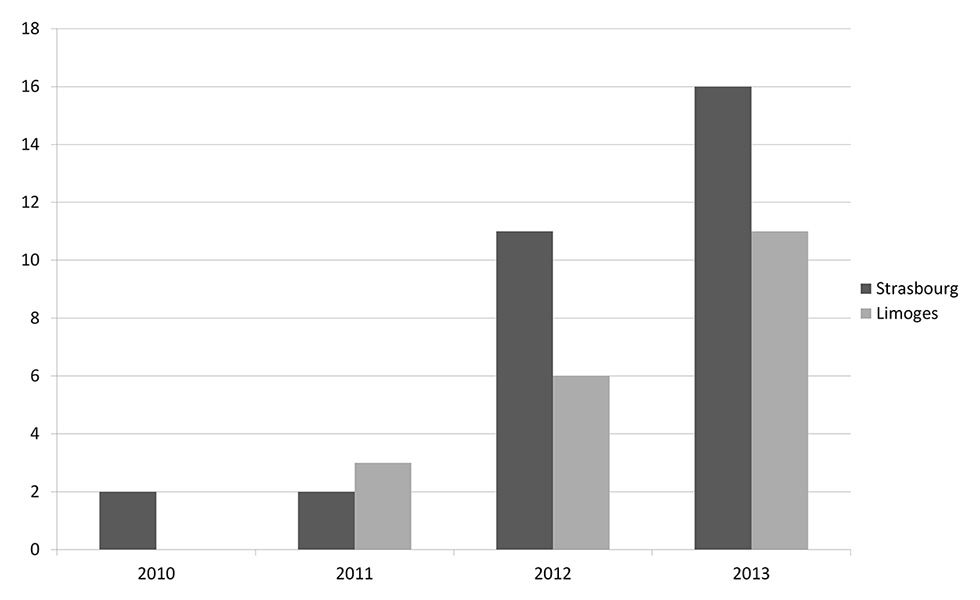
Figure 1
Number of Moraxella osloensis samples over time in the teaching hospitals of Limoges and Strasbourg.
DOI: https://doi.org/10.4414/smw.2015.14185
Moraxellaspp.are normal commensals of the upper respiratory tract in humans. The most frequently pathogenic species is M. catarrhalis and other species are not usually involved in human diseases. There have been case reports of endocarditis, septic arthritis, meningitis, bacteraemia, etc. with different types of Moraxella [1].Moraxella are nonmotile, Gram-negative coccobacilli. They are aerobic, oxidase-positive, fastidious organisms. Moraxella species are an unusual cause of endocarditis. With development of new microbiological techniques such as matrix-assisted laser desorption/ionisation and time-of-flight mass spectrometry (MALDI-TOF), new genera of bacteria are being found in samples of infected tissues. Thus, bacteria encountered rarely before the era of MALDI-TOF could emerge owing to these new techniques. Moraxella osloensis, for example, was first reported in 1967 but fewer than 40 cases have been described in the literature [2]. We report here two cases of recently diagnosed endocarditis due to M. osloensis; only one other case has been reported in the last 50 years [3].
A 75-year-old man with no past medical history complained of increasing dyspnoea for a year which was classified as grade III in December 2013. Transthoracic echography (TTE) revealed aortic regurgitation (4/4) associated with a 45% ejection fraction without any signs of infection or vegetation. There was no fever or inflammatory syndrome. Valve replacement surgery was planned and 13 teeth were extracted prior to this surgery to avoid secondary infection. In February 2014 a biological valve was implanted. No blood culture was performed prior to this surgery as the patient did not have a raised temperature. Bacteriological analysis of the removed valve revealed M. osloensis. The bacterium grew after specific parts of the valve were inoculated into blood culture vials (BacT/ALERT®, BioMérieux, France) and on chocolate agar. Identification was performed with MALDI-TOF (VITEX MS®, Biomérieux). This system does not give a score of accuracy but identification was considered to be correct by the system. The identification was confirmed with use of 16SRNA sequencing technology. Finally, histopathology confirmed the diagnosis of endocarditis. Antibiotic susceptibility showed that the strain was susceptible to amoxicillin, cefalotin, gentamicin and kanamycin. Minimum inhibitory concentration (MIC) for amoxicillin was 0.047 mg/l. The patient was treated with a combination of amoxicillin and gentamicin for 5 days, followed by intravenous amoxicillin for 4 weeks. During hospitalisation a B-cell chronic lymphocytic leukaemia was discovered. Patient follow-up after antibiotic treatment did not show any relapse of infection and TTE detected a correctly functioning biological valve.
A 51-year-old man consulted for an aortic abscess found accidentally during a follow-up cardiac echography. This man had a complicated medical history with two previous incidents of endocarditis that occurred on a prosthetic mitral valve (nutritionally deficient streptococcus and Staphylococcus lugdunensis), Hodgkin’s lymphoma and a kidney transplant. As a result of this abscess a Ross procedure (pulmonary autograft) was performed. All blood cultures (two) performed before surgery remained sterile but they were sampled while the patient was receiving antibiotics (BACTEC 9240®, Becton Dickinson). Bacteriological analysis of the removed prosthetic valve revealed M. osloensis. Identification was performed by use of MALDI-TOF (Bruker), with a good score (1.916), and was confirmed using 16SRNA sequencing. MICs were not determined as the bacterium was fully susceptible to antibiotics. Follow-up was complicated with multiple infections and kidney failure. The patient was treated with cefotaxime for 6 weeks and survived.
M. osloensis was first described in Oslo by Bövre and Henriksen in 1967 [4]. This strain is nutritionally undemanding and accumulates poly-B,hydroxybutyrate as a carbon stock [2].

Figure 1
Number of Moraxella osloensis samples over time in the teaching hospitals of Limoges and Strasbourg.
To our knowledge, these are the second and third cases of endocarditis due to M. osloensis reported in the literature. The first one was described by Stryker et al. in 1982 [3]. The clinical presentation of their patient corresponded to the classical clinical picture of endocarditis, with an association of fever, heart murmur, kidney failure and macroscopic haematuria. The diagnosis was confirmed by histopathology performed on the removed prosthetic valve and growth in blood culture of M. osloensis. Unfortunately, the patient died.
Literature analysis revealed that since the first description of this bacterium, fewer than 40 cases of infection due to this genus have been described [5]. It can invade a wide range of tissues and reported cases included: bacteraemia, eye infections, indwelling catheter infections, meningitis, bone and joint infections, pneumonia and peritonitis. Interestingly, a vast majority of these infections, as in both our cases, occurred in immunocompromised patients, including cases with solid tumours or haematological malignancies or transplant recipients. For Limoges, among the 21 cases, 61.9% occurred in immunosuppressed patients. Immunosuppression was due to haematological malignancies (7), cirrhosis (2), dialysis (2), kidney transplant (1) and pancreas cancer (1).
Because of the low number of cases, there are no guidelines for treatment, but these strains are generally susceptible to amoxicillin, cephalosporins, aminoglycosides, fluoroquinolones and carbapenems [6].
Because of the rarity of this infection, the first case (case 1) led us to look for other cases, not only in our hospital but also in other French teaching hospitals. Five other hospitals were asked to examine their bacteriological files for this bacterium and, in the event of positivity, to establish whether or not the strain was involved in endocarditis. The retrospective review period varied from 3 to 10 years. In two of them (Brest and Nantes) the bacterium was not found at all. In Rennes the strain was found six times and in Poitiers three times, but without endocarditis. The Strasbourg hospital retrieved one case of endocarditis (case 2) and 31 other positive samples. In Limoges, we found 20 other cases (in addition to case 1), but none with endocarditis. Other types of positive samples, from Limoges and Strasbourg, were mainly represented by blood culture (21 samples – 41%) and among them 6 occurred with the presence of intravascular catheters. Other sites of specimens, for the most representative, were lungs (4), dialysis fluid (4), bone and joints (3), ear-nose-throat (3), ascites (2), cerebrospinal fluid (1).
The entire population of these towns (all with a teaching hospital) represents about 3 162 000 inhabitants. We obviously cannot calculate an incidence, but only 61 cases over several years for this population makes this infection quite unusual, but interestingly, there has been a rise in the number of cases per year (fig. 1). We think that it is explained not by an increase in the number of infections, but rather by the use of new techniques such as MALDI-TOF, which result in better identification. Thus prior to access to this technique, strains were probably either misidentified or incompletely categorised, which explains this sudden rise. Indeed, at least in Limoges, MALDI-TOF technic has been used since November 2011, which coincides with the rise. However, in Strasbourg the technic has been used since 2009.
Unlike the case reported by Stryker et al., none of our patients showed clinical signs of endocarditis or even sepsis. Blood cultures remained sterile and the bacteriological diagnosis was made by the culture of the removed valves (classical culture and molecular biology using 16SRNA sequencing). Histopathology also revealed the presence of vegetations and thus remains an important examination for this type of diagnosis. The negativity of blood cultures for case 2 can be explained, because at the time of sampling blood, the patient was receiving antibiotics. For case 1, as the patient did not present any signs of infection, no blood cultures were performed. The difference between the results at each centre can probably be explained by the fact that care of patients differs from one hospital to another. However, with improvement of microbiological technics with more powerful blood culture analysis, MALDI-TOF as a tool for identification and 16SRNA sequencing as a confirmation tool, identification of bacteria in infectious diseases will probably be easier, if antibiotics are not prescribed without justification, thus leaving the possibility of positive cultures.
In conclusion, M. osloensis is probably not a clinically emerging pathogen but rather a bacteriological pathogen emerging owing to new techniques. Even if it is sensitive to antibiotics, it remains a bacterium that can cause lethal diseases such as endocarditis that moreover seem to occur in immunocompromised patients. Physicians caring for such patients must keep in mind this strain, which could become a true emerging clinical pathogen.
Acknowledgments: We thank Dr R. Le Berre (Brest), Pr D. Boutoille (Nantes), Pr F. Roblot (Poiters), Pr P. Tattevin (Rennes) for the time they spent examining the bacteriology lab files for M. osloensis as well as patient files for possible endocarditis.
1 Murphy T. Moraxella (Branhamella) catarrhalis and other Gram-negative Cocci. Principles and Practice of Infectious Diseases. 2005. p. 2529–36.
2 Baumann P, Doudoroff M, Stanier RY. Study of the Moraxella group. I. Genus Moraxella and the Neisseria catarrhalis group. J Bacteriol. 1968;95(1):58–73.
3 Stryker TD, Stone WJ, Savage AM. Renal failure secondary to Moraxella osloensis endocarditis. Johns Hopkins Med J. 1982;150(6):217–9.
4 Bövre K, Henriksen SD. A new Moraxella species, Moraxella osloensis, and a nonliquefaciens, revised description of Moraxella. Intern J Syst Bacteriol. 1967;17:127–35.
5 Dien Bard J, Lewinski M, Summanen PH, Deville JG. Sepsis with prolonged hypotension due to Moraxella osloensis in a non-immunocompromised child. J Med Microbiol. 2011;60:138–41.
6 Han XY, Tarrand JJ. Moraxella osloensis blood and catheter infections during anticancer chemotherapy: clinical and microbiologic studies of 10 cases. Am J Clin Pathol. 2004;121(4):581–7.
Disclosure statement: No financial support and no other potential conflict of interest relevant to this article was reported.