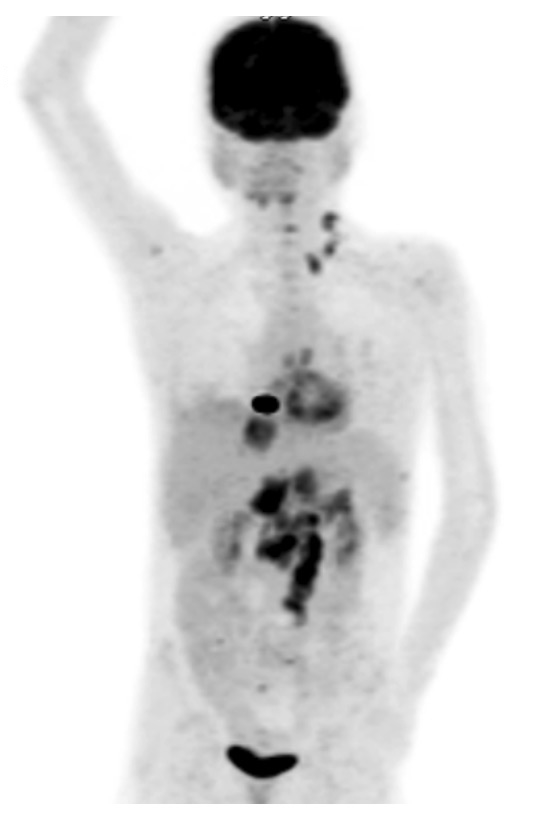
Figure 1
Positron emission tomography-computed tomography scan showing fluorodeoxyglucose uptake in the liver, bones and lymph nodes (highlighted Virchow’s node in the left supraclavicular area).
DOI: https://doi.org/10.4414/smw.2015.14165
Cystic fibrosis (CF) is one of the most common genetic disorders. CF is caused by autosomal recessive mutations of the cystic fibrosis transmembrane regulator (CFTR), which cause dysfunction of epithelial membranes within the gastrointestinal and respiratory systems [1]. Following improvement in disease management, survival of CF patients has increased significantly in past decades. The median predicted survival for CF patients in the United States has increased by almost 10 years, from age 31.3 years in 2002 to 41.1 years in 2012 (Cystic Fibrosis Foundation, www.cfff.org). According to a prediction model, children born with CF in 2000 have a median survival of 50 years [2, 3]. However, owing to chronic airway infection, haemoptysis, development of pulmonary hypertension and respiratory failure, CF patients remain one of the most important groups undergoing lung transplantation (LTx) [4]. LTx is the ultimate therapy for end-stage CF lung disease [5] and we and others have demonstrated a survival benefit for CF patients undergoing this therapy [6–8]. The overall cancer risk in nontransplanted CF patients is similar to the background risk of the normal population, with a standardised incidence ratio (SIR) of 1.1. In transplanted CF patients more tumours have been observed than expected (SIR 2.7). In both groups, the risk is particularly high for gastrointestinal tumours (SIR 3.5 in nontransplanted and SIR 17.3 in transplanted patients) [9–11].
In this context, our group has previously described the case of a lung transplanted CF patient developing pancreatic cancer [12]. Here we extend and discuss this series with two additional CF patients who developed adenocarcinoma of the gastrointestinal tract following LTx.
A 42-year-old male CF patient developed metastatic cholangiocarcinoma 5 years following bilateral LTx. Diagnosis of CF was made at the age of 4 years (unknown genetic). As a result of progressive CF lung disease, he underwent LTx in 2002 (at the age of 34 years). Apart from pancreatic insufficiency, the medical history, with regards to the gastrointestinal tract, was remarkable for a cholecystectomy in 1988 which was performed owing to gallstone-induced cholecystitis.
Maintenance immunosuppression after LTx at our centre consisted of a triple combination therapy with prednisone (PDN), ciclosporin (CSA) and mycophenolate mofetil (MMF). Because of the development of chronic lung allograft dysfunction (CLAD) 3 years after LTPL, CSA was switched to tacrolimus (TAC) and extracorporeal photopheresis (ECP) was started. Seven years after transplantation, the patient developed recurrent episodes of fever and elevation of C-reactive protein levels without signs of infection. Abdominal ultrasound and magnetic resonance imaging (MRI) studies showed a 5x4 cm lesion of the left liver lobe with stenosis of the left ductus hepaticus. After the exclusion of distant metastasis using positron emission tomography-computed tomography (PET-CT), a resection of the extrahepatic bile ducts with roux-en-Y hepaticojejunostomy and left extended hemihepatectomy were performed. The histology of the resected specimen revealed a moderately differentiated adenocarcinoma of the extrahepatic bile duct (cholangiocarcinoma; pT3, pN1, G2, cM0).
In this scenario, an interdisciplinary panel judged adjuvant chemotherapy not to be a valuable option because of the high risk of a relapse under immunosuppressive therapy.
A follow-up CT chest scan 7 months after tumour resection showed bilateral lung lesions, whose radiological appearance was compatible with pulmonary metastasis. Palliative chemotherapy using gemcitabine and, when liver and bone lesions developed, second-line therapy with oxaliplatin and capecitabine was initiated. Unfortunately, the patient died 18 months after tumour surgery as a result of tumour progression and liver failure. In the last 12 months the median trough (C0) TAC level was 11.3 ng/ml (target level 8–10 ng/ml), the MMF dose 500 mg BID and the PDN dose 7.5 mg ID (maintenance dose 0.1 mg/kg/day, body weight 75 kg),
A 27-year-old female CF patient developed metastatic gastric adenocarcinoma 9½ years after bilateral LTx. The diagnosis of CF (homozygous F508del) was made at the age of 9 months because of recurrent pulmonary infections. After the age of 6 years, CF lung disease progressed and, finally, the patient underwent LTx at the age of 17 in 2004. Maintenance immunosuppression consisted of a triple combination therapy with PDN, CSA and MMF. Three years after LTx a persistent gastric fistula following gastrostomy tube removal resulted in an excision of the fistula and a subtotal gastrectomy. An upper gastrointestinal endoscopy performed 4 years later showed discrete inflammation and fibrosis of the residual stomach tissue. The patient suffered repeatedly from severe constipation owing to episodes of distal intestinal obstructive syndrome (DIOS).

Figure 1
Positron emission tomography-computed tomography scan showing fluorodeoxyglucose uptake in the liver, bones and lymph nodes (highlighted Virchow’s node in the left supraclavicular area).
In August 2013, an abdominal ultrasound was performed because of diffuse epigastric pain, which showed several hepatic lesions. The MRI revealed coeliac, retroperitoneal and hilar hepatic lymphadenopathy. PET-CT detected fluorodeoxyglucose (FDG)-enriching lesions in the bones and in the supraclavicular lymph nodes (“Virchow’s node”, fig. 1). Fine needle aspiration of the latter showed adenocarcinoma cells, probably of gastrointestinal origin. Endoscopy confirmed the presence of a gastric tumour, which was found to be a poorly differentiated adenocarcinoma on biopsy. Probably as a result of paraneoplastic thrombophilia, the patient developed multiple venous thromboses and a fatal event of pulmonary embolism with fulminant right heart failure led to death. In the previous 12 months, the median trough (C0) CSA level was 44 μmg/l (target level calculated from the area under the concentration-time curve [AUC]: 50–70 μg/l), the MMF dose 1000 mg BID and the PDN dose 5 mg ID (maintenance dose 0.1 mg/kg/day, body weight 35 kg).
This case was described previously [12]. Briefly, an 18-year-old female CF patient developed metastatic pancreatic cancer 6 years after LTx. She was diagnosed with CF at the age of 7 weeks because of failure to thrive (unknown genetic). In 1999, she underwent bilateral LTx at the age of 12 owing to advanced CF lung disease. Maintenance immunosuppression consisted of triple combination therapy with PDN, CSA and MMF. Therapy with somatropin was started in 2002 because of growth retardation and low serum levels of insulin-like growth factor-1 (IGF-1).
In April 2004, the patient was hospitalised owing to symptomatic choledocholithiasis. Symptoms and cholestasis resolved with conservative therapy. Nine months later, she again developed upper abdominal pain and elevated cholestatic parameters. Abdominal CT revealed dilated intra- and extrahepatic bile ducts, an enlarged pancreatic head and an atrophic pancreatic corpus/tail. Transhepatic cholangiography with insertion of a percutaneous drain was performed and detected a massive dilatation of the ductus choledochus with a long distal stenosis. The patient underwent laparotomy; rapid section diagnosis of the pancreatic head revealed an adenocarcinoma. Pancreaticoduodenectomy was performed. The histology of the resected specimen confirmed a poorly differentiated ductal pancreatic adenocarcinoma with involvement of regional lymph nodes (pT3, pN1, cMO). One month later, liver lesions occurred and fine needle aspiration obtained malignant cells. Palliative chemotherapy with gemcitabine was initiated. After four cycles of chemotherapy, the patient decided to stop therapy and died shortly after. In the last 12 months, the median trough (C0) CSA level was 191 μg/l (target level calculated from the AUC: 160–190 μg/l), the MMF dose 500 mg BID and the PDN dose 5 mg ID (maintenance dose 0.1 mg/kg/day, body weight 43 kg).
In our cohort of 100 CF patients undergoing lung transplantation between 1992 and 2009 [13], three patients developed digestive tract cancer. This observation suggests an excess of cancer in an organ system known to be affected by CF. The reasons for the organ-specific increase of cancer rate in CF patients remain unclear.
The first case of a CF patient with gastrointestinal cancer was described in 1982, an adult with an extrahepatic biliary tract cancer [14]. The largest dataset that is currently available on the association between CF and cancer was published in 2013 [9], in which Maisonneuve et al. followed more than 40,000 CF patients who were registered at any of the 110 centres in the United States accredited by the Cystic Fibrosis Foundation during the period from 1990 to 2009. The overall cancer risk in non-transplanted patients was similar to the expected incidence rates in the general population observed by the National Cancer Institute (SIR 1.1). Following transplantation (92% of transplanted CF patients received a lung transplant), the risk of developing a solid cancer was, however, found to be increased. (SIR 2.7) This study confirms findings from previous studies [10] that reported an increased risk (SIR 17.3) of digestive tract cancer in CF patients, especially following transplantation.
Colon cancer represents the most common malignancy in CF patients, both in non-transplanted and in lung transplanted patients (SIR 6.2 and SIR 30.1) [9–11, 15, 16]. The immunosuppressive medications used after LTx can cause adverse gastrointestinal reactions including diarrhoea and intestinal infections [15]. These mechanisms may contribute to intestinal carcinogenesis. However, after LTx, in non-CF transplant recipients, colon cancer is less frequently observed than in CF transplant recipients [15]. Analysis of national databases in the UK and US from recipients of LTx for any indication showed higher incidences of lung and bronchial cancer and posttransplant lymphoproliferative disorder (PTLD) than colorectal cancer [17, 18]. Of interest, CF patients who underwent screening colonoscopy before the age of 50 years had a higher prevalence of both adenomas and advanced adenomas as compared with non-CF patients in the same age group [16, 19]. In all of these studies, retrospective risk calculation is potentially limited by the inclusion of older CF patients that might bear genotypes encoding for milder pulmonary phenotypes and a lower risk to develop cancers.
The factors that predispose CF patients to develop more adenomas than the general population and more gastrointestinal cancers following LTx than lung transplanted patients with other diagnoses are unknown, but it has been suggested that the localisation and expression of the CFTR gene might play a role [11]. Profound changes in the biology of epithelial cells and physiology of mucosal surfaces due to CFTR deficiency have been described [16]. In human foetal tissues, the highest levels of CFTR messenger RNA are seen in defined areas of the developing pancreas, liver, gall bladder and intestine [20]. The importance of the CFTR gene in the development of cancer is highlighted by the observation that homozygous mutations in the allelic variant F508del carry a higher risk for CF patients to develop colon cancer [10]. Moreover, mutations within the CFTR gene cause abnormal mucous production in exocrine glands, which, in turn, leads to obstruction with resultant duct dilatation and tissue damage. The resulting inflammation of the pancreatic tissue may represent a procarcinogenic stimulus [12]. It remains unclear, however, whether other factors contributed to the development of malignancies in our young female patient, such as the long-term application of exogenous somatropin. An increased risk for cholangiocarcinoma is also found among patients with gallstone disease, and gallstones are frequently found in patients with CF. However, only few cases of cholangiocarcinoma in patients with CF and especially after lung transplantation are known [9, 21, 22]. Whether in our second case of a patient with gastric adenocarcinoma, the mechanical stress and the consecutive inflammation following subtotal gastrectomy resulted in carcinogenic transformation is unclear, but this provides a potential explanation.
Of interest, a multicentre trial demonstrated antitumour effects by switching from calcineurin inhibitors to sirolimus, an inhibitor of mammalian target of rapamycin (mTOR), in kidney transplant recipients with squamous cell skin cancer [23]. In addition in-vitro studies targeting the mTOR pathway for colorectal cancer have provided promising perspectives [24]. Whether a switch from a calcineurin to an mTOR inhibitor might be a therapeutic strategy in lung transplanted CF patients with intestinal cancers remains, however, unclear at the moment.
In conclusion we report three cases of adenocarcinoma of the gastrointestinal tract in CF patients following LTx. These data highlight the need for an increased awareness of such complications in all lung transplant recipients presenting with abdominal symptoms. Moreover, these cases emphasise that patients under immunosuppressive therapies following lung transplantation are expected to develop posttransplant lymphoproliferative disorder but, as shown previously [9] and confirmed by these case series, there is an increased risk for the development of solid cancers in the gastrointestinal tract. Although such an association has been suggested by studies that showed an increase in the rate of colon cancer in lung transplanted CF patients as compared with non-CF patients, the case of a gastric cancer is a novel observation. As mentioned, our group has already published the case report on the CF patient with a pancreatic cancer (case 3), which, to the best of our knowledge is still the only published case [12].
Routine colonoscopy in CF patients is a controversial issue and is not recommended according to the European consensus of 2005 [25]. As such, it is not performed at our institution. During pretransplant evaluation in our centre, we perform upper and lower gastrointestinal endoscopy as screening for gastrointestinal cancer in all patients older than 50 years with suggestive symptoms or a positive faecal occult blood test. This case series should increase the awareness of physicians for the increased risk of gastrointestinal tumours in lung transplanted CF patients. In conclusion, the transplant community might consider whether endoscopic investigations should be performed as a baseline investigation before lung transplantation in all CF patients [10].
1 Ratjen F, Döring G. Cystic fibrosis. The Lancet. 2003;361(9358):681–9.
2 Dodge JA, Lewis PA, Stanton M, Wilsher J. Cystic fibrosis mortality and survival in the UK: 1947–2003. Eur Respir J. 2007;29(3):522–6.
3 O’Sullivan BP, Freedman SD. Cystic fibrosis. The Lancet. Elsevier Ltd; 2009;373(9678):1891–904.
4 Yusen RD, Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, et al. The Registry of the International Society for Heart and Lung Transplantation_ Thirtieth Adult Lung and Heart-Lung Transplant Report – 2013; Focus Theme_ Age. HEALUN. Elsevier; 2013;32(10):965–78.
5 A B. Update on cystic fibrosis: selected aspects related to lung transplantation. 2003:1–8.
6 Liou TG, Adler FR, Cahill BC, FitzSimmons SC, Huang D, Hibbs JR, et al. Survival effect of lung transplantation among patients with cystic fibrosis. JAMA. 2001;286(21):2683–9.
7 Aurora P, Whitehead B, Wade A, Bowyer J, Whitmore P, Rees PG, et al. Lung transplantation and life extension in children with cystic fibrosis. The Lancet. 1999;354(9190):1591–3.
8 Hofer M, Benden C, Inci I, Schmid C, Irani S, Speich R, et al. True survival benefit of lung transplantation for cystic fibrosis patients: the Zurich experience. J Heart Lung Transplant. 2009;28(4):334–9.
9 Maisonneuve P, Marshall BC, Knapp EA, Lowenfels AB. Cancer Risk in Cystic Fibrosis: A 20-Year Nationwide Study From the United States. JNCI Journal of the National Cancer Institute. 2013;105(2):122–9.
10 Maisonneuve P, FitzSimmons SC, Neglia JP, Campbell PW, Lowenfels AB. Cancer risk in nontransplanted and transplanted cystic fibrosis patients: a 10-year study. JNCI Journal of the National Cancer Institute. 2003;95(5):381–7.
11 Neglia JP, FitzSimmons SC, Maisonneuve P, Schöni MH, Schöni-Affolter F, Corey M, et al. The risk of cancer among patients with cystic fibrosis. Cystic Fibrosis and Cancer Study Group. N Engl J Med. 1995;332(8):494–9.
12 Petrowsky H, Schuster H, Irani S, Schäfer M, Jochum W, Schmid C, et al. Pancreatic cancer in cystic fibrosis after bilateral lung transplantation. Pancreas. 2006;33(4):430–2.
13 Inci I, Stanimirov O, Benden C, Kestenholz P, Hofer M, Boehler A, et al. Lung transplantation for cystic fibrosis: a single center experience of 100 consecutive cases. Eur J Cardiothoracic Surg. 2012;41(2):435–40.
14 Abdul-Karim FW, King TA, Dahms BB, Gauderer MW, Boat TF. Carcinoma of extrahepatic biliary system in an adult with cystic fibrosis. Gastroenterology. 1982;82(4):758–62.
15 Meyer KC, Francois ML, Thomas HK, Radford KL, Hawes DS, Mack TL, et al. Colon cancer in lung transplant recipients with CF: Increased risk and results of screening. J Cyst Fibros. 2011;10(5):366–9.
16 Billings JL, Dunitz JM, McAllister S, Herzog T, Bobr A, Khoruts A. Early Colon Screening of Adult Patients With Cystic Fibrosis Reveals High Incidence of Adenomatous Colon Polyps. J Clin Gastroenterol. 2013:1.
17 Sampaio MS, Cho YW, Qazi Y, Bunnapradist S, Hutchinson IV, Shah T. Posttransplant malignancies in solid organ adult recipients: an analysis of the U.S. National Transplant Database. Transplantation. 2012;94(10):990–8.
18 Collett D, Mumford L, Banner NR, Neuberger J, Watson C. Comparison of the incidence of malignancy in recipients of different types of organ: a UK Registry audit. Am J Transplant. 2010;10(8):1889–96.
19 Ferlitsch M, Reinhart K, Pramhas S, Wiener C, Gal O, Bannert C, et al. Sex-specific prevalence of adenomas, advanced adenomas, and colorectal cancer in individuals undergoing screening colonoscopy. JAMA. American Medical Association; 2011;306(12):1352–8.
20 Tizzano EF, Chitayat D, Buchwald M. Cell-specific localization of CFTR mRNA shows developmentally regulated expression in human fetal tissues. Hum Mol Genet. 1993;2(3):219–24.
21 Tesluk H, McCauley K, Kurland G, Ruebner BH. Cholangiocarcinoma in an adult with cystic fibrosis. J Clin Gastroenterol. 1991;13(4):485–7.
22 Naderi ASA, Farsian FN, Lee WM. Cholangiocarcinoma after lung transplantation in a patient with cystic fibrosis. Eur J Gastroenterol Hepatol. 2008;20(11):1115–7.
23 Euvrard S, Morelon E, Rostaing L, Goffin E, Brocard A, Tromme I, et al. Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med. 2012;367(4):329–39.
24 Wang X-W, Zhang Y-J. Targeting mTOR network in colorectal cancer therapy. World J Gastroenterol. 2014;20(15):4178–88.
25 Kerem E, Conway S, Elborn S, Heijerman H, Consensus Committee. Standards of care for patients with cystic fibrosis: a European consensus. 2005. pp. 7–26.
Disclosures: One of three cases discussed has been published by our group before. There are no potential conflicts of interest.