
Figure 1
Management protocol of acute non-traumatic hip pain applied in our institution between 1999 and 2007.
ANA = antinuclear antibodies; ASO = antistreptolysin O; ER = emergency room; RF = rheumatoid factor;
DOI: https://doi.org/10.4414/smw.2015.14176
Children presenting with acute nontraumatic hip pathology can display a wide variety of nonspecific symptoms, such as a limp or abnormal gait, pain, refusal to bear weight or decreased movement of the involved joint. These complaints represent a diagnostic problem for paediatricians and emergency physicians, not only because of the broadness of these complaints but also because the differential diagnosis varies from quite harmless conditions such as transient synovitis of the hip to more severe problems such as Legg-Calvé-Perthes’ disease (LCP), slipped capital femoral epiphysis (SCFE) and life-threatening conditions such as septic arthritis of the hip [1] . The most common cause of hip pain and limp in children is transient synovitis of the hip (TSH) [1, 2]. Commonly, the age of presentation is between 4 to 10 years, and the boy to girl ratio is 2:1 [2, 3]. Symptom resolution without any specific treatment in a matter of days is an essential feature of diagnosis [2, 3]. However, the symptoms of TSH are nonspecific and may be present in many other potentially serious hip conditions [4, 5]. Because of the potential risk of missing a serious condition in this situation, acute nontraumatic limping children are promptly referred and often over-investigated [6, 7]. In cases where no definite diagnosis is made during initial management, it is not clear whether further investigations, such as a rheumatological panel, to exclude all other possible diagnoses are necessary. A few studies have aimed to establish which parameters are most relevant in clinical decision-making for acute nontraumatic hip pathology in order to make the correct diagnosis [8, 9]. In our institution, a strict investigation protocol for children presenting with an acutely irritable hip in the emergency room was applied systematically between 1999 and 2007 (fig. 1). The primary aim of this study was to assess whether the investigation model used at our hospital could exclude all hip pathologies, in the case of an inconclusive initial survey at the emergency department. Secondly, it was intended to assess which investigations were truly necessary in this setting. Finally, we wanted to elaborate a comprehensive and adapted protocol for the management of a child presenting an acutely irritable hip.

Figure 1
Management protocol of acute non-traumatic hip pain applied in our institution between 1999 and 2007.
ANA = antinuclear antibodies; ASO = antistreptolysin O; ER = emergency room; RF = rheumatoid factor;
We retrospectively reviewed the medical records of all children under the age of 16 years who were managed according to a specific protocol for acute nontraumatic hip pain used in our institution between January 1999 and April 2007. The protocol included anteroposterior and Lauenstein hip views, hip ultrasonography, white blood cell (WBC) count, measurement of C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR), as well as a panel of rheumatoid tests including antinuclear antibodies (ANA), rheumatoid factor (RF), antistreptolysin O (ASO) titres and Lyme disease serology. All children were admitted to our paediatric orthopaedic ward. Inclusion criteria were: age under 16 years, presentation at the emergency department with an acute, new-onset, nontraumatic limp, hip pain or refusal to bear weight; absence of bone and joint involvement on hip radiography; and blood sample results inconclusive for a definite diagnosis in the emergency department. Exclusion criteria were cases where a definite diagnosis was clearly made during initial management in the emergency room.
Children included in the protocol were prescribed complete avoidance of weight bearing and lower-limb nonadhesive skin traction. Nonsteroidal anti-inflammatory and myorelaxing drugs were used for pain relief and to improve the children’s comfort. Once symptom-free, supervised ambulation was resumed and patients were allowed to leave the ward. Children were systematically re-evaluated 6 weeks after hospital discharge with a clinical check-up and radiological (anteroposterior and Lauenstein) views.
The study received institutional review board approval (Mat-Ped 07-009R) and was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki.
Patients were diagnosed with TSH if an ultrasound-confirmed hip effusion was present, complete resolution of symptoms occurred without any specific treatment, and no other pathology of the hip was identified during follow-up.
In the event of negative hip ultrasonography for hip effusion, together with spontaneous resolution of symptoms during follow-up and no other identified hip pathology, the diagnosis of irritable hip of unknown origin was given.
Data collected included patient age and gender, duration of symptoms prior to admission, results of clinical, radiological and laboratory findings, results of clinical and radiological follow-up 6 weeks after hospital discharge, and the definitive diagnosis. Weight-bearing status was determined from the clinical examination, and delay between limp onset and presentation was recorded in days. Fever was defined as an oral temperature >38.5 °C. The values of ANA, ASO and RF were interpreted according to our laboratory references, i.e., greater than 6 U/ml for RF, 1:80 for ANA, and above 200 U/ml for ASO. At ultrasonography, the presence and amount of articular effusion was reported in mm of space between the femoral neck and the joint capsule.
Descriptive statistics were used to present our demographic data. Unpaired Student's test was performed to compare subgroups of patients on laboratory data for values of CRP, WBC count, ESR, and ASO titre. Statistical analysis was performed using SPSS (Version 16.0; SPSS Inc., Chicago, Illinois). Differences were considered significant at p <0.05.
Between January 1999 and April 2007, 440 patients were admitted at our paediatric orthopaedic ward with a chief complaint of nontraumatic hip pain or a limp. Among these patients, 74 were given a definite diagnosis based on clinical, laboratory and radiological work-up at time of presentation at the emergency room. There were 2 cases of septic arthritis, 46 of Perthes’ disease, 2 of juvenile arthritis, 20 of SCFE, 1 Kawasaki’s disease, 1 myositis ossificans, 1 fibrous dysplasia of the proximal femur and 1 blood effusion of the hip due to haemophilia. The remaining 366 patients with nondiagnostic initial work-ups were managed under the specific protocol as described in the methods section and fulfilled the inclusion criteria for this study. Among these patients, 51 (14%) had another episode of hip pain requiring readmission to the hospital during the study period. In total, 417 hospitalisation episodes for hip pain without a definite diagnosis at the emergency room were collected. Among this group, the diagnosis of TSH was subsequently confirmed in 383 (91.8%) cases. After admission on the ward, one patient presented clinical and biological parameters (CRP of 51 mg/l and WBC count of 24.0 x 103/mm3) compatible with septic arthritis, and was treated accordingly despite negative hip joint aspiration. One patient had Lyme arthritis diagnosed on positive Lyme's serology, and the remaining 32 patients had a hip pain or limp of unknown origin defined as irritable hips of unknown origin with a negative ultrasound for hip effusion (table 1). Among these 32 cases of irritable hip of unknown origin, full recovery was observed at follow-up. Demographic data of patients who underwent the specific protocol are shown in table 1.
| Table 1: Patients’ demographic data (417 cases). | |
| Males/females | 289/128 |
| Age in years (range) | 5.6 (1‒13) |
| Diagnosis Transient synovitis (%) Septic arthritis (%) Lyme arthritis (%) Irritable hip of unknown origin | 383 (91.8) 1 (0.2) 1 (0.2) 32 (7.7) |
Among the 383 patients with transient synovitis of the hip, 192 (51%) had symptoms for less than 24 hours prior to admission. When symptoms were present for more than 24 hours, mean symptom duration was 2.1 days (range 1–21 days) before medical attention was sought. Among patients (417) who underwent the specific "irritable hip protocol", limping was present in 392 cases (94%), and 69 (16.5%) were absolutely unable to bear weight. In 23 (5%) cases, no information was found concerning the weight-bearing status. Only 5 (1.2%) patients presented an oral temperature greater than 38.5 °C at initial presentation.
One hundred and seventy (40%) patients had an upper respiratory infection within 2 weeks preceding symptom onset.
WBC count greater than >12.0 x 103/mm3was present in 47 (11.3%) children. Seventy-two patients had a CRP greater than 10 mg/dl with a median value of 15 ± 21.9 mg/dl (5‒86). CRP was lower than 10 mg/dl in 345 patients (83%). ESR was greater than 40 mm/h in 8 (2.4%) patients (table 2). ASO titres were tested in 403 patients and were above 200 U/ml in 87 (22%) cases. When abnormal, the median value was 400 U/ml, ranging from 300 to 800 U/ml (table 2). Lyme serology testing was performed in 323 patients and results were normal for all patients but one.
ANA titres were assessed in 402 patients and were negative in 341 cases (86%). Among the positive results, ANA titres were higher than 1:80 in 25 cases (6%). Rheumatoid factor was tested in 388 patients and was positive in 60 (15%) cases; rheumatoid factor was above 6 U/ml in 60 (15%) patients (table 3). Among children with abnormal results from the rheumatological panel, 38 underwent ophthalmological examination in order to rule out uveitis and all were normal.
Indirect signs of effusion were seen in 82 (19.6%) children on the standard pelvic radiographs, while no osseous abnormalities were noted. A hip ultrasound was performed in all patients. Hip effusion was observed in 385 (92.3%) cases with a mean value of 6.7 mm (range 2–19 mm).
Four hundred and two patients (96.4%) were seen for a clinical and radiological follow-up 6 weeks after hospital discharge and 15 were lost to follow-up. Three hundred and ninety six children were asymptomatic and had a normal hip X-ray. One patient had persistent pain despite a normal radiological aspect of the hip at 6 weeks follow-up. He required further follow-up, and hip X-rays at 12 weeks showed early signs of LCP disease.
Five patients had an asymmetrical aspect of the femoral head on hip X-rays and required additional follow-up despite entire resolution of clinical symptoms. Eventually, all these patients had a complete and spontaneous resolution of radiographic anomalies.
| Table 2: Laboratory results. | ||||
| Total* | Transient synovitis of the hip | Irritable hip with unknown origin | p-value | |
| CRP < 10 mg/dl (%) 10–20 mg/dl (%) > 20 mg/dl (%) Range | n = 415 345 (83) 47 (11) 23 (6) 5‒86 | n = 383 319 (83) 45 (11.7) 19 (5) 5‒86 | n = 32 26 (81) 2 (6) 4 (12.5) 5‒61 | p = 0.3 |
| WBC count >12.0 x 109 cells/l (%) Range | n = 415 47 (11) 3.4‒22.1 | n = 383 44 (11.5) 3.4‒17 | n = 32 3 (9.4) 4.3‒22.1 | p = 0.73 |
| ESR <5 mm/h (%) 5–40 mm/h (%) >40 mm/h (%) Range | n = 316 24 (7.5) 285 (90) 7 (2.5) 2‒61 | n = 296 22 (7.5) 267 (90) 7 (2.3) 2‒61 | n = 20 2 (10) 18 (90) 0 2‒24 | p = 0.17 |
| ASO titre <200 U/ml (%) >200 U/ml (%) Range | n = 401 316 (78) 85 (22) 0‒800 | n = 371 293 (79) 78 (21) 0‒800 | n = 30 23 (67) 7 (23) 0‒400 | p = 0.61 |
| ASO = antistreptolysin 0; CRP = C-reactive protein; ESR = erythrocyte sedimentation rate; WBC = white blood cell *The two patients diagnosed with septic arthritis and Lyme disease were excluded from data analysis. | ||||
| Table 3: Rheumatological panel results. | |||
| Total | Transient synovitis of the hip | Irritable hip with unknown origin | |
| ANA titre Negative (%) >1:80 (%) Median (SD) | n = 401 342 (86) 59 (14) 160 (387) | n = 371 316 55 160 (400) | n = 30 26 4 120 (113) |
| RF <6 U/ml (%) >6 U/ml (%) | n = 387 327 (85) 60 (15) | n = 359 303 (84) 56 (16) | n = 28 24 (86) 4 (14) |
| ANA = antinuclear antibodies; RF = rheumatoid factor; SD = standard deviation *The two patients diagnosed with septic arthritis and Lyme disease were excluded from data analysis. | |||
The diagnosis of TSH in children with acute nontraumatic hip pain or limp is often challenging. Clinical manifestations of TSH may be suggestive of other, more serious hip disorders, such as septic arthritis, LCP or acute SCFE, and must be ruled out [9]. In order to overcome the possibility of leaving a potentially serious condition undiagnosed after initial work-up at the emergency room, a strict policy of running a systematic clinical, radiological and laboratory work-up has been established in our institution. Moreover, all patients were seen 6 weeks after hospital discharge for clinical and radiological follow-up. Results of the current study show that children presenting with acute, nontraumatic hip pain or limp at the emergency room without a clear diagnosis at time of presentation were subsequently found to have TSH in the great majority of cases (92%) at the end of diagnostic work-up. These results confirm that most children complaining of a limp will turn out to have TSH, which may be adequately managed without any specific therapeutic measures and patients can in most cases be followed up on an outpatient basis [6, 7]. In addition, among the patients included in our study, conditions such as septic arthritis, SCFE or LCP disease were not diagnosed on the basis of the specific investigation work-up. This may be as a result of the fact that these diagnoses were made upon initial presentation at the emergency department, based on routine investigations.
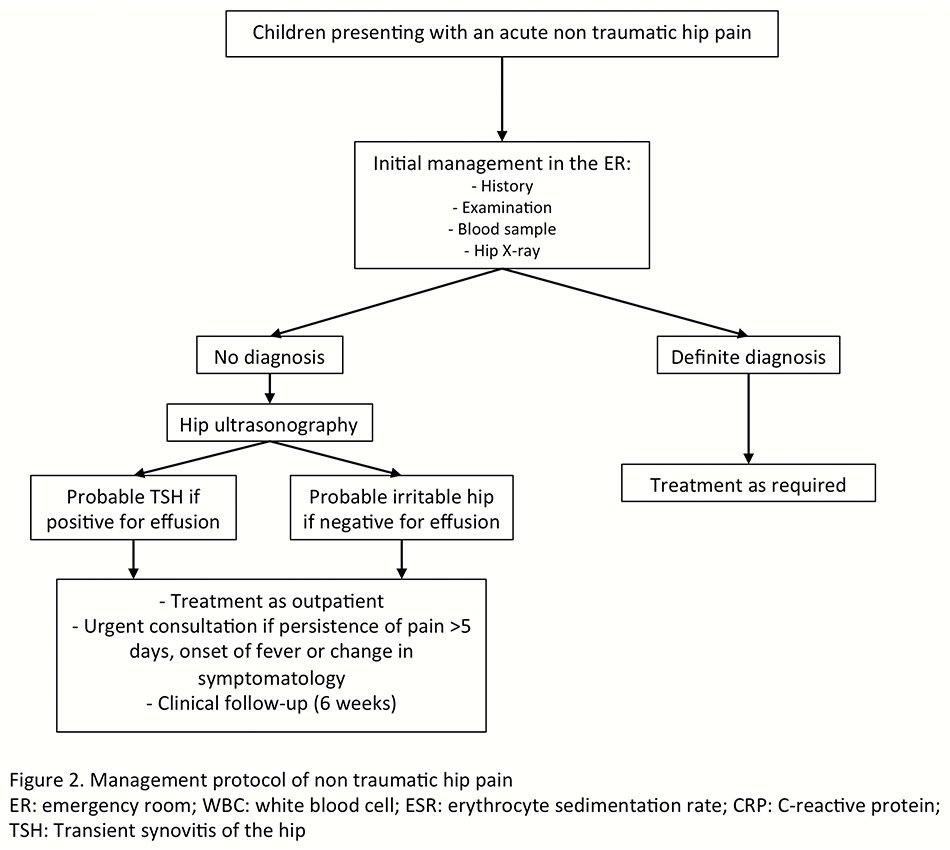
Figure 2
Management protocol of nontraumatic hip pain.
ER = emergency room; TSH = transient synovitis of the hip
Serum inflammatory parameters may be slightly raised in TSH [3]. As a recent viral infection (most commonly an upper respiratory tract infection) has been postulated as a precipitating factor [10], ESR and CRP values may be discretely elevated. However, raised inflammatory markers are paramount predictors of septic arthritis of the hip [8, 9]. Kocher et al. compared data from children with septic arthritis and TSH, and concluded that there were four predictive factors for septic arthritis of the hip: a history of fever (oral temperature higher than 38.5 °C), nonweight-bearing status, ESR >40 mm/h, and a WBC count >12.0 x 103/mm3[9, 11]. Caird et al. added CRP level of >20mg/l to these four factors [8]. On the basis of the Caird algorithm, 70% of the investigated children had no predictor, 24% one predictor, 19% two predictors, 1% three predictors, and none had four or five predictors (table 4). The only patient who was considered to have septic arthritis was found to have only two predictive factors according to Kocher and three factors according to Caird, corresponding to a probability of septic arthritis of 40% and 82.6%, respectively [8, 9]. These algorithms should be used with caution, especially in children under 4 years, since it has been shown that children between 6 and 48 months of age are susceptible to Kingella kingae osteoarticular infection despite normal laboratory values [12, 13].
Consistently with current literature, our results show that radiographs of the hip are often unremarkable in patients with TSH and may show only indirect signs of effusion [14, 15]. The main reason for radiographic evaluation is to rule out osseous lesions such as LCP disease, acute or chronic SCFE, or bone tumours, and should be obtained early in the diagnostic workup. Nevertheless, it is important to note that these hip disorders do not present with a similar history and clinical findings to TSH. Children with TSH have a complete resolution of symptoms in a week or less, even if residual symptoms may occasionally persist 7 to 10 days after the initial presentation [2, 16]. When compared with patients with TSH, patients with Perthe’s disease have a considerably longer duration of hip pain [17]. Analogously, children with chronic SCFE usually present with a history of limping for several weeks and poorly localised pain in the hip, groin, thigh, or knee. Moreover, these latter children are often older than those with TSH.
Ultrasound of the hip may help to confirm or exclude hip effusion in children with suspicion of transient synovitis or septic arthritis [14, 18, 19]. However care must be taken not to misinterpret false-negative studies performed too early in the course of septic arthritis [20]. Moreover, ultrasound is not discriminative in differentiating septic arthritis and TSH and should not be considered a safe diagnostic tool to confirm or exclude hip septic arthritis in children [21, 22]. In fact, ultrasound served mainly to confirm the localisation of symptoms to the hip and to detect the presence of effusion. In the present study, a hip ultrasound was performed in all children presenting with suspicion of TSH. A hip effusion was present in 92% of cases. All negative ultrasound cases were treated as TSH and had a 6-week follow-up. All had complete resolution of symptoms and no diagnostic entity could explain their initial clinical presentation.
In our series, ASO titres was higher than normal values in 22% of children (table 2), but we did not encounter any cases of post-streptococcal reactive arthritis (PRSA) or acute rheumatic fever (ARF). The incidence of ARF in the United States and Western Europe has dramatically decreased and PSRA is currently more prevalent [23]. PSRA is a distinct clinical entity and differs from the typical arthritis associated with rheumatic fever [24]. Patients suffering from PRSA usually have ongoing arthritis 6 weeks after symptom onset [25] , which may require more than 50 days to resolve [26]. Thus, the clinical course of PRSA is characteristic and radically different from that of TSH. Finally, all clinical findings in PRSA usually subside before the ASO titres reach their peak value. We conclude that streptococcal antibody tests should be performed only in patients who had a documented β-haemolytic group A streptococcal throat infection or when the children have an unusually long history of hip pain, compatible with PRSA.
Lyme disease was screened in all patients in our study. Only one patient had positive serology and was successfully treated with antibiotic therapy. Lyme arthritis in children is characterised by brief, often recurrent attacks of oligoarthritis [27, 28]. Of all joints, the knee is the most commonly involved [27, 28]. Lyme arthritis of the hip has been rarely reported [28, 29]. Unlike TSH, isolated hip involvement is very unusual in Lyme arthritis, further helping the distinction between these two entities [29]. Systematic serological screening for Lyme disease is certainly not recommended in children presenting with acute atraumatic hip pain. However, Lyme arthritis should be considered in the differential diagnosis of transient synovitis of the hip in children residing in areas in which Lyme disease exists.
Another goal of the screening protocol used in our institution was to exclude a rheumatological disorder such as juvenile idiopathic arthritis (JIA). In order to achieve this, ANA and RF were assessed. Among patients with positive rheumatological tests, no child was diagnosed with JIA according to the International League of Associations for Rheumatology criteria [30], as none were symptomatic at 6 weeks follow-up. In our study, abnormal values for ANA and RF were found in 8.3% and 14% of patients, respectively. These values are comparable to those observed in a normal population [31]. In fact, none of these parameters are specific to JIA and are present in only 30%–50% of patients with JIA [32, 34]. Retrospectively, measurement of ANA and RF seems questionable in cases of acute hip pain. However, to our knowledge, this has never been assessed and the results presented here support this affirmation. We suggest that these parameters should not be tested routinely in cases of acute hip pain or limp, but should only be performed 6 weeks after onset of articular pain in presence of criteria for JIA.
Finally, all children admitted for suspicion of TSH in our hospital were seen 6 weeks after hospital discharge for clinical and radiological follow-up. All children but one presented with a complete remission of symptoms. The latter presented recurrence of intermittent pain to the groin and was diagnosed with LCP disease, and follow-up was prolonged accordingly. During follow-up, an asymmetrical aspect of the femoral head was found in five asymptomatic patients. These radiographic findings resolved in subsequent checks. In all remaining patients, complete resolution of symptoms occurred and all standard radiographs were normal. These results suggest that the use of radiographs during follow-up should be considered only in the event of recurrent or persistence of pain or limp and should not be performed routinely.
The design of our study is comparable to previous studies and led to the same conclusion, that most children with acute nontraumatic hip pain or limp may be managed as outpatients and specific investigations should not be done routinely [6, 35].
This study confirms that a vast majority of children with acute hip pain or limp suffer from TSH, a benign and self-limiting condition. Children with suspicion of TSH may therefore be treated on an outpatient basis, provided that severe hip disorders, such as septic arthritis of the hip or acute SCFE, have been ruled out. Therapeutic measures such as skin traction are probably unnecessary and the use of anti-inflammatory and pain managing medication alone may help to alleviate the symptoms. With respect to this particular point, we have currently changed our practice, as hospitalisation and skin traction are no longer used in our department. Specific investigations are probably not fundamental, especially if symptoms have been present for less than a few days. The consequences of such common practice may lead to unnecessary costs as well as patient and parental distress. Thus, initial investigations should, in our opinion, include WBC count, CRP and ESR, and X-rays imaging of the hip (fig. 2). In the absence of a definite diagnosis, we suggest hip ultrasonography should be performed. In the presence of hip effusion without suspicion of septic arthritis, patients could be considered to have a probable transient synovitis of the hip. In the absence of hip effusion, children may be considered to have a so-called irritable hip. However, other diagnoses, such as osteomyelitis, should be kept in mind and excluded in the case of persistence of symptoms. In both situations, children may be treated on an outpatient basis and parents must be told to seek medical care urgently in the event of any change in symptomatology, onset of fever, or persistence of pain. Children should be referred to their paediatrician or paediatric orthopaedist for a check-up 6 weeks after the initial workup only in the case of persistence of symptoms. Also, radiographic views are unnecessary during follow-up for TSH in the asymptomatic child. Finally, screening for rheumatological diseases, acute rheumatic fever, post-streptococcal reactive arthritis, or Lyme disease is not justified at the time of presentation at the emergency room.
1 Krul M, van der Wouden JC, Schellevis FG, van Suijlekom-Smit LW, Koes BW. Acute non-traumatic hip pathology in children: incidence and presentation in family practice. Fam Pract. 2010;27(2):166–70.
2 Landin LA, Danielsson LG, Wattsgard C. Transient synovitis of the hip. Its incidence, epidemiology and relation to Perthes' disease. J Bone Joint Surg Br. 1987;69(2):238–42.
3 Haueisen DC, Weiner DS, Weiner SD. The characterization of "transient synovitis of the hip" in children. J Pediatr Orthop. 1986;6(1):11–7.
4 Eich GF, Superti-Furga A, Umbricht FS, Willi UV. The painful hip: evaluation of criteria for clinical decision-making. Eur J Pediatr. 1999;158(11):923–8.
5 Fischer SU, Beattie TF. The limping child: epidemiology, assessment and outcome. J Bone Joint Surg Br 1999;81(6):1029–34.
6 Taylor GR, Clarke NM. Management of irritable hip: a review of hospital admission policy. Arch Dis Child 1994;71(1):59–63.
7 Bickerstaff DR, Neal LM, Brennan PO, Bell MJ. An investigation into the etiology of irritable hip. Clin Pediatr (Phila) 1991;30(6):353–6.
8 Caird MS, Flynn JM, Leung YL, Millman JE, D'Italia JG, Dormans JP. Factors distinguishing septic arthritis from transient synovitis of the hip in children. A prospective study. J Bone Joint Surg Am. 2006;88(6):1251–7.
9 Kocher MS, Zurakowski D, Kasser JR. Differentiating between septic arthritis and transient synovitis of the hip in children: an evidence-based clinical prediction algorithm. J Bone Joint Surg Am. 1999;81(12):1662–70.
10 Kastrissianakis K, Beattie TF. Transient synovitis of the hip: more evidence for a viral aetiology. Eur J Emerg Med. 2010;17(5):270–3.
11 Kocher MS, Mandiga R, Zurakowski D, Barnewolt C, Kasser JR. Validation of a clinical prediction rule for the differentiation between septic arthritis and transient synovitis of the hip in children. J Bone Joint Surg Am. 2004;86-A(8):1629–35.
12 Ceroni D, Cherkaoui A, Ferey S, Kaelin A, Schrenzel J. Kingella kingae osteoarticular infections in young children: clinical features and contribution of a new specific real-time PCR assay to the diagnosis. J Pediatr Orthop. 2010;30(3):301–4.
13 Yagupsky P, Porsch E, St Geme JW, 3rd. Kingella kingae: an emerging pathogen in young children. Pediatrics. 2011;127(3):557–65.
14 Terjesen T, Osthus P. Ultrasound in the diagnosis and follow-up of transient synovitis of the hip. J Pediatr Orthop 1991;11(5):608–13.
15 Royle SG. Investigation of the irritable hip. J Pediatr Orthop. 1992;12(3):396–7.
16 Hardinge K. The etiology of transient synovitis of the hip in childhood. J Bone Joint Surg Br. 1970;52(1):100–7.
17 Adams JA. Transient Synovitis of the Hip Joint in Children. J Bone Joint Surg Br. 1963;45:471–6.
18 Futami T, Kasahara Y, Suzuki S, Ushikubo S, Tsuchiya T. Ultrasonography in transient synovitis and early Perthes' disease. J Bone Joint Surg Br. 1991;73(4):635–9.
19 Wingstrand H. Transient synovitis of the hip in the child. Acta Orthop Scand Suppl. 1986;219:1–61.
20 Gordon JE, Huang M, Dobbs M, Luhmann SJ, Szymanski DA, Schoenecker PL. Causes of false-negative ultrasound scans in the diagnosis of septic arthritis of the hip in children. Journal of pediatric orthopedics. 2002;22(3):312–6.
21 Zamzam MM. The role of ultrasound in differentiating septic arthritis from transient synovitis of the hip in children. J Pediatr Orthop B. 2006;15(6):418–22.
22 Zawin JK, Hoffer FA, Rand FF, Teele RL. Joint effusion in children with an irritable hip: US diagnosis and aspiration. Radiology. 1993;187(2):459–63.
23 Gordis L. The virtual disappearance of rheumatic fever in the United States: lessons in the rise and fall of disease. T. Duckett Jones memorial lecture. Circulation. 1985;72(6):1155–62.
24 Uziel Y, Perl L, Barash J, Hashkes PJ. Post-streptococcal reactive arthritis in children: a distinct entity from acute rheumatic fever. Pediatr Rheumatol Online J. 2011;9(1):32.
25 Riise OR, Lee A, Cvancarova M, Handeland KS, Wathne KO, Nakstad B, et al. Recent-onset childhood arthritis--association with Streptococcus pyogenes in a population-based study. Rheumatology (Oxford). 2008;47(7):1006–11.
26 Simonini G, Taddio A, Cimaz R. No evidence yet to change American Heart Association recommendations for poststreptococcal reactive arthritis: comment on the article by van Bemmel et al. Arthritis Rheum. 2009;60(11):3516–8; author reply 3518–9.
27 Gerber MA, Zemel LS, Shapiro ED. Lyme arthritis in children: clinical epidemiology and long-term outcomes. Pediatrics. 1998;102(4 Pt 1):905–8.
28 Steere AC, Malawista SE, Snydman DR, Shope RE, Andiman WA, Ross MR, et al. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three connecticut communities. Arthritis Rheum. 1977;20(1):7–17.
29 Saulsbury FT. Lyme arthritis presenting as transient synovitis of the hip. Clinical pediatrics. 2008;47(8):833–5.
30 Cleary AG, Sills JA, Davidson JE: Revision of the proposed classification criteria for juvenile idiopathic arthritis: Durban, 1997. J Rheumatol. 2000;27(6):1568.
31 McGhee JL, Kickingbird LM, Jarvis JN. Clinical utility of antinuclear antibody tests in children. BMC Pediatr. 2004;4:13.
32 Serra CR, Rodrigues SH, Silva NP, Sztajnbok FR, Andrade LE. Clinical significance of anticardiolipin antibodies in juvenile idiopathic arthritis. Clin Exp Rheumatol. 1999;17(3):375–80.
33 Berntson L, Andersson Gare B, Fasth A, Herlin T, Kristinsson J, Lahdenne P, et al. Incidence of juvenile idiopathic arthritis in the Nordic countries. A population based study with special reference to the validity of the ILAR and EULAR criteria. J Rheumatol. 2003;30(10):2275–82.
34 Wong KO, Bond K, Homik J, Ellsworth JE, Karkhaneh M, Ha C, et al. In: Antinuclear Antibody, Rheumatoid Factor, and Cyclic-Citrullinated Peptide Tests for Evaluating Musculoskeletal Complaints in Children. edn. Rockville (MD). 2012.
35 Mattick A, Turner A, Ferguson J, Beattie T, Sharp J. Seven year follow up of children presenting to the accident and emergency department with irritable hip. J Accid Emerg Med. 1999;16(5):345–7.
Disclosures: No financial support and no other potential conflict of interest relevant to this article was reported.