Differential influence of maternal and fetal pregnancy factors on the in-vitro induction of human regulatory T cells: a preliminary study
DOI: https://doi.org/10.4414/smw.2015.14172
Natalie
Marcoli, Monika
Østensen, Bettina
Portmann-Lanz, Daniel
Surbek, Peter
Villiger, Frauke
Förger
Summary
PROBLEM: Given the important role of regulatory T cells (Treg) for successful pregnancy, the ability of soluble maternal and fetal pregnancy factors to induce human Treg was investigated.
METHOD OF STUDY: Peripheral blood mononuclear cells (PBMCs) or isolated CD4+CD25‒ cells were cultured in the presence of pooled second or third trimester pregnancy sera, steroid hormones or supernatants from placental explants, and the numbers and function of induced CD4+CD25+FOXP3+ Treg were analysed.
RESULTS: Third trimester pregnancy sera and supernatants of early placental explants, but not sex steroid hormones, induced an increase of Tregs from PBMCs. Early placental supernatant containing high levels of tumour necrosis factor-α, interferon-γ, interleukins -1, -6 and -17, soluble human leucocyte antigen-G, and transforming growth factor-β1, increased the proportion of Treg most effectively and was able to induce interleukin-10-secreting-Treg from CD4+CD25‒cells.
CONCLUSIONS: Compared with circulating maternal factors, placental- and fetal-derived factors appear to exert a more powerful effect on numerical changes of Treg, thereby supporting fetomaternal tolerance during human pregnancy.
Introduction
Successful pregnancy is a challenge for the maternal immune system. Tolerance mechanisms have to become operative in order to protect the semiallogeneic fetus against immune attacks from the mother. Among the factors orchestrating tolerance during pregnancy, regulatory T cells (Treg) play a pivotal role [1]. The development and function of the Treg lineage depends on the expression of the transcription factor forkhead box P3 (FOXP3). In humans, about 1%–2% of T helper cells (CD4+ T cells) in the peripheral blood are Treg [2]. They originate in the thymus as naturally occurring Treg and can also be induced in the periphery from CD4+CD25‒ cells under certain conditions, including T cell receptor-mediated stimulation, tolerogenic dendritic cells, human leucocyte antigen-G (HLA-G) or transforming growth factor-beta (TGF-β) [3–5]. The suppressive activity of Treg is mediated either in a cell-cell contact mediated fashion via cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) or by the secretion of cytokines such as TGF-β or interleukin (IL)-10 [6, 7].
During normal murine pregnancy, the pool of Treg cells expands locally at the fetomaternal interface and systemically compared with nonpregnant mice [8]. Treg cells exposed to paternal antigens suppress maternal alloresponses to protect against fetal rejection, whereas in miscarriage-prone mice the number and function of Treg are diminished [8, 9]. Similar to the animal data, studies performed in human pregnancy have shown an expansion of Treg at the fetomaternal interface, resulting in higher percentages of Treg at the decidual level compared with those in the peripheral blood [10, 11]. Moreover, reduced local Treg levels are associated with pregnancy pathologies such as spontaneous abortion [10]. In human peripheral blood, however, the frequency of Treg varies depending on how these cells are characterised by means of flow cytometry. If Treg are defined as CD4+CD25+ T cells, which includes Treg and activated T cells, there is an increase of these cells during normal human pregnancy, peaking in the second trimester and decreasing after delivery [12]. By contrast, when Treg are defined as CD4dimCD25high cells or as CD4+CD25+FOXP3+CD127‒ cells the frequency of circulating Treg during pregnancy is unaltered or even diminished [13, 14].
In regard to the mechanisms that play a role in Treg expansion during pregnancy different factors have been investigated. The hormones oestradiol and progesterone, which increase markedly during gestation, have been suggested as possible explanations for the pregnancy-related Treg expansion [15, 16]. Other important factors that contribute to Treg expansion during gestation are fetal antigens. Until now, the expansion of Treg specific to fetal antigens and its importance in a successful allogeneic pregnancy have mainly been shown in the mouse model [8, 17, 18].
In this study we aimed to investigate the nature of factors contributing to the numerical changes of human Treg cells during gestation. By exposing peripheral blood mononuclear cells (PBMCs) and isolated CD4+CD25- T cells from nonpregnant healthy women to pregnancy sera and supernatants of placental tissue, the abilities of maternal and fetal factors to induce numerical changes of Treg were compared.
Materials and methods
Collection of peripheral blood and serum samples
The recruitment of healthy women and the experimental design were approved by the Ethics Board of the Canton of Bern. Blood samples and placental tissue were obtained after informed consent of the participating women.
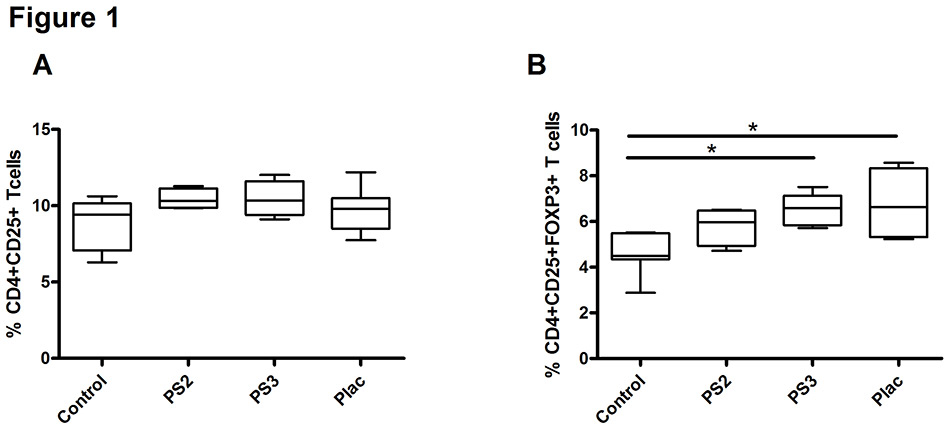
Figure 1
Pregnancy sera and placental factors augment the proportion of regulatory T (Treg) cells among peripheral blood mononuclear cells.
Effect of pregnancy sera and supernatant from early placental explants on the percentage of CD4+CD25+ T cells (A) or CD4+CD25+FOXP3+ T cells (B) among peripheral blood mononuclear cells from healthy women (n = 4). Data are expressed as boxplots with whiskers from minimum to maximum; the statistical analysis was performed using the Wilcoxon signed rank test. An asterisk indicates p <0.05.
Control = nonpregnant human serum; PS2 = pooled second trimester pregnancy sera; PS3 = pooled third trimester pregnancy sera; Plac = supernatant from early placental explants
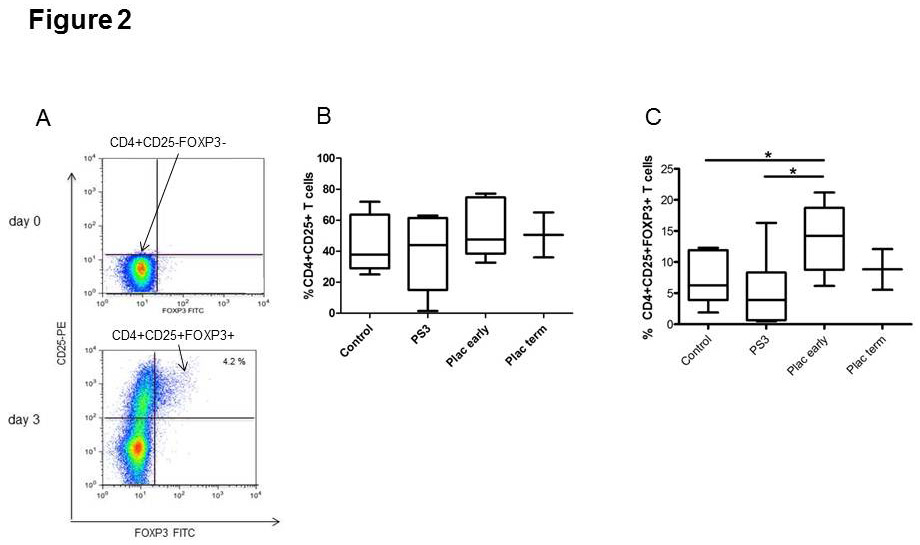
Figure 2
Induction of regulatory T (Treg) cells from CD4+CD25‒ T cells.
FACS staining after cell sorting. Cells are gated on CD4+ cells. The upper plot shows CD4+CD25‒FOXP3‒ T cells that were used at day 0 for the induction assay. The lower plot shows the induction of CD4+CD25+FOXP3+ cells after 3 days’ exposure to supernatant from early placental explants (A). Effect of pregnancy serum and supernatant from early or term placental explants on the percentage of CD4+CD25+ T cells (B) and CD4+CD25+FOXP3+ T cells (C) in cell cultures with sorted CD4+CD25‒ T cells from healthy women (n = 6; for cultures with term placenta n = 2). Data are expressed as boxplots with whiskers from minimum to maximum and statistical analysis was performed using Wilcoxon signed rank test. An asterisk indicates p <0.05.
Control = nonpregnant human serum; PS3 = pooled third trimester pregnancy sera; Plac early = supernatant from early placental explants; Plac term = supernatant from term placental explants
Heparinised venous blood or buffy coat from female blood donors was obtained from 24 healthy, nonpregnant women aged between 25 and 45 years. All donors were nonsmoking healthy volunteers working in the Inselspital and taking neither hormonal contraceptives nor any other medication.
Human AB serum was obtained from the Swiss blood bank in Bern. The autologous donor serum was obtained from venous blood. Serum samples from pregnant, healthy women – who had been recruited in a cross-sectional manner at the second trimester (n = 10, gestational weeks 14–24) and at the third trimester (n = 10, gestational weeks 26–34) – were pooled and stored at –20 °C until further use.
Four first trimester placentas (gestational weeks 7–12) were obtained from electively aborted fetuses of healthy women who had opted for pregnancy termination for psychosocial reasons. Four term placentas were obtained from healthy women (range, gestational age week 37–40) after normal pregnancy and vaginal delivery.
Isolation of early and term placental explants and preparation of placental supernatant
Placental villi from early and term placenta tissue samples (2 x 2 cm in size) were dissected into 2–4 mm3 fragments free of visible vessels and washed three times in sterile PBS to remove blood cells. The explants were cultured in 10-cm diameter dishes precoated with collagen type 1 (BD Bioscience) in DMEM/F12 nutrient mixture 1:1 (Gibco, USA) supplemented with 15% KnockOut serum replacement (Gibco, USA), 1% Glutamax and antibiotic/antimycotic solution (Invitrogen, CA, USA). The explants were incubated in a 95% humidified atmosphere at 37 °C and comprising 5% CO2. After adherence of the explants (after 5–7 days) to the culture dish, the culture medium was changed and collected every 3 days for 2 weeks. After collection the supernatant was immediately stored at –20 °C. The collected and pooled placental supernatant from each sample was concentrated 10X using sterilised Vivaspin ultracentrifugation tubes (Sartorius Stedim Biotech, Germany) with a 5kDa filter cut-off. It was then stored in 1 ml aliquots at –20 °C until further use.
Antibodies used for flow cytometry
For cell surface staining, anti-CD3 PE (clone HIT-3A); anti-CD4 PerCP (clone SK3), anti-CD25 APC and anti-CD25PE (clone 2A3) were used (all from BD Biosciences Pharmingen, USA). For the intracellular staining, anti-Foxp3 FITC and APC (clone PCH101) were utilised with the eBioscience Fix/Perm Buffer kit. After surface and intracellular staining of PBMCs and sorted cells, acquisition was done through FACs Calibur (BD;USA) and analysed using Flowjo software (Treestar, USA). Cells were gated on CD4+ T cells.
Cell culture, generation of regulatory T cells and suppression assay
PBMCs were isolated from either heparinised venous blood or buffy coats through density gradient centrifugation using Histopaque-1077 (Sigma Aldrich Company, St. Louis, MO, USA). After washing, PBMCs were cultured in RPMI-1640 (Invitrogen, CA, USA) supplemented with 1% sodium pyruvate, 1% nonessential amino acids, 1% kanamycin and 1% glutamax at 37 °C with 5% CO2. Cells were cultured in the presence of 10% nonpregnant human serum, 10% pregnancy serum, or 10% of supernatant from early or term placental explants. Hormones at pregnancy levels (10 ng/ml of 17β-oestradiol, 10 ng/ml of oestriol, 200 ng/ml of progesterone; all hormones were from Sigma Aldrich) or recombinant human TGF-β1 (R&D systems, UK) were separately added to the culture medium supplemented with nonpregnant human serum.
In order to induce Treg from PBMCs, isolated PBMCs (1 x 106cells) were cultured for 3 days in the presence of the aforementioned different pooled sera or placental supernatants, as well as in the presence of hormones or recombinant TGF-β1. In all experiments with PBMCs, 10 IU of recombinant human IL-2 (R&D systems, UK) were added.
In order to induce Treg from CD4+CD25‒ T cells, CD4+CD25‒ T cells were sorted by use of flow cytometry (FACS Aria, Becton and Dickinson) and cultured (1 x 106cells) for 3 days in the presence of the aforementioned different pooled sera, placental supernatants and hormones. For polyclonal activation, CD4+CD25‒ T cells were cultured with 1 μg/ml of plate-bound anti-CD3 (clone OKT-3), 5 μg/ml of anti-CD28 (BD Biosciences; USA) and 10 IU of recombinant human IL-2.
The percentages of CD4+CD25+ T cells and CD4+CD25+Foxp3+ T cells in the induction assays were analysed by means of flow cytometry.
Treg induced from sorted CD4+CD25‒ T cells as described above were further investigated in a suppression assay. First, induced Treg were isolated using the CD4+CD25+CD127dimisolation kit (Miltenyi Biotec, Germany). CD4+CD25‒ T cells were isolated from PBMCs from the same donor using CD4 and CD25 microbeads (Miltenyi Biotec, Germany). Isolated CD4+CD25‒ and CD4+CD25+CD127dim Treg (25,000 cells : 25,000 cells) were cultured alone or co-cultured in a 1:1 ratio and stimulated with 1 μg/ml immobilised anti-CD3 and 5 μg/ml of anti-CD28. After 72 hours, H3-thymidine was incorporated, and the cells were harvested after 18 hours in order to measure cell proliferation. The percentage of suppression was calculated by use of the following formula: 1 ‒ (cpm incorporated in the cocultured cells / cpm of CD4+CD25‒ cells cultured alone) x 100.
Cytokine profile and HLA-G
The cytokine concentration in pooled pregnancy sera, pooled nonpregnant sera, pooled placental supernatants, and supernatants from the induction assays was analysed for tumour necrosis factor-α (TNF-α), interferon-γ (IFN-γ), IL-1, IL-6, IL-17 and IL-10 using a customised bioplex assay (Bio-Rad Laboratories, CA, USA). The samples were prepared and run according to the manufacturer’s protocol, then read and analysed using the Bioplex manager software (Bio-Rad).
TGF-β1 and soluble HLA-G were measured in the samples described above by use of sandwich enzyme-linked immunosorbent assay. For TGF-β1, the detection and capture antibodies were obtained from BD Biosciences. The recombinant human proteins used for the standard curve were obtained from R&D systems UK. The nonclassical major histocompatibility complex class I molecule HLA-G was determined using sHLA-G kit (Biovendor, CZ) according to the manufacturer’s protocol. The samples were read at 450 nm using Spectramax 190 and analysed using Softmax Pro software.
Statistical analysis
Significant differences of related samples were calculated using the nonparametric Wilcoxon signed-rank test. For all tests a significance level of p <0.05 was applied.
Results
Effect of pregnancy sera and placental supernatant on the proportion of Treg
We first determined whether soluble pregnancy factors are able to increase the percentage of CD4+CD25+FOXP3+ Treg in PBMCs of nonpregnant, healthy women. Cells were cultured in the presence of pooled sera from the second or third trimester of normal pregnancy, representing mainly the sum of maternally derived pregnancy factors, or in the presence of filtered supernatant from early placental explants, representing mainly fetal factors. The analysis showed that pregnancy sera and placental supernatant induced a slight, insignificant, increase in the percentage of CD4+CD25+ activated T cells (fig. 1A). However, the percentage of Treg rose significantly in the presence of third trimester pregnancy sera (p = 0.028) and early placental supernatants (p = 0.018) (fig. 1B).
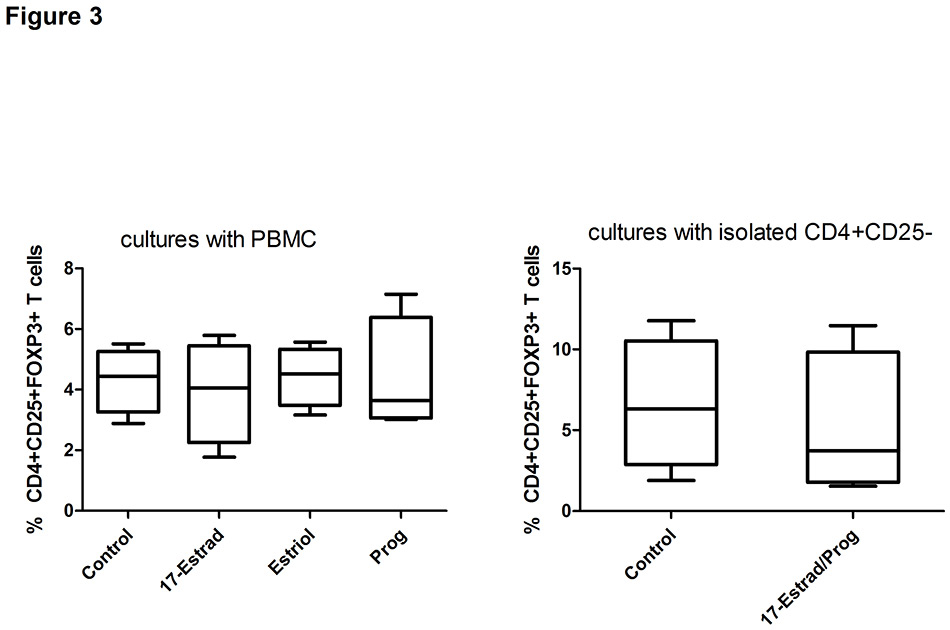
Figure 3
Effects of hormones on the proportion of regulatory T cells (Treg).
Effect of 17-oestradiol, oestriol and progesterone used at pregnancy levels on the proportion of Treg among PBMC (A) and CD4+CD25‒ T cells (B) from healthy women (n = 4). Data are expressed as boxplots with whiskers from minimum to maximum and statistical analysis was performed using Wilcoxon signed rank test.
Control = non-pregnant human serum; 17-Estrad = 17β-oestradiol; Prog = progesterone; 17-Estrad/Prog = combination of 17β-oestradiol and progesterone; PBMC = peripheral blood mononuclear cell
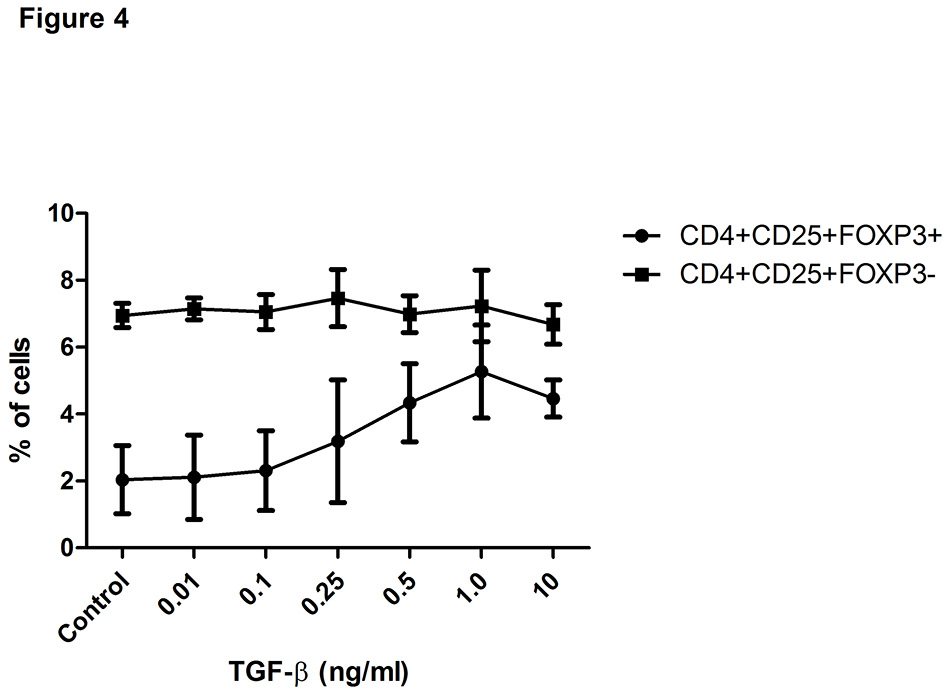
Figure 4
Transforming growth factor-β (TGF-β) increases the proportion of regulatory T cells (Treg).
Displayed is the effect of increasing doses of TGF-β1 on the percentages of CD4+CD25+FOXP3+ and CD4+CD25+FOXP3- T cells among PBMC of healthy women (n = 2). Data are expressed as dots, error bars representing mean ± SEM.
Control = nonpregnant human serum
We then investigated the induction of Treg from isolated CD4+CD25‒ T cells (fig. 2A, upper panel), which were exposed separately to three different conditions: pregnancy sera, supernatant from early placental explants, and supernatant from term placental explants. After 3 days, both the percentage of induced CD4+CD25+FOXP3+ Treg (fig. 1A, lower panel) and the percentage of activated T cells (CD4+CD25+) were analysed. No difference in the percentage of activated T cells was observed (fig. 2B). By contrast, the percentage of CD4+CD25+FOXP3+ cells was significantly higher among cells cultured in the presence of supernatant from early placental explants than among those cultured in control sera (p = 0.028) or third trimester sera (p = 0.028) (fig. 2A lower panel and fig. 2C).
Analysis of single factors of pregnancy sera and placental supernatants
Since pregnancy is associated with a marked increase of sex steroid hormones in the maternal circulation, the effect of 17β-oestradiol, oestriol and progesterone on the proportion of Treg was analysed. Though the hormones were applied at concentrations found during the third trimester, there was no effect on the percentage of Treg (fig. 3), neither in cultures with PBMC nor in cultures with isolated CD4+CD25‒ T cells.
Furthermore, pregnancy serum and supernatant from placental explants were analysed for factors that could play a role in cell activation and induction or expansion of Treg (table 1). Unlike the control sera and third trimester pregnancy sera, supernatant of early placental explants contained substantial amounts of proinflammatory cytokines such as TNF-α, IFN-γ, IL-1, IL-6 and IL-17 and showed the highest levels of soluble HLA-G. In addition, both supernatant of early placental explants and pregnancy sera displayed elevated levels of active TGF-β1. In contrast to supernatant of early placental explants, supernatant of term placental explants showed lower levels of TGF-β1 and lower levels of sHLA-G but higher levels of TNF-α and of IL-6. Interestingly, TGF-β1 concentrations at levels present in sera of the third trimester and in supernatants of early placental explants were able to augment the proportion of Treg in a dose-dependent manner (p <0.05 for the difference between CD4+CD25+FOXP3+ cells in control medium or with 1 ng/ml TGF-β1, fig. 4).
Cytokine secretion and suppressive function of regulatory T cells induced from the pool of CD4+CD25‒ T cells
To analyse the function of Treg induced from isolated CD4+CD25‒ T cells, the cell culture supernatants were analysed for various secreted cytokines. Proinflammatory cytokines such as IFN-γ, TNF-α and IL-17 could be detected in similar amounts in cell cultures exposed to each control sera, pregnancy sera, and the supernatant of placental explants (fig. 5 A–C). In contrast, the concentration of IL-10 was significantly elevated in the culture supernatants of Treg induced in the presence of supernatant of early placental explants relative to cultures in pregnancy sera (p = 0.046) or control sera (p = 0.046) (fig. 5D). Thus, the elevated amounts of IL-10 corresponded to the increased percentage of Treg induced in cell cultures of CD4+CD25‒ T cells which were exposed to supernatants of early placental explants.
Next, we analysed the suppressive function of induced Treg that were generated in the induction assay from sorted CD4+CD25‒ T cells of nonpregnant healthy women. As shown in figure 6, isolated Treg, defined as CD4+CD25+CD127lowcells, that were induced in the presence of pregnancy serum or supernatant of early placental explants were able to suppress the proliferation of autologous CD4-CD25‒ T effector cells (mean suppression rates: 97% with control sera, 62% with pregnancy sera, 51% with placental supernatant).
|
Table 1:Cytokines and soluble HLA-G in sera and placental supernatant. |
| |
Control sera+
|
Pregnancy sera
|
Placental supernatants
|
Placental supernatant
|
| |
|
3rd trimester
++
|
Early*
|
Term*
|
|
TNF-α (pg/ml) |
Below** |
Below** |
344.5 |
666.6 |
|
IFN-γ (pg/ml) |
Below** |
Below** |
2692.8 |
1120.4 |
|
IL-1(pg/ml) |
Below** |
Below** |
3.6 |
7.6 |
|
IL-6 (pg/ml) |
0.05 |
0.05 |
2769.7 |
4338.1 |
|
IL-17 (pg/ml) |
Below** |
Below** |
153.5 |
29.8 |
|
IL-10 (pg/ml) |
Below** |
Below** |
2.1 |
1.5 |
|
TGF-β1 (pg/ml) |
7.3 |
971.3 |
865.9 |
287 |
|
sHLA-G (U/ml) |
22.7 |
73.6 |
270.4 |
11.8 |
| + Pooled female non-pregnant sera
++ Pooled third trimester pregnancy sera
* Mean of two supernatants from early or term placental explants
** Below = below detection limit
IFN-γ = interferon-gamma; IL-6 = interleukin 6; sHLA-G = soluble human leucocyte antigen-G; TGF-β1 = transforming growth factor-beta1; TNF-α = tumour necrosis factor-alpha |
Discussion
Our study shows that factors present in the maternal circulation during pregnancy and in the placenta augment the proportion of CD4+CD25+FOXP3+ T cells that show a cytokine profile and suppressive activity characteristic of regulatory T cells. By analysing the effects of pregnancy serum and supernatant of placental explants on numerical changes of Treg, we compared the relevance of maternal versus fetal factors. We showed that supernatant of early placental explants was the most powerful factor, since it generated increased percentages of Treg not only from PBMCs but also from isolated CD4+CD25‒ T cells. By contrast, pregnancy serum containing predominantly maternal pregnancy factors was able to increase the percentage of Treg among PBMC, but had no effect on the induction of Treg from isolated CD4+CD25‒ T cells.
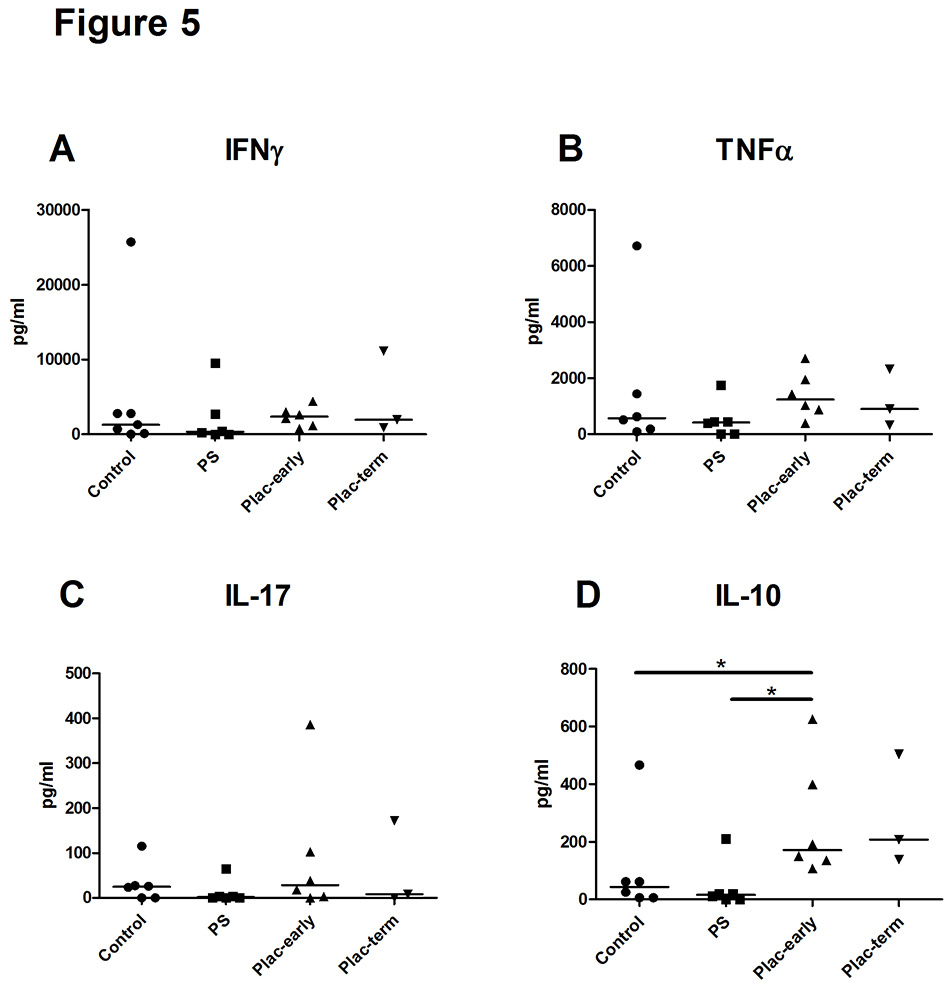
Figure 5
Cytokine secretion of regulatory T (Treg) cells induced by pregnancy serum or placental supernatant.
Cytokine concentrations (picograms per millilitre) in culture supernatants of Treg induced from sorted CD4+CD25‒ T cells of healthy donors in the presence of pregnancy serum or supernatant from early or term placental explants. Statistical analysis was performed using Wilcoxon signed rank test. Asterisk indicates p <0.05.
Control = non-pregnant human serum, PS3 = pooled third trimester pregnancy serum; Plac-early = supernatant of early placental explants; Plac-term = supernatant of term placental explants: IFNγ = interferon-γ; IL = interleukin; TNFα = tumour necrosis factor-α
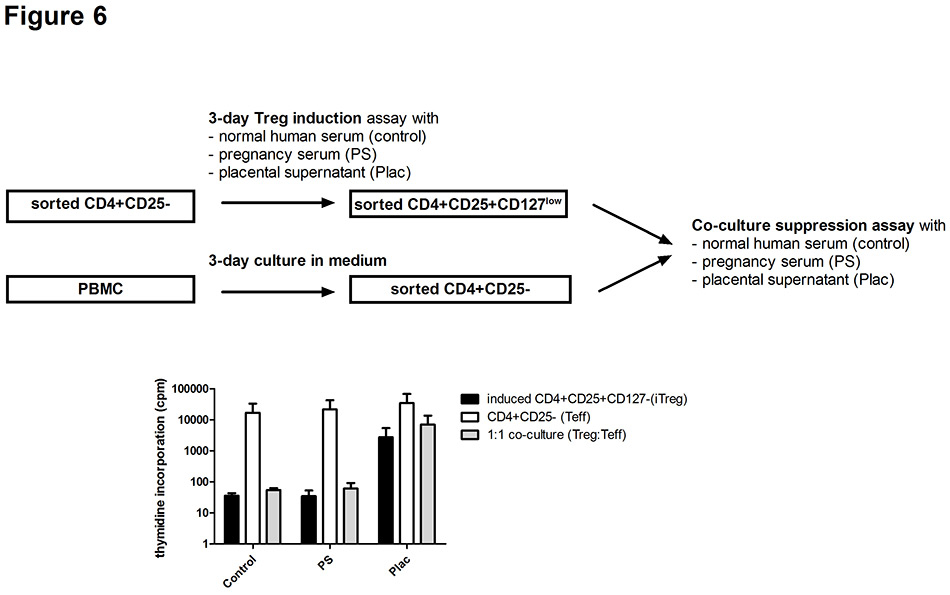
Figure 6
Suppressive function of regulatory (Treg) T cells induced by soluble pregnancy factors.
Treg cells induced from CD4+CD25‒ T cells (iTreg) of two healthy donors in the presence of different soluble pregnancy factors were isolated and used in a co-culture suppression assay with autologous CD4+CD25‒ T cells (Teff). iTreg and Teff cells cultured alone or in a 1:1 setting were analysed for proliferation and for the suppressive capacity of Treg. Data are expressed as mean ± SEM.
Control = nonpregnant human serum; PS3 = pooled third trimester pregnancy serum; Plac = supernatant of early placental explants.
The effect of pregnancy serum was likely related to the increased concentration of TGF-β1, but not related to oestrogen or progesterone. This contradicts previous studies that found an increase in Treg both in mice treated with oestrogen and in human male CD4+CD25+ cells cultured with pregnancy doses of oestradiol combined with activation through CD3/CD28 [15, 16]. Differences in study design and in the culture conditions may account for the disagreement. To detect the effect of hormones on Treg expansion, a longer culture duration or a different stimulation modus than used in our study might be necessary.
Even though fetal antigens are released into the maternal circulation during pregnancy, the placenta remains the main source of fetal antigens [19]. In our study, supernatants of early placental explants served as a source of fetal antigens. The measurement of soluble HLA-G, which is primarily derived from fetal trophoblast cells [20], was used to demonstrate the prominence of fetal antigens in placental supernatants as compared with pregnancy sera or sera of nonpregnant women. Fetal antigens are the most relevant factors driving the expansion of fetus-specific Treg that accumulate in the placenta during murine pregnancy and even persist after delivery [18, 21]. In contrast to thymus-derived Treg, these peripherally induced fetus-specific Treg are essential for successful pregnancy and are regulated by a conserved noncoding DNA sequence of the foxp3 locus, namely CNS 1 [21]. CNS1 contains binding sites for the Smad3 protein which are activated by TGF-β underlining the importance of this particular cytokine for the differentiation of peripheral Treg from CD4+CD25- naïve T cells [21, 22]. Hence, fetal antigens and TGF-β are important for the expansion of peripheral Treg during pregnancy. High levels of TGF-β produced by trophoblast cell lines were recently found to be an important factor for the induction of Treg from CD4+CD25- T cells [23]. Our analysis showed that both pregnancy serum and supernatant of placental explants contained similar concentrations of TGF-β, yet only placental supernatant was able to generate CD4+CD25+FOXP3+ Treg from isolated CD4+CD25‒ T cells. Our data therefore support the Treg-inducing/expanding effect of additional factors such as fetal antigens, which are more prevalent in placental supernatant than in pregnancy serum.
In the comparison of supernatants from early versus term placental explants, a significant increase of Treg from sorted CD4+CD25‒ T cells could be detected only in cultures with supernatant from early placental explants. This phenomenon might be due to the fact that HLA-G as a marker of extravillous trophoblast cells (EVT cells) is abundantly expressed by first trimester placenta, because EVT cells are important for appropriate trophoblast invasion into decidual intersitium and spiral arteries in successful pregnancy [24]. During the course of pregnancy, in particular in the third trimester, HLA-G-expressing EVT cells and soluble HLA-G decrease significantly [25, 26].
Therefore, the larger proportion of HLA-G-expressing EVT cells in first trimester placental explants relative to term placental explants might be responsible for the more powerful Treg expansion by the supernatant of early placental explants. In addition, the placental protein 14, also known as glycodelin, is found in higher concentrations in the first trimester decidua than in late pregnancy decidua [27]. This placental glycoprotein has been shown to expand Treg cells and could therefore be an additional factor for the more pronounced effects of supernatant from early placental explants [28].
In contrast to pregnancy serum, supernatant of early placental explants contained both anti- and proinflammatory cytokines. This mirrors the cytokine milieu present at the fetomaternal interface during early normal pregnancy and underlines the fact that that a certain amount of inflammation is necessary for successful placentation [29–31]. Proinflammatory cytokines do play a role in cell activation as well as in the development of INFγ and TNF-α secreting Th1 cells or IL-17-secreting Th17 subsets [32, 33]. On the other hand, it has been shown that proinflammatory cytokines such as IL-6 and TNF-α render T effector cells resistant to the suppressive activity of Treg [34]. However, during pregnancy pro- and anti-inflammatory cytokines need to be well balanced since a predominance of either Th1 cells or Th17 cells has been associated with recurrent miscarriage or pre-eclampsia [35]. Compared with third trimester sera or nonpregnant sera, supernatant of early placental explants did not induce an increase of activated CD4+CD25+ T cells, Th1 cells or Th17 cells, but rather augmented the percentage of CD4+CD25+FOXP3+ Treg cells. This predominance of Treg is of interest, since the cytokines IL-1β and IL-6 present in supernatant of early placental explants are known to be required for the differentiation of human Th17 cells [36]. However, elevated concentrations of TGF-β inhibit the development of Th17 cells and promote the induction of Treg [37].
In our experiments with PBMC, an increase in the percentage of Treg could be seen in cell cultures stimulated with 10 IU IL-2 and exposed to either third trimester pregnancy sera or supernatant of early placental explants. In these PBMC cultures, antigen-presenting cells (APCs), including dendritic cells and macrophages, could play an important role in Treg cell induction and expansion as shown by others [38, 39]. In this respect, HLA-G and cytokines such as IL-10, TGF-β, and IFN-γ are among the factors that induce tolerogenic APCs [5, 40, 41]. During human pregnancy, tolerogenic APCs emerge especially at the fetomaternal interface, facilitating the induction and expansion of Treg [42]. One important mechanism by which tolerogenic monocytes from first trimester decidua induce Treg is the expression of indoleamine 2,3-dioxygenase [43].
The main limitation of our study is the small sample size. The representative character of our findings might therefore be limited. However, recent findings about the significance of human placental-derived factors for the expansion of Treg support our results [44].
In conclusion, it appears in our study that a combination of cytokines and fetal antigens present in supernatant of first trimester placental explants showed the most powerful impact on the frequency of CD4+CD25+FOXP3+ Treg cells, whereas maternal circulating factors were less effective. However, extended studies are needed to clarify which placenta-derived molecules exert antigen-specific Treg expansion and function, thereby supporting fetomaternal immune tolerance during human pregnancy.
References
1 Guleria I, Sayegh MH. Maternal acceptance of the fetus: true human tolerance. J Immunol. 2007;178(6):3345–51. Epub 2007/03/07.
2 Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167(3):1245–53. Epub 2001/07/24.
3 Walker MR, Kasprowicz DJ, Gersuk VH, Benard A, Van Landeghen M, Buckner JH, et al. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25- T cells. J Clin Invest. 2003;112(9):1437–43. Epub 2003/11/05.
4 Ohkura N, Kitagawa Y, Sakaguchi S. Development and maintenance of regulatory T cells. Immunity. 2013;38(3):414–23. Epub 2013/03/26.
5 Carosella ED, Gregori S, LeMaoult J. The tolerogenic interplay(s) among HLA-G, myeloid APCs, and regulatory cells. Blood. 2011;118(25):6499–505. Epub 2011/10/01.
6 Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J Immunol. 2002;168(3):1080–6. Epub 2002/01/22.
7 Wahl SM, Swisher J, McCartney-Francis N, Chen W. TGF-beta: the perpetrator of immune suppression by regulatory T cells and suicidal T cells. J Leukoc Biol. 2004;76(1):15–24. Epub 2004/02/18.
8 Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5(3):266–71. Epub 2004/02/06.
9 Zenclussen AC, Gerlof K, Zenclussen ML, Sollwedel A, Bertoja AZ, Ritter T, et al. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am J Pathol. 2005;166(3):811–22. Epub 2005/03/04.
10 Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod. 2004;10(5):347–53. Epub 2004/03/05.
11 Tilburgs T, Roelen DL, van der Mast BJ, van Schip JJ, Kleijburg C, de Groot-Swings GM, et al. Differential distribution of CD4(+)CD25(bright) and CD8(+)CD28(-) T-cells in decidua and maternal blood during human pregnancy. Placenta. 2006;27(Suppl A):S47–53. Epub 2006/01/31.
12 Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004;112(1):38–43. Epub 2004/04/21.
13 Mjosberg J, Svensson J, Johansson E, Hellstrom L, Casas R, Jenmalm MC, et al. Systemic reduction of functionally suppressive CD4dimCD25highFoxp3+ Tregs in human second trimester pregnancy is induced by progesterone and 17beta-estradiol. J Immunol. 2009;183(1):759–69. Epub 2009/06/19.
14 Wegienka G, Havstad S, Bobbitt KR, Woodcroft KJ, Zoratti EM, Ownby DR, et al. Within-woman change in regulatory T cells from pregnancy to the postpartum period. J Reprod Immunol. 2011;88(1):58–65. Epub 2010/10/22.
15 Polanczyk MJ, Carson BD, Subramanian S, Afentoulis M, Vandenbark AA, Ziegler SF, et al. Cutting edge: estrogen drives expansion of the CD4+CD25+ regulatory T cell compartment. J Immunol. 2004;173(4):2227–30. Epub 2004/08/06.
16 Prieto GA, Rosenstein Y. Oestradiol potentiates the suppressive function of human CD4 CD25 regulatory T cells by promoting their proliferation. Immunology. 2006;118(1):58–65. Epub 2006/04/25.
17 Schumacher A, Wafula PO, Bertoja AZ, Sollwedel A, Thuere C, Wollenberg I, et al. Mechanisms of action of regulatory T cells specific for paternal antigens during pregnancy. Obstet Gynecol. 2007;110(5):1137-45. Epub 2007/11/06.
18 Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490(7418):102–6. Epub 2012/10/02.
19 Kumpel BM, Manoussaka MS. Placental immunology and maternal alloimmune responses. Vox Sang. 2012;102(1):2–12.
20 Hunt JS, Petroff MG, McIntire RH, Ober C. HLA-G and immune tolerance in pregnancy. FASEB J. 2005;19(7):681–93. Epub 2005/04/29.
21 Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 2012;150(1):29–38. Epub 2012/07/10.
22 Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198(12):1875–86. Epub 2003/12/17.
23 Ramhorst R, Fraccaroli L, Aldo P, Alvero AB, Cardenas I, Leiros CP, et al. Modulation and recruitment of inducible regulatory T cells by first trimester trophoblast cells. Am J Reprod Immunol. 2012;67(1):17–27. Epub 2011/08/09.
24 Dahl M, Hviid TV. Human leucocyte antigen class Ib molecules in pregnancy success and early pregnancy loss. Hum Reprod Update. 2012;18(1):92–109. Epub 2011/11/25.
25 Zhu X, Han T, Yin G, Wang X, Yao Y. Expression of human leukocyte antigen-G during normal placentation and in preeclamptic pregnancies. Hypertens Pregnancy. 2012;31(2):252–60. Epub 2011/12/14.
26 Steinborn A, Varkonyi T, Scharf A, Bahlmann F, Klee A, Sohn C. Early detection of decreased soluble HLA-G levels in the maternal circulation predicts the occurrence of preeclampsia and intrauterine growth retardation during further course of pregnancy. Am J Reprod Immunol. 2007;57(4):277–86. Epub 2007/03/17.
27 Julkunen M, Rutanen EM, Koskimies A, Ranta T, Bohn H, Seppala M. Distribution of placental protein 14 in tissues and body fluids during pregnancy. BJOG. 1985;92(11):1145–51. Epub 1985/11/01.
28 Ochanuna Z, Geiger-Maor A, Dembinsky-Vaknin A, Karussis D, Tykocinski ML, Rachmilewitz J. Inhibition of effector function but not T cell activation and increase in FoxP3 expression in T cells differentiated in the presence of PP14. PloS one. 2010;5(9):e12868. Epub 2010/10/05.
29 Ostensen M, Forger F, Villiger PM. Cytokines and pregnancy in rheumatic disease. Ann N Y Acad Sci. 2006;1069:353–63.
30 Champion H, Innes BA, Robson SC, Lash GE, Bulmer JN. Effects of interleukin-6 on extravillous trophoblast invasion in early human pregnancy. Mol Hum Reprod. 2012;18(8):391–400.
31 Chaouat G, Zourbas S, Ostojic S, Lappree-Delage G, Dubanchet S, Ledee N, et al. A brief review of recent data on some cytokine expressions at the materno-foetal interface which might challenge the classical Th1/Th2 dichotomy. J Reprod Immunol. 2002;53(1-2):241–56.
32 Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383(6603):787–93. Epub 1996/10/31.
33 Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–32. Epub 2005/10/04.
34 Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nature Med. 2007;13(4):423–31. Epub 2007/03/27.
35 Saito S, Nakashima A, Shima T, Ito M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol. 2010;63(6):601–10. Epub 2010/05/12.
36 Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454(7202):350–2. Epub 2008/05/13.
37 Crome SQ, Wang AY, Levings MK. Translational mini-review series on Th17 cells: function and regulation of human T helper 17 cells in health and disease. Clin Exp Immunol. 2010;159(2):109–19. Epub 2009/11/17.
38 Belkaid Y, Oldenhove G. Tuning microenvironments: induction of regulatory T cells by dendritic cells. Immunity. 2008;29(3):362–71. Epub 2008/09/19.
39 Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8(10):1086–94. Epub 2007/09/18.
40 Kosiewicz MM, Alard P. Tolerogenic antigen-presenting cells: regulation of the immune response by TGF-beta-treated antigen-presenting cells. Immunol Res. 2004;30(2):155–70. Epub 2004/10/13.
41 von Rango U. Fetal tolerance in human pregnancy – a crucial balance between acceptance and limitation of trophoblast invasion. Immunol Lett. 2008;115(1):21–32. Epub 2007/12/07.
42 Hsu P, Santner-Nanan B, Dahlstrom JE, Fadia M, Chandra A, Peek M, et al. Altered decidual DC-SIGN+ antigen-presenting cells and impaired regulatory T-cell induction in preeclampsia. Am J Pathol. 2012;181(6):2149–60. Epub 2012/10/16.
43 Vacca P, Cantoni C, Vitale M, Prato C, Canegallo F, Fenoglio D, et al. Crosstalk between decidual NK and CD14+ myelomonocytic cells results in induction of Tregs and immunosuppression. Proc Natl Acad Sci U S A. 2010;107(26):11918–23. Epub 2010/06/16.
44 Svensson-Arvelund J, Mehta RB, Lindau R, Mirrasekhian E, Rodriguez-Martinez H, Berg G, et al. The human fetal placenta promotes tolerance against the semiallogeneic fetus by inducing regulatory T cells and homeostatic M2 macrophages. J Immunol. 2015;194(4):1534–44.





