multiple functions in more than 180 biochemical reactions in the human body including electron transport in redox reactions (cytochromes, sulphuric proteins), redox catalytic functions (cytochrome p450, catalase, peroxidase) and reversible storage and transport of O2 (haemoglobin, myoglobin). It also plays an important role in the production of neurotransmitters, and is essential in synaptogenesis and myelinisation. Moreover oxidative phosphorylation is the most critical biochemical pathway in which iron is involved [8, 12].

Figure 1
Iron compartments.
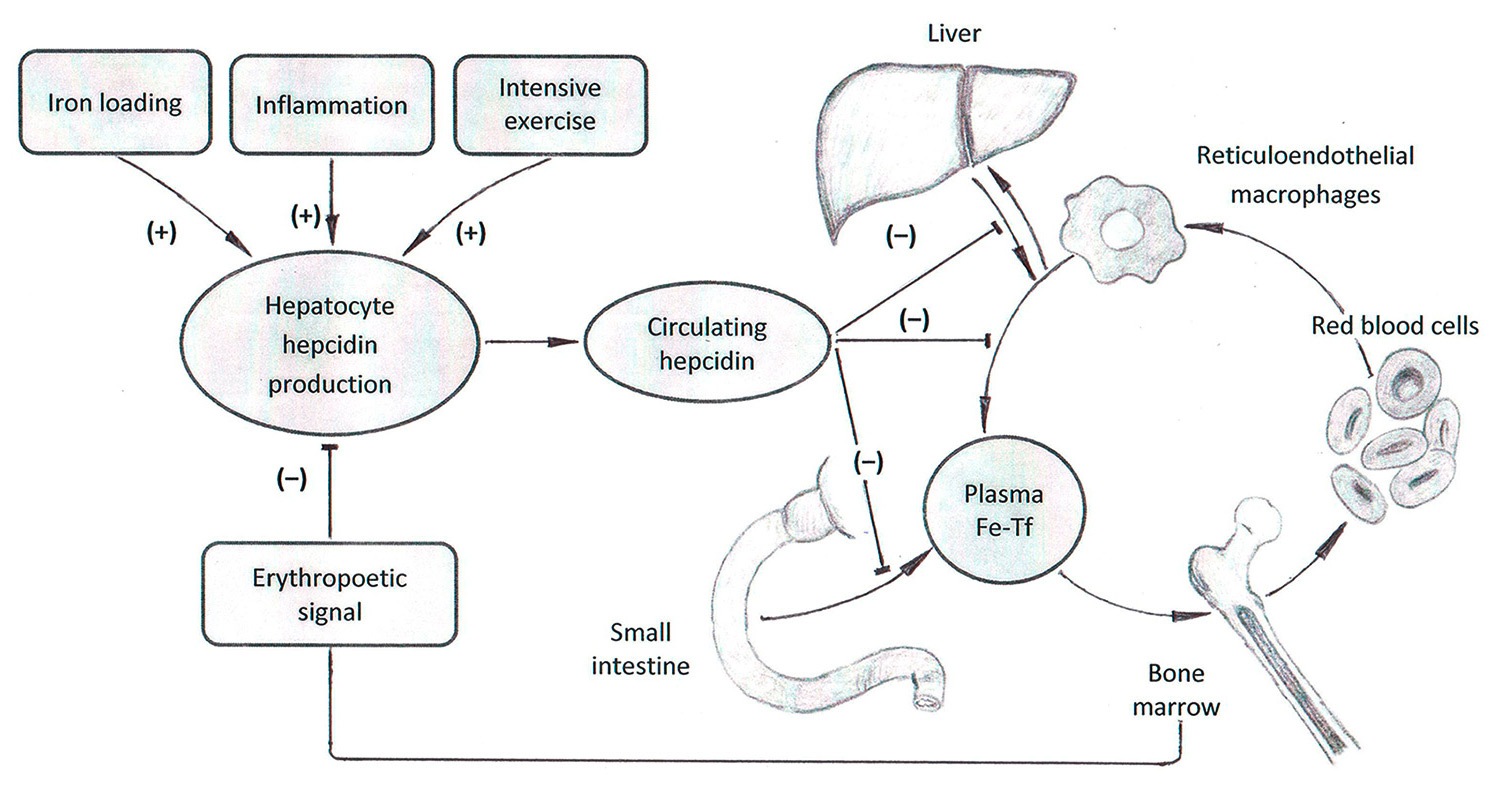
Figure 2
Hepcidin effects, adapted after Young and Zaritsky. Republished with permission of Americal Society of Nephrology, from Young B, Zaritsky J. Hepcidin for clinicians. Clin J Am Soc Nephrol. 2009;4:1384–7; permission conveyed through Copyright Clearance Center, Inc.
Figure 1 gives an overview of iron distribution in the human body. The total body content of iron amounts to approximately 4 g in men and 2.5 g in women. This iron is divided up between three active sites, firstly haemoglobin, myoglobin and enzymes. The rest (20% of total) remains as inactive, depot iron in the form of ferritin and haemosiderin. Finally, 0.2% of the total iron exists as transport iron in the form of transferrin. In adolescents the relative amount of iron in the different compartments is comparable but may vary slightly depending on body size and initiation of menses (fig. 1) [8].
The usual loss of iron (1 mg per day in males and 2 mg per day in females) due to gastrointestinal epithelial shedding and menstruation is compensated by absorption in the small intestine [13, 14]. Of the ingested iron of 10–14 mg, the enterocytes absorb only about 0.5–2 mg (5–15%) [14, 15]. Nevertheless, during increased losses (e.g. microischaemia in sports, bleeding, haemolysis) and elevated demand (e.g. growth with the building up of the adult haemoglobin mass, pregnancy), adequate uptake is guaranteed through an up to four-fold elevated intestinal absorption as long as sufficient iron is provided by nutritional intake [14].
The absorbed iron is stored in ferritin in the cytoplasm of the enterocytes [12]. To export the iron to the plasma, the iron is carried out by ferroportin on the basolateral surface of the enterocytes. There, iron is bound to transferrin and transported to the liver where it is stored as ferritin or transferred to the iron consuming tissues e.g. bone marrow.
Ferroportin is important in the tight regulation of iron homeostasis. The main regulatory constituent is hepcidin [16]. Synthesised in hepatocytes, hepcidin regulates iron export out of the storing cells. In phases of high iron loading and in response to inflammatory processes the synthesis of hepcidin is increased, leading to the internalisation of ferroportin on enterocytes, which in turn blocks iron transportation into the circulation. The same mechanism leads to a blockade of iron within the macrophage system, thus preventing the transfer of iron from macrophages to erythroblasts, the precursors of erythrocytes [12] (fig. 2).
Any type of exercise will also cause some kind of inflammation in the body, as this inflammation and the subsequent repair mechanisms are the basis of adaptation to training. The size of the inflammatory response depends on the type, intensity and duration of the training. Several markers of iron metabolism are affected by the inflammatory cascade, as they are part of the acute-phase response [18]. Concerning iron metabolism, intensive training has been shown to lead to distinct increases in hepcidin [5, 19, 20]. This leads to a block of iron absorption, disruption of iron transfer from macrophages to erythroblasts and may possibly induce iron deficiency. Hepcidin synthesis is, on the other hand, suppressed by erythropoetic activity and anaemia. This allows increased intestinal absorption and utilisation of iron from the macrophages and hepatocytes under conditions of elevated iron loss or increased demand [21, 22].
Up to now the main mechanism by which sport causes an increase of iron loss was explained by microischaemia of the gut during excessive training [23, 24]. Losses through excessive sweating [25] and possible blood loss in the urinary tract [26] are in absolute terms not relevant. Foot-strike haemolysis, which describes the mechanical destruction of red blood cells in the foot of the running athlete does exist [27, 28], but as the salvage of blood constituents is complete there is no loss of iron. Newer results show that there is the additional mechanism explained above where participation in intensive sport triggers hepcidin bursts, causing blockage of iron [20].
Figure 3 describes the different stages of progressive iron deficiency: When iron losses exceed absorption or absorption falls below demand, initially iron stores will deplete, resulting in a reduced ferritin level. At a certain point, the stored iron is too low to provide the tissues with sufficient iron. This will induce the production of zinc-protoporphyrin (ZnPP) and an increase of soluble transferrin receptor (sTfR). As haemoglobin, mean cellular volume (MCV), and mean cellular haemoglobin (MCH) are still normal, this condition is called nonanaemic iron deficiency (NAID). NAID is defined as a deficiency of iron without affecting haematopoiesis.
If iron balance remains negative, the youngest red cells will be insufficiently haemoglobinised and thus appear as hypochromic and microcytic, with lower MCH and MCV than the entire cell population. If progression of iron deficiency continues, MCH and MCV will drop below the lower limit of the normal range (28 pg and 80 fl, respectively) and iron deficiency with microcytosis and/or hypochromia (IDMH) develops. IDMH is defined as an iron deficiency affecting haematopoiesis. In this case, ferritin is <30 mcg/l, the red cell indices are mostly, but not always, affected and the concentration of haemoglobin is still normal (men >140 g/l, women >120 g/l) [29].
Ultimately, haemoglobin concentrations will drop below the lower limit of the normal range and frank iron deficient anaemia (IDA) is established [29]. In IDA ferritin and haemoglobin are lowered and the red cell indices are reduced or normal [29].
A further particular situation is the functional iron deficiency encountered in patients with anaemia of chronic disorder, or tumour or haemodialysis patients. The iron demand is higher than the iron supply out of the iron stores. In consequence hypochromic reticulocytes and erythrocytes are formed, the haemoglobin in reticulocytes (CHr or Ret-He, table 1) falls <28 pg [30, 31]. This situation is a result of elevated interleukin-6 and hepcidin levels seriously impairing iron turnover and may therefore occur with normal or even elevated ferritin values, reflecting normal iron stores [21, 31].
The basic measurements are shown in figure 4: these are haemoglobin, haematocrit, and erythrocyte count, with calculation or measurement of the red cell indices MCV and MCH. Table 1 gives an overview and explains cut off values of relevant tests to diagnose NAID, IDMH and IDA.
Ferritin is the most widely used parameter in the evaluation of iron deficiency. Since ferritin also acts as an acute phase protein each inflammatory process should be excluded by the patient history. Additionally, it should be considered that even heavy/powerful sporting activities might increase acute phase reactants [37, 40–42]. Depending on duration and intensity of the activity, ferritin values may stay normal, show a rise of 27% [40] with a return to baseline within a day or, in the case of ultramarathons, be double as high as the prerace value and return to baseline only after 6 days [42, 43].
Free serum iron has a high daytime and high variability between one person and another. Morning values are at a peak more than twice as high as values measured 12 hours later, so it cannot be used to represent iron in the body. Furthermore, free serum iron is lowered with acute phase reactions and elevated in cases of haemolysis after blood sampling. Nowadays it is an obsolete marker and should be used only to calculate transferrin saturation or in situations of acute iron intoxication [29]. Transferrin saturation has some benefit for classification of iron deficiency. A cut off of 20% and below is accepted as definition of iron deficiency [29]. As free serum iron is needed for its calculation, one must be aware of this limitation, particularly in inflammation where transferrin saturation may be between 10 and 20% without iron deficiency.
The indirect markers ZnPP [38, 39] and sTfR [29, 37] may be additionally useful to define IDMH and differentiate NAID from a functional iron deficiency. The sTfR was thought to be an excellent parameter because it is not influenced by inflammation and exercise. But it is mostly elevated during increased erythropoesis, so it may be of only additional value. ZnPP is reentering routine testing now and gives additional information to judge the iron status of erythropoesis.
Hepcidin has been established as a key regulator of iron absorption from the enterocytes and release from the macrophages; however, it has not yet entered routine testing [16, 21].
| Table 1: Relevant tests to define anemia, NAID, IDMH and IDA. | ||
| Parameter | Cut-off | Comment/Cave |
| Hb | Women: >120 g/l Men: >140 g/l | Defines diagnosis of anaemia |
| MCH | >28 pg | Categorises anaemia |
| MCV | >80 fl | Categorises anaemia (as a result of a certain instability after blood sampling, less useful than MCH) |
| %HYPO Percentage of hypochromic erythrocytes [32, 33] | %HYPO <10 | Indicates iron deficiency and its influence on haematopoiesis at an earlier stage, particularly IDMH. Iron deficiency, affecting erythropoiesis, is followed by an increase in %HYPO within 1–2 weeks. In patients with a low ferritin, means empty iron stores, a normal %HYPO would indicate that the erythropoiesis is not yet affected. Limitation: availability of the method |
| Reticulocyte count, absolute | 20–100 (×109/l) Reference values of the manufacturer need to be respected. | Assesses red blood cell production |
| Reticulocyte indices [30, 31, 34, 35] | MCVr, 92–120 fl Mean cellular volume of reticulocytes in fl CHr 28–35 pg [31] Amount of Hb in reticulocytes (measured in Advia 120) Ret-He 28–35 pg [36] Amount of Hb in reticulocytes (measured in Sysmex NE 2100) | A lowering of the MCVr and even more specificly the CHr and Ret-He are very early indicators of the iron demand of erythropoiesis. CHr or Ret-He react quickly within 48–72 h to an increased demand or lowered supply, compared with, e.g., MCV and MCH which react only within weeks. Limitation: availability of the method |
| Ferritin | 30 mcg/l | The most widely used parameter for IDA. Limitations: as an acute phase protein, ferritin is increased during inflammation and infection, after intensive exercise, in pregnancy and with liver damage, see also CRP |
| CRP | <3 mg/l | Acute phase protein, indicating infection and inflammation |
| Transferrin saturation | >20% | <20% indicates an iron deficiency Limitations: acute phase reactions lower the transferrin saturation without iron deficiency. Moreover, as free serum iron is used for the calculation, transferrin saturation may also vary. |
| sTfR | Reference values of the manufacturer need to be respected, as they differ substantially! Woman: 0.75–1.5 mg/l Men: 0.75–1.75 mg/l | Indirect marker to define IDMH and NAID. Similar sensitivity as ZnPP for NAID and IDMH. Not influenced by inflammation and exercise [ 37] |
| ZnPP [38, 39] | <50 mcmol/mol Hb excludes iron deficient erythropoiesis, >100 mcmol/mol Hb indicates iron deficient erythropoiesis | Indirect marker to define IDMH and NAID. Early marker of NAID and increases to higher values in IDMH [29]. Not as much influenced by inflammation as ferritin |
| Hepcidin | Not entered routine laboratory testing yet | Key regulator of iron absorption from erythrocytes [21] Elevation impairs haematopoiesis [14, 22] |
| CRP = C-reactive protein; Hb = haemoglobin; IDA =iron deficiency with anaemia; IDMH = iron deficiency with microcytosis and/or hypochromia; MCH = mean cellular haemoglobin; MCV = mean cellular volume; NAID = nonanaemic iron deficiency; sTfR = solublle transferrin receptor; ZnPP = zinc protoporphyrin | ||
| Table 2: Randomised blinded interventional trials investigating NAID and performance. | |||||
| First author | Performance measurement | n / Study population | Inclusion criteria | Intervention | Results |
| Burden 2014 [63] | VO2 max Time to exhaustion Running economy Haemoglobin mass | 15 Runners 9 F, VO2 max 64.5 ml/kg·min 6 M VO2 max 76.7 ml/kgmin | Females: Hb >120 g/l Fer <30 mcg/l Males: Hb >120 g/l Fer <40 mcg/l | i.v. 500 mg Fe-carboxymaltose vs placebo Testing at BL, after 7 days and after 4 wk Blood sampling as above and one day after injection | – Fer ↑, traSat ↑, serum iron ↑ in Tx, Fer → in P – Hepcidin ↑ from day 1 up to four weeks in Tx – Hb mass →, VO2 max →, running economy →, time to exhaustion → in Tx and P |
| Garvican 2014 [64] | Treadmill running with VO2 max, time to exhaustion Haemoglobin mass | 27 Highly trained distance runners 13 M, 14 F 4 randomised treatments: LG oral, LG i.v., CG oral, CG i.v. | Low group (LG) Fer <35 mcg/l and trSat <20% or Fer <15 mcg/l Control group (CG) Fer <65 mcg/l | Oral: 105 g elemental iron 2×/d in LG; 1×/d in CG i.v.: 2–4 injections Fe-carboxymaltose due to iron status (mean i.v. CG 375 – mean i.v. LG 550 mg) BL testing and after 6 wk | – Fer ↑ with oral and i.v. treatment, but significantly ↑ with i.v. – Hb → in all groups – In LG i.v.: Hb mass ↑, VO2 max ↑ and time to exhaustion ↑ Authors named CG SUB group, having a suboptimal iron status. But there was no change in Hb mass, VO2 max and time to exhaustion in this group. |
| Della-Valle 2014 [65] | 4 km time trial VO2 peak | 40 Female rowers At the beginning of a season! | Hb >120 g/l Fer <20 mcg/l | Oral 100 mg FeSO4/d vs placebo for 6 wk Testing at BL and after 6 wk of training | – Fat free mass ↑ and VO2 peak ↑ in Tx and P – Fer ↑ in Tx – Lower lactate response during first half of time trial and after 5 min recovery in Tx – Energy expenditure in Tx ↑, lactate response ↓ in first half of time trial in Tx, and 5 min after time trial↓ |
| Waldvogel 2012 [66] | Chester step test (r = 0.92 to VO2 max) Fatigue (VAS 10) | 154 Female blood donors | Hb >120 g/l Fer ≤30 mcg/l | Oral 80 mg FeSO4/d vs placebo for 4 wk BL (1 wk after donation of 450 ml blood) and after 4 wk. | In Tx Hb ↑, Fer ↑ compared with P No significant effect for fatigue, aerobic capacity ( step test), mood disorder, quality of life |
| McClung 2009 [67] | 2 mile running time Profile of mood state | 219 Female soldiers during basic combat training Three groups: IDA (P 17 / Tx 18) ID (P 14 / Tx 14) Normal (P 51 / Tx 52) | No inclusion criteria. IDA defined as: Hb <120 g/l and ≥2 of: Fer <12 mcg/l, traSat <16%, RDW >15% ID defined as ≥2 of iron crit. | Oral 100 mg FeSO4/d vs placebo Testing at BL and after 8 wk of basic combat training | – RDW ↑, sTfr↑, Fer↓ in Tx and P. – Decrement in iron status ↓ in Tx – In soldiers with IDA and Tx – 2 mile running time↓ but not in P and Normal, – POMS↑ in Tx, P and Normal, but only in IDA vigour scores of POMS↑ |
| Hinton 2007 [68] | VO2 max 60 min submax. cycle ergometer test (at 60% VO2 max) | 20 Recreationally trained (3 M, 17 F) | Hb >120 g/l F Hb >130 g/l M, Fer <16 mcg/l, sTfr >8 mg/l, or sTfr / log Fer index >4.5 | Oral 30 mg elemental iron as FeSO4/d vs placebo Testing at BL and after 6 wk | – Fer↑ in Tx, Hb and haematocrit → – In P ventilatory threshold↓, in Tx ventilatory threshold→ – Energetic efficiency during submaximal tes ↑ in Tx |
| Brownlie 2004 [69] | 15 km time trial on cycle ergometer VO2 max | 41 Untrained women Training of 30 min/d, 5×/wk for the final 4 wk | Hb >120 g/dl, Fer <16 mcg/l | Oral 100 mg FeSO4/d vs placebo Testing at BL and after 6 wk | Time in time trial↓, percentage of VO2 max ↓ , work rate ↓ in Tx when sTfr >8 mg/l No difference with normal sTfr |
| Brownlie 2002 [70] | VO2 max | 41 Untrained women Training of 30 min/d, 5×/wk for the final 4wk | Hb >120 g/dl, Fer <16 mcg/l | Oral 50 mg FeSO4/d vs placebo for 6wk Testing at BL and after 6 wk | – Fer ↑, traSat ↑ in Tx – VO2 max ↑in Tx and P, but VO2 max was significantly higher in Txr |
| Friedman 2001 [71] | Treadmill VO2 max Anaerobic capacity (O2 consumption) Haemoglobin mass | 40 Young elite athletes | Hb >135 g/l M, Hb >117 g/l F Fer <20 mcg/l | Oral 200 mg elemental iron/d vs placebo for 12 wk Testing at BL and after 12 wk | – Fer ↑ in Tx, whereas P → Venous Hb and Hb mass → in Tx and P VO2 max ↑ and O2 consumption ↑ inTx |
| Hinton 2000 [72] | 15 km time trial on cycle ergometer | 42 Women Training of 30 min/d, 5×/wk for the final 4 wk | Hb >120 g/l Fer <16 mcg/l | Oral 100 mg FeSO4/d vs placebo for 6 wk Testing at BL and after 6 wk | – Fer ↑ and Hb ↑ in Tx – 15 km time ↓ in Tx and P, but significantly ↓ in Tx (p = 0.04) |
| ↑ = increased significantly; → = no change; ↓ = lowered significantly; BL = baseline; CG = control group; F = female; Fer = ferritin; Hb = haemoglobin; ID = iron deficiency; IDA = iron deficiency anaemia; LG = low group; M = male; NAID = nonanaemic iron deficiency; P = placebo group; POMS = profile of mood state; RDW = red cell distribution width; SUB = group with suboptimal iron status; traSat = transferrin saturation; Tx = treatment group; VAS = visual analogue scale | |||||
Anaemia is defined by a lowered haemoglobin concentration in a venous blood sample. This definition neglects the fact that anaemia is the real reduction of the total haemoglobin mass in the body, a so-called absolute anaemia (see fig. 4). In contrast, relative or dilutional pseudoanaemia is defined as a lowered haemoglobin and haematocrit owing to a distinctly elevated plasma volume with normal red cell mass and total haemoglobin mass. The other blood parameters, particularly ferritin, MCV and MCH are within the normal range and performance is not affected. This plasma volume increase is considerable and is due to repeated bouts of physical training over the years. It magnitude depends on the intensity and duration of the effort. In about 10–15% of mainly endurance athletes, especially when training time exceeds 10 hours per week, this dilutional pseudoanaemia can be observed [44]. The same dilutional effect is found in ultralong endurance disciplines or sports events lasting several days (e.g. road cycling tours). As seen in figure 5, in male and female athletes with dilutional pseudoanaemia the increase in plasma volume causes a slight lowering of the haematocrit while the red cell mass stays high [42, 45–48].
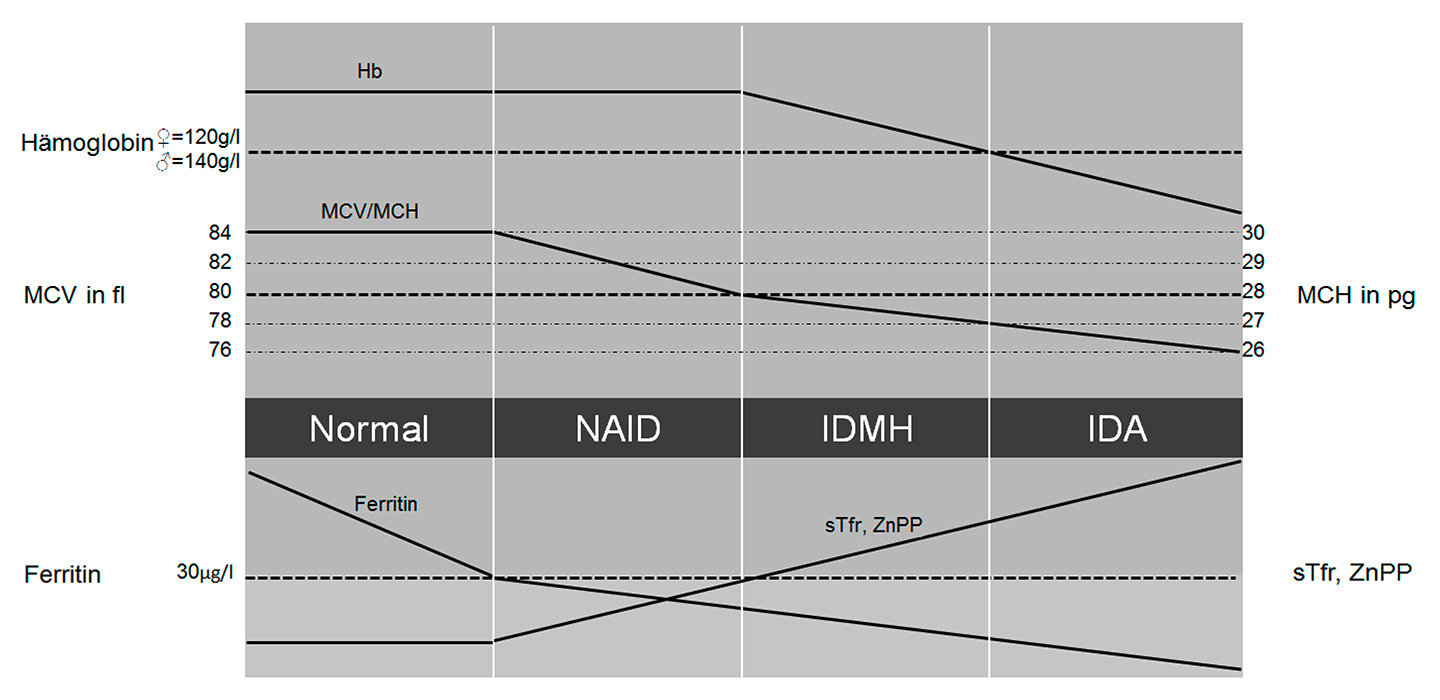
Figure 3
Stages of iron deficiency.
Hb = haemoglobin; IDA = iron deficiency anaemia; IDMH = iron deficiency with microcytosis and/or hypochromia; MCH = mean cellular haemoglobin; MCV = mean cellular volume; NAID = nonanaemic iron deficiency; sTfr = soluble transferrin receptor; ZnPP = zinc protoporphyrin
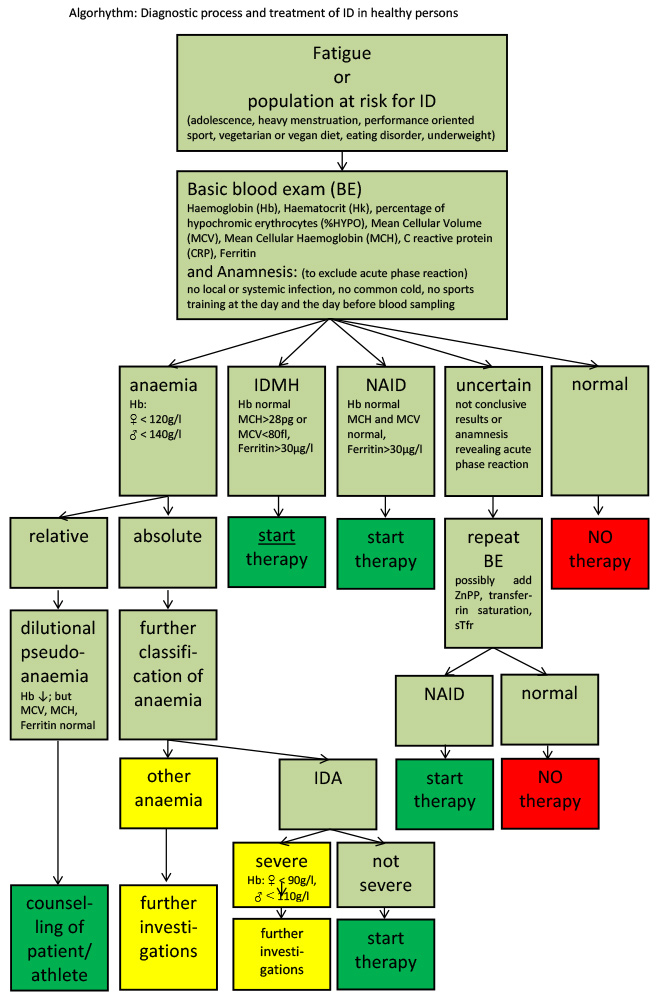
Figure 4
Overview of diagnosis and indications for treatment in adults.
CRP = C-reactive protein; Hb = haemoglobin; Hk = haematocrit;IDA = iron deficiency with anaemia; IDMH =iron deficiency with microcytosis and/or hypochromia; MCH = mean cellular haemoglobin; MCV = mean cellular volume; NAID = nonanaemic iron deficiency; ; ZnPP = zinc protoporphyrin
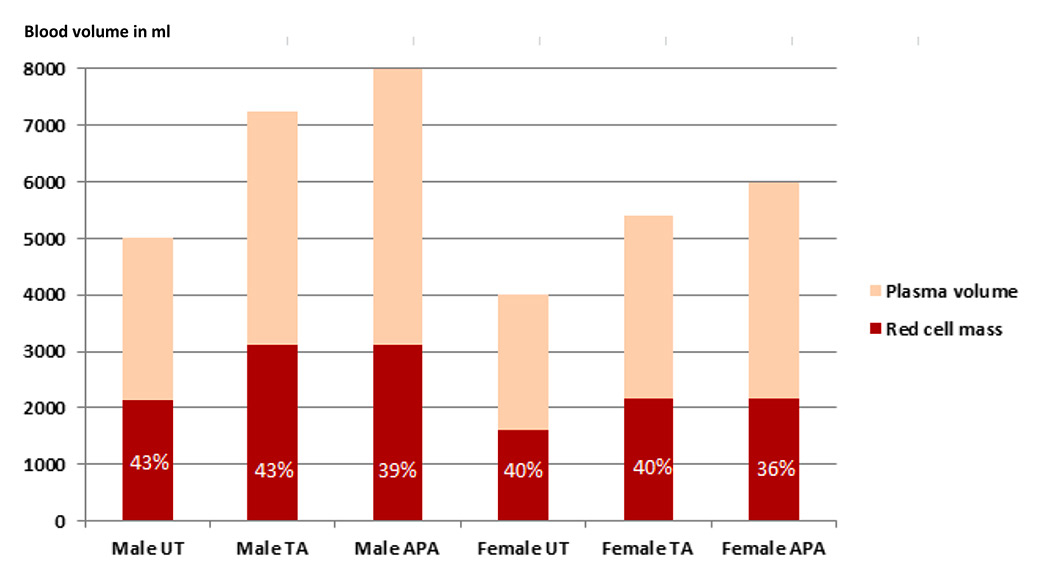
Figure 5
Total blood volume of male and female untrained, top athletes and athletes with dilutional pseudoanaemia in ml [44, 46, 49].
APA = athlete with dilutional pseudoanaemia; TA = top athlete; UT = untrained In the column of the red cell mass the haematocrit in percentage is shown in white letters.
This is why top level endurance athletes show a high correlation between haemoglobin mass and endurance capacity, but a low correlation between haematocrit or haemoglobin concentration and endurance capacity [49, 50]. Therefore, the haemoglobin mass is the important factor for endurance capacity, while having a high plasma volume accompanied by a low haematocrit is by no means a disadvantage [51–53]. A larger intravascular volume has beneficial effects on performance, as it improves thermoregulation, increases cardiac preload and enhances the body´s ability to dilute metabolites. The exact mechanism for this adaptation remains to be explored, and shifts in proteins and electrolytes are thought to play a role [54]. Exercise induced plasma volume shifts are usually reversible within days after cessation of the physical effort [40].
A dilutional pseudoanaemia cannot be ruled out by a venous blood examination only, but in clinical practice serial measurements of the basic blood examination are helpful. These include measurements during the training pause at the end of the season (haematocrit, Hk↑), during the season (Hk↓), once immediately after an intensive and long lasting training session (Hk↑) and 24–48 h after this session (Hk↓). If these serial measurements do not reveal the expected variations of the haematocrit (showing at least normal values in the training pause at season’s end) the measurement of haemoglobin mass to confirm the dilutional pseudoanaemia may be considered with, for example, the CO rebreathing method [48–50].
Unfortunately, top level road cyclists 15 years ago often showed excessive haematocrit levels of over 50%, exceptionally up to 60%. Based on the confessions of several riders, these values could only be reached by mostly systematic doping procedures with mainly erythropoietin, growth hormone and testosterone. During longer lasting competitions blood transfusions (retransfusion of own blood, collected several months before) [55] were given to boost oxygen carrying capacity. Through awareness of these doping procedures, health control tests with blood sampling were introduced, with upper limits of the haematocrit (e.g. the 50% rule of the Union de Cyclisme International [56]) or haemoglobin concentration (e.g. 170 g/l for males, 160 g/l for females of the Fédération International de Ski [57]) to protect athletes from fatal thrombotic events. These were followed by newer doping tests (e.g. EPO-tests, growth hormone) and antidoping strategies such as the biological passport [58]. Several blood samples collected over the year provide an individual blood profile and allow individual reference values to be established for each athlete [59]. This biological passport proposed by the WADA (World antidoping agency) gives a much closer follow-up and is used in cases of violation of the antidoping code [58].
Iron is, in addition to its function in oxygen transport, a key component of the enzymatic system of the respiratory chain. These presumably distinct roles were investigated in early animal studies [60–62]. In these studies, the authors aimed at differentiating between a decline in performance due to anaemia and a decline due to enzymatic impairment. Using an animal model in a cross-over setting with iron depletion and cross-over transfusion to correct for anaemia, it was shown that not only anaemia but also iron depletion without anaemia led to a significantly decreased number of mitochondria and reduced activity of the respiratory enzymes. The authors therefore postulated that iron depletion without anaemia affected oxidative capacity, whereas anaemia affected mostly oxygen transport [60–62].
At present the cut-off for ferritin is still debated with values ranging from 15 mcg/l from the World Health Organization [73] to 16–32 mcg/l [74, 75] in studies that used the “gold standard procedure of bone marrow staining”. It has to be noted that studies investigating this matter were not all conclusive and some presented controversial results [76, 77].
If not only erythropoiesis but also clinical symptoms of an iron deficiency such as fatigue (but not yet performance) are considered, the cut-off may be slightly higher. Recently Krayenbuehl et al. showed in their double-blind randomised study with intravenous iron administration in nonanaemic premenopausal women with low ferritin and fatigue an improvement in mood state in the ferritin group <15 mcg/l [78]. Earlier, Verdon et al. analysed fatigue and mood state with oral therapy in a family doctor’s office setting. They found that nonanaemic women with ferritin concentrations below 50 mcg/l improved with oral supplementation [79]. Vaucher et al came to comparable results [80]. But their proposed cut-off of 50 mcg/l must be interpreted with caution because of methodological concerns (e.g. assessment of stool coloration by iron not blinded, definition of iron deficiency based on a limited number of variables).
A few studies have integrated a performance measure (table 2). McClung et al showed in female military recruits treated with oral iron or placebo an improvement in running performance in recruits with IDA. Those with NAID showed an improved mood state, but no change in performance. Since then, several other studies have shown a performance impairment in nonanaemic iron-depleted endurance athletes [68–70, 72, 81]. A limiting factor of all these studies is the influence of training or mild changes in haematological parameters of other origin, both of which are difficult to quantify. Garvican et al very recently performed a study with 27 highly trained distance runners (13 male and 14 female athletes) with measurement of haemoglobin mass and endurance performance. They compared in a randomised controlled setting intravenous iron versus 6 weeks of oral treatment with one group having clearly deficient iron stores (ferritin <15 mcg/l or ferritin <35 mcg/l and transferrin saturation <20%) and the other with better iron stores but a ferritin level of <65 mcg/l. Both forms of supplementation substantially increased ferritin levels, but haemoglobin concentration did not change in any group. Haemoglobin mass increased in the iron deficiency group with intravenous treatment accompanied by an increase in VO2max and run time to exhaustion, whereas the group with ferritin <65 mcg/l did not show any changes in these parameters [53, 64].
As multiple muscle enzymes are affected other functions related to performance have been investigated. Brutsaert et al. found an increase in knee extensor strength after iron supplementation in nonanaemic iron-depleted volunteers using a randomised controlled study design, which points towards a role of iron in resistance to fatigue and adaptation to training [82]. Very recently Della Valle et al investigated female rowers with NAID under oral iron supplementation for 6 weeks in a double-blind design. They were able to show an improvement of iron status and a better energetic efficiency in the iron group [65].
Based on the cited results and according to several review articles, a ferritin cut-off of 30 mcg/l in adults seems to be most plausible [8, 83–90].
The first step in therapy of iron deficiency is the correction of the nutritional iron intake. In nutrition, haem iron (mainly in meat) and free iron as Fe2+ or Fe3+ exist. Oral uptake studies show that the uptake of haem iron is much better than the uptake of free iron [91]. For the latter, uptake of Fe2+ is better than uptake of Fe3+[92]. Meat, liver, poultry or fish contain haem iron as well as free iron. A vegetarian diet contains only free iron. The bioavailability of iron is very variable and depends much on actual iron stores [93], ranging from 5 to 15%. In the case of iron deficiency, a significant increase in iron bioavailability up to 35% can be observed [94]. Furthermore, iron uptake in the intestinal tract is influenced by different nutritional factors including enhancers and inhibitors. Substances enhancing iron uptake are vitamin C, peptides from partially digested muscle tissue, fermented food, organic acids such as malate or citrate. Substances inhibiting iron uptake are phytates, oxalates, polyphenols (black tea and coffee), peptides from partially digested vegetable proteins and calcium [83, 84]. The nutritional intake should be 14 mg per day. General recommendations for an optimal dietary iron intake in sports include an adequate energy intake, especially for athletes with low body mass index as they suffer more frequently from iron deficiency [95]. Whether catabolism related to low energy intake influences the hepcidin regulation and down-regulates iron uptake remains open to debate. In general, regular consumption of meat, poultry or fish, at least 5 times per week is recommended as it is the main contributor of nutritional iron intake. Complementary eating of wholemeal products and daily legumes and green vegetables is suggested. Furthermore, it is beneficial to replace tea and coffee by a glass of orange or citrus fruit juice with an iron containing meal as vitamin C enhances iron uptake [84, 94]. For vegetarians the goal is to reach a high load of iron through their vegetable diet. Even if nutrition is important in iron homeostasis in the human organism, IDA cannot be corrected by nutrition alone, as this would mean eating kilograms of iron containing products (e.g. liver).
Usually dietary counselling and oral iron therapy are combined. Oral preparations differ in the amount and type of iron (Fe2+or Fe3+), their complex forming substrate and in their galenical form. Novel products combine iron with vitamin C. In a dose-finding study in elderly patients with IDA, Rimon et al compared three doses of oral iron: 15 mg, 50 mg and 150 mg. They were able to show that iron supplementation at a level of the recommended daily allowances (RDA, 15 mg of elemental iron) already led to significant increases of the iron status. In these anaemic elderly patients, the doses of 50 mg and 150 mg of elemental iron did not show further benefit, but had significantly more side effects particularly in the highest dose group [96]. As there is further evidence that oral iron loading increases circulating hepcidin [97], the recommended dosage of oral iron should not be too high. In a recent comparison of oral iron supplementation in a randomised controlled trial looking at iron status and performance in active women, 100 mg of FeSO4 (approximately 20 mg of elemental iron) was shown to be effective [98]. We therefore recommend supplementation of 40 to 60 mg of elemental iron once daily. Oral iron is in general well tolerated and efficient [99]. Side effects of oral therapy are usually not serious and include nausea, dyspepsia, constipation or diarrhoea [83, 84, 100]. Some individuals with an existing tendency to constipation benefit from drinking additional fruit juice to prevent heavy constipation. Otherwise compliance will be seriously affected.
When oral therapy fails or immediate restoration is needed i.v. therapy should be considered. At the moment two different preparations are available in Switzerland, comprising iron saccharose and ferric carboxymaltose [100]. The dosage is dependent on the severity of the iron deficiency. In one application usually 200 mg Fe-saccharose, 500 to 100 mg Fe-carboxymaltose can be administered.
The main advantage of i.v. therapy is the immediate correction of the iron deficiency and restoration of the empty iron stores. Generally, compliance with i.v. iron supplements is good. Side effects may encompass a transient disturbance of taste, headache, dizziness, myalgia and fever, but severe adverse reactions such as hypotonic and anaphylactoid reactions, tachycardia and arrhythmia, dyspnoea and bronchospasm may also be observed, albeit very rarely [100].
Moreover, transient and usually asymptomatic hypophosphataemia is frequently observed after the administration of Fe-carboxymaltose [101].
It is still under discussion whether hypophosphataemia may possibly be a cofactor for cardiac events [101]. Nowadays severe side effects are rare. For Fe-saccharose and Fe-carboxymaltose there has been no fatal outcome reported in Switzerland. This is in contrast to the previously used iron-dextran products, which had a much higher rate of severe and fatal side effects. In 2013, another type of i.v. iron, Fe-oxytol, was withdrawn owing to severe hypersensitivity reactions in four patients, including one fatal outcome, observed 9 months after approval by the regulatory authorities in Switzerland [102, 103]. Even with the use of the new preparations, severe adverse reactions cannot be excluded, and the administration of i.v. iron preparations is only recommended in settings where resuscitation skills are available and observation of the patient for 30 minutes after the end of the i.v. administration can be guaranteed [104, 105].
In general, the frequency of side effects seems to be lower when the i.v. iron is applied by infusion instead of slow bolus injection. Importantly, the exact dilution given by the manufacturer needs to be respected. Further, the physician needs to respect the WADA antidoping regulations concerning administration of infusion in elite sports: “Intravenous infusions and/or injections of more than 50 ml per 6 hour period are prohibited except for those legitimately received in the course of hospital admissions or clinical investigations” [106].
| Table 3: Critical cut-offs for differentiation of iron deficiency in children and adolescents. Adapted from Herklotz et al. [29]. | ||||
| Definition | Haemoglobin | MCV/MCH | Ferritin | CRP |
| NAID | Normal | Normal | Normal | |
| IDMH | Normal | 6–12y: MCV <76 fl or no MCH <25 pg or no | 6–12y: <15 mcg/l | |
| 12–15 y: MCV <78 fl or no MCH <26 pg or no | 12–15y: <20 mcg/l | |||
| 15–18 y: MCV <79 fl or no MCH <26.5 pg or no | 15–18y: <30 mcg/l | |||
| IDA | 6–12 y: <112 g/l | 6–12y: MCV <76 fl MCH <25 pg | ||
| 12–15y: M <125 g/l F <120 g/l | 12–15y: MCV <78 fl or no MCH <26 pg or no | |||
| 15–18y: M <130 g/l F <120 g/l | 15–18 y: MCV <79 fl or no MCH <26.5 pg or no | |||
| CRP = C-reactive protein; IDA = iron deficiency anaemia; IDMH = iron deficiency with microcytosis and/or hypochromia; MCV = mean cellular haemoglobin; MCH = mean corpuscular haemoglobin; NAID = nonanaemic iron deficiency | ||||
In sports a regular check of blood parameters is necessary, especially in endurance athletes. To monitor efficacy of therapeutic measures we recommend repeating the basic blood tests 6 to 8 weeks after the start of the nutritional measures, oral therapy or i.v. iron administration.
Depending on the blood results, therapy will be continued or modified with the aim of reaching or to keeping the iron stores in the regular range. Treatment approaches may be combined and athletes with repeatedly low iron stores may benefit from intermittent oral substitution to preserve iron stores, e.g. substitution with 14 to 28 m elemental iron per day or 40–60 mg elemental iron two to three times per week. In vegetarian athletes a similar approach to preventing iron deficiency is recommended: 40 to 60 mg elemental iron three times per week instead of meat intake or a daily supplementation with at least 14 or 28 mg/day of iron will usually cover iron demand.
As iron homeostasis is exclusively and meticulously controlled by the iron uptake through the intestinal tract [107] and as there is no pathway to eliminate iron in cases of overload, iron supplementation has to be done always carefully. In extreme cases, chronic iron overload may lead to secondary haemochromatosis. Furthermore, excessive supplementation of oral or i.v. iron is thought to increase oxidative stress and production of free radicals [108, 109] and oxidative stress is suggested to play a role in cancer genesis [110, 111]. This must be critically appraised as acute exercise leads to oxidative stress [112, 113].
It has been shown in mice that oral iron supplementation enhances colonic tumour development [114]. Data in humans suggest that iron may increase the risk for colorectal cancer [115]. A recent meta-analysis showed on the one hand a tendency toward a positive association between high intake of haem iron and cancer risk. On the other hand high levels of biomarkers of iron stores implied a lower cancer risk [116]. Further prospective and experimental studies are needed to evaluate the possible influence of iron in carcinogenesis.
A British study reported an iron deficiency prevalence of 21% in adolescents between 11–18 years [117, 118]. Although likely to be higher in the athletic population, data about young athletes concerning iron deficiency are sparse [119–121].
Total iron requirements in children and adolescents are distinctly increased because of additional iron needs for the expansion of the total blood volume and mean haemoglobin mass, as well as for the enhancement in lean body mass during growth [122]. In adolescent females the onset of menarche is associated with an increase of iron requirement. The mean total iron requirement for adolescents reaches 1.8 mg/d for boys and 2.2 mg/d for girls (in females with heavy periods it is considerably more), which corresponds to more than double that in the preadolescent period [123, 124]. Otherwise risk factors for iron deficiency, both in younger and older athletes, are the same [20, 23, 24, 125].
Haematological normal values in children and adolescents are different from adults and should always be considered (see table 3). We recommend defining the lower level of normal for ferritin as 15 mcg/l for children between 6–12 years, 20 mcg/l between 12–15 years and 30 mcg/l for 15–18 year old adolescents [126].
As in adults, in a case of NAID dietary counselling is the first step, often combined with oral therapy. Iron requirements (RDA) are 8 mg/d for 9–13 year-old children and 11 mg/d for male and 15 mg/d for female adolescents older than 13 years. Careful management, especially of the menstruating teenage girl and the vegetarian athlete, is warranted [127]. If iron deficiency results in IDMH or IDA, further supplementation should be considered. Comparable to the therapy in adults, oral substitution can be with either Fe2+ or Fe3+ preparations. The dosage for iron preparations is 5 mg/kg/d for 3 months in two or three daily doses. The Fe2+ preparation is recommended as medication of choice because of better bioavailability. As in adult athletes, measurement of haemoglobin, red cell indices, ferritin and CRP after 6–8 weeks of treatment is necessary in order to observe responsiveness and compliance with the treatment.
Iron deficiency in sports is frequent and relevant as all stages of iron deficiency ‒ IDA, IDMH and NAID ‒ affect physical performance.
To diagnose iron deficiency, haemoglobin, haematocrit, MCV, MCH and ferritin are first-line parameters to assess. For a valid interpretation of the results it is necessary to exclude acute-phase reactions that may interfere, such as training sessions and infectious diseases (patient history and measurement of CRP). In unclear situations a second measurement of the same parameters or the additional measurement of zinc-protoporphyrin, soluble transferrin receptor and transferrin saturation may be helpful. In high level endurance athletes basic blood examinations should be made two to three times a year.
Ferritin values below <15 mcg/l are very specific for empty iron stores. Ferritin values from 15 to 30 mcg/l correspond to low iron stores. A ferritin value of 30 mcg/l should be taken as a reasonable cut-off for adult men and women and older adolescents (15 years and older). For younger adolescents from 12 to 15 years a cut-off of 20 mcg/l and for children from 6 to 12 years a cut-off of 15 mcg/l is recommended. In adult elite sports altitude training represents a special situation with an increased need for iron. In this case a ferritin value of 50 mcg/l prior to altitude training should be aimed for. Every case of severe iron deficiency warrants an extended work up, and this is also true in sports.
Therapy of iron deficiency in sports consists of the nutritional counselling including sufficient energy intake and 5 times/week haem iron intake (meat, poultry, fish) with the addition of legumes and green vegetables (e.g. spinach, fennel) usually combined with oral iron supplementation. Daily oral iron preparations in a dosage of 40 to 60 mg of elemental iron are appropriate, as the not severe but disturbing gastrointestinal side effects are related to iron dosage and preparation. Consider enhancers (vitamin C) and prevent inhibitors (coffee, black tea, phytates, calcium) of iron uptake to increase absorption. Athletes and patients with repeatedly low ferritin values profit from intermittent oral substitution to preserve iron stores, e.g. two iron tablets per week (containing 40 to 60 mg elemental iron) as maintenance therapy. In cases of plausible nonresponders, incompatibility with oral therapy, a concomitant disease (e.g. depression) or low iron stores before an altitude training camp, i.v. iron therapy should be considered.
Long-term daily oral iron intake or i.v. supplementation in the presence of normal or even high ferritin values is not recommended and may be harmful.
Acknowledgements: We thank all those who supported us to write this article. These include Prof. Dr. med. Arno Schmidt-Trucksäss, Prof. Dr. med. Roland von Känel, Dr. med. Kerstin Warnke and Dr. med. Christian Schlegel.
1 Proposed nutrient and energy intakes for the European community: a report of the Scientific Committee for Food of the European community. Nutr Rev. 1993;51:209–12.
2 Egli I. M. MARIA ANDERSSON*, INES M. EGLI*, MICHAEL B. ZIMMERMANN. at <http://www.rosenfluh.ch/rosenfluh/articles/download/1318/Eisenmangel.pdf>
3 Schleiffenbaum BE, et al. Unexpected high prevalence of metabolic disorders and chronic disease among young male draftees – the Swiss Army XXI experience. Swiss Med Wkly. 2006;136:175–84.
4 Sandström G, Börjesson M, Rödjer S. Iron deficiency in adolescent female athletes – is iron status affected by regular sporting activity? Clin J Sport Med. 2012;22:495–500.
5 Latunde-Dada G. O. Iron metabolism in athletes- achieving a gold standard. Eur J Haematol. (2012). doi:10.1111/ejh.12026
6 Dubnov G, et al. High prevalence of iron deficiency and anemia in female military recruits. Mil Med. 2006;171:866–9.
7 Cippa P, Krayenbühl P-A. Eisenmangel: Es geht nicht nur um Anämie. Schweiz Med Forum. 2014;11–12.
8 Martius F. Eisenmangel ohne Anämie – ein heisses Eisen. in Schweiz Med Forum. 2009;9:294–9.
9 Streuli R. A. Ferrum bonum et laudabile (lucrosumque). in Schweiz Med Forum. 2008;8:563.
10 Colombani PC, Mannhart C. Energie- und Nährstoffaufnahme im Schweizer Spitzensport – eine erste Bestandsaufnahme zu Beginn des zweiten Jahrtausends. 2003;7–16.
11 Mettler S, Zimmermann M. B. Iron excess in recreational marathon runners. Eur J Clin Nutr. 2010;64490–4.
12 Ganz T. Molecular Control of Iron Transport. JASN. 2007;18:394–400.
13 Löffler G. in S.416 ff (Springer Verlag).
14 Ganz T, Nemeth E. Iron metabolism: interactions with normal and disordered erythropoiesis. Cold Spring Harb Perspect Med. 2012;2:a011668.
15 Yip R. in Present Knowledge in Nutrition, Bowman BA, Russell RM 311 – 328 (ILSI Press, Washington DC, 2001).
16 Nemeth E, Ganz T. Regulation of iron metabolism by hepcidin. Annu Rev Nutr. 2006;26:323–42.
17 Young B, Zaritsky J. Hepcidin for clinicians. Clin J Am Soc Nephrol. 2009;4:1384–7.
18 Taylor C, et al. Hematologic, iron-related, and acute-phase protein responses to sustained strenuous exercise. J Appl Physiol. 1987;62:464–9.
19 Peeling P, Dawson B, Goodman C, Landers G, Trinder D. Athletic induced iron deficiency: new insights into the role of inflammation, cytokines and hormones. Eur J Appl Physiol. 2008;103:381–91.
20 Peeling P. Exercise as a mediator of hepcidin activity in athletes. Eur J Appl Physiol. 2010;110:877–83.
21 Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003;102:783–8.
22 Newlin MK, et al. The effects of acute exercise bouts on hepcidin in women. Int J Sport Nutr Exerc Metab. 2012;22:79–88.
23 Gaudin C, Zerath E, Guezennec CY. Gastric lesions secondary to long-distance running. Dig Dis Sci. 1990;35:1239–43.
24 Peters HP, De Vries WR, Vanberge-Henegouwen GP, Akkermans LM. Potential benefits and hazards of physical activity and exercise on the gastrointestinal tract. Gut. 2001;48:435–9.
25 Waller MF, Haymes EM. The effects of heat and exercise on sweat iron loss. Med Sci Sports Exerc. 1996;28:197–203.
26 Jones GR, Newhouse I. Sport-related hematuria: a review. Clin J Sport Med. 1997;7:119–25.
27 Miller BJ, Pate RR, Burgess W. Foot impact force and intravascular hemolysis during distance running. Int J Sports Med. 1988;9:56–60.
28 Telford RD, et al. Footstrike is the major cause of hemolysis during running. J Appl Physiol. 2003;94:38–42.
29 Herklotz R, Huber A. Labordiagnose von Eisenstoffwechselstörungen. in Schweiz Med Forum. 2010;10:500–7.
30 Brugnara C, Laufer MR, Friedman AJ, Bridges K, Platt O. Reticulocyte hemoglobin content (CHr): early indicator of iron deficiency and response to therapy. Blood. 1994;83:3100–1.
31 Thomas C, Thomas L. Biochemical markers and hematologic indices in the diagnosis of functional iron deficiency. Clin Chem. 2002;48:1066–76.
32 Macdougall IC. What is the most appropriate strategy to monitor functional iron deficiency in the dialysed patient on rhEPO therapy? Merits of percentage hypochromic red cells as a marker of functional iron deficiency. Nephrol Dial Transplant. 1998;13:847–9.
33 Onofrio G d’, Zini G, Ricerca BM, Mancini S, Mango G. Automated measurement of red blood cell microcytosis and hypochromia in iron deficiency and beta-thalassemia trait. Arch Pathol Lab Med. 1992;116:84–9.
34 Onofrio G d’, et al. Simultaneous measurement of reticulocyte and red blood cell indices in healthy subjects and patients with microcytic and macrocytic anemia. Blood. 1995;85:818–23.
35 Brugnara C, Zurakowski D, DiCanzio J, Boyd T, Platt O. Reticulocyte hemoglobin content to diagnose iron deficiency in children. JAMA. 1999;281:2225–30.
36 Franck S, Linssen J, Messinger M., Thomas L. Potential utility of Ret-Y in the diagnosis of iron-restricted erythropoiesis. Clin Chem. 2004;50:1240–2.
37 Schumacher YO, Schmid A, König D, Berg A. Effects of exercise on soluble transferrin receptor and other variables of the iron status. Br J Sports Med. 2002;36:195–9.
38 Magge H, Sprinz P, Adams WG, Drainoni M-L, Meyers A. Zinc protoporphyrin and iron deficiency screening: trends and therapeutic response in an urban pediatric center. JAMA Pediatr. 2013;167:361–7.
39 Baart AM, et al. High prevalence of subclinical iron deficiency in whole blood donors not deferred for low hemoglobin. Transfusion. 2013;53:1670–7.
40 Voss SC, et al. Variability of serum markers of erythropoiesis during 6 days of racing in highly trained cyclists. Int J Sports Med. 2014;35:89–94.
41 Cordova A, Monserrat J, Villa G, Reyes E, Soto MA-M. Effects of AM3 (Inmunoferon) on increased serum concentrations of interleukin-6 and tumour necrosis factor receptors I and II in cyclists. J Sports Sci. 2006;24:565–73.
42 Dickson DN, Wilkinson RL, Noakes TD. Effects of ultra-marathon training and racing on hematologic parameters and serum ferritin levels in well-trained athletes. Int J Sports Med. 1982;3:111–7.
43 Fallon KE, Sivyer G, Sivyer K, Dare A. The biochemistry of runners in a 1600 km ultramarathon. Br J Sports Med. 1999;33:264–9.
44 Friedmann B. Standards der Sportmedizin Sportleranämie. DEUTSCHE ZEITSCHRIFT FÜR SPORTMEDIZIN 2001;52.
45 Shaskey DJ, Green GA. Sports haematology. Sports Med. 2000;29:27–38.
46 Bärtsch P, Mairbäurl H, Friedmann B. Pseudo-anemia caused by sports. Ther Umsch. 1998;55:251–5.
47 Nichols AW. Nonorthopaedic problems in the aquatic athlete. Clin Sports Med. 1999;18:395–411, viii.
48 Heinicke K, et al. Blood volume and hemoglobin mass in elite athletes of different disciplines. Int J Sports Med. 2001;22:504–12.
49 Steiner T, Wehrlin JP. Does hemoglobin mass increase from age 16 to 21 and 28 in elite endurance athletes? Med Sci Sports Exerc. 2011;43:1735–43.
50 Steiner T, Wehrlin JP. Comparability of haemoglobin mass measured with different carbon monoxide-based rebreathing procedures and calculations. Scand J Clin Lab Invest. 2011;71:19–29.
51 Hinrichs T, et al. Total hemoglobin mass, iron status, and endurance capacity in elite field hockey players. J Strength Cond Res. 2010;24:629–38.
52 Wehrlin JP. Live high-train low for 24 days increases hemoglobin mass and red cell volume in elite endurance athletes. J Appl Physiol. 2006;100:1938–45.
53 Garvican LA, et al. Intravenous Iron Supplementation in Distance Runners with Low or Suboptimal Ferritin. Med Sci Sports Exerc. (2013). doi:10.1249/MSS.0b013e3182a53594.
54 Fellmann N. Hormonal and plasma volume alterations following endurance exercise. A brief review. Sports Med. 1992;13:37–49.
55 Hamilton T, et al. Die Radsport-Mafia und ihre schmutzigen Geschäfte: Der Insider-bericht über die Welt des Profiradsports: eine minutiöse Beichte, die erstmals das ganze ... Armstrongs Schlüsselrolle darin aufzeigt. (2012).
56 40_ans.pdf. at <http://oldsite.uci.ch/english/health_sante/docs/40_ans.pdf>
57 Bad blood : Nature News. at <http://www.nature.com//news/2006/060224/full/news060220-18.html>
58 Athlete Biological Passport (ABP) Operating Guidelines | World Anti-Doping Agency. at <https://www.wada-ama.org/en/resources/athlete-biological-passport/athlete-biological-passport-abp-operating-guidelines>
59 Saugy M, Lundby C, Robinson N. Monitoring of biological markers indicative of doping: the athlete biological passport. Br J Sports Med. 2014;48:827–32.
60 Davies KJ, Maguire JJ, Brooks GA, Dallman PR, Packer L. Muscle mitochondrial bioenergetics, oxygen supply, and work capacity during dietary iron deficiency and repletion. Am J Physiol. 1982;242:E418–427.
61 Davies KJ, et al. Distinguishing effects of anemia and muscle iron deficiency on exercise bioenergetics in the rat. Am J Physiol. 1984;246:E535–543.
62 Finch CA, et al. Iron deficiency in the rat. Physiological and biochemical studies of muscle dysfunction. J Clin Invest. 1976;58:447–53.
63 Burden RJ, et al. Impact of Intravenous Iron on Aerobic Capacity and Iron Metabolism in Elite Athletes. Med Sci Sports Exerc. (2014). doi:10.1249/MSS.0000000000000568
64 Garvican LA, et al. Intravenous iron supplementation in distance runners with low or suboptimal ferritin. Med Sci Sports Exerc. 2014;46:376–85.
65 DellaValle DM, Haas JD. Iron supplementation improves energetic efficiency in iron-depleted female rowers. Med Sci Sports Exerc. 2014;46:1204–15.
66 Waldvogel S, et al. Clinical evaluation of iron treatment efficiency among non-anemic but iron-deficient female blood donors: a randomized controlled trial. BMC Med. 2012;10;8.
67 McClung JP, et al. Randomized, double-blind, placebo-controlled trial of iron supplementation in female soldiers during military training: effects on iron status, physical performance, and mood. Am J Clin Nutr. 2009;90:124–31.
68 Hinton PS, Sinclair LM. Iron supplementation maintains ventilatory threshold and improves energetic efficiency in iron-deficient nonanemic athletes. Eur J Clin Nutr. 2007;61:30–9.
69 Brownlie T, 4th, Utermohlen V, Hinton PS, Haas JD. Tissue iron deficiency without anemia impairs adaptation in endurance capacity after aerobic training in previously untrained women. Am J Clin Nutr. 2004;79:437–43.
70 Brownlie T, 4th, Utermohlen V, Hinton PS, Giordano C, Haas JD. Marginal iron deficiency without anemia impairs aerobic adaptation among previously untrained women. Am J Clin Nutr. 2002;75:734–42.
71 Friedmann B, Weller E, Mairbaurl H, Bärtsch P. Effects of iron repletion on blood volume and performance capacity in young athletes. Med Sci Sports Exerc. 2001;33:741–6.
72 Hinton PS, Giordano C, Brownlie T, Haas J. D. Iron supplementation improves endurance after training in iron-depleted, nonanemic women. J Appl Physiol. 2000;88:1103–11.
73 WHO. WHO | Iron deficiency anaemia: assessment, prevention and control. WHO at <http://www.who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/WHO_NHD_01.3/en/>
74 Hallberg L, et al. Screening for iron deficiency: an analysis based on bone-marrow examinations and serum ferritin determinations in a population sample of women. Br J Haematol. 1993;85:787–98.
75 van Tellingen O. The importance of drug-transporting P-glycoproteins in toxicology. Toxicol Lett. 2001;120:31–41.
76 Magnusson B, Hallberg L, Rossander L, Swolin B. Iron metabolism and ‘sports anemia’. I. A study of several iron parameters in elite runners with differences in iron status. Acta Med Scand. 1984,216:149–55.
77 Thomason RW, Almiski MS. Evidence that stainable bone marrow iron following parenteral iron therapy does not correlate with serum iron studies and may not represent readily available storage iron. Am J Clin Pathol. 2009;131:580–5.
78 Krayenbuehl P-A, Battegay E, Breymann C, Furrer J, Schulthess G. Intravenous iron for the treatment of fatigue in nonanemic, premenopausal women with low serum ferritin concentration. Blood. 2011;118:3222–7.
79 Verdon F, et al. Iron supplementation for unexplained fatigue in non-anaemic women: double blind randomised placebo controlled trial. BMJ. 2003;326:1124.
80 Vaucher P, Druais P-L, Waldvogel S, Favrat B. Effect of iron supplementation on fatigue in nonanemic menstruating women with low ferritin: a randomized controlled trial. CMAJ. 2012;184:1247–54.
81 Gropper SS, Bader-Crowe DM, McAnulty LS, White BD, Keith RE. Non-anemic iron depletion, oral iron supplementation and indices of copper status in college-aged females. J Am Coll Nutr. 2002;21:545–52.
82 Brutsaert TD, et al. Iron supplementation improves progressive fatigue resistance during dynamic knee extensor exercise in iron-depleted, nonanemic women. Am J Clin Nutr. 2003;77:441–8.
83 Clénin GE. Eisen im Sport – oft zu wenig, gelegentlich aber auch zu viel! Schweizerische Zeitschrift für Ernährungsmedizin. 2006;21–25.
84 Mettler S. Ferrum – ein Mineralstoff im Sport. SCHWEIZERISCHE ZEITSCHRIFT FUR SPORTMEDIZIN UND SPORTTRAUMATOLOGIE 2004;52:105–14.
85 Mast AE, Blinder MA, Gronowski AM, Chumley C, Scott MG. Clinical utility of the soluble transferrin receptor and comparison with serum ferritin in several populations. Clin Chem. 1998;44:45–51.
86 Lamanca JJ, Haymes EM. Effects of low ferritin concentration on endurance performance. Int J Sport Nutr. 1992;2:376–85.
87 Yu D, Huo J, Xie L, Wang L. Meta-analysis of studies on cut-off value of serum ferritin for identifying iron deficiency. Wei Sheng Yan Jiu. 2013;42:228–35.
88 Pitsis GC, Fallon KE, Fallon SK, Fazakerley R. Response of soluble transferrin receptor and iron-related parameters to iron supplementation in elite, iron-depleted, nonanemic female athletes. Clin J Sport Med. 2004;14:300–4.
89 Fallon KE. Screening for haematological and iron-related abnormalities in elite athletes-analysis of 576 cases. J Sci Med Sport. 2008;11:329–36.
90 Fallon KE. Utility of hematological and iron-related screening in elite athletes. Clin J Sport Med. 2004;14:145–52.
91 Bothwell TH, Charlton RW, Cook JD, Finch CA. IRon metabolism in man. Oxford, UK: Blackwell Scientific Publications (1979).
92 Miret S, Simpson RJ, McKie AT. Physiology and molecular biology of dietary iron absorption. Annu Rev Nutr. 2003;23:283–301.
93 Zimmermann MB, Biebinger R, Egli I, Zeder C, Hurrell RF. Iron deficiency up-regulates iron absorption from ferrous sulphate but not ferric pyrophosphate and consequently food fortification with ferrous sulphate has relatively greater efficacy in iron-deficient individuals. Br J Nutr. 2011;105:1245–50.
94 Monsen ER. Iron nutrition and absorption: dietary factors which impact iron bioavailability. J Am Diet Assoc. 1988;88:786–90).
95 Hercberg S, Preziosi P, Galan P. Iron deficiency in Europe. Public Health Nutr. 2001;4:537–45.
96 Rimon E, et al. Are we giving too much iron? Low-dose iron therapy is effective in octogenarians. Am J Med. 2005;118:1142–7.
97 Zimmermann M.B, et al. Plasma hepcidin is a modest predictor of dietary iron bioavailability in humans, whereas oral iron loading, measured by stable-isotope appearance curves, increases plasma hepcidin. Am J Clin Nutr. 2009;90:1280–7.
98 DellaValle DM. Iron supplementation for female athletes: effects on iron status and performance outcomes. Curr Sports Med Rep. 2013;12:234–9.
99 Cancelo-Hidalgo MJ, et al. Tolerability of different oral iron supplements: a systematic review. Curr Med Res Opin. 2013;29:291–303.
100 Arzneimittel-Kompendium der Schweiz. (Documed, 2012).
101 Ritzmann, P. Eisencarboxymaltose. pharma-kritik 2010;32:29–31.
102 Rienso®, Lösung zur intravenösen Injektion (Ferumoxytol) – Swissmedic –. at <https://www.swissmedic.ch/zulassungen/00153/00189/00200/00497/index.html?lang=de>
103 Rienso, Lösung zur intravenösen Injektion – Swissmedic –. at <https://www.swissmedic.ch/marktueberwachung/00135/00166/00707/index.html?lang=de>
104 Arzneimittelinformation. at <http://www.swissmedicinfo.ch/>
105 Arzneimittelinformation. at <http://www.swissmedicinfo.ch/>
106 Prohibited List. World Anti-Doping Agency at <http://www.wada-ama.org/en/World-Anti-Doping-Program/Sports-and-Anti-Doping-Organizations/International-Standards/Prohibited-List/>
107 Demarmels Biasiutti F. Die Regulation des Eisenstoffwechsels. Schweiz Med Forum. 2009;630–2.
108 Koskenkorva-Frank TS, Weiss G, Koppenol WH, Burckhardt S. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radic Biol Med. 2013;65:1174–94.
109 Kohgo Y, Ikuta K, Ohtake T, Torimoto Y, Kato J. Body iron metabolism and pathophysiology of iron overload. Int J Hematol. 2008;88:7–15.
110 Steinboeck F, et al. The relevance of oxidative stress and cytotoxic DNA lesions for spontaneous mutagenesis in non-replicating yeast cells. Mutat Res. 2010;688:47–52.
111 Nowsheen S, et al. Accumulation of oxidatively induced clustered DNA lesions in human tumor tissues. Mutat Res. 2009;674:131–6.
112 Pingitore A, et al. Exercise and oxidative stress: Potential effects of antioxidant dietary strategies in sports. Nutrition. 2015;31:916–22.
113 Powers SK, Nelson WB, Hudson MB. Exercise-induced oxidative stress in humans: cause and consequences. Free Radic Biol Med. 2011;51:942–50.
114 Chua ACG, et al. Dietary iron enhances colonic inflammation and IL-6/IL-11-Stat3 signaling promoting colonic tumor development in mice. PLoS ONE. 2013;8:e78850.
115 Wurzelmann JI, Silver A, Schreinemachers DM, Sandler RS, Everson RB. Iron intake and the risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 1996;5:503–7.
116 Fonseca-Nunes A, Jakszyn P, Agudo A. Iron and cancer risk – a systematic review and meta-analysis of the epidemiological evidence. Cancer Epidemiol Biomarkers Prev. 2014;23:12–31.
117 Heath A-LM, Fairweather-Tait SJ. Clinical implications of changes in the modern diet: iron intake, absorption and status. Best Pract Res Clin Haematol. 2002;15:225–41.
118 Rossander-Hulthen L, Hallberg L. in Iron Nutrition in Health and Disease (Hallberg L.&Asp N.G.) 149–156 (John Libbey&Co; London, UK, 1996).
119 Constantini NW, Eliakim A, Zigel L, Yaaron M, Falk B. Iron status of highly active adolescents: evidence of depleted iron stores in gymnasts. Int J Sport Nutr Exerc Metab. 2000;10:62–70.
120 Rowland TW. Iron deficiency in the young athlete. Pediatr Clin North Am. 1990;37:1153–63.
121 Spodaryk K. Iron metabolism in boys involved in intensive physical training. Physiol Behav. 2002;75:201–6.
122 Fairweather-Tait S. in Iron Nutrition in Health and Disease (Hallberg L.&Asp N.G.) 137–148 (John Libbey&Co; London, UK, 1996).
123 Beard JL. Iron requirements in adolescent females. J Nutr. 2000;130:440S–442S.
124 Hallberg L. in Iron Nutrition in Health and Disease (Hallberg L.&Asp N.G.) 165–182 (John Libbey&Co; London, UK).
125 Raunikar RA, Sabio H. Anemia in the adolescent athlete. Am J Dis Child. 1992;146:1201–5.
126 Herklotz R, Lüthi I, Ottiger C, Huber AR. Referenzbereiche in der Hämatologie. Ther UmscH. 2006;63:5–24.
127 Dietary Reference Intakes (DRIs): Estimated Average Requirements for Groups - 5_Summary Table Tables 1-4.pdf. at http://www.iom.edu/Activities/Nutrition/SummaryDRIs/~/media/Files/Activity%20Files/Nutrition/DRIs/5_Summary%20Table%20Tables%201-4.pdf
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.