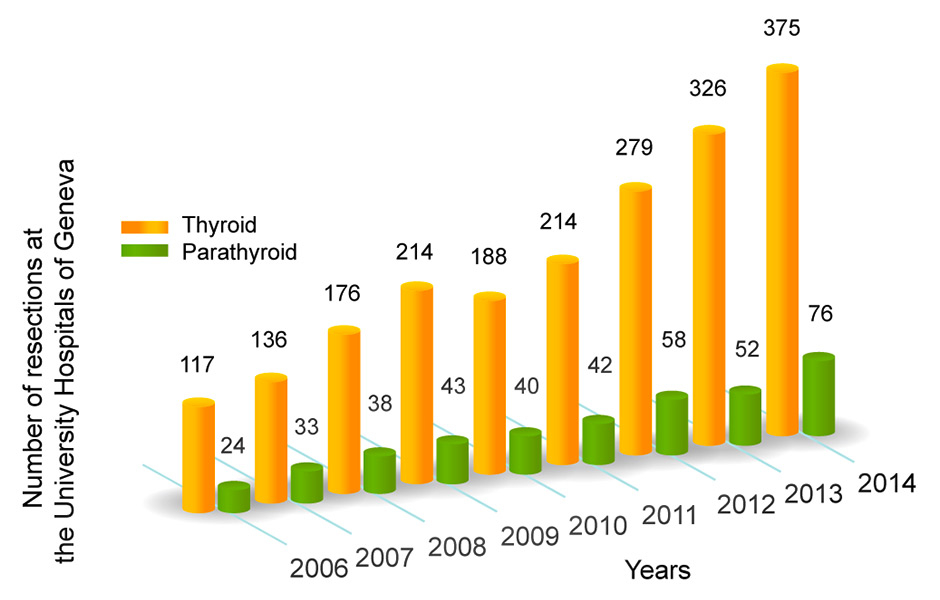
Figure 1
Number of operations on the thyroid and parathyroid glands performed in our centre during the last 8 years.
DOI: https://doi.org/10.4414/smw.2015.14144
Celsus and Galen were the first to describe cervical masses and goitres in the first and second century. The first attempts to explain the origin of goitres was made by Abulkasim during the eleventh century. Between the eleventh and thirteenth centuries, treatments of these conditions with products derived from the sea were initiated. In 1656, the term “glandula thyroidea” was introduced by Thomas Wharton (UK), and the scientific basis of thyroid surgery was initiated by Lorenz Heister. In 1850, the mortality rate of thyroid surgery was around 40%. Surgery was prohibited by the French Academy of Medicine and in 1866 in the United States of America, David Gross stated: “Can the thyroid gland, when in a state of enlargement, be removed with a reasonable hope of saving the patient? Experience emphatically answers NO .… If a surgeon should be so foolhardy as to undertake it … every step of the way will be environed with difficulty, every stroke of his knife will be followed by a torrent of blood, and lucky will it be for him if his victim lives long enough to enable him to finish his horrid butchery. No honest and sensible surgeon would ever engage in it!” [1].
The main advances in the role and physiology of the thyroid gland were made from 1850. In only a few decades, the mortality rate of the thyroid surgery decreased drastically from 40% to less than 1%. This decrease was made possible by improvements in surgical technique, as well as anaesthesia and antiseptic progress. In 1886, at the French Congress of Surgery, Jacques-Louis Reverdin from Geneva published his original work upon 22 cases of goitre operated through vertical incisions. He described for the first time postoperative myxoedema, which is now a very well-known postoperative hypothyroid state. This work, as well as similar works from Germany, Austria, France and Switzerland, finally led Theodor Kocher from Bern to obtain the Nobel Prize in Medicine for the understanding of the function of the thyroid gland. Jacques-Louis Reverdin was also one of the first authors to describe this postoperative hypothyroidism leading to cretinism in children [2, 3]. However, this was more scientifically studied and reported by Kocher, which is probably one of the main reasons he received the Nobel Prize. Both of them began to understand that the volume of thyroid left in place was probably more important than the amount removed during surgery and they started to perform enucleation, or unilateral or subtotal thyroidectomies instead of total thyroidectomies.
Thyroid surgery is closely linked to the parathyroid glands. In 1850, the curator of the Natural History Museum of London, Sir Richard Owen, discovered the parathyroid glands during dissection of a dead rhinoceros. In 1880, Ivar Sandström in Upsala, Sweden, described for the first time the parathyroid glands in humans but unfortunately his work was refused by most of the scientific journals. Then he managed to publish his work in a local Swedish journal [4]. In 1890, Eugene Gley discovered the function of parathyroid glands and established a link between parathyroid gland removal, tetany and hypocalcaemia. He discovered that this condition was treatable with supplementation with parathyroid extracts or with calcium [5]. In 1907, William S. Halsted stated that: “It seems incredible that the loss of such small bodies could also lead to catastrophic effects” [6]. The concept of parathyroid transplantation was initiated by Eugene Gley. However, the first autotransplantation during thyroidectomy, was performed in 1982 by Anton von Eiselberg in Vienna [7].
Today, thyroid surgery is a common surgery: about 45,000 thyroidectomies are performed per year in France, 60,000 in Germany and 4,000 in Switzerland. Thyroid surgery has become very safe with a mortality of almost 0% and a very low complication rate. In our centre, the number of thyroidectomies has more than tripled in the last decade (fig. 1).
The overall incidence of thyroid nodule on ultrasound in the general population is 30% and 50% at 40- and 60-years-old, respectively, with a gender distribution of 3:1 (female:male) [8]. Despite a marked increase in the incidence of thyroid cancer in the last 30 years, the mortality rate remains stable or has even decreased in some countries such as Switzerland (fig. 2). This incidence increase is thought to be mainly due to the improvement of screening techniques. Indeed, this increase is particularly evident for small papillary thyroid cancer [9]. Mortality is near zero for carcinomas of less than 1–2 cm in diameter and the risk of distant metastasis is extremely low for cancers <2 cm [10]. The main issue is, therefore, to identify clearly cancerous or suspicious nodules that need surgical treatment and to avoid surgery in nonsuspicious ones. For this purpose, algorithms have been developed and published for the investigation of thyroid nodules, using different tests such as ultrasound and fine needle aspiration, principally [11, 12].

Figure 1
Number of operations on the thyroid and parathyroid glands performed in our centre during the last 8 years.
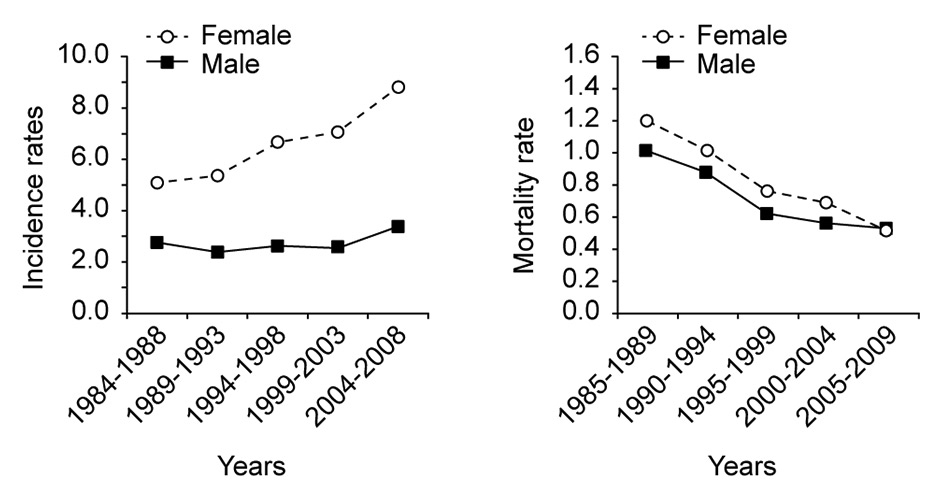
Figure 2
Incidence and mortality rate of thyroid cancer in Switzerland. Adapted from the National Institute for Cancer Epidemiology and Registration, http://www.nicer.org
For papillary thyroid cancer, the extent of surgery on the thyroid gland and on the lymph nodes has been and still is a matter of debate, because of the specific risk-benefit ratio of each procedure. In brief, removing more lymph nodes could reduce the risk of recurrence, particularly in high-risk patients, but increases the risk of complications. The risk of recurrence can be estimated from knowledge of the patient and the tumour characteristics, but one of the remaining problems is that precise tumour characteristics are known only after the surgery. Therefore, the tumour aggressiveness has to be estimated using data available preoperatively. The risk of complications is highly dependent upon the surgical expertise and experience of the surgical team, and the risk-benefit ratio has, therefore, to be evaluated in an individual and local fashion, and is hence difficult to publish in international guidelines. However, general rules can be applied. Currently, total thyroidectomy is usually recommended for papillary thyroid cancers >1 cm. Lymph node dissection (central and/or lateral) is recommended in all patients with proven invaded lymph nodes found either preoperatively (by ultrasound, sometimes with fine needle aspiration) or during surgical exploration. Prophylactic lymph node dissection (without clearly invaded lymph nodes) is never indicated for the lateral compartment (fig. 3, levels III and IV, sometimes with levels IIB and IIA) but can/should be performed at the time of initial surgery in the central compartment (fig. 3, level VI) in high-risk patients and perhaps also in intermediate-risk patients if the complication rate is low. All patients with thyroid cancer should be discussed in a multidisciplinary team, in order to give them the best chance of cure and to avoid unnecessary complications due to too extensive surgery.
For follicular thyroid cancer, the treatment is similar although there are some differences, particularly concerning lymph node dissection.
Medullary thyroid cancer (MTC) management has experienced the most dramatic changes in the last 40 years, following the discovery of the RET proto-oncogene mutations leading to familial MTC, either alone (familial medullary thyroid cancer) or in association with other endocrine tumours in the multiple endocrine neoplasia syndromes type 1a, 1b and 2. There is a well characterised genotype–phenotype correlation leading to precise (but changing!) recommendations for each type of mutation. For instance, the recommended age for prophylactic thyroidectomy (with or without associated lymph node dissection, depending on the value of calcitonin) is different for each mutation. In conclusion, each patient with MTC should benefit from specialised multidisciplinary consultation including genetic counselling. This topic is complex and could be the subject of a review by itself.
Unfortunately, the progresses made in the treatment of anaplastic thyroid cancer has not led to a significant improvement in the dismal prognosis of this type of tumour. Current treatment should include, when possible, surgery and chemoradiation.
The management of the hyperthyroid state and large goitres has not undergone major changes in recent decades. Goiters of big size/volume, apart from being a cosmetic problem, may also cause compression problems such as respiratory, upper digestive tract compression and recurrent laryngeal nerve (RLN) compression. When hyperthyroidism cannot be treated or when treatment cannot be continued any longer (for various reasons such as patient’s choice or drug intolerance, for instance) a more radical treatment is usually proposed: either radioiodine, as is typical in the United States, or surgery as is typical in European countries. We tend to operate on more patients with Graves’ disease, probably because the regulations concerning radioactivity have become stricter, leading to a longer in-hospital stay, and because the complications of surgery have become very low.
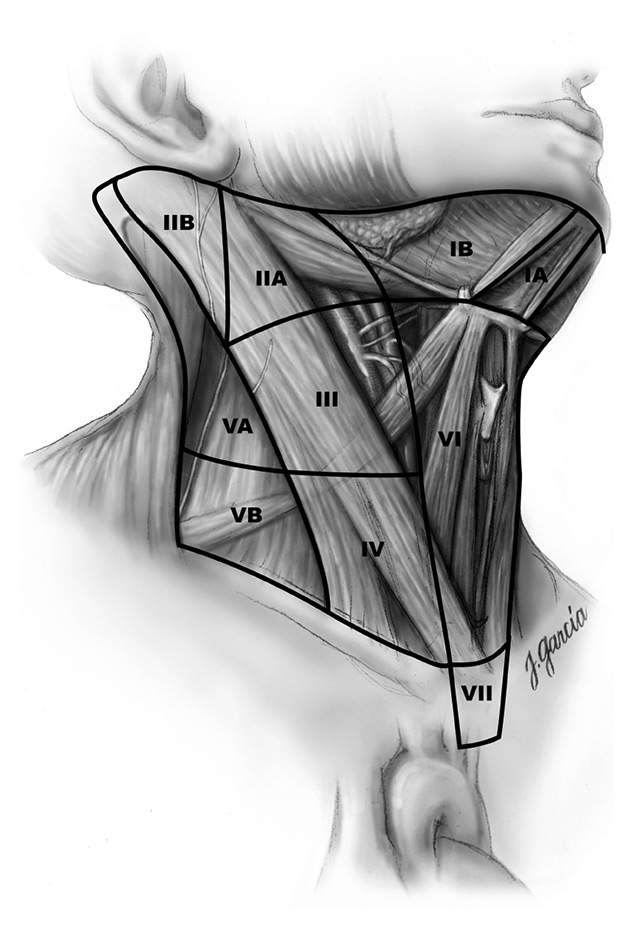
Figure 3
Schematic right anterior oblique view indicating levels of the neck and upper mediastinum relevant to neck dissection in thyroid cancer. Reprinted with permission from Carty SE, et al., Consensus statement on the terminology and classification of central neck dissection for thyroid cancer. Thyroid. 2009;19(11):1153–8. The publisher for this copyrighted material is Mary Ann Liebert, Inc. publishers.
Intrathoracic goitres remain a classical indication for surgery because of the difficulty to follow the intrathoracic part and because of the risk of compression of important structures in the chest (vessels and trachea). Most of intrathoracic goitres, even the largest ones, can be removed via a standard cervical incision.
Thyroid surgery can lead to three main complications which are described and discussed below: haemorrhage, RLN palsy and postoperative hypocalcaemia.
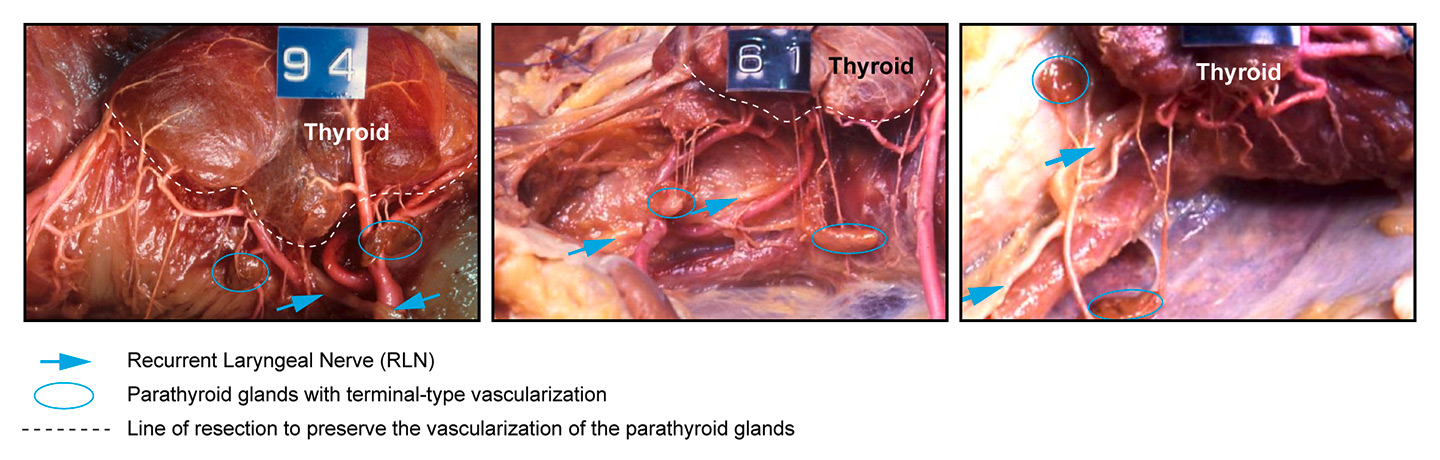
Figure 4
Anatomical preparations demonstrating the thyroid gland, and the parathyroid glands in different anatomic locations with their terminal-type vascularisation, and the recurrent laryngeal nerve. Adapted from Delattre JF, Variations in the parathyroid glands. Number, situation and arterial vascularisation. Anatomical study and surgical application J Chir (Paris). 1982;119(11):633–41.
In the past, haemorrhage used to be much feared and was an integral part of the high mortality rate linked to thyroid surgery. Nowadays, intraoperative haemorrhage is extremely rare. Haemostasis is very well performed by use of classical clamp and tie techniques, or by adoption of new devices using thermofusion or ultrasonic coagulation. These devices facilitate the dissection and allow proper immediate and late haemostasis. All these “new” devices create a heat wave around the area that has been coagulated and proper use of them should avoid lesions to the neighbouring structures like parathyroid glands and RLN.
The occurrence of delayed bleeding (after the wound has been closed) is still not nil and may lead to a suffocating haematoma. Most often, postoperative bleeding occurs within the first 6 hours after surgery but it may occur well after this time. In European countries, an overnight stay is usually recommended after thyroidectomy [13] whereas in the United States, close monitoring during the first 6–12 hours postoperatively allows outpatient surgery.
The vocal cords are very important for the quality and tone of voice and also for avoiding voice fatigue even if the tone is correct. Vocal cords are also essential as a sphincter protecting the airway during swallowing; if the vocal cords are open or not properly closed, there is a clear risk of broncho-aspiration that can lead to pulmonary infection, particularly in fragile patients.
Vocal cord paralysis due to thyroid surgery can have different functional consequences depending on the open or closed position of the vocal cords. In patients with vocal cords paralysis in the open position, the patient’s voice will be breathy and the sphincter function will be ineffective, leading to a high risk of broncho-aspiration. Additionally, in this abducted position there will be no possibility of producing an efficient Valsalva manoeuvre, which can lead to ineffective cough or constipation. In patients with vocal cords paralysed in the closed position, the tone and the quality of the voice are almost normal. The sphincter function remains effective. Patients may experience some dyspnoea on exertion by halving the tracheal surface; they can feel voice fatigue despite the fact that the tone is correct. Clinical diagnosis of vocal cords paralysis in the closed position may be very difficult, which may explain an underreported rate of vocal cords paralysis in some series in which no objective diagnostic tool was used for the diagnosis of laryngeal nerve palsy, such as routine laryngoscopy or routine use of neuromonitoring [14–16].
In most cases, vocal cords lesions are due to stretching of the RLN. In 2009, Dionigi et al. evaluated 434 patients after thyroid surgery providing 825 nerves at risk. The authors showed RLN palsy in 6.7% postoperatively compared with 0.7% at 12 months after surgery. They concluded that recovery was possible in almost 90% of cases [17], which is also our experience. In some cases, vocal cords lesions are due to a transection of the RLN, either voluntarily in some cases of invasive thyroid cancer or, rarely, inadvertently. In these situations, there is no possibility of full recovery of the vocal cords mobility and management usually begins with conservative speech training followed, if necessary, by vocal cords medialisation procedures to bring the paralysed vocal cords in a paramedian position.
If a RLN has been cut, the surgeon should suture it, if possible. The main objective will be the preservation of muscle trophicity, even if correct vocal cord movements will never be achieved, probably because it is impossible to adapt correctly ad- and abductor fibres that are contained in the nerve.
Neuromonitoring of the RLN is a new tool available for thyroid surgeons. The intubation probe is equipped with electrodes that are placed between the vocal cords by the anaesthesiologist. With a stimulator probe, the vagal nerves and the RLNs can be stimulated, providing a sound and a graphic signal, which allow the surgeon to test the integrity of the nerve. It can also be used to make sure that an identified structure is really the nerve (and not a vessel for instance) and can sometimes help to find the nerve, for instance in scar tissue in reoperative surgery. This technique is called intermittent monitoring, as the RLN is monitored when the surgeon applies the stimulation probe on the nerve. Using this technology, surgeons demonstrated that when a RLN is bifid (in about 20–30% of the patients, the RLN separates in two or more branches before its entry in the larynx), the anterior branch is always the motor branch and the posterior branch is a sensory branch [18]. This physiological aspect was unknown before the introduction of neuromonitoring. This is probably one of the explanations of RLN palsies that occurred when the surgeon identified the RLN only visually; in some patients, surgeons probably identified the posterior branch of a RLN, considering that it was the RLN and cut everything that was in front of that, not recognising that there was another branch (the motor one) that was still more anterior. In the literature, the use of the neuromonitoring did not show statistical evidence of decreasing the risk of definitive RLN paralysis, but showed a trend to improving outcomes for the most at-risk interventions [16]. Only one randomised trial showed a lower transient RLN paralysis without effect on definitive paralysis [19].
A current development of this technique is continuous monitoring of the RLN. In this case, a stimulation probe is placed on the vagus nerve at the beginning of the operation and the nerve is stimulated automatically at fixed intervals (every second for instance). The system is calibrated at the beginning of the operation and an alarm is given when the latency and/or the amplitude of the response is significantly decreased; the idea behind this is that the surgeon should get an alarm when the nerve is put under too much tension so that the tension can be released in time. Of course, it will not avoid an inadvertent section of the RLN; however, as discussed above, most of the RLN lesions are due to overstretching because of surgical manoeuvres around the nerve. Continuous monitoring is a new technology and its prospective evaluation is still ongoing. One study evaluating 102 nerves at risk demonstrated that modification of the surgical manoeuvre resulted in a 73% resolution of the adverse electromyogram (EMG) changes and therefore potentially avoided vocal cords paralysis. These results suggested that identification of such EMG events could be very useful in the per-operative surgical management of the RLN [20]. Continuous monitoring of the RLN is a promising technique; however, it needs special equipment and a surgical dissection of the vagus nerve that is usually not necessary during standard thyroid surgery. Moreover, it leads to a number of alarms that the surgeon should analyse intraoperatively in order to know whether it is a “true” alarm, (then something should be done to correct the problem) or a “false” alarm (and then the surgery can be pursued without interruption). These alarm managements are quite new for surgeons, who need to get used to them. Anaesthesiologists are familiar with these problems: it is estimated that 95% of the alarms given by the anaesthesiology equipment are in fact false ones due to “technical” problems (like electrode displacement) which do not require any change in the management of the patient.
Neuromonitoring of the RLN can help to identify the RLN and can perhaps decrease the risk of RLN lesions (even if this has not been demonstrated significantly). However, one of its main impacts is the change in operative strategy in the case of bilateral thyroidectomy. Before the era of neuromonitoring, most surgeons always began the operation on the same side (as a personal habit, some surgeons began with the left side, others with the right side) or some began the operation on the smaller side because it is then a little bit easier to access the bigger side. Nowadays, it is well accepted that surgery should begin on the cancerous/bigger side, so that if a loss of the neuromonitoring signal occurs following the dissection of the first side, the surgeons can stop the operation to avoid the risk of a bilateral RLN palsy. Most of RLNs will recover with time and the surgery of the contra-lateral side can then be scheduled safely or, in some situations, alternative treatment options can be discussed (radioiodine treatment for Graves’ disease for instance) [15, 21]. With these safety measures, the risk of a catastrophic bilateral RLN paralysis should be very close to 0%.
Hypocalcaemia is another major complication after thyroid surgery and is almost always due to hypoparathyroidism (in some hyperthyroid patients, hypocalcaemia can occur with normal parathyroid hormone levels). In the literature, parathyroid glands are usually described in standard positions, but in fact they are found in quite variable positions. The parathyroid glands have a terminal type vascularisation, which means that during surgery it is essential to keep them in place and to keep their blood supply intact (fig. 4). The only way to avoid hypoparathyroidism is by making a careful total thyroidectomy, which requires a good knowledge of embryology, anatomy and parathyroid vascularisation. Endocrine surgeons usually say that we should “fight for every parathyroid as if it was the last one”. Despite meticulous dissection by an experienced surgeon, 10% to 30% of patients undergoing total thyroidectomy will experience transient hypocalcaemia and, 1% to 3% will experience definitive hypoparathyroidism [22, 23].
The main risk factors for hypoparathyroidism are reoperations, central neck dissection, goitre size and some inflammatory states like thyroiditis and Graves’ disease. The individual risk is probably given by the specific anatomy of the parathyroid glands and their blood supply, but this cannot be anticipated before surgery. Hypocalcaemia is characterised by tingling (“pins and needles”, mostly in the well innervated muscles like the small muscles in the hands or around the mouth, due to neuromuscular hyperexcitability associated with hypocalcaemia), tetany, laryngospasm and sometimes psychosis and hallucinations. The treatment of transient hypocalcaemia remains easy with oral calcium and active Vitamin D (1,25(OH)2D3) supplementation such as Rocaltrol® as the kidney needs parathyroid hormone to activate the vitamin D. In some cases, patients need intravenous calcium substitution, particularly in winter when there is an additional lack of vitamin D. In order to minimise the hospital length of stay, some authors recommend looking for a deficiency of Vitamin D in a preoperative setting and correcting it preoperatively.
Long term hypocalcaemia can lead to serious complications such as extra-osseous tissue calcifications (brain, cataracts, kidney, vessels), development of kidney stones and to a significantly decrease of the quality of life [24]. This disease is most troublesome for young women owing to high calcium needs during pregnancy and breastfeeding. The long-term management of hypoparathyroid patients aims at avoiding hypocalcaemic symptoms and avoiding complications due to relatively high calcium X phosphorus product and high urinary calcium excretion.
In conclusion, hypoparathyroidism prevention needs surgeons’ continuing education, training and specialisation. A French prospective study, the CATHY study, ranged surgeons depending on transient or definitive hypocalcaemia rates following thyroidectomies. This study showed a decrease of performance at the beginning of early training and at the end of the professional career, and emphasised the need for continuous training for surgeons [22, 25]. Finally, it is capital to take into account that for most patients, what is left around the thyroid gland is at least as important as what is removed; sometimes performing subtotal thyroidectomy instead of total thyroidectomy may be a better surgical choice, in order to preserve parathyroid function.
We are far away from the historical vertical incisions performed initially to operate on the thyroid gland. These vertical incisions were aesthetically not good at all. Nowadays, the incision not only follows the lines and/or neck folds but also its size has become much smaller. Some surgeons tend slightly to lateralise the incision when a unilateral lobectomy is performed, thereby minimising the incision size and possibly leading to less visibility of the lateral scar as compared with a central scar. Miccolli et al. in Pisa, Italy, developed a minimally invasive approach using a <2 cm incision in the neck (MIVAT, minimally invasive video-assisted thyroidectomy) [26]. This approach is used in different centres but has not gained a very wide acceptance because there is still a visible scar in the neck (and sometimes a 2-cm skin incision that has undergone a lot of tension will not heal better than a 4–5 cm incision that has undergone less tension). Furthermore, this technique requires at least two experienced surgeons and one or two more assistants, which is not the standard surgical team outside of Italy. Owing to the remaining scar in the neck, and owing to the exposed situation of the neck, many authors have tried extracervical approaches, particularly in Asia [27]. Many approaches have been described: infraclavicular, around the breast nipples, axillary [28], combined approaches such as post-auricular and axillary [29], etc., all under endoscopic or robotic vision. At the University Hospital of Geneva, upon patient request, we sometimes perform transaxillary robotic thyroidectomy using the Da Vinci robot. There is no visible scar following surgery with the Da Vinci robot; however, the size of the axillary scar and the large dissection necessary to reach the operating region of interest remain important and therefore this technique cannot be called “minimally invasive”. The vision and exposure on the first side is very good and the dissection of the RLN and the parathyroid glands can be done safely. However, the vision and exposure of the contralateral side is still difficult with the current equipment; the contralateral RLN cannot always be identified, leading some surgeons to make bilateral axillary incisions and others to accept a less than total thyroidectomy in some patients when the RLN cannot be identified. This may be a result of lack of experience on our side, as experienced robotic surgeons report bilateral thyroidectomies without significant problems. Studies on the extracervical approaches were mainly done in Asia and results cannot always be extrapolated to European countries because the population (body mass index, diet, physical characteristics) and pathologies (size of tumours resected, goitre) studied are completely different [30]. For instance one study in Korea from the largest robotic centre in the world, reported that the mean size of the cancer was 0.66 cm on 3,000 thyroidectomies performed for papillary thyroid cancer. In the US and Europe, and in our centre, it is extremely rare to resect a thyroid gland for a cancer that has been diagnosed as such and measures <1 cm [30]. The complexity of those new procedures introduces new complications, unknown in classical thyroid surgery, such as brachial plexus injury, and external and internal jugular vein, carotid artery or tracheal lesions [31]. Additionally, extracervical approaches usually require longer intervention time (>4 hours for a bilateral axillobreast approach with two surgical teams) as compared with classical thyroid surgery and require more expensive equipment. Moreover, patients experience more pain, and hospitalisation is longer and more expensive than with classical thyroid surgery [32, 33]. In conclusion, the future of these approaches remains, at the moment, uncertain and always subject to the explicit request of the patient.
Thyroid surgery is currently a very safe surgery that has undergone significant improvements during recent decades. It remains a stressful operation from the beginning to the end because of the very delicate structures around the thyroid gland that have to be preserved. Results are immediately visible and audible, and may have important long-term consequences for the patients. Specialised training, experience, use of modern equipment and standardisation of the patient tracks are the keys to modern thyroid surgery. Efforts are being made to avoid a neck scar, but currently the standard remains a small transverse incision in the neck.
1 Terris DJ, et al. Incisions in thyroid and parathyroid surgery. GW Randolph, 2nd Edition, Elsevier/Saunders, Philadelphia, in Surgery and Parathyroid Surgery. 2013;42:403–6.
2 Reverdin JL. Contribution à l’étude du mixoedème consecutive à l’extirpation totale ou partielle du corps de la thyroid. Rev Med Suisse Romande. 1887;7(5):275–91. French.
3 Reverdin JL. Contribution à l’étude du mixoedème consecutive à l’extirpation totale ou partielle du corps de la thyroid. Rev Med Suisse Romande. 1887;7(6):318–30. French.
4 Sandström I. On a new gland in man and fellow animals, Uppsala Lak Foren Forh. 1880;14:441–71.
5 Gley E. Sur Les fonctions du corps thyroide. CR Soc Biol.1891;43:841–7.
6 Halsted WS, Evans HM. The Parathyroid Glandules. Their Blood Supply and their Preservation in Operation upon the Thyroid Gland. Ann Surg. 1907;46(4):489–506.
7 Von Eiselberg A. Uber erfolgreiche Einheilung der Katzen Schildriise in die Baueehdecke und Auftreten von Tetanie nach der Extirpation. Wien Klin Wochenschr. 1892;5:81–5. German.
8 Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993;328:553–9.
9 Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States 1973–2002. JAMA. 2006;295(18):2164–7.
10 Machens A, Holzhausen HJ, Dralle H. The prognostic value of primary tumor size in papillary and follicular thyroid carcinoma. Cancer. 2005;103(11):2269–73.
11 Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Revised American thyroid association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11).
12 Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedüs L, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocr Pract. 2010;16(1):1–43.
13 Menegaux F. Thyroïdectomie ambulatoire: recommandations de l’association francophone de chirurgie endocrinienne (AFCE). Enquête sur les pratiques actuelles. J Visc Surg. 2013;150:185–92. French.
14 Dralle H, Sekulla C, Haerting J, Timmermann W, Neumann HJ, Kruse E, et al. Risk factors of paralysis and functional outcome after recurrent laryngeal nerve monitoring in thyroid surgery. Surgery. 2004;136:1310–22.
15 Randolph GW, Dralle H, et al. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope. 2011;121(1):S1–16.
16 Dralle H, Sekulla C, Lorenz K, Brauckhoff M, Machens A. Intraoperative monitoring of the recurrent laryngeal nerve in thyroid surgery. World J Surg. 2008;32:1358–66.
17 Dionigi G, Boni L, Rovera F, Rausei S, Castelnuovo P, Dionigi R. Postoperative laryngoscopy in thyroid surgery: proper timing to detect recurrent laryngeal nerve injury. Langenbecks Arch Surg. 2010;395(4):327–3.
18 Serpell JW, Yeung MJ, Grodski S. The motor fibers of the recurrent laryngeal nerve are located in the anterior extralaryngeal branch. Ann Surg. 2009;249(4):648–52.
19 Ferrari CC, Spampatti S, Leotta A, Rausei S, Rovera F, Boni L, et al. Web-based information on intraoperative neuromonitoring in thyroid surgery. Inter J of Surg. 2013;11(1):S40–1.
20 Phelan E, Schneider R, Lorenz K, Dralle H, Kamani D, Potenza A, et al. Continuous vagal IONM prevents recurrent laryngeal nerve paralysis by revealing initial EMG changes of impending neuropraxic injury: a prospective, multicenter study. Laryngoscope. 2014;124(6):1498–505.
21 Sadowski SM, Soardo P, Leuchter I, Robert JH, Triponez F. Systematic use of recurrent laryngeal nerve exchange the operative neuromonitoring. Planned strategy in bilateral thyroidectomy. Thyroid. 2013;23(3):329–33.
22 Duclos A, Peix JL, Colin C, Kraimps JL, Menegaux F, Pattou F, et al. Influence of experience on performance of individual surgeons in thyroid surgery a prospective cross sectional multicenter study. BMJ. 2012;344.
23 Bergenfelz A, Jansson S, Kristoffersson A, Mårtensson H, Reihnér E, Wallin G, et al. Complications to thyroid surgery: results as reported in a database from a multicenter audit comprising 3,660 patients. Langenbecks Arch Surg. 2008;393:667–73.
24 Arlt W, Fremerey C, Callies F, Reincke M, Schneider P, Timmermann W, et al. Well-being, mood and calcium homeostatis in patients with hypoparathyroidism receiving standard treatment with calcium and vitamin D. Eur J Endocrinol. 2002;146(2):215–22.
25 Duclos A, Carty MJ, Peix JL, Colin C, Lipsitz SR, Kraimps JL, et al. Development of a charting method to monitor the individual performance of surgeons at the beginning of their career. PLoS One. 2012;7(7):e41944.
26 Miccoli P, Minuto MN, Ugolini C, Pisano R, Fosso A, Berti P. Minimally invasive video-assisted thyroidectomy for benign thyroid disease: an evidence-based review. World J Surg. 2008;32(7):1333–40.
27 Slotema ET, Sebag F, Henry JF. What is the evidence for endoscopic thyroidectomy in the management of benign thyroid disease? World J Surg. 2008;32(7):1325–32.
28 Ikeda Y, Takami H, Sasaki Y, Takayama J, Kan S, Niimi M. Minimally invasive video-assisted thyroidectomy and lymphadenectomy for micropapillary carcinoma of the thyroid. J Surgical Oncol. 2002;80(4):218–21.
29 Lee KE, Kim HY, Park WS, Choe JH, Kwon MR, Oh SK, et al. Postauricular and axillary approach endoscopic neck surgery: A new technique. World J Surg. 2009;33(4):767–72.
30 Ban EJ, Yoo JY, Kim WW, Son HY, Park S, Lee SH, et al. Surgical complications after robotic thyroidectomy for thyroid carcinoma: a single center experience with 3,000 patients. Surg Endosc. 2014;28(9):2555–63.
31 Patel D, Kebebew E. Pros and cons of robotic transaxillary thyroidectomy. Thyroid. 2012;22(10):984–5.
32 Miyano G, Lobe TE, Wright SK. Bilateral transaxillary endoscopic total thyroidectomy. J Pediatr Surg. 2008;43(8):299–303.
33 Tan C, Cheah WK, Delbridge L. “Scarless” (in the Neck) endoscopic thyroidectomy (SET): an evidence-based review of published techniques. World J Surg. 2008;32(7):1349–57.
Disclosures: No financial support and no other potential conflict of interest relevant to this article was reported.