Figure 1
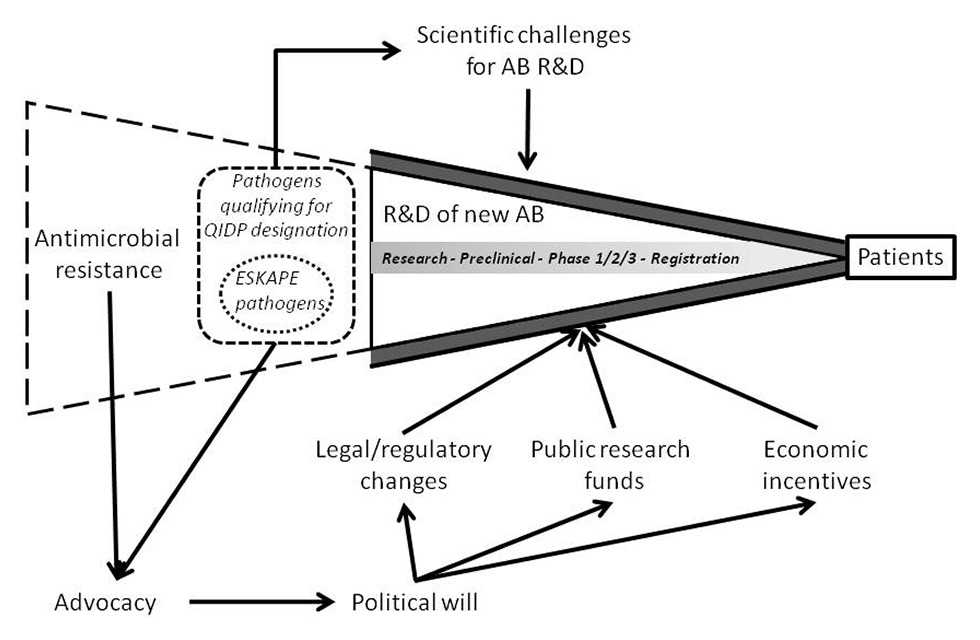
Essential determinants impacting the research and development (R&D) of new antibiotics.
QIDP = qualified infectious disease product.
DOI: https://doi.org/10.4414/smw.2015.14167
List of abbreviations
ABSSSI: acute bacterial skin and skin structure infections
AMR: antimicrobial resistance
ANRESIS: Swiss Antibiotic Resistance Surveillance database
BARDA: Biomedical Advanced Research and Development Authority
BLI: β-lactamase inhibitor
CABP: community-acquired bacterial pneumonia
CHMP: Committee for Medicinal Products for Human Use
cIAI: complicated intra-abdominal infection
CRE: carbapenem-resistant Enterobacteriaceae
cUTI: complicated urinary tract infections
EFPIA: European Federation of Pharmaceutical Industries and Associations
EMA: European Medicines Agency
ESBL: extended spectrum β-lactamase
EU: European Union
FDA: Food and Drug Administration
FNIH: Foundation for the National Institutes of Health
GAIN: Generating Antibiotic Incentives Now
Gram‒: Gram-negative.
Gram+: Gram-positive
HAP: hospital-acquired pneumonia
IMI: Innovative Medicine Initiative
JPI-AMR: Joint Programming Initiative on Antimicrobial Resistance
KPC: Klebsiella pneumoniae carbapenemase
MDR: multi-drug resistant
MRSA: meticillin resistant Staphylococcus aureus
NA: not applicable
ND4BB: New Drugs for Bad Bugs
NIBR: Novartis Institute for Biomedical Research
PDR: pan-drug resistant
QIDP: Qualified Infectious Disease Product
R&D: research and development
SME: small and medium enterprises
VAP: ventilator-associated pneumonia
VRSA: vancomycin-resistant Staphylococcus aureus
WAAAR: World Alliance against Antimicrobial Resistance
WHO: World Health Organization
XDR: extensively drug resistant
Although antimicrobial resistance (AMR) existed well before humans started to use antibiotics to treat infections, the menace of a post-antibiotic era threatening modern day medicine is closer to reality than ever before [1, 2]. Based on modelling studies, the impact of AMR was recently quantified as potentially causing the death of 300 million people during the next 35 years and having so much impact as to decrease the world gross domestic product by 2–3.5% compared with what it should be by 2050 [3, 4]. Although these crude predictions are based on large uncertainty and may overestimate the future health-economic impact of AMR [5], experts and policy makers agree that AMR should be considered a serious public health threat.
Both common and rare pathogens found in hospitals and in the community have seen their resistance rates increase dramatically in recent decades. As reported by the World Health Organization (WHO), more than 50% of Escherichia coli, Klebsiella pneumoniae and Staphylococcus aureus are reported as resistant to commonly used antibiotics in many parts of the world [6]. Several levels of acquired resistance to antibacterial agents have been defined. Multidrug resistant (MDR) pathogens are resistant to at least one antibiotic in three or more antibiotic classes, extensively drug resistant (XDR) pathogens are resistant to at least one antibiotic in all but one or two antibiotic classes and pan-drug resistant (PDR) pathogens are resistant to all antibiotics in all clinically relevant antibiotic classes [7]. The causes of such a rise in resistance are multiple, from natural evolution to antibiotics overuse in patients and farm animals [8]. In Switzerland, the overall prevalence of MDR among E. coli isolates at a single hospital in 2011 was still low (6.5%) compared with the numbers reported by the WHO and was similar in the community (5.7%), hospital (7.8%) and specialised outpatient clinic (5.3%) settings [9]. However, using data from the Swiss Antibiotic Resistance Surveillance database (ANRESIS) [10], Kronenberg et al. also showed that the prevalence of MDR increased significantly between 2004 and 2011 from 1% to 5.8% for E. coli and from 1.1% to 4.4% for K. pneumoniae[11].
Unfortunately, development of new antibiotics against these resistant bacteria did not progress at the same speed, and even lagged behind. This worrisome trend leaves physicians with a limited therapeutic arsenal for an increasing number of resistant pathogens and the absence of therapy for some PDR pathogens. Although a few new antibiotics might be available in Switzerland in the next couple of years, these new agents are not based on completely new mechanisms of action as they do not attack new bacterial targets. Nevertheless, through modified economic incentives and updated regulatory guidance, R&D of new antibiotics is slowly taking off again, as we describe in this narrative review.
During the flourishing years of antibiotic development in the 1940s and 50s, 12 different classes of antibiotics were discovered, but only seven have been discovered since, the last one being the lipopeptides in the 1980s [12]. Despite this discovery void, the panel of antibiotics available for clinicians to treat susceptible infections still remains large. Many of these antibiotic compounds belong to a known class, but were chemically modified from the original compound to increase the number of susceptible pathogens or to be insensitive to a particular mechanism of resistance. This flourishing era was followed by a steep decline in numbers of new antibiotics approved during the 1990s and 2000s. Indeed, difficulties in the development of new antibiotics spanning the whole spectrum of drug development appeared: drug discovery challenges [12], regulatory hurdles [13, 14], difficulties in conducting clinical trials [15] and economic disincentives [16, 17]. It also led large pharmaceutical companies to desert this area for more lucrative and less scientifically and economically challenging therapeutic areas, like cardiology and oncology [18]. Although this inverse trend (increasing AMR rates / decreasing availability of efficient antibiotics) has been known for a long time, many alerts and calls to action were needed to raise the awareness around the problem and trigger policy initiatives.
The starting point to raising awareness around the AMR issue and setting priorities for R&D of new antibiotics was to define resistant pathogens for which the highest unmet medical need exists or, in other words, resistant pathogens that have the potential to pose the most serious threat to public health. A first list of six key pathogens was created in 2008 under the acronym “ESKAPE pathogens” [19]: Enterococcus faecium, S. aureus, K. pneumoniae, Acinetobacter baumanii, Pseudomonas aeruginosa,andEnterobacter species. In 2014, the United States Food and Drug Administration (FDA) released a final list of a total of 21 target pathogens (table 1) with high unmet medical need [20]. As highlighted in the table, the unmet need is highest for XDR or PDR strains [21]. Importantly, this list shows that AMR is not a problem restricted to the healthcare setting only, but that common community-acquired infections such as urinary tract infections, gonorrhoea and tuberculosis are increasingly caused by MDR or XDR pathogens.
Since January 2012, when the Generating Antibiotic Incentives Now (GAIN) act came into effect in the United States, development of a new antibiotic agent active against one or several pathogens on this list allows a Qualified Infectious Disease Product (QIDP) designation to be obtained. This offers several incentives such as priority review of the new drug application file by the FDA and an economic incentive in the form of 5 additional years of market exclusivity if a marketing authorisation is obtained. Other economic incentives have been put in place recently in the United States. Funds from the Biomedical Advanced Research and Development Authority (BARDA), which are public, nonrefundable funds given to companies to develop specific compounds for potential bioterrorism threats, are now extended to compounds active against MDR, XDR and PDR pathogens [22]. Consequently, the overall United States budget for 2016 to fight AMR almost doubled to about 1.2 billion dollars.
| Table 1: Qualified infectious disease product (QIDP) qualifying pathogens: pathogens that have the highest unmet medical need. | ||||
| QIDP qualifying pathogen names [20] | Type of infection | |||
| Bacteria | Gram | Opportunistic | Hospital acquired | Community acquired |
| Acinetobacter species1 | Gram‒ | X | X | |
| Burkholderia cepaciacomplex | Gram‒ | X | X | |
| Campylobacter species | Gram- | X | X | |
| Clostridium difficile 1 | Gram+ | X | X | |
| Enterobacteriaceae 1 (especially Citrobacter, Enterobacter cloacae, Klebsiella pneumoniae, Escherichia coli, Proteus vulgaris, Salmonella, Serratia marcescens, Shigella) | Gram‒ | X | X | X |
| Enterococcus species | Gram+ | X | X | |
| Helicobacter pylori | Gram‒ | X | ||
| Mycobacterium tuberculosiscomplex1 | NA | X | X | |
| Neisseria gonorrhoeae 1 | Gram‒ | X | ||
| Neisseria meningitidis | Gram‒ | X | ||
| Nontuberculous mycobacteria species | NA | X | X | |
| Pseudomonas species1 | Gram‒ | X | X | |
| Staphylococcus aureus 1, 2 | Gram+ | X | X | X |
| Streptococcus agalactiae (group B) | Gram+ | X | ||
| Streptococcus pneumoniae | Gram+ | X | X | |
| Streptococcus pyogenes (group A) | Gram+ | X | ||
| Vibrio cholerae | Gram‒ | X | ||
| Fungi | ||||
| Aspergillus species | NA | X | ||
| Candida species | NA | X | X | |
| Coccidioides species | NA | X | ||
| Cryptococcus species | NA | X | ||
| 1 Key unmet need due to high and increasing prevalence of XDR or PDR strains [21] 2 Unmet need primarily for blood, bone and prosthesis infections and not for skin infection. NA = Not applicable. | ||||
The crisis of AMR needs to be tackled from various angles and most importantly, there needs to be a global political will leading to policy, legal and regulatory changes if our society wants to keep up with the issue. Essential determinants impacting R&D of new antibiotics are displayed in figure 1. Thanks to the mobilisation and lobbying activities of several independent initiatives, awareness of the AMR problem has finally risen since 2010 and the second step of concerted action is advancing. The initiatives that have played a key role in raising global awareness on AMR include the independent global network ReAct [23], the World Alliance against Antimicrobial Resistance (WAAAR) [24] and Antibiotic Action [25].

Figure 1
Essential determinants impacting the research and development (R&D) of new antibiotics.
QIDP = qualified infectious disease product.
Although the bulk of multidrug resistance might lie outside of these regions, the United States and the European Union (EU) are key regions for pharmaceutical companies to register a new drug. New regulatory guidance for infections caused by bacteria with high unmet medical need (i.e., in this case MDR, XDR and PDR bacteria) have been issued by the European Medicines Agency (EMA) and the FDA [26, 27], with the aim to facilitate the approval of new antibiotics. A new risk/benefit balance must be found between approving new antibiotics with limited clinical data, because of the high unmet need, and ensuring patients are treated with new antibiotics that are safe and efficacious. This kind of trade-off has been successfully applied in drug development, for example, to orphan diseases [28, 29]. Furthermore, potential new regulatory pathways being discussed for new antibiotic agents include (1) the Limited Population Drug Approval mechanism [30] and (2) the tiered approach in which different amounts of clinical data are required on the basis of the level of unmet medical need and pathogen-based indications are pursued rather than conventional disease-based indications [31].
Methodological investigations on how to ameliorate trial design for antibiotics are also being addressed by different consortia involving the academic, private and regulatory sectors, such as the Foundation for the National Institutes of Health (FNIH) biomarkers consortium [32] and the COMBACTE project [33], part of the Innovative Medicine Initiative (IMI)’s New Drug for Bad Bugs (ND4BB) programme [34]. IMI is a public-private partnership between the EU and the European Federation of Pharmaceutical Industries and Associations (EFPIA). Its ND4BB programme launched in 2013 aims to promote R&D of new antibiotics: the TRANSLOCATION and ENABLE projects focus on the discovery stage, the COMBACTE, COMBACTE-MAGNET, COMBACTE-CARE project include clinical trials with new compounds and the sharing of clinical development costs from phases I to III, and the DRIVE-AB project, which started in October 2014, aims to develop new economic models for antibiotics while preserving their use [35]. With a total budget for infectious diseases of over 710 million euros [36], such public-private partnerships are an important contribution to a wealth of measures that can be implemented to support the R&D of new antibacterials. Rapid diagnostic test development is another key area and has been recently incentivised through a prize [37].
Other initiatives include the Joint Programming Initiative on Antimicrobial Resistance (JPI-AMR) in the EU [38] and an independent review on AMR commissioned by the United Kingdom government in July 2014 [39]. The latter initiative aims to deliver a list of priority action items that should be agreed internationally to tackle AMR. Last but not least, the recent WHO action plan on antimicrobial resistance (adopted in May 2015) [40] will without a doubt foster attention and trigger a series of actions at a global level.
What is the current pipeline status and are any new antibiotic agents likely to reach Swiss pharmacies in the next 2 years? First, as listed in table 2, five systemic compounds have been approved since May 2014 in the United States (ceftolozane+tazobactam [41], oritavancin [42], tedizolid [43], dalbavancin [42] and ceftazidime+avibactam [44]). All had QIDP designation, meaning they are active against at least one of the pathogens with high unmet medical need (table 1). Four of these five new antibiotics or antibiotic combinations have pending marketing authorisations at the EMA and three of them (ceftolozane+tazobactam, oritavancin and tedizolid) have been submitted to the Swiss Agency for Therapeutic Products (Swissmedic). One new antibiotic agent, ceftobiprole [45], was approved in the EU at the end of 2013 and in Switzerland at the end of 2014, after unusually long regulatory approval delays [46]. Importantly, four of these six compounds have activity mainly against Gram-positive pathogens and will, therefore, be useful for treatment of meticillin-resistant Staphylococcus aureus (MRSA) infections. As MRSA infection rates are decreasing in many parts of Europe [47, 48], these new compounds are enlarging our arsenal but clearly not tackling the key emerging resistant Gram-negative pathogens. Only ceftolozane+tazobactam, which has a submission pending in Switzerland, partially covers MDR Gram-negative pathogens such as extended spectrum β-lactamase (ESBL)-producing strains.
Importantly for MDR Gram-negative infections, ceftazidime-avibactam was approved in January 2015 in the United States for complicated intra-abdominal infections (cIAI) and complicated urinary tract infections (cUTI), but restricted to patients who have limited treatment options [49]. Avibactam is the first new β-lactamase inhibitor (BLI) approved in two decades. Using the new regulatory guidance and QIDP designation registration process, the antibiotic combination was approved on the basis of data for ceftazidime alone, supplemented by in-vitroand phase II data for ceftazidime+avibactam in the targeted indications [49]. Phase III trials are planned to be completed in 2015 [50]. Although the label is currently restricted because of the limited availability of data, it will help to preserve use of the drug and development of resistance. It remains to be seen whether, once data from ongoing phase III or postmarketing studies are available for broad spectrum antibiotics such as ceftazidime+avibactam, pharmaceutical companies will try to expand the label, leaving it to countries’ public health systems to decide upon and put in place conservation measures for these precious new antibiotics.
| Table 2: Late-stage pipeline: systemic antibiotics recently approved, in registration or in phase III of clinical development. | ||||||
| Drug (brand name) - Company | Antibiotic class | Activity spectrum/resistant pathogens targeted | Phase and indication1 | Regulatory status | ||
| US | European Union | Switzerland | ||||
| Ceftazidime+ avibactam [44] (AvycazTM) ‒ AstraZeneca/Actavis | Cephalosporin + new BLI | Gram‒, including MDR P. aeruginosa, ESBL-producing strains and KPC | Approved February 2015 for cIAI in combination with metronidazole, and for cUTI in patients who have limited or no alternative treatment options, in phase III for HAP/VAP and cIAI | Approved February 2015 | Not submitted yet | Not submitted yet |
| Ceftolozane+ tazobactam [41] (ZerbaxaTM) ‒ Cubist Pharmaceuticals / Merck Sharp & Dohme | Cephalosporin + BLI | Gram‒, including carbapenem, piperacillin+tazobactam and ceftazidime-resistant Pseudomonas aeruginosa, ESBL-producing strains | Approved for cUTI and cIAI, in phase III for VAP and phase I for paediatric use | Approved December 2014 | Under review since August 2014 | Under review since September 20142 |
| Ceftobiprole medocaril [45] (Zevtera®/Mabelio®) – Basilea Pharmaceutica/Quintiles | Cephalosporin | Gram+ and ‒, including MRSA, VRSA, penicillin- and ceftriaxone-resistant Streptococcus pneumoniae, Enterobacteriaceae, P. aeruginosa | Approved for CABP and HAP, excluding VAP | Not submitted (additional phase III data required | Approved October 2013 | Approved December 2014 |
| Oritavancin [42] (OrbactivTM) – The Medicines Company | Glycopeptide | Gram+, including MRSA | Approved for ABSSSI, in phase I for paediatric use | Approved August 2014 | Approved May 2015 | Under review2 |
| Tedizolid phosphate [43] (SivextroTM) – Cubist Pharmaceuticals / Merck Sharp & Dohme | Oxazolidinone | Gram+, including MRSA and linezolid-resistant MRSA | Approved for ABSSSI, in phase III for HAP/VAP and for ABSSSI in adolescents | Approved June 2014 | Approved March 2015 | Under review since second quarter 20142 |
| Dalbavancin [42] (DalvanceTM/XydalbaTM) – Actavis / Durata Therapeutics | Glycopeptide | Gram+, including MRSA | Approved for ABSSSI, in phase III for CABP and phase I and III for paediatric use | Approved May 2014 | Approved March 2015 | Unknown |
| Meropenem+RPX7009 [54, 55] (CarbavanceTM) – The Medicines Company | Carbapenem + new class of BLI | Gram‒, including CRE and particularly KPC | Phase III for cUTI and infections caused by CRE3 | NA | NA | NA |
| Eravacycline [56] – Tetraphase Pharmaceuticals | Tetracycline | Gram+ and ‒, including CRE, ESBL-producing strains, MDR Acinetobacter baumanii, VRE, MRSA | Phase III for cUTI and cIAI4 | NA | NA | NA |
| Plazomicin [57] ‒ Achaogen | Aminogylcoside | Gram‒, including CRE | Phase III for bloodstream infection and nosocomial pneumonia caused by CRE5 | NA | NA | NA |
| Delafloxacin [51] – Melinta Therapeutics | Fluoroquinolone | Gram+ and ‒, including MRSA | Phase III for ABSSSI | NA | NA | NA |
| Solithromycin [52] – Cempra Pharmaceuticals | Macrolide | Gram+, including macrolide-resistant strains | Phase III for CABP and uncomplicated gonorrhoea, in phase I for paediatric use | NA | NA | NA |
| 1 Information retrieved from clinicaltrials.gov as of March 2015. 2 Personal communication. 3 Completion of trial expected in 2016; clinicaltrial.gov identifiers: NCT02168946 and NCT02166476. 4 Completion of trial expected in 2015; clinicaltrial.gov identifiers: NCT01978938 and NCT0184485. 5 Completion of trial expected in 2017; clinicaltrial.gov identifiers: NCT01970371. ABSSSI = acute bacterial skin and skin structure infections; BLI =: β-lactamase inhibitor; CABP = community-acquired bacterial pneumonia; cIAI = complicated intra-abdominal infections; CRE = carbapenem-resistant Enterobacteriaceae; cUTI = complicated urinary tract infections; ESBL = extended spectrum β-lactamase; Gram+ = Gram-positive; Gram‒ = Gram-negative; HAP = hospital-acquired pneumonia; KPC = Klebsiella pneumoniae carbapenemase; MRSA = meticillin-resistant Staphylococcus aureus; VAP = ventilator-acquired pneumonia; VRSA = vancomycin-resistant Staphylococcus aureus | ||||||
As of June 2015, six compounds are in phase III for the systemic treatment of severe bacterial infections. They all have QIDP designation. While delafloxacin [51] and solithromycin [52] will most likely play a role in management of Gram-positive infections [53], four of the six other compounds that could be available in Switzerland within the next 3–5 years have activity against MDR Gram-negative pathogens with key unmet medical needs (table 2 and [21]): ceftazidime+avibactam [44], meropenem+RPX7009 [54, 55], eravacycline [56] and plazomicin [57]. Notably, three of these are also active against carbapenem-resistant Enterobacteriaceae (meropenem+RPX7009, eravacycline and plazomicin).
In Switzerland, there are a few published small-scale outbreaks [58] and reports of imported cases of carbapenem-resistant Enterobacteriaceae (CRE) from endemic countries [58–63]. Thus, the need for new therapeutic agents is so far very limited, but this will likely change in the coming years as more and more infections with CRE will occur with the global spread of XDR/PDR Gram-negative bacteria [64]. As current treatment options for CRE are limited to, for example, colistin, fosfomycin, tigecycline or combination therapies [65], compounds active against such XDR bacteria such as eravacycline, plazomicin or meropenem+RPX7009 will hopefully be available to treat patients by that time.
The need is very different in Switzerland for ESBL-producing strains, which are now widely present in the community. Indeed, local studies have shown that carriage of ESBLs is observed in 5.8% of screened healthy people [66] and 4.8% of patients at admission [67]. Most ESBL-producing Enterobacteriaceae are resistant to fluoroquinolones and cotrimoxazole, limiting orally available therapeutic options for more severe ESBL infections. Fosfomycin and nitrofurantoin remain highly active for uncomplicated UTI, which led to new Swiss treatment guidelines for uncomplicated UTI: the recommended first-line antibiotic changed from a quinolone agent to fosfomycin or nitrofurantoin [68, 69].
As listed in the inventory of antibiotics in clinical development kept by the Pew Charitable Trusts antibiotics and innovation project [70], there are currently 37 antibiotics overall in clinical development from phase I to registration for systemic bacterial infections (including Clostridium difficile infection). Ten compounds are in phase I and 18 in phase II. All of these have QIDP designation, meaning that they are active against at least one QIDP-qualifying pathogen. Based on published calculated failure rates for all therapeutic areas [71, 72], only 1 in 9 or 10 compounds entering phase I can be expected to obtain market approval. This ratio could be even smaller for innovative antibiotics displaying new mechanisms of action.
As mentioned earlier, pharmaceutical companies massively left the antibiotics field in the 1990s. Importantly, some of the large pharmaceutical companies that had left are back in the antibiotics R&D field. In Switzerland, the two largest Swiss pharmaceutical companies Novartis and Hoffmann-La Roche have restarted R&D activities for antibiotics. Roche started a partnership in 2013 with Polyphor to develop RG7929 (POL7080), a compound active against MDR Gram-negative bacteria and in particular MDR P. aeruginosa [73]. Two phase II clinical trials with this compound are currently recruiting patients (NCT02096315, NCT02096328). In January 2015 Roche also partnered with two other companies to develop the new BLI RG6080 (OP0595), which is currently in phase I [74]. As announced in September 2014 [75], Novartis restarted in-house research activities conducted at the Novartis Institute for Biomedical Research (NIBR) on C. difficile, P. aeruginosa and S. aureus [76].
Smaller Swiss companies are not lagging behind and this is important as most of today’s antibiotic innovation stems from small and medium enterprises, especially in the early phases of research and preclinical development. Basilea Pharmaceutica had ceftobiprole recently approved in the EU and Switzerland and has an active antibiotics pipeline with BAL30072, a monosulfactam compound active against MDR Gram-negative infections currently in phase I. Debiopharm entered into the antibiotics field in 2013, focusing on inhibitors of the new staphylococcal target FabI. Two of their compounds, Debio 1452 and its prodrug Debio 1450 are in phase II and I of clinical development, respectively.
Other promising compounds in early clinical development with activity against MDR Gram-negative pathogens include avibactam in combination with aztreonam or ceftaroline and the novel BLI relebactam in combination with imipenem-cilastatin. More details on some of these compounds are reviewed in Butler et al. [77]. Importantly, we must emphasise that all of the above-listed compounds with activity spectrum against Gram-negative MDR pathogen are modified compounds of existing classes. There is a clear need to discover new bacterial targets or new natural compounds. In this regard, the approach of Ling et al. recently described a new technique to discover natural antibacterial compounds from soil bacteria and the discovery of teixobactin, a new inhibitor of the synthesis of peptidoglycan [78].
AMR threatens modern day medicine. Five new systemic antibiotics have been approved since May 2014 in the United States, which is good news but clearly not enough to fight all resistant infections. Four of these have pending marketing authorisations at EMA and three of them at Swissmedic (ceftolozane+tazobactam, oritavancin and tedizolid). One new antibiotic, ceftobiprole, was approved in the EU at the end of 2013 and in Switzerland at the end of 2014. These new antibiotics expand the arsenal of treatments available to clinicians, but do not have new mechanisms of action and are not active against CRE, the MDR Gram-negative pathogens for which there is the highest unmet medical need. There are currently 37 antibiotics in clinical development from phase I to registration, among which some compounds have activity against CRE and could be available in a couple of years if they prove safe and efficacious.
Since 2010, awareness around the AMR issue has dramatically increased and actions have started to be called for or implemented. R&D of new antibiotics is a key component of all programmes aiming at fighting AMR, but it has lagged owing to a plethora of scientific, regulatory and economic challenges. During past years, regulatory guidance has been updated and economic incentives have started to be put in place. This is encouraging both large and small pharmaceutical companies to slowly re-enter the antibiotics field. As R&D is a long process, the real effect of all these measures and restarting R&D activities will only be assessable in 5‒10 years in terms of new antibiotics available to treat patients infected with MDR, XDR and PDR pathogens. As AMR is a global issue, coordinated action will now become key in order to optimise the use of resources to develop new antibiotics, as well as to allow their appropriate distribution (where they are most needed in terms of patterns of AMR) and use (to prevent development of AMR).
Acknowledgments:The authors would like to thank Chantal Morel for useful discussions. Disclosures: The research leading to this review has received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement n° 115523 [Combatting Bacterial Resistance in Europe – COMBACTE] and n° 115618 [Driving re-investment in R&D and responsible antibiotic use – DRIVE-AB], resources of which are composed of financial contribution from the European Union’s 7th Framework Programme (FP7/2007-2013) and EFPIA companies’ in kind contribution. SH has received peer-reviewed research grants funded by Pfizer and B. Braun and is a member of the advisory boards of Destiny Pharma, bioMérieux, Novartis and DaVolterra.
1 Carlet J, Collignon P, Goldmann D, Goossens H, Gyssens IC, Harbarth S, et al. Society’s failure to protect a precious resource: antibiotics. Lancet. 2011;378(9788):369–71. Epub 2011/04/12.
2 CDC. Antibiotic resistance threats in the United States. Report, 2013.
3 The Lancet. Antimicrobial resistance: in terms politicians understand. Lancet. 2014;384(9961):2173.
4 UK Review on Antimicrobial Resistance. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. 2014.
5 Graves N, Harbarth S, Beyersmann J, Barnett A, Halton K, Cooper B. Estimating the cost of health care-associated infections: mind your p’s and q’s. Clin Infect Dis. 2010;50(7):1017–21. Epub 2010/02/25.
6 World Health Organization. Antimicrobial resistance: Global report on surveillance. 2014.
7 Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–81. Epub 2011/07/29.
8 Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, et al. Antibiotic resistance-the need for global solutions. Lancet Infect Dis. 2013;13(12):1057–98.
9 Seiffert SN, Hilty M, Kronenberg A, Droz S, Perreten V, Endimiani A. Extended-spectrum cephalosporin-resistant Escherichia coli in community, specialized outpatient clinic and hospital settings in Switzerland. J Antimicrob Chemother. 2013;68(10):2249–54.
10 ANRESIS. Swiss Centre for Antibiotic resistance [08.03.2015]. Available from: http://www.anresis.ch/.
11 Kronenberg A, Hilty M, Endimiani A, Muhlemann K. Temporal trends of extended-spectrum cephalosporin-resistant Escherichia coli and Klebsiella pneumoniae isolates in in- and outpatients in Switzerland, 2004 to 2011. Euro Surveill. 2013;18(21).
12 Silver LL. Challenges of antibacterial discovery. Clinical microbiology reviews. 2011;24(1):71–109.
13 Bax R, Green S. Antibiotics: the changing regulatory and pharmaceutical industry paradigm. J Antimicrob Chemother. 2015;70(5):1281-4.
14 Rex JH, Goldberger M, Eisenstein BI, Harney C. The evolution of the regulatory framework for antibacterial agents. Ann N Y Acad Sci. 2014;1323:11–21.
15 Nambiar S, Laessig K, Toerner J, Farley J, Cox E. Antibacterial drug development: challenges, recent developments, and future considerations. Clin Pharmacol Ther. 2014;96(2):147–9.
16 Spellberg B, Bartlett J, Wunderink R, Gilbert DN. Novel Approaches Are Needed to Develop Tomorrow’s Antibacterial Therapies. Am J Respir Crit Care Med. 2015;191(2):135–40.
17 So AD, Gupta N, Brahmachari SK, Chopra I, Munos B, Nathan C, et al. Towards new business models for R&D for novel antibiotics. Drug Resist Updat. 2011;14(2):88–94.
18 Kinch MS, Patridge E, Plummer M, Hoyer D. An analysis of FDA-approved drugs for infectious disease: antibacterial agents. Drug Discov Today. 2014;19(9):1283–7.
19 Rice LB. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis. 2008;197(8):1079–81.
20 US eCFR Title 21, Chapter I, Subchapter D, §317.2 List of qualifying pathogens that have the potential to pose a serious threat to public health. 2014 [12.01.2015]. Available from: http://www.ecfr.gov/cgi-bin/retrieveECFR.
21 Spellberg B, Shlaes D. Prioritized current unmet needs for antibacterial therapies. Clin Pharmacol Ther. 2014;96(2):151–3.
22 BARDA. Biomedical Advanced Research and Development Authority 2014 [03.03.2015]. Available from: http://www.phe.gov/about/barda/Pages/default.aspx.
23 ReAct. [05.01.2015]. Available from: http://www.reactgroup.org/#.
24 WAAAR. The WAAAR declaration against antibiotic resistance [05.01.2015]. Available from: http://www.ac2bmr.fr/index.php/en/waaar-declaration.
25 Antibiotic Action. [05.01.2015]. Available from: http://antibiotic-action.com/.
26 CHMP. Addendum to the guideline on the evaluation of medicinal products indicated for treatment of bacterial infections. EMA/CHMP/351889/2013. London, UK, 2013.
27 CDER. Draft guidance for industry: antibacterial therapies for patients with unmet medical need for the treatment of serious bacterial diseases. UCM359184. Silver Spring, MD, USA: US Department of Health and Humans Services, Food and Drug Administration, 2013 UCM359184.
28 Hall AK, Carlson MR. The current status of orphan drug development in Europe and the US. Intractable Rare Dis Res. 2014;3(1):1–7.
29 Milne CP. Prospects for rapid advances in the development of new medicines for special medical needs. Clin Pharmacol Ther. 2014;95(1):98–109.
30 IDSA. White paper: recommendations on the conduct of superiority and organism-specific clinical trials of antibacterial agents for the treatment of infections caused by drug-resistant bacterial pathogens. Clin Infect Dis. 2012;55(8):1031–46.
31 Rex JH, Eisenstein BI, Alder J, Goldberger M, Meyer R, Dane A, et al. A comprehensive regulatory framework to address the unmet need for new antibacterial treatments. Lancet Infect Dis. 2013;13(3):269–75.
32 Talbot GH, Powers JH, Fleming TR, Siuciak JA, Bradley J, Boucher H, et al. Progress on developing endpoints for registrational clinical trials of community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections: update from the Biomarkers Consortium of the Foundation for the National Institutes of Health. Clin Infect Dis. 2012;55(8):1114–21.
33 COMBACTE. Combating bacterial resistance in Europe [03.03.2015]. Available from: https://www.combacte.com/.
34 ND4BB. New Drugs for Bad Bugs [03.03.2015]. Available from: http://www.imi.europa.eu/content/nd4bb.
35 DRIVE-AB [10.03.2015]. Available from: http://drive-ab.eu/.
36 Infectious disease leads in first phase of Europe’s IMI effort. Nat Med. 2014;20(1):5.
37 Longitude Prize. UK 2014 [03.03.2015]. Available from: http://www.longitudeprize.org/.
38 Joint Programming Initiative on Antimicrobial Resistance (JPI-AMR). [05.01.2015]. Available from: http://www.jpiamr.eu/.
39 UK review on antimicrobial resistance. [05.01.2015]. Available from: http://amr-review.org/.
40 WHO. Draft global action plan on antimicrobial resistance. December 2014 Contract No.: EB136/20.
41 Zhanel GG, Chung P, Adam H, Zelenitsky S, Denisuik A, Schweizer F, et al. Ceftolozane/tazobactam: a novel cephalosporin/beta-lactamase inhibitor combination with activity against multidrug-resistant gram-negative bacilli. Drugs. 2014;74(1):31–51.
42 Zhanel GG, Calic D, Schweizer F, Zelenitsky S, Adam H, Lagace-Wiens PR, et al. New lipoglycopeptides: a comparative review of dalbavancin, oritavancin and telavancin. Drugs. 2010;70(7):859–86.
43 Kisgen JJ, Mansour H, Unger NR, Childs LM. Tedizolid: a new oxazolidinone antimicrobial. Am J Health Syst Pharm. 2014;71(8):621–33.
44 Zhanel GG, Lawson CD, Adam H, Schweizer F, Zelenitsky S, Lagace-Wiens PR, et al. Ceftazidime-avibactam: a novel cephalosporin/beta-lactamase inhibitor combination. Drugs. 2013;73(2):159–77.
45 Dauner DG, Nelson RE, Taketa DC. Ceftobiprole: A novel, broad-spectrum cephalosporin with activity against methicillin-resistant Staphylococcus aureus. Am J Health Syst Pharm. 2010;67(12):983–93.
46 Chahine EB. Ceftobiprole: Farewell or just a delay? Am J Health Syst Pharm. 2010;67(12):981.
47 de Kraker ME, Jarlier V, Monen JC, Heuer OE, van de Sande N, Grundmann H. The changing epidemiology of bacteraemias in Europe: trends from the European Antimicrobial Resistance Surveillance System. Clin Microbiol Infect. 2013;19(9):860–8.
48 Lee AS, Cooper BS, Malhotra-Kumar S, Chalfine A, Daikos GL, Fankhauser C, et al. Comparison of strategies to reduce meticillin-resistant Staphylococcus aureus rates in surgical patients: a controlled multicentre intervention trial. BMJ Open. 2013;3(9):e003126.
49 FDA approves new antibacterial drug Avycaz [Internet]. February 25, 2015 [cited 20.03.2015]. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm435629.htm
50 Actavis Receives U.S. FDA Approval for AVYCAZ™ (CEFTAZIDIME-AVIBACTAM) [Internet]. [cited 10.03.2015]. Available from: http://www.actavis.com/NEWS/News/Thomson-Reuters/Actavis-Receives-U-S-FDA-Approval-for-AVYCAZ-CEFT
51 Bassetti M, Della Siega P, Pecori D, Scarparo C, Righi E. Delafloxacin for the treatment of respiratory and skin infections. Expert Opin Investig Drugs. 2015;24(3):433–42.
52 Oldach D, Clark K, Schranz J, Das A, Craft JC, Scott D, et al. Randomized, double-blind, multicenter phase 2 study comparing the efficacy and safety of oral solithromycin (CEM-101) to those of oral levofloxacin in the treatment of patients with community-acquired bacterial pneumonia. Antimicrob Agents Chemother. 2013;57(6):2526–34.
53 Liapikou A, Cilloniz C, Mensa J, Torres A. New antimicrobial approaches to gram positive respiratory infections. Pulm Pharmacol Ther. 2015;32:137-43.
54 Drawz SM, Papp-Wallace KM, Bonomo RA. New beta-lactamase inhibitors: a therapeutic renaissance in an MDR world. Antimicrob Agents Chemother. 2014;58(4):1835–46.
55 Shlaes DM. New beta-lactam-beta-lactamase inhibitor combinations in clinical development. Ann N Y Acad Sci. 2013;1277:105–14.
56 Solomkin JS, Ramesh MK, Cesnauskas G, Novikovs N, Stefanova P, Sutcliffe JA, et al. Phase 2, randomized, double-blind study of the efficacy and safety of two dose regimens of eravacycline versus ertapenem for adult community-acquired complicated intra-abdominal infections. Antimicrob Agents Chemother. 2014;58(4):1847–54.
57 Walkty A, Adam H, Baxter M, Denisuik A, Lagace-Wiens P, Karlowsky JA, et al. In vitro activity of plazomicin against 5,015 gram-negative and gram-positive clinical isolates obtained from patients in canadian hospitals as part of the CANWARD study, 2011–2012. Antimicrob Agents Chemother. 2014;58(5):2554–63.
58 Lemmenmeier E, Kohler P, Bruderer T, Goldenberger D, Kleger GR, Schlegel M. First documented outbreak of KPC-2-producing Klebsiella pneumoniae in Switzerland: infection control measures and clinical management. Infection. 2014;42(3):529–34.
59 Seiffert SN, Perreten V, Johannes S, Droz S, Bodmer T, Endimiani A. OXA-48 carbapenemase-producing Salmonella enterica serovar Kentucky isolate of sequence type 198 in a patient transferred from Libya to Switzerland. Antimicrob Agents Chemother. 2014;58(4):2446–9.
60 Seiffert SN, Marschall J, Perreten V, Carattoli A, Furrer H, Endimiani A. Emergence of Klebsiella pneumoniae co-producing NDM-1, OXA-48, CTX-M-15, CMY-16, QnrA and ArmA in Switzerland. Int J Antimicrob Agents. 2014;44(3):260–2.
61 Poirel L, Schrenzel J, Cherkaoui A, Bernabeu S, Renzi G, Nordmann P. Molecular analysis of NDM-1-producing enterobacterial isolates from Geneva, Switzerland. J Antimicrob Chemother. 2011;66(8):1730–3.
62 Poirel L, Bonnin RA, Boulanger A, Schrenzel J, Kaase M, Nordmann P. Tn125-related acquisition of blaNDM-like genes in Acinetobacter baumannii. Antimicrob Agents Chemother. 2012;56(2):1087–9.
63 Abbas M, Cherkaoui A, Fankhauser C, Schrenzel J, Harbarth S. [Epidemiology and clinical implications of carbapenemase-producing bacteria in Switzerland]. Revue medicale suisse. 2012;8(338):882–4, 6–9. French.
64 Wernli D, Haustein T, Conly J, Carmeli Y, Kickbusch I, Harbarth S. A call for action: the application of The International Health Regulations to the global threat of antimicrobial resistance. PLoS Med. 2011;8(4):e1001022.
65 Lee CS, Doi Y. Therapy of Infections due to Carbapenem-Resistant Gram-Negative Pathogens. Infection & chemotherapy. 2014;46(3):149–64.
66 Geser N, Stephan R, Korczak BM, Beutin L, Hachler H. Molecular identification of extended-spectrum-beta-lactamase genes from Enterobacteriaceae isolated from healthy human carriers in Switzerland. Antimicrob Agents Chemother. 2012;56(3):1609–12.
67 Pasricha J, Koessler T, Harbarth S, Schrenzel J, Camus V, Cohen G, et al. Carriage of extended-spectrum beta-lactamase-producing enterobacteriacae among internal medicine patients in Switzerland. Antimicrob Resist Infect Control. 2013;2:20.
68 Bonkat G, Muller G, Braissant O, Frei R, Tschudin-Suter S, Rieken M, et al. Increasing prevalence of ciprofloxacin resistance in extended-spectrum-beta-lactamase-producing Escherichia coli urinary isolates. World J Urol. 2013;31(6):1427–32.
69 Meier S, Weber R, Zbinden R, Ruef C, Hasse B. Extended-spectrum beta-lactamase-producing Gram-negative pathogens in community-acquired urinary tract infections: an increasing challenge for antimicrobial therapy. Infection. 2011;39(4):333–40.
70 The Pew Charitable Trusts Antibiotics and Innovation project [13.02.2015]. Available from: http://www.pewtrusts.org/en/projects/antibiotics-and-innovation.
71 Hay M, Thomas DW, Craighead JL, Economides C, Rosenthal J. Clinical development success rates for investigational drugs. Nature biotechnology. 2014;32(1):40–51.
72 Paul SM, Mytelka DS, Dunwiddie CT, Persinger CC, Munos BH, Lindborg SR, et al. How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nat Rev Drug Discov. 2010;9(3):203–14.
73 Roche and Polyphor join efforts to combat multi-drug-resistant bacterial infections [Internet]. 2013. Available from: http://www.roche.com/media/media_releases/med-cor-2013-11-04.htm
74 Roche, Meiji and Fedora join forces to tackle increasing bacterial resistance to antibiotics [Internet]. January 2015. Available from: http://www.roche.com/med-cor-2015-01-13-e.pdf
75 Novartis. Responding to the Superbug Alarm 2014 [08.03.2015]. Available from: http://www.novartis.com/newsroom/feature-stories/2014/09/responding-to-the-superbug-alarm.shtml.
76 Novartis Institute for Biomedical Research (NIBR) - Infectious Diseases. [19.12.2014]. Available from: http://nibr.com/research/disease/infectious.shtml.
77 Butler MS, Blaskovich MA, Cooper MA. Antibiotics in the clinical pipeline in 2013. J Antibiot. 2013;66(10):571–91.
78 Ling LL, Schneider T, Peoples AJ, Spoering AL, Engels I, Conlon BP, et al. A new antibiotic kills pathogens without detectable resistance. Nature. 2015;517(7535):455–9.