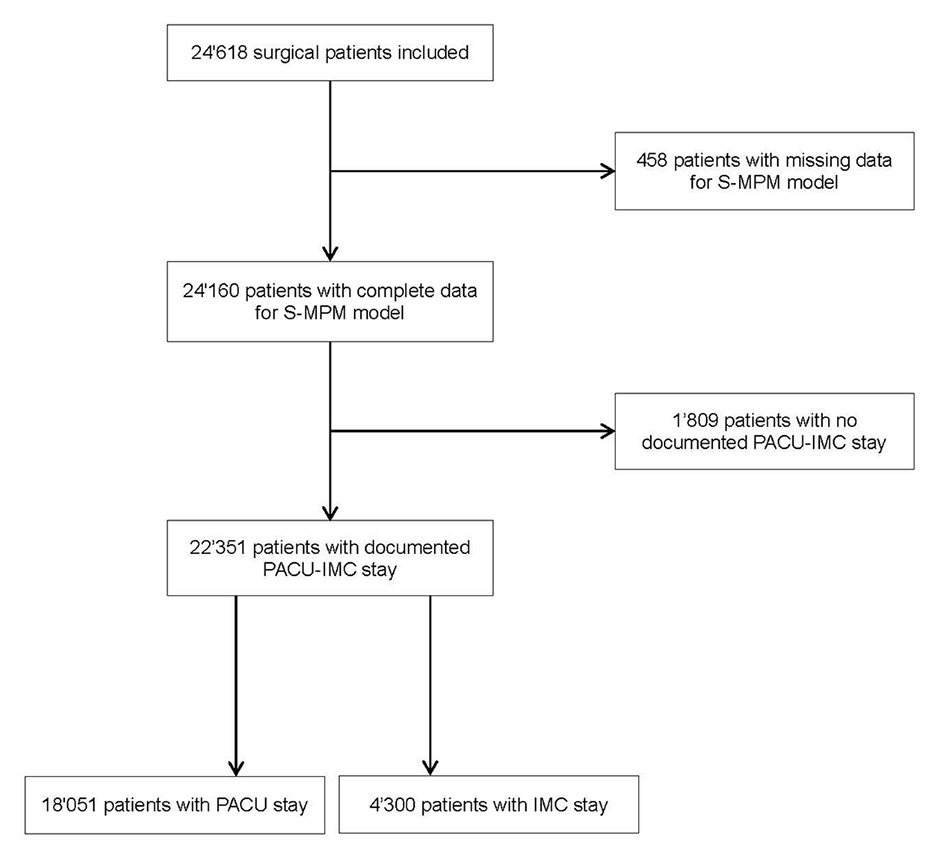
Figure 1
Flow chart of surgical patients included in the study.
ICM = intermediate care unit; PACU = post anaesthesia care unit
DOI: https://doi.org/10.4414/smw.2015.14205
Postinterventional in-hospital death in cardiac or noncardiac surgery and interventional medicine is a reality, even in high-quality hospitals, and is frequently inadequately anticipated. Therefore, postinterventional mortality is considered as a quality and safety indicator for anaesthesia, and surgical and nonsurgical interventions. In the Netherlands, based on a population-based study involving 3.7 million surgical procedures including adults with elective, open, surgical procedures from 1991 to 2005, the 30-day death rate was 1.9% [1]. There is increasing evidence that this mortality is related mainly to three risk factors: the physical status of the patient or physiological reserve (contributing most), complexity of the interventional procedure (procedural risk) and emergency intervention [2]. Even after minor surgical interventions the 30-day postoperative mortality may be high if the patients have considerable comorbidities: 8.5% for patients with preoperative nonischaemic heart failure, 8.1% with ischaemic heart failure, 5.7% with atrial fibrillation and 2.3% with coronary artery disease [3].
Postoperative complications have been identified more recently as a further, major contributor to short- and long-term mortality [4–8]. In elderly patients, pulmonary, cardiac and renal complications were associated with decreased long-term survival [9]. The first 48 hours after surgery were identified as the critical period in high-risk patients with the highest mortality rate and, therefore, a stay in the intensive care unit (ICU) or intermediate care unit (IMC) during this period should be considered [10]. Hospitals with a safe practice [11], an infrastructure including adequate nurse staffing levels [12] and a low postinterventional failure-to-rescue-rate [13, 14] observed decreased postinterventional mortality rates.
Feasible and innovative safety estimations are needed to permit decision makers to guide peri-interventional quality policy. A method of safety estimation is to assess the observed-to-expected mortality using validated models based on the hypothesis that a favourable observed-to-expected mortality ratio is evidence of high patient safety of an institution.
The main objective was to investigate the peri-operative patient safety in a large primary and tertiary care hospital in Switzerland using the observed-to-expected 30-day in-hospital death rates of surgical patients, and to identify risk factors associated with the observed mortality in patients undergoing surgical interventions.
Ethical approval for this study (N°12-241) was provided by the Ethics Committee of Geneva University Hospitals, Switzerland (Chairman: Prof. S. Lacroix). The Ethics Committee waived the requirement for written informed consent for this single centre, retrospective study. The STROBE Statement checklist (supplement) for cohort studies was used to develop this manuscript.

Figure 1
Flow chart of surgical patients included in the study.
ICM = intermediate care unit; PACU = post anaesthesia care unit
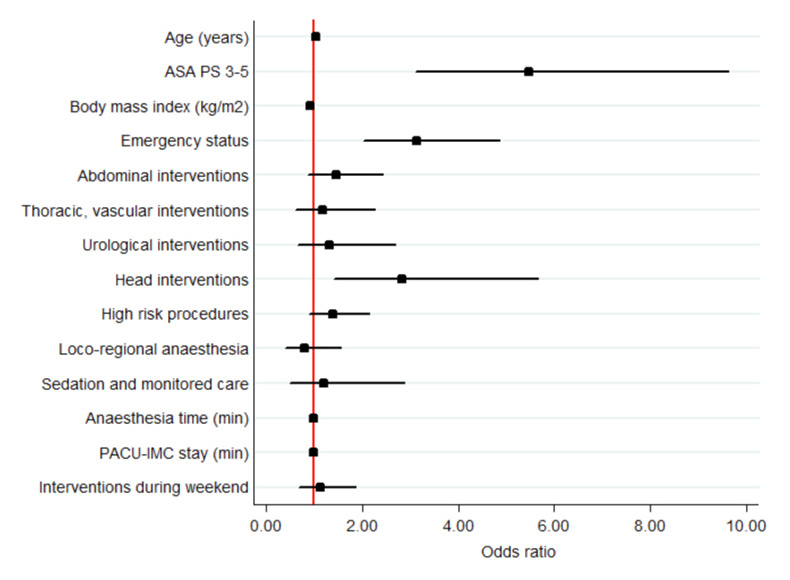
Figure 2
Odd ratios for 30-day in-hospital death in all patients (see also table 3a, multivariate models). ASA PS 3-5 was assessed versus ASA PS 1-2; emergency (nonelective) status versus elective; abdominal, thoracic – vascular, urological, head intervention versus orthopaedic; high-risk procedures versus low- and intermediate-risk procedures; locoregional anaesthesia, sedation and monitored care versus general and combined anaesthesia; interventions during weekend versus interventions during weekdays.
ASA PS = American Society of Anesthesiologists Physical Status score; ICM = intermediate care unit; PACU = post anaesthesia care unit

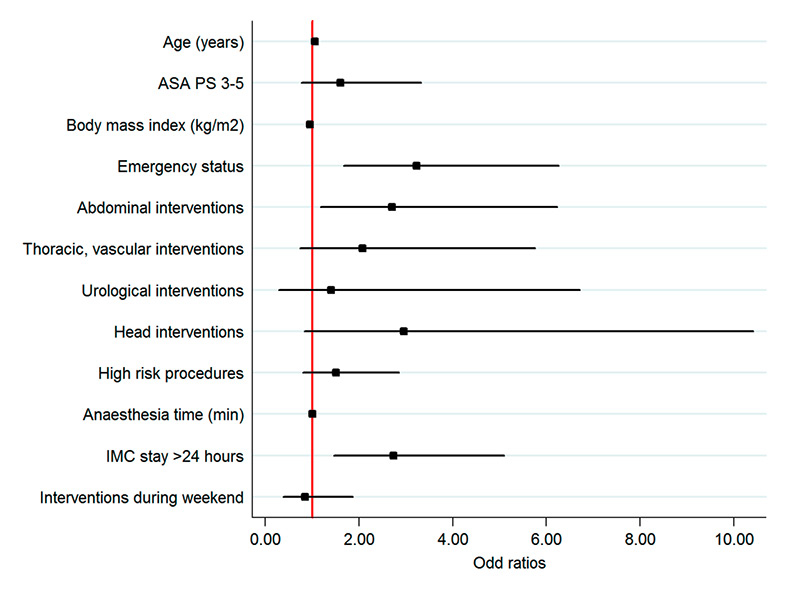
Figure 3
Odd ratios for 30-day in-hospital death in patients with PACU stay (A) and in patients with IMC stay (B) (multivariate model). PACU stay 3 hours to ≤6 hours was assessed versus PACU stay <3 hours; IMC stay >24 hours versus IMC stay 6 hours to ≤24 hours. ASA PS 3‒5 was assessed versus ASA PS 1–2; emergency (nonelective) status versus elective; abdominal, thoracic – vascular, urological, head intervention versus orthopaedic; high-risk procedures versus low- and intermediate-risk procedures; locoregional anaesthesia, sedation and monitored care versus general and combined anaesthesia; interventions during weekend versus interventions during weekdays.
ASA PS = American Society of Anesthesiologists Physical Status score; ICM = intermediate care unit; PACU = post anaesthesia care unit
Geneva University Hospitals, Switzerland are a primary and tertiary care centre with all types of surgical, medical, gynaecological, obstetric, paediatric and radiological departments with 1 908 beds, representing 48 112 admissions and 671 709 hospital-days with 26 533 anaesthetics in 2011.
Our study was undertaken in this single-university hospital in Switzerland where strategies to decrease risk have been routine for several years. All surgical interventions were performed using an adapted surgical safety checklist. The implementation process was accompanied with an observational study [15]. Furthermore, quality improvement initiatives were performed to improve identification of patients and surgery sites [16], as well as peri-operative infection control interventions by infection control specialists [17, 18].
The Geneva University Hospital has had a combined Post Anaesthesia Care Unit – Intermediate Care Unit (PACU-IMC) for many years. A quality improvement programme was performed in the last few years [19]. About 9 000 adult patients, with about 48 000 hours of PACU-IMC stay, are admitted, electively and nonelectively, every year to the centralised PACU-IMC after surgical, radiological or endoscopic procedure under anaesthesia. The PACU-IMC offers intermediate critical care for high-risk patients who may require temporary noninvasive ventilation, haemodynamic support including catecholamines and continuous invasive monitoring. These high-risk patients stay typically >6 hours in PACU-IMC and are treated in the “slow” subunit (IMC patients). The decision for IMC stay depends on preoperative risk stratification (American Society of Anesthesiologists Physical Status; ASA PS), surgical risk and on a medical triage procedure of patients staying in the PACU. This medical triage process is a systematic assessment of all patients in the PACU with an adverse event and is performed by the physician in charge of PACU-IMC. Patients with a haemodynamic, respiratory, cognitive or consciousness abnormality that may need a prolonged period of surveillance and/or an invasive treatment are transferred to the IMC subunit. Therefore, all high-risk surgical patients can be identified at different times during the peri-interventional pathway. All other surgical patients, the large majority, are treated postoperatively in the PACU.
We retrospectively included all surgical patients from the period July 2008 to June 2011 (3 years) who had received an anaesthetic, were aged above 18 years, and had stayed in the PACU-IMC immediately after their intervention. Exclusion criteria were patients admitted to the ICU (about 1 250 admissions of about 1 000 patients per year), patients who underwent paediatric and gynaecological interventions, patients with nonoperating-room anaesthesia and patients transferred from surgical wards to the PACU for monitoring and advanced care. Therefore, we included typical, noncardiac, surgical patients who are treated in most Swiss hospitals even in absence of an ICU.
We searched in the computerised patient data system of the anaesthetic information system and the hospital administrative databases for pre-, intra- and postoperative parameters. The anaesthetic information system is a computerised patient information system used for all patients who undergo in-hospital or ambulatory surgery with an anaesthesiologist, and includes the age and sex of the patient, ASA PS score[20], the anaesthetic and surgical techniques employed, general information such as timing, duration and sequence of procedures, length of stay in the PACU-IMC, and emergency status. The hospital administrative database is used for financial purposes and includes administrative information. It does not contain data on postoperative complications or the causes of death. The database is managed by the Unit for Medico-Economic Investigations. These outcome data are extracted from patient discharge reports. For this study, we merged the two datasets using a unique patient identification number. Mortality data was double-checked against medical records.
The primary outcome was the observed 30-day in-hospital mortality rate. For each patient we calculated the expected probabilities of in-hospital death using the validated Surgical Mortality Probability Model (S-MPM) [2]. The S-MPM, which was published in 2012, exhibits superior calibration and nearly identical discrimination to the full 35-variable American College of Surgeons National Surgical Quality Program model and is, therefore, appropriate for quality and safety studies [21].
We included established risk factors for postoperative mortality: ASA PS, procedure risk and emergency [2]. We investigated age, body mass index, types of surgery, type of anaesthesia, weekend intervention, anaesthesia time (as an indirect estimate of technical difficulties during the intervention), and PACU-IMC stay duration (as an indirect estimate of early postoperative complications) as possible further independent risk factors for postoperative mortality.
We considered the ASA PS classification as surrogate comorbidity score because it is routinely used in our hospital and is validated as a predictor of postoperative anaesthesia-related complications and mortality [20]. The procedural risk was defined based on the Physiological and Operative Severity Score for the enUmeration of Mortality and Morbidity (POSSUM) (minor, intermediate, major and major +) which is used in all patients with anaesthesia in our hospital [22]; for this study low risk included minor interventions, intermediate risk included intermediate interventions and high risk major and major + interventions. We recorded and aggregated surgical procedures into the categories “emergency” (or nonelective), whenever the surgical procedure had to be performed outside of the scheduled operating programme, and “elective” based on our hospital data reporting systems. The type of anaesthesia was recorded in five categories. For the purpose of this investigation we classified general anaesthesia and combined anaesthesia together in one category, as well as monitored care and sedation. Furthermore, we regrouped interventions, in particular, into thoracic and vascular surgery, head surgeries (neurosurgery, ear nose and throat, and maxillofacial surgery).
Anaesthetic interventions, surgical procedures and patient characteristics were described using frequency, percentages or medians with interquartile range (IQR). First, we analysed all patients with a surgical procedure excluding patients with missing data (ASA PS, emergency or elective surgery and procedure risk). Second, we analysed separately patients with a documented PACU or an IMC stay.
Descriptive statistics were used for surviving patients versus nonsurviving patients (for all patients, and for patients with a documented PACU or an IMC stay, separately). Differences between groups were assessed using parametric student t-test or nonparametric Wilcoxon signed-rank test if not normally distributed, with an alpha threshold of 5% for quantitative variables; and by χ² tests with an alpha threshold of 5% for qualitative variables.
We used the equation of the validated S-MPM to estimate expected probabilities of in-hospital death at 30 days (for all patients, and for patients with a documented PACU or an IMC stay, separately).
Univariate and multivariate logistic regressions were performed to adjust for differences between survivors and nonsurvivors (for all patients, and for patients with a documented PACU or an IMC stay, separately). We divided the ASA PS into two separate categories: patients with no or minor comorbidities (ASA PS 1-2) and patients with major comorbidities (ASA PS 3-5). We stratified procedural risk into low-risk (minor and intermediate interventions) and high-risk surgery for the regression models. We divided day of intervention into weekdays and weekends. We calculated a crude and an adjusted odds ratio (OR) with 95% confidence interval (95% CI); an alpha threshold of 5% was used to identify risk factors of postoperative in-hospital 30-day mortality. Each multivariable linear regression model included all variables with a p<0.20 in the univariable model, or clinically relevant variables (confounders). A p-value of less than 0.05 was considered statistically significant.
We performed two further multivariate analyses for patients with a documented PACU or an IMC stay but we coded PACU and IMC stay depending on the duration of stay: PACU stay was stratified in the following two classes: PACU stay from >3 hours to ≤6 hours versus PACU stay from 0 hour to ≤3 hours; IMC stay was stratified in the following two classes: IMC stay >24 hours versus IMC stay from 6 hours to ≤24 hours. The ORs of these two models were presented by forest plots.
We used STATA software (version 12.0/IC 2011; StataCorp, College Station, Texas, USA) for all analyses.
| Table 1a: Patient and intervention characteristics of all patients. | ||
| Patients with missing data n = 458 | Included patients n = 24 160 | |
| n (%) | n (%) | |
| Sex: | ||
| Male | 270 (59.0) | 13 343 (55.2) |
| Female | 188 (41.0) | 10 817 (44.8) |
| ASA PS: | ||
| ASA PS 1 | 109 (23.8) | 4 678 (19.4) |
| ASA PS 2 | 217 (47.4) | 13 409 (55.5) |
| ASA PS 3 | 122 (26.6) | 5 617 (23.3) |
| ASA PS 4 | 10 (2.2) | 447 (1.9) |
| ASA PS 5 | 0 (0.0) | 9 (0.0) |
| Age (years), median (IQR) | 58 (38–73) | 58 (41–73) |
| Body mass index (kg/m2), median (IQR) | 24 (22–28) | 25 (22–29) |
| Emergency status: | ||
| Elective | 219 (47.8) | 16 286 (67.4) |
| Emergency | 239 (52.2) | 7 874 (32.6) |
| Type of intervention: | ||
| Orthopaedic | 235 (51.3) | 9 931 (41.1) |
| Abdominal | 88 (19.2) | 7 089 (29.3) |
| Thoracic, vascular | 34 (7.4) | 3 109 (12.9) |
| Urological | 52 (11.4) | 3 203 (13.3) |
| Head | 49 (10.7) | 828 (3.4) |
| Procedural risk: | ||
| Low risk | NA | 6 975 (28.9) |
| Intermediate risk | NA | 12 831 (53.1) |
| High risk | NA | 4 354 (18.0) |
| Type of anaesthesia: | ||
| General and combined | 388 (84.7) | 20 919 (86.6) |
| Locoregional | 56 (12.2) | 2 437 (10.1) |
| Sedation and monitored care | 14 (3.1) | 804 (3.3) |
| Times: | ||
| Anaesthesia duration (min), median (IQR) | 150 (100–220) | 175 (125–241) |
| Surgery duration (min), median (IQR) | 85 (50–145) | 86 (50–136) |
| PACU-IMC: | ||
| PACU-IMC stay (min), median (IQR) | 263 (150–460) | 160 (104–282) |
| Day of intervention: | ||
| Monday | 63 (13.8) | 3 769 (15.6) |
| Tuesday | 74 (16.2) | 4 286 (17.7) |
| Wednesday | 67 (14.7) | 4 678 (19.4) |
| Thursday | 69 (15.1) | 4 283 (17.7) |
| Friday | 84 (18.4) | 4 411 (18.3) |
| Saturday | 46 (10.0) | 1 397 (5.8) |
| Sunday | 54 (11.8) | 1 431 (5.5) |
| ASA PS = American Society of Anesthesiologists Physical Status score; IMC = Intermediate Care Unit; IQR = interquartile range; NA = not available; PACU = Post Anaesthesia Care Unit | ||
| Table 1b:Patient and intervention characteristics of patients with a PACU or an IMC stay. | ||
| PACU n = 18 051 | IMC n = 4 300 | |
| n (%) | n (%) | |
| Sex: | ||
| Male | 9 925 (55.0) | 2 376 (55.3) |
| Female | 8 126 (45.0) | 1 924 (44.7) |
| ASA PS: | ||
| ASA PS 1 | 3 959 (21.9) | 388 (9.0) |
| ASA PS 2 | 10 466 (58.0) | 2 006 (46.7) |
| ASA PS 3 | 3 431 (19.0) | 1 702 (39.6) |
| ASA PS 4 | 193 (1.1) | 200 (4.7) |
| ASA PS 5 | 2 (0.0) | 4 (0.1) |
| Age (years), median (IQR) | 57 (40–71) | 65 (48–77) |
| Body mass index (kg/m2), median (IQR) | 25 (22–29) | 25 (22–29) |
| Emergency status: | ||
| Elective | 15 786 (87.5) | 2 436 (56.7) |
| Emergency | 2 265 (12.6) | 1 864 (43.4) |
| Type of intervention: | ||
| Orthopaedic | 7 697 (42.6) | 1 499 (34.9) |
| Abdominal | 5 034 (27.9) | 1 565 (36.4) |
| Thoracic, vascular | 2 177 (12.1) | 726 (16.9) |
| Urological | 2 656 (14.7) | 280 (6.5) |
| Head | 487 (2.7) | 230 (5.4) |
| Procedural risk: | ||
| Low risk | 5 702 (31.6) | 681 (15.8) |
| Intermediate risk | 10 084 (55.9) | 1 867 (43.4) |
| High risk | 2 265 (12.6) | 1 752 (40.7) |
| Type of anaesthesia: | ||
| General and combined | 15 632 (86.6) | 3 880 (90.2) |
| Locoregional | 1 869 (10.4) | 298 (6.9) |
| Sedation and monitored care | 550 (3.1) | 122 (2.8) |
| Times | ||
| Anaesthesia duration (min), median (IQR) | 167 (120–225) | 235 (161–338) |
| Surgery duration (min), median (IQR) | 79 (47–124) | 125 (72–205) |
| PACU stay (min), median (IQR) | 135 (95–194) | – |
| IMC stay (min), median (IQR) | – | 827 (490–1 160) |
| Day of intervention: | ||
| Monday | 2 762 (15.3) | 726 (16.9) |
| Tuesday | 3 306 (18.3) | 674 (15.7) |
| Wednesday | 3 513 (19.5) | 835 (19.4) |
| Thursday | 3 353 (18.6) | 645 (15.0) |
| Friday | 3 243 (18.0) | 836 (19.4) |
| Saturday | 996 (5.5) | 276 (6.4) |
| Sunday | 878 (4.9) | 308 (7.2) |
| ASA PS = American Society of Anesthesiologists Physical Status score; IMC = intermediate care unit; IQR = interquartile range; PACU = post anaesthesia care unit | ||
The database included 24 618 patients; 458 patients had missing data (1.9%) (table 1a, fig. 1). The median anaesthesia time was 175 min (IQR 125–241) and the median PACU-IMC stay duration was 160 min (IQR 104‒282). Median age was 58 years (IQR 41‒73); 6 073 (25.1%) patients had an impaired physical status (ASA PS 3‒5), 4 354 (18.0%) had a high procedural risk, and 7 874 (32.6%) were treated as an emergency (table 1a). Most patients were treated on weekdays (21 427 [88.7%]).
For 1 809 (7.5%) patients no documentation of a PACU or IMC stay could be found (fig. 1); 18 051 out of 22 351 patients (80.8%) were treated postoperatively in the PACU and 4 300 (19.2%) patients in the IMC. The case-mix was different between patients treated in PACU and in IMC (table 1b), with older patients, more comorbid patients, more patients with high-risk surgery and more patients with emergency surgery in the IMC.
The observed 30-day mortality of all patients was 0.7% (176 deaths) (table 2a); the expected 30-day mortality was 1.2% using the S-MPM.
The observed 30-day mortality of the patients with PACU stay was 0.4% (73 deaths) (table 2b); the expected 30-day mortality was 0.8% using the S-MPM. The observed 30-day mortality of the patients with IMC stay was 2.3% (97 deaths) (table 2b); the expected 30-day mortality was 2.4% using the S-MPM.
Nonsurvivors were older (80 years, IQR 70‒87), 86.9% had an ASA PS of 3‒5 and 66.5% an emergency intervention i (table 2a and 3a, fig. 2). Head surgery had a significantly higher death rate (21/828; 2.5%) compared with other surgeries (155/23 177; 0.7%). In nonsurvivors, the median body mass index was significantly lower (24 kg/m2 [IQR 20‒26] vs 25 kg/m2 [IQR 22‒29]).
The procedural risk, the type and time of anaesthesia and the day of intervention were not independent risk factors of mortality. Duration of PACU-IMC stay was an independent risk factor.
Similar independent risk factors for mortality in both units were: age, emergency surgery, and duration of PACU-IMC stay (table 3b, fig. 3).
Further independent risk factors of mortality for patients with a PACU stay were ASA PS and head surgery, and an independent protective factor was higher body mass index.
A further independent risk factor of mortality for patients with IMC stay was abdominal surgery.
| Table 2a: Characteristics of survivors and nonsurvivors (30-day in-hospital mortality). | ||||
| Survivors | Non-Survivors | Death rate | ||
| n (%) | n (%) | p-value | (%) | |
| n = 23 984 | n = 176 | 0.7 | ||
| Sex: | 0.523 | |||
| Male | 13 250 (55.3) | 93 (52.8) | 0.7 | |
| Female | 10 734 (44.8) | 83 (47.2) | 0.8 | |
| ASA PS: | <0.0001 | |||
| ASA PS 1 | 4 678 (19.5) | 0 (0.0) | ||
| ASA PS 2 | 13 386 (55.8) | 23 (13.0) | 0.2 | |
| ASA PS 3 | 5 505 (23.0) | 112 (63.6) | 2.0 | |
| ASA PS 4 | 407 (1.7) | 40 (22.7) | 9.8 | |
| ASA PS 5 | 8 (0.1) | 1 (0.5) | 13.0 | |
| Age (years), median (IQR) | 58 (41–72) | 80 (70–87) | <0.0001 | |
| Body mass index (kg/m2), median (IQR) | 25 (22–29) | 24 (20–26) | <0.0001 | 0.0 |
| Emergency status: | <0.0001 | |||
| Elective | 16 227 (67.7) | 59 (33.5) | 0.4 | |
| Emergency | 7 757 (32.3) | 117 (66.5) | 1.5 | |
| Type of intervention: | <0.0001 | |||
| Orthopaedic | 9 874 (41.2) | 57 (32.4) | 0.6 | |
| Abdominal | 7 027 (29.3) | 62 (35.2) | 0.9 | |
| Thoracic, vascular | 3 088 (12.9) | 21 (11.9) | 0.7 | |
| Urological | 3 188 (13.3) | 15 (8.5) | 0.5 | |
| Head | 807 (3.4) | 21 (11.9) | 2.6 | |
| Procedural risk: | <0.0001 | |||
| Low risk | 6 951 (29.0) | 24 (13.6) | 0.3 | |
| Intermediate risk | 12 753 (53.2) | 78 (44.3) | 0.6 | |
| High risk | 4 280 (17.9) | 74 (42.1) | 1.7 | |
| Type of anaesthesia: | 0.091 | |||
| General and combined | 20 770 (86.6) | 149 (84.7) | 0.7 | |
| Locoregional | 2 421 (10.1) | 16 (9.1) | 0.7 | |
| Sedation and monitored care | 793 (3.3) | 11 (6.3) | 1.4 | |
| Times: | ||||
| Anaesthesia duration (min), median (IQR) | 175 (125–241) | 212 (136–287) | 0.003 | |
| Surgery duration (min), median (IQR) | 85 (50–136) | 105 (56–157) | 0.061 | |
| PACU-IMC stay (min), median (IQR) | 159 (104–279) | 527 (219–1 153) | <0.0001 | |
| Day of intervention: | 0.001 | |||
| Monday | 3 738 (15.6) | 27 (15.3) | 0.7 | |
| Tuesday | 4 257 (17.8) | 25 (14.2) | 0.6 | |
| Wednesday | 4 670 (19.5) | 25 (14.2) | 0.5 | |
| Thursday | 4 250 (17.7) | 28 (15.9) | 0.7 | |
| Friday | 4 373 (18.2) | 32 (18.2) | 0.7 | |
| Saturday | 1 373 (5.7) | 20 (11.4) | 1.5 | |
| Sunday | 1 321 (5.5) | 19 (10.8) | 1.4 | |
| ASA PS = American Society of Anesthesiologists Physical Status score; IMC = intermediate care unit; IQR = interquartile range; PACU = post anaesthesia care unit | ||||
| Table 2b:Characteristics of survivors and nonsurvivors (30-day in-hospital mortality) with a PACU or an IMC stay. | ||||||||
| PACU | PACU | PACU | IMC | IMC | IMC | |||
| Survivors | Nonsurvivors | Death rate | Survivors | Nonsurvivors | Death rate | |||
| p-value | (%) | n (%) | n (%) | p-value | (%) | |||
| n = 17 978 | n = 73 | 0.4 | n = 4 203 | n = 97 | 2.3 | |||
| Sex: | 0.614 | 0.592 | ||||||
| Male | 9 887 (55.0) | 38 (52.1) | 0.4 | 2 325 (55.3) | 51 (52.6) | 2.2 | ||
| Female | 8 091 (45.0) | 35 (48.0) | 0.4 | 1 878 (44.7) | 46 (47.4) | 2.4 | ||
| ASA PS: | <0.0001 | <0.0001 | ||||||
| ASA PS 1 | 3 959 (22.0) | 0 (0.0) | 0.0 | 388 (9.2) | 0 (0.0) | 0.0 | ||
| ASA PS 2 | 10 458 (58.2) | 8 (11.0) | 0.8 | 1 992 (47.4) | 14 (14.4) | 0.7 | ||
| ASA PS 3 | 3 381 (18.8) | 50 (68.5) | 1.5 | 1 642 (39.1) | 60 (61.9) | 3.0 | ||
| ASA PS 4 | 178 (1.0) | 15 (20.6) | 8.4 | 177 (4.2) | 23 (23.7) | 13.0 | ||
| ASA PS 5 | 2 (0.0) | 0 (0.0) | 0.0 | 4 (0.1) | 0 (0.0) | 0.0 | ||
| Age (years), median (IQR) | 57 (40–71) | 78 (70–87) | <0.0001 | 64 (47–76) | 82 (70–87) | <0.0001 | ||
| Body mass index (kg/m2), median (IQR) | 25 (22–29) | 24 (19–26) | 0.001 | 25 (22–29) | 24 (21–27) | 0.012 | ||
| Emergency status: | <0.0001 | <0.0001 | ||||||
| Elective | 12 771 (71.0) | 28 (38.4) | 0.2 | 2 407 (52.3) | 29 (29.9) | 1.2 | ||
| Emergency | 5 207 (29.0) | 45 (61.6) | 0.9 | 1 796 (42.7) | 68 (70.1) | 3.7 | ||
| Type of intervention: | <0.0001 | 0.002 | ||||||
| Orthopaedic | 7 662 (42.6) | 35 (48.0) | 0.5 | 1 478 (35.2) | 21 (21.7) | 1.4 | ||
| Abdominal | 5 024 (28.0) | 10 (13.7) | 0.2 | 1 514 (36.0) | 51 (52.6) | 3.3 | ||
| Thoracic, vascular | 2 170 (12.1) | 7 (9.6) | 0.3 | 714 (17.0) | 12 (12.4) | 1.7 | ||
| Urological | 2 645 (14.7) | 11 (15.1) | 0.4 | 276 (6.6) | 4 (4.1) | 1.4 | ||
| Head | 477 (2.7) | 10 (13.7) | 2.1 | 221 (5.3) | 9 (9.3) | 3.9 | ||
| Procedural risk: | 0.006 | 0.036 | ||||||
| Low risk | 5 686 (31.6) | 16 (21.9) | 0.3 | 673 (16.0) | 8 (8.3) | 1.2 | ||
| Intermediate risk | 10 044 (55.9) | 40 (54.8) | 0.4 | 1 829 (43.5) | 38 (39.2) | 2.1 | ||
| High risk | 2 248 (12.5) | 17 (23.3) | 0.8 | 1 701 (40.5) | 51 (52.6) | 2.9 | ||
| Type of anaesthesia: | 0.012 | 0.779 | ||||||
| General and combined | 15 576 (86.6) | 56 (76.7) | 0.4 | 3 791 (90.2) | 89 (91.8) | 2.3 | ||
| Locoregional | 1 858 (10.3) | 11 (15.1) | 0.6 | 293 (7.0) | 5 (5.2) | 1.7 | ||
| Sedation and monitored care | 544 (3.0) | 6 (8.2) | 1.1 | 119 (2.8) | 3 (3.1) | 2.5 | ||
| Times: | ||||||||
| Anaesthesia duration (min), median (IQR) | 167 (120–225) | 175 (115–236) | 0.937 | 235 (161–338) | 250 (170–315) | 0.763 | ||
| Surgery duration (min), median (IQR) | 79 (47–123) | 87 (44–126) | 0.882 | 125 (72–205) | 129 (170–315) | 0.825 | ||
| PACU stay (min), median (IQR) | 135 (95–194) | 205 (118–281) | <0.0001 | 823 (487–1 157) | 1 067 (625–1 710) | <0.0001 | ||
| Day of intervention: | 0.015 | 0.159 | ||||||
| Monday | 2 751 (15.3) | 11 (15.1) | 0.4 | 703 (16.7) | 14 (14.4) | 2.0 | ||
| Tuesday | 3 295 (18.3) | 11 (15.1) | 0.3 | 662 (15.8) | 10 (10.3) | 1.5 | ||
| Wednesday | 3 504 (19.5) | 9 (12.3) | 0.3 | 831 (19.8) | 17 (17.5) | 2.0 | ||
| Thursday | 3 342 (18.6) | 11 (15.1) | 0.3 | 626 (14.9) | 16 (16.5) | 2.5 | ||
| Friday | 3 228 (18.0) | 15 (20.6) | 0.5 | 814 (19.4) | 16 (16.5) | 1.9 | ||
| Saturday | 985 (5.5) | 11 (15.1) | 1.1 | 268 (6.4) | 9 (9.3) | 3.3 | ||
| Sunday | 873 (4.9) | 5 (6.9) | 0.6 | 299 (7.1) | 15 (15.5) | 4.8 | ||
| ASA PS = American Society of Anesthesiologists Physical Status score; IMC = intermediate care unit; IQR = interquartile range; PACU = post anaesthesia care unit | ||||||||
| Table 3a:Risk factors of 30-day in-hospital mortality for all included patients. | ||||
| Mortality | Univariate models | Multivariate models | ||
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| ASA PS: | ||||
| ASA PS 1–2 | 1 | 1 | ||
| ASA PS 3–5 | 20.30 (13.08–31.50) | <0.0001 | 5.48 (3.12–9.63) | <0.0001 |
| Age (years) | 1.08 (1.06–1.09) | <0.0001 | 1.05 (1.03–1.06) | <0.0001 |
| Body mass index (kg/m2) | 0.90 (0.87–0.94) | <0.0001 | 0.92 (0.89–0.96) | <0.0001 |
| Emergency status: | ||||
| Elective | 1 | 1 | ||
| Emergency | 3.34 (2.47–4.51) | <0.0001 | 3.15 (2.04–4.86) | <0.0001 |
| Type of intervention: | ||||
| Orthopaedic | 1 | 1 | ||
| Abdominal | 1.53 (1.07–2.19) | 0.021 | 1.45 (0.87–2.43) | 0.152 |
| Thoracic, vascular | 1.18 (0.71–1.95) | 0.522 | 1.17 (0.60–2.27) | 0.644 |
| Urological | 0.82 (0.46–1.44) | 0.482 | 1.32 (0.65–2.68) | 0.437 |
| Head | 4.51 (2.72–7.47) | <0.0001 | 2.83 (1.41–5.67) | 0.003 |
| Procedural risk: | ||||
| Low and intermediate risk | 1 | 1 | ||
| High risk | 3.34 (2.47–4.51) | <0.0001 | 1.38 (0.89–2.14) | 0.145 |
| Type of anaesthesia: | ||||
| General and combined | 1 | 1 | ||
| Locoregional | 0.92 (0.55–1.55) | 0.756 | 0.80 (0.41–1.56) | 0.511 |
| Sedation and monitored care | 1.93 (1.04–3.58) | 0.036 | 1.20 (0.50–2.89) | 0.691 |
| Times: | ||||
| Anaesthesia duration (min) | 1.001 03 (1.000 55–1.001 52) | <0.0001 | 1.000 39 (0.999 48–1.001 30) | 0.406 |
| PACU-IMC stay (min) | 1.000 76 (1.000 64–1.000 89) | <0.0001 | 1.000 49 (1.000 30–1.000 68) | <0.0001 |
| Day of intervention: | ||||
| Weekday | 1 | 1 | ||
| Weekend | 2.25 (1.57–3.22) | <0.0001 | 1.13 (0.68–1.86) | 0.639 |
| ASA PS = American Society of Anesthesiologists Physical Status score; CI = confidence interval; IMC = intermediate care unit; OR = odds ratio; PACU = post anaesthesia care unit | ||||
| Table 3b:Risk factors of 30-day in-hospital mortality for patients with a PACU or an IMC stay. | ||||||||
| PACU | IMC | |||||||
| Mortality | Univariate models | Multivariate models | Univariate models | Multivariate models | ||||
| OR (95% IC) | p-value | OR (95% IC) | p-value | OR (95% IC) | p-value | OR (95% IC) | p-value | |
| ASA PS: | ||||||||
| ASA PS 1‒2 | 1 | 1 | 1 | 1 | ||||
| ASA PS 3‒5 | 32.90 (15.77–68.62) | <0.0001 | 14.54 (5.81–36.36) | <0.0001 | 7.74 (4.38–13.68) | <0.0001 | 1.55 (0.74–3.23) | 0.243 |
| Age (years) | 1.08 (1.06–1.10) | <0.0001 | 1.03 (1.01–1.05) | 0.001 | 1.06 (1.05–1.08) | <0.0001 | 1.05 (1.03–1.08) | <0.0001 |
| Body mass index (kg/m2) | 0.88 (0.82–0.93) | <0.0001 | 0.91 (0.86–0.96) | 0.001 | 0.93 (0.88–0.98) | 0.011 | 0.95 (0.89–1.00) | 0.072 |
| Emergency status: | ||||||||
| Elective | 1 | 1 | 1 | 1 | ||||
| Emergency | 3.94 (2.46–6.32) | <0.0001 | 2.83 (1.56–5.11) | 0.001 | 3.14 (2.03–4.88) | <0.0001 | 3.42 (1.75–6.67) | <0.0001 |
| Type of intervention: | ||||||||
| Orthopaedic | 1 | 1 | 1 | 1 | ||||
| Abdominal | 0.44 (0.22–0.88) | 0.021 | 0.73 (0.31–1.73) | 0.472 | 2.37 (1.42–3.96) | 0.001 | 2.91 (1.27–6.70) | 0.012 |
| Thoracic, vascular | 0.71 (0.31–1.59) | 0.402 | 0.96 (0.38–2.43) | 0.936 | 1.18 (0.59–2.42) | 0.645 | 2.07 (0.74–5.77) | 0.164 |
| Urological | 0.91 (0.46–1.80) | 0.786 | 1.23 (0.54–2.79) | 0.621 | 1.02 (0.35–2.99) | 0.971 | 1.43 (0.30–6.86) | 0.654 |
| Head | 4.60 (2.26–9.32) | <0.0001 | 3.03 (1.32–6.96) | 0.009 | 2.87 (1.30–6.34) | 0.009 | 3.22 (0.91–11.40) | 0.070 |
| Procedural risk: | ||||||||
| Low and intermediate risk | 1 | 1 | 1 | 1 | ||||
| High risk | 2.12 (1.23–3.66) | 0.007 | 1.06 (0.54–2.05) | 0.871 | 1.63 (1.09–2.44) | 0.017 | 1.50 (0.79–2.83) | 0.215 |
| Type of anaesthesia: | ||||||||
| General and combined | 1 | 1 | 1 | |||||
| Locoregional | 1.65 (0.86–3.15) | 0.132 | 0.80 (0.37–1.73) | 0.575 | 0.73 (0.29–1.80) | 0.491 | – | – |
| Sedation and monitored care | 3.08 (1.32–7.15) | 0.009 | 1.64 (0.62–4.35) | 0.318 | 1.07 (0.33–3.44) | 0.905 | – | – |
| Times: | ||||||||
| Anaesthesia duration (min) | 0.999 584 (0.99 696–1.002 21) | 0.756 | – | – | 1.000 56 (0.999 73–1.001 40) | 0.186 | 1.000 14 (0.998 856–1.001 43) | 0.831 |
| PACU stay (min) (≤6 hours) | 1.007 44 (1.004 72–1.010 15) | <0.0001 | 1.003 24 (1.000 03–1.006 45) | 0.048 | – | – | – | – |
| IMC stay (min) (>6 hours) | – | – | – | – | 1.000 43 (1.000 26–1.000 59) | <0.0001 | 1.000 48 (1.000 25–1.000 71) | <0.0001 |
| Day of intervention: | ||||||||
| Weekday | 1 | 1 | 1 | 1 | ||||
| Weekend | 2.44 (1.40–4.25) | 0.002 | 1.34 (0.69–2.59) | 0.388 | 1.90 (1.17–3.08) | 0.009 | 0.88 (0.40–1.93) | 0.754 |
| ASA PS = American Society of Anesthesiologists Physical Status score; CI = confidence interval; IMC = intermediate care unit; OR = odds ratio; PACU = post anaesthesia care unit | ||||||||
The in-hospital mortality rate was 0.7% in this high-standard peri-operative care environment. The expected 30-day mortality rate of surgical patients was 1.2% based on the validated S-MPM. The identified risk factors associated with overall mortality were classical risk factors such as age, ASA PS and emergency surgery; head surgery and PACU-IMC stay duration were further independent risk factors. The procedural risk for surgery was not an independent risk factor for mortality. We identified an increased body mass index as a potential protective factor of mortality.
A lower than expected in-hospital death rate was observed, suggesting a low rate of failure to rescue and early correction of complications in the peri-operative period in the investigated hospital and, therefore, safe practice [11]. This safe peri-operative pathway including permanently open units for postoperative monitoring may also have an impact on the hospital readmission rate, since postoperative mortality and readmission rates are associated [23].
Compared with the findings of the Dutch population-based study with a 30-day all-cause death rate of 1.9% [1], this study has a lower observed 30-day mortality rate (0.7%); in contrast to the Dutch study our cohort included not only patients with elective, open surgery but also nonelective and nonopen surgical procedures. As in the Dutch study, we observed an association between postoperative mortality and the type of surgery: in particular, we observed higher risks of mortality for abdominal and head surgery.
Postoperative mortality is an established quality and safety indicator of peri-operative care [24]; however, this indicator gives no clues for improvement possibilities. Associated risk factors of 30-day mortality, in particular modifiable risk factors, may be more useful. In the peri-operative setting pre-, intra- or postoperative risk factors are normally tested. In this cohort, intraoperative factors, except emergency status, may have played a minor role because the procedural risk of the surgical intervention, day of intervention, and type and duration of anaesthesia were not independent risk factors.
Duration of PACU stay and IMC stay were independent risk factors for observed mortality. A prolonged postoperative stay in a monitored unit is probably a surrogate marker for early postoperative adverse events or complications needing prolonged treatment with monitored care; despite this prolonged care some complications were fatal. This observation corresponds to the established evidence that postoperative complications have a strong impact on 30-day mortality [25]. Similar risk factors were observed in a recent investigation including older patients with hip fracture repair [26]; however, the patients with increased risks were in an ICU. Prolonged ICU stay was observed to be an independent risk factor for mortality. A prolonged PACU stay may be a surrogate marker of patients with palliative surgery; however, no data were available on palliative conditions of the patients included.
The population with head surgery mainly included patients after surgical treatment of acute or chronic subdural haematoma evacuation in older patients. This may explain the high mortality rate of this specific subgroup of our cohort [27].
Our investigation supports the hypothesis of the obesity paradox [28]: despite increased risk of morbidity (atelectasis, hyperglycaemia, infection), these patients have a decreased risk of postoperative mortality. However, this association is probably not causal and causes of this phenomenon are highly speculative as the unexpected association of low body mass index with higher mortality might reflect frailty in older or sicker patients.
The rigorous selection of the patients as well as the period of analysis of 3 years, which resulted in a valuable sample, enabled reliable reporting of all deaths after surgical intervention requiring anaesthesia and PACU-IMC stay. A further strength of this large retrospective study is the calculation of the expected mortality based on a validated model [2], allowing comparability with other data bases, in particular with the American College of Surgeons National Surgical Quality Improvement Program database. However, a possible limitation is the validation process, which was performed retrospectively in US patients; we cannot completely rule out that ASA PS, procedural risk and emergency estimation might be calculated differently and that a Swiss model would give slightly different result.
This study has several limitations. First, this purely observational study with potential unmeasured bias allows hypothesis generation on peri-operative care only. Despite this limitation, the study adds some new aspects in an under-researched, out-of-ICU domain and may trigger further research. Second, the results of this single centre study performed in a Swiss primary and tertiary care hospital cannot be generalised, even though we have excluded surgeries needing ICU; only hospitals with similar structures and pathways may observe similar mortality results. However, with the standardised use of S-MPM, comparability may be simplified and may be used as a basis for other safety and quality investigations on peri-operative care. Third, postoperative complications and their degree of severity were not available [29]. We used the duration of PACU-IMC stay as a surrogate of early postoperative complications. This may be an oversimplification. Further studies should include these complications; the identification of these adverse events may permit more detailed analyses of modifiable major risks leading to prolonged IMC stay.
In conclusion, a favourable observed-to-expected mortality ratio for surgical patients was found in this high-standard care environment with well-established strategies to decrease risks for several years. Therefore, we suspected that our quality improvement initiatives performed in recent years, including the increased performance in our postoperative monitoring units may have contributed to this favourable result. Head surgeries and early postoperative complications expressed as a prolonged PACU-IMC stay were identified as potential new and supplementary risk factors of postoperative mortality in a large Swiss university hospital.
| Item No | Recommendation | |
| Title and abstract | 1 | (a) Indicate the study’s design with a commonly used term in the title or the abstract |
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found | ||
| Introduction | ||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported |
| Objectives | 3 | State specific objectives, including any prespecified hypotheses |
| Methods | ||
| Study design | 4 | Present key elements of study design early in the paper |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection |
| Participants | 6 | (a) Cohort study – Give the eligibility criteria, and the sources and methods of selection of participants. Describe methods of follow-up Case-control study – Give the eligibility criteria, and the sources and methods of case ascertainment and control selection. Give the rationale for the choice of cases and controls Cross-sectional study – Give the eligibility criteria, and the sources and methods of selection of participants |
| (b)Cohort study – For matched studies, give matching criteria and number of exposed and unexposed Case-control study – For matched studies, give matching criteria and the number of controls per case | ||
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable |
| Data sources/ measurement | 8* | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group |
| Bias | 9 | Describe any efforts to address potential sources of bias |
| Study size | 10 | Explain how the study size was arrived at |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding |
| (b) Describe any methods used to examine subgroups and interactions | ||
| (c) Explain how missing data were addressed | ||
| (d) Cohort study – If applicable, explain how loss to follow-up was addressed Case-control study – If applicable, explain how matching of cases and controls was addressed Cross-sectional study – If applicable, describe analytical methods taking account of sampling strategy | ||
| (e) Describe any sensitivity analyses | ||
| Results | ||
| Participants | 13* | (a) Report numbers of individuals at each stage of study – e.g. numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analysed |
| (b) Give reasons for non-participation at each stage | ||
| (c) Consider use of a flow diagram | ||
| Descriptive data | 14* | (a) Give characteristics of study participants (e.g. demographic, clinical, social) and information on exposures and potential confounders |
| (b) Indicate number of participants with missing data for each variable of interest | ||
| (c) Cohort study – Summarise follow-up time (e.g., average and total amount) | ||
| Outcome data | 15* | Cohort study – Report numbers of outcome events or summary measures over time |
| Case-control study –Report numbers in each exposure category, or summary measures of exposure | ||
| Cross-sectional study –Report numbers of outcome events or summary measures | ||
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (e.g., 95% confidence interval). Make clear which confounders were adjusted for and why they were included |
| (b) Report category boundaries when continuous variables were categorized | ||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | ||
| Other analyses | 17 | Report other analyses done – e.g. analyses of subgroups and interactions, and sensitivity analyses |
| Discussion | ||
| Key results | 18 | Summarise key results with reference to study objectives |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence |
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results |
| Other information | ||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based |
| * Give information separately for cases and controls in case-control studies and, if applicable, for exposed and unexposed groups in cohort and cross-sectional studies. | ||
| Note: An Explanation and Elaboration article discusses each checklist item and gives methodological background and published examples of transparent reporting. The STROBE checklist is best used in conjunction with this article (freely available on the Web sites of PLoS Medicine at http://www.plosmedicine.org/, Annals of Internal Medicine at http://www.annals.org/, and Epidemiology at http://www.epidem.com/). Information on the STROBE Initiative is available at http://www.strobe-statement.org . | ||
Acknowledgements:Assistance with the study: We thank Manuela Farinas for her help to identify the patients of interest. We thank also Eleanor Galtrey for the language editing of this manuscript.
1 Noordzij PG, Poldermans D, Schouten O, Bax JJ, Schreiner FA, Boersma E. Postoperative mortality in The Netherlands: a population-based analysis of surgery-specific risk in adults. Anesthesiology. 2010;112(5):1105–15.
2 Glance LG, Lustik SJ, Hannan EL, Osler TM, Mukamel DB, Qian F, et al. The Surgical Mortality Probability Model: derivation and validation of a simple risk prediction rule for noncardiac surgery. Ann Surg. 2012;255(4):696–702.
3 van Diepen S, Bakal JA, McAlister FA, Ezekowitz JA. Mortality and readmission of patients with heart failure, atrial fibrillation, or coronary artery disease undergoing noncardiac surgery: an analysis of 38 047 patients. Circulation. 2011;124(3):289–96.
4 Khuri SF, Henderson WG, DePalma RG, Mosca C, Healey NA, Kumbhani DJ. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005;242(3):326–41.
5 Silber JH, Rosenbaum PR, Trudeau ME, Chen W, Zhang X, Kelz RR, et al. Changes in prognosis after the first postoperative complication. Med Care. 2005;43(2):122–31.
6 Eber MR, Laxminarayan R, Perencevich EN, Malani A. Clinical and economic outcomes attributable to health care-associated sepsis and pneumonia. Arch Intern Med. 2010;170(4):347–53.
7 Gooiker GA, Dekker JW, Bastiaannet E, van der Geest LG, Merkus JW, van de Velde CJ, et al. Risk factors for excess mortality in the first year after curative surgery for colorectal cancer. Ann Surg Oncol. 2012;19(8):2428–34.
8 Kamphues C, Bova R, Schricke D, Hippler-Benscheidt M, Klauschen F, Stenzinger A, et al. Postoperative complications deteriorate long-term outcome in pancreatic cancer patients. Ann Surg Oncol. 2012;19(3):856–63.
9 Manku K, Bacchetti P, Leung JM. Prognostic significance of postoperative in-hospital complications in elderly patients. I. Long-term survival. Anesth Analg. 2003;96(2):583–9.
10 Cavaliere F, Conti G, Costa R, Masieri S, Antonelli M, Proietti R. Intensive care after elective surgery: a survey on 30-day postoperative mortality and morbidity. Minerva Anestesiol. 2008;74(9):459–68.
11 Brooke BS, Dominici F, Pronovost PJ, Makary MA, Schneider E, Pawlik TM. Variations in surgical outcomes associated with hospital compliance with safety practices. Surgery. 2012;151(5):651–9.
12 Aiken LH, Sloane DM, Bruyneel L, Van den Heede K, Griffiths P, Busse R, et al. Nurse staffing and education and hospital mortality in nine European countries: a retrospective observational study. Lancet. 2014;383(9931):1824–30.
13 Sheetz KH, Waits SA, Krell RW, Campbell DA, Jr., Englesbe MJ, Ghaferi AA. Improving mortality following emergent surgery in older patients requires focus on complication rescue. Ann Surg. 2013;258(4):614–7; discussion 7–8.
14 Silber JH, Kaestner R, Even-Shoshan O, Wang Y, Bressler LJ. Aggressive treatment style and surgical outcomes. Health Serv Res. 2010;45(6 Pt 2):1872–92.
15 Lubbeke A, Hovaguimian F, Wickboldt N, Barea C, Clergue F, Hoffmeyer P, et al. Effectiveness of the surgical safety checklist in a high standard care environment. Med Care. 2013;51(5):425–9.
16 Garnerin P, Ares M, Huchet A, Clergue F. Verifying patient identity and site of surgery: improving compliance with protocol by audit and feedback. Qual Saf Health Care. 2008;17(6):454–8.
17 Zingg W, Cartier V, Inan C, Touveneau S, Theriault M, Gayet-Ageron A, et al. Hospital-wide multidisciplinary, multimodal intervention programme to reduce central venous catheter-associated bloodstream infection. PLoS One. 2014;9(4):e93898.
18 Uckay I, Lubbeke A, Harbarth S, Emonet S, Tovmirzaeva L, Agostinho A, et al. Low risk despite high endemicity of methicillin-resistant Staphylococcus aureus infections following elective total joint arthroplasty: a 12-year experience. Ann Med. 2012;44(4):360–8.
19 Eichenberger AS, Haller G, Cheseaux N, Lechappe V, Garnerin P, Walder B. A clinical pathway in a post-anaesthesia care unit to reduce length of stay, mortality and unplanned intensive care unit admission. Eur J Anaesthesiol. 2011;28(12):859–66.
20 Wolters U, Wolf T, Stutzer H, Schroder T. ASA classification and perioperative variables as predictors of postoperative outcome. Br J Anaesth. 1996;77(2):217–22.
21 Khuri SF, Daley J, Henderson W, Hur K, Gibbs JO, Barbour G, et al. Risk adjustment of the postoperative mortality rate for the comparative assessment of the quality of surgical care: results of the National Veterans Affairs Surgical Risk Study. J Am Coll Surg. 1997;185(4):315–27.
22 Copeland GP. The POSSUM system of surgical audit. Arch Surg. 2002;137(1):15–9.
23 Tsai TC, Joynt KE, Orav EJ, Gawande AA, Jha AK. Variation in surgical-readmission rates and quality of hospital care. N Engl J Med. 2013;369(12):1134–42.
24 Dimick JB, Welch HG, Birkmeyer JD. Surgical mortality as an indicator of hospital quality: the problem with small sample size. JAMA. 2004;292(7):847–51.
25 Ferraris VA, Bolanos M, Martin JT, Mahan A, Saha SP. Identification of patients with postoperative complications who are at risk for failure to rescue. JAMA Surg. 2014;149(11):1103–8.
26 Gottschalk A, Hubbs J, Vikani AR, Gottschalk LB, Sieber FE. The Impact of Incident Postoperative Delirium on Survival of Elderly Patients After Surgery for Hip Fracture Repair. Anesth Analg. 2015.
27 Berghauser Pont LM, Dammers R, Schouten JW, Lingsma HF, Dirven CM. Clinical factors associated with outcome in chronic subdural hematoma: a retrospective cohort study of patients on preoperative corticosteroid therapy. Neurosurgery. 2012;70(4):873–80; discussion 80.
28 Hutagalung R, Marques J, Kobylka K, Zeidan M, Kabisch B, Brunkhorst F, et al. The obesity paradox in surgical intensive care unit patients. Intensive Care Med. 2011;37(11):1793–9.
29 Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13.
Disclosure statement: No financial support and no other potential conflict of interest relevant to this article was reported.