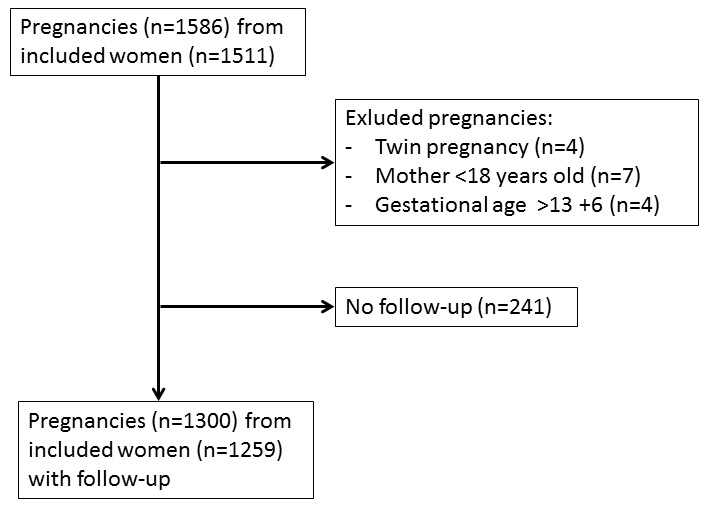
Figure 1
Flow chart of the study. The participating women were subsequently recruited by nine obstetricians based in private practices.
DOI: https://doi.org/10.4414/smw.2015.14175
Preeclampsia is a pregnancy complication unique to humans [1]. It is one of the hypertensive disorders of pregnancy characterised by endothelial dysfunction, high blood pressure, and proteinuria arising in the second half of gestation, but its exact definition and diagnostic criteria remain a subject of debate [1–3].
The International Society for the Study of Hypertension in Pregnancy (ISSHP) has proposed unified criteria for diagnosing preeclampsia: correctly measured de novo hypertension (systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg) after 20 weeks of gestation that returns to normal postpartum, and proteinuria of ≥300 mg/day, 1+ on dipstick or a protein/creatinine ratio of ≥30 mg protein / mmol creatinine in a spot urine analysis [4]. There are also other consensus definitions such as the one proposed by the World Health Organization (WHO) [5]. In order to predict or diagnose preeclampsia, several serum and ultrasound markers are either under investigation or underway to being used in clinical routine [6–12].
According to severity, preeclampsia can be divided into mild and severe forms. It can also be classified according to the time of onset. Early-onset preeclampsia is defined as developing, or leading to delivery, before 34 gestational weeks, whereas late-onset preeclampsia occurs at week 34 or later [13, 14]. Earlier onset is associated with more severe disease and worse outcomes for both mother and child [14]. Sometimes a distinction is made between term and preterm preeclampsia, the latter developing before 37 weeks of gestation [15].
Risks for developing preeclampsia include nulliparity, prior preeclampsia, preexisting hypertension, renal disease, gestational or preexisting diabetes mellitus, a family history of preeclampsia, maternal age of ≥35 years, multiple gestation, obesity and a long interpregnancy interval [1, 16–18]. A change in paternity is a risk factor according to some authors but not to others [1, 18, 19]. Smoking has been shown to be a protective factor [18, 19]; however, if the disease still develops, it is more severe and has poorer outcomes than in nonsmokers [20]. There are inconsistent data as to whether maternal age of <20 years is a predisposing factor [1, 18, 21]. Black race is often found to be a risk factor [16]; the association of other races with heightened risk of preeclampsia is not clear [22–24].
Preeclampsia is one of the leading causes of maternal and perinatal morbidity and mortality, together with eclampsia causing over 50,000 maternal deaths a year worldwide [25]. Preeclampsia complicates around 2.2% of pregnancies [26]. The incidence varies greatly between countries, even inside Europe: figures based on the whole pregnant population range from 2.2% in the Netherlands to 4.5% in Norway, while in Finnish nulliparae aged 35 and over the incidence of preeclampsia is as high as 9.4% [27–29]. So far, there are no prospective data about the incidence of preeclampsia in Switzerland. The aim of this study was to assess the incidence of preeclampsia among Swiss women and compare it with findings from other countries.
Study participants were recruited in the context of the PRADO study (PRedictive mArkers for the Diagnosis Of preeclampsia). The primary aim of this study was to identify biomarkers for recognition of women at high risk of developing preeclampsia. As a secondary aim, the PRADO study provided the unique opportunity to describe the epidemiological characteristics of preeclampsia in Switzerland. Nine obstetricians based in private practices in the French- and German-speaking regions of the Swiss Plateau and the Jura Mountains consecutively recruited study participants between July 2008 and January 2011. On the occasion of an antenatal visit at the end of the first trimester, pregnant women were invited to participate in the study. Inclusion criteria were: gestational age between 6+0 and 13+6 weeks since the last menstrual period verified by use of ultrasound; blood sample provided at gestational age 6+0 to 13+6 weeks; maternal age 18–45 years; singleton pregnancy. Exclusion criteria were: multifetal pregnancy; gestational age below 6+0 or above 13+6 weeks; maternal age under 18 or over 45 years; mental retardation or other mental disorders that raised doubts regarding the patient’s willingness to participate in the study; lack of a blood sample at the specified enrolment period. A flow chart illustrating patient inclusion is given in figure 1. Written informed consent was obtained from all patients who agreed to participate in the study. The study was compliant with the principles of the Declaration of Helsinki and approved by the local institutional review board (Ethics Commission of the Canton of Bern).

Figure 1
Flow chart of the study. The participating women were subsequently recruited by nine obstetricians based in private practices.
On the occasion of the antenatal visit at the end of the first trimester, each participant’s personal history and anthropometric measurements were collected, including information on parity, smoking status, blood pressure, body mass index (BMI), age and ethnicity. Further, ultrasound measurements were recorded, such as nuchal translucency and crown-rump length. Gestational age since the last menstrual period was determined from ultrasound measurements. After birth or termination of pregnancy, the participants were followed-up for occurrence of preeclampsia, defined as blood pressure >140/90 mm Hg or a rise of >30 mm Hg in systolic and >20 mm Hg in diastolic, proteinuria ≥300 mg / 24 h, with or without clinical symptoms such as oedema, headaches and stomach aches, and brisk reflexes. This follow-up information was recorded on a questionnaire, which was systematically sent to the participating obstetricians. If no response for follow-up was obtained 11 months after the anticipated birth date (which was calculated at the study centre for each study participant) the obstetrician was contacted by telephone to obtain the missing information. Early onset preeclampsia was defined as preeclampsia with delivery before gestational week 34. Eclampsia was defined as tonic-clonic seizures unrelated to epilepsy and metabolic disorders. HELLP syndrome was defined as the occurrence of haemolysis, elevated liver enzymes, and thrombocytopenia. Other characteristics such as birth weight, placental weight, presence of malformations, presence of intrauterine growth restriction (IUGR; based on two measurements at least 2 weeks apart providing evidence of the fetus not growing according to the percentile curve), mode of birth, gestational age at birth, and Apgar scores (1, 5 and 10 minutes after birth) were also provided. IUGR was assessed according to individual follow-up schemes for ultrasound measurements of the participating obstetricians.
Statistical analysis was based on the included women and not on completed pregnancies. Gestational weeks were obtained from the calculated day of conception estimated by use of ultrasound at inclusion. The data are presented as medians and interquartile ranges (IQRs) or mean ± standard deviation, depending on the assessment with the Kolmogorov-Smirnov test. Proportions are given with a 95% confidence interval (CI). Proportions were compared by means of Fisher’s exact test, whereas tests for trends in proportions are evaluated with the Cochran-Armitage test. Medians were compared using the Mann-Whitney U-test, whereas means are compared with Student’s t-test. Incidences are calculated for the date of enrolment into the study. Birth weights according to presence or absence of preeclampsia were compared as percentiles adjusted for gestational age. For all comparisons, p-values <0.05 were considered to be significant; p-values up to 0.20 were reported numerically, whereas p-values >0.2 were reported as not significant. All calculations are performed with MedCalc software version 12.2.1 (MedCalc software, Mariakerke, Belgium).
The study population consisted of 1,511 women. Sixty women had two pregnancies during the study period; therefore a total of 1,571 pregnancies were included in the study cohort. The women presented at median gestational week 12+3 (IQR 12+0, 12+6). The baseline characteristics of the study population are given in table 1. Follow-up data were available for 1,300 pregnancies resulting in a follow-up rate of 82.75%. It can be seen in table 1, that the women with and without follow-up exhibit significant (but clinically unimportant) differences only in BMI and the frequency of Asian descent. Four women were excluded because of a twin gestation, seven women were under 18 years old upon enrolment and four women provided the sample at a gestational age above 13+6 weeks. In order to minimise the influence of missing follow-up data and owing to the fact that no meaningful differences between women with and without follow-up could be detected, the analysis was confined to women with follow-up.
The final analysis included 1,300 pregnancies, 30 of which were preeclamptic. This reveals a preeclampsia incidence of 2.31% (95% CI 1.62%–3.28%). Two of those women had early-onset preeclampsia (0.15%; 0.05%–0.55%). Four preeclampsia patients developed eclampsia, yielding a rate of 0.31% (0.12%–0.79%), whereas three patients (0.23%; 0.08%–0.67%) progressed to HELLP syndrome. Fifteen pregnancies did not result in the birth of a living baby, one of which was from a woman with preeclampsia.
Women with preeclampsia had significantly earlier delivery than women without preeclampsia (median 37.50 weeks, IQR 36.75–38.21 vs 39.43 weeks, IQR 38.42‒40.29; p<0.001). Of the pregnancies affected by preeclampsia, 6.67% (2.04%–21.42%; 2/30) showed an early onset, with birth before gestational week 34. Thirty percent (16.68%–48.04%; 9/30) of preeclamptic women and 6.69% (5.45%–8.20%; 85/1,270) of healthy women had a preterm birth (before 37 completed weeks of gestation) (p <0.001); 0.23% (0.09%–0.69%; 3/1,270) of healthy women and none of the preeclampsia patients had a post-term birth (at least 42 gestational weeks).
The incidence of preeclampsia was highest in nulliparous women (19/480; 3.96%, 95% CI 2.56%–6.10%) and decreased further from 1.60% (0.79%–3.27%; 7/437) in second pregnancies to 0.92% (0.33%–2.65%; 3/327) in third or later pregnancies. There was a significant decrease of preeclampsia incidence with increasing parity (p for trend = 0.003). Preeclampsia developed in 25.93% (13.22%–44.87%; 7/27) of women with a prior history of preeclampsia and in 0.41% (0.02%–1.20%; 3/726) of those who had had previous pregnancies without preeclampsia (p <0.001).
Women with preeclampsia were not significantly older than women without preeclampsia, as seen in table 2. Women up to 24 years old had an incidence of preeclampsia of 2.65% (1.17%–6.03%; 5/189). The incidence was 2.58% (1.42%–4.68%; 10/388) in the age group 25–29, 2.30% (1.30%–4.06%; 11/479) in women aged 30‒34 and 1.87% (0.76%–4.69%; 4/214) in the group aged 35–39 years. There were no preeclampsia cases at the age of 40 or more (n = 27). There was neither a significant trend nor significant differences in preeclampsia incidence among these different age groups.
There was a significant difference in BMI between women with and without preeclampsia (p = 0.047), as seen in table 2. Women with normal weight (BMI 18.5–24.99 kg/m2) had a 1.99% (1.23%–3.21%; 16/804) incidence of preeclampsia. Underweight women exhibited an incidence of 2.44% (0.58%–12.57%; 1/41). The incidence of preeclampsia at higher BMI was 2.70% (1.39%–5.24%; 8/296) in women with a BMI between 25 and 29.99 kg/m2 and 3.88% (1.71%–8.75%; 5/129) in women with a BMI of 30 or more. There was neither a significant trend nor significant differences in preeclampsia incidence among these different BMI groups.
Women with preeclampsia had significantly higher systolic (p = 0.045) and diastolic (p = 0.015) blood pressure than women without preeclampsia. At inclusion, systolic blood pressure was >140 mm Hg in 47/1,270 (3.70%, 95% CI 2.80%–4.89%) pregnancies without preeclampsia and 3/30 (10%; 95% CI 3.63%–25.75%) (p = 0.09) in pregnancies with preeclampsia, whereas diastolic blood pressure was >90 mm Hg in 14/1,270 (1.10%; 95% CI% 0.66%–1.84%) pregnancies without preeclampsia and in none of the women with preeclampsia. There were no statistically significant differences in preeclampsia incidence between smokers (1.47%; 2/136) and nonsmokers (2.41%; 27/1,121). Nuchal translucency was similar in pregnancies with and without preeclampsia (1.2 mm, IQR 1.0–1.5 vs 1.2 mm, IQR 1.0–1.4). There were 30 women with diabetes. None of them developed preeclampsia.
Neonates born to mothers with preeclampsia had significantly lower birth weight (median 2,953 g, IQR 2,570–3,380) than neonates of mothers without preeclampsia (median 3,300 g, IQR 3,010 –3,630) (p <0.001). Comparing the birth weight percentiles related to gestational age in neonates of mothers with preeclampsia to those of neonates born to mothers without preeclampsia revealed no such statistical difference (p = 0.20). This suggests that the difference in birth weight was mainly due to earlier deliveries in cases of preeclampsia, although other reasons leading to lower birth weight, which were not investigated in the present study, could also have contributed to this difference. Apgar scores of babies born to mothers with preeclampsia tended to be lower at 1 minute after birth (median 9, IQR 8–9; p = 0.08) but not after 5 (median 10, IQR 9‒10; p = 0.58) and 10 minutes (median 10, IQR 10‒10; p = 0.58). The fetuses of three preeclampsia patients (10%; 3.63%‒25.75%) and 68 controls (5.35%; 4.25%‒6.73%) suffered from intrauterine growth restriction. This difference was statistically not significant.
| Table 1: Maternal characteristics presented as median (interquartile range) or number of pregnancies (percentage) in women with and without follow-up. Characteristics were obtained from pregnancies at gestational age 10+3 until 13+6 weeks. | |||
| Characteristic | Follow-up (n =1,300) | No follow-up (n = 271) | p-value |
| Age at sampling (years) | 30 (27–33) | 30 (26–33) | NS |
| <20 | 14 (1.1%) | 2 (0.7%) | NS |
| 20–24 | 175 (13.5%) | 35 (12.9%) | NS |
| 25–29 | 388 (29.8%) | 89 (32.8%) | NS |
| 30–34 | 479 (36.8%) | 96 (35.4%) | NS |
| 35–39 | 214 (16.5%) | 44 (16.2%) | NS |
| 40–45 | 27 (2.1%) | 5 (1.8%) | NS |
| Unknown | 3 (0.2%) | 0 (0%) | NS |
| Previous pregnancies (n) | 1 (0–2) | 1 (0–2) | NS |
| 480 (36.9%) | 91 (33.6%) | NS | |
| 1 | 437 (33.6%) | 88 (32.5%) | NS |
| 2 | 213 (16.4%) | 46 (17.0%) | NS |
| 3 | 69 (5.3%) | 14 (5.2%) | NS |
| 4 | 30 (2.3%) | 4 (1.5%) | NS |
| 5+ | 15 (1.2%) | 2 (0.7%) | NS |
| Unknown | 56 (4.3%) | 26 (9.6%) | 0.0009 |
| Body mass index (kg/m2) | 23.33 (21.18-26.07) | 23.72 (21.77–26.89) | 0.0339 |
| <18.5 | 41 (3.2%) | 5 (1.8%) | NS |
| 18.5–24.99 | 804 (61.8%) | 162 (59.8%) | NS |
| 25–29.99 | 296 (22.8%) | 64 (23.6%) | NS |
| 30–34.99 | 83 (6.4%) | 25 (9.2%) | 0.1120 |
| 35+ | 46 (3.5%) | 8 (3.0%) | NS |
| Unknown | 30 (2.3%) | 7 (2.6%) | NS |
| Smokers (%) | 136 (10.5%) | 28 (10.3%) | NS |
| Diabetics (%) | 30 (2.3%) | 2 (0.7%) | 0.1511 |
| Ethnicity | |||
| Caucasian | 1186 (91.2%) | 241 (88.9%) | NS |
| African | 17 (1.3%) | 5 (1.8%) | NS |
| Asian | 30 (2.3%) | 13 (4.8%) | 0.0374 |
| Other | 7 (0.5%) | 0 (0%) | NS |
| Unknown | 60 (4.6%) | 12 (4.4%) | NS |
| SBP (mm Hg) | 117 (109–126) | 117 (108–125) | NS |
| DBP (mm Hg) | 70 (64–77) | 71 (64–78) | NS |
| Previous preeclampsia | 16 (1.2%) | 4 (1.5%) | NS |
| DBP = diastolic blood pressure; NS = not statistically significant; SBP = systolic blood pressure | |||
| Table 2: Maternal characteristics presented as median (interquartile range) or number of pregnancies (percentage) in followed-up women with and without preeclampsia. Characteristics were obtained from pregnancies at gestational age 10+3 until 13+6 weeks. | |||
| Characteristic | Preeclampsia cases (n = 30) | Normal pregnancies (n = 1,270) | p-value |
| Age at sampling (years) | 29.5 (26.25–34) | 30 (27–33) | NS |
| <20 | 1 (3.3%) | 13 (1.0%) | NS |
| 20–24 | 4 (13.3%) | 171 (13.5%) | NS |
| 25–29 | 10 (33.3%) | 378 (29.8%) | NS |
| 30–34 | 11 (36.7%) | 468 (36.9%) | NS |
| 35–39 | 4 (13.3%) | 210 (16.5%) | NS |
| 40–45 | 0 (0%) | 27 (2.1%) | NS |
| Unknown | 0 (0%) | 3 (0.2%) | NS |
| Previous pregnancies (n) | 0 (0–1) | 1 (0–2) | 0.003 |
| 19 (63.3%) | 461 (36.3%) | 0.004 | |
| 1 | 7 (23.3%) | 430 (33.9%) | NS |
| 2 | 2 (6.7%) | 211 (16.6%) | NS |
| 3 | 0 (0%) | 69 (5.4%) | NS |
| 4 | 1 (3.3%) | 29 (2.3%) | NS |
| 5+ | 0 (0%) | 15 (1.2%) | NS |
| Unknown | 1 (3.3%) | 55 (4.3%) | NS |
| Body mass index (kg/m2) | 24.60 (22.11–28.27) | 23.27 (21.16–26.04) | 0.047 |
| <18.5 | 1 (3.3%) | 40 (3.1%) | NS |
| 18.5–24.99 | 16 (53.3%) | 788 (62.0%) | NS |
| 25–29.99 | 8 (26.7%) | 288 (22.7%) | NS |
| 30–34.99 | 3 (10.0%) | 80 (6.3%) | NS |
| 35+ | 2 (6.7%) | 44 (3.5%) | NS |
| Unknown | 0 (0%) | 30 (2.4%) | NS |
| Smokers (%) | 2 (6.7%) | 134 (10.6%) | NS |
| Diabetics (%) | 0 (0%) | 30 (2.4%) | NS |
| Ethnicity | |||
| Caucasian | 27 (90.0%) | 1159 (91.3%) | NS |
| African | 1 (3.3%) | 16 (1.3%) | NS |
| Asian | 1 (3.3%) | 29 (2.3%) | NS |
| other | 0 (0%) | 7 (0.6%) | NS |
| Unknown | 1 (3.3%) | 59 (4.6%) | NS |
| SBP (mm Hg) | 123 (108–134) | 117 (109–126) | 0.045 |
| DBP (mm Hg) | 75 (68–81) | 70 (64–77) | 0.015 |
| Previous preeclampsia | 4 (13.3%) | 12 (0.9%) | 0.0003 |
| DBP = diastolic blood pressure; NS = not statistically significant; SBP = systolic blood pressure | |||
This study suggests that the incidence of preeclampsia in Switzerland is in line with the frequencies observed elsewhere in the world. When looking at European countries, the incidence in Switzerland is at the lower end of the reported frequencies. About 10% of all observed cases progressed to eclampsia, whereas another 10% progressed to HELLP syndrome. About 7% of all preeclampsia cases were early-onset preeclampsia. Nulliparity and prior history of preeclampsia were more frequently seen in pregnancies with preeclampsia than in pregnancies without preeclampsia. BMI, as well as systolic and diastolic blood pressure, were higher in pregnancies subsequently developing preeclampsia. As a result of a statistical type II error arising from a relatively low number of preeclampsia cases, some further associations between predisposing risk factors and the occurrence of preeclampsia might have been missed. Analogously, a type I error may also have occurred in the analysis of the observed associations. Neonates of women with preeclampsia were delivered earlier and tended to show lower 1-minute Apgar scores compared with neonates born from uncomplicated pregnancies.
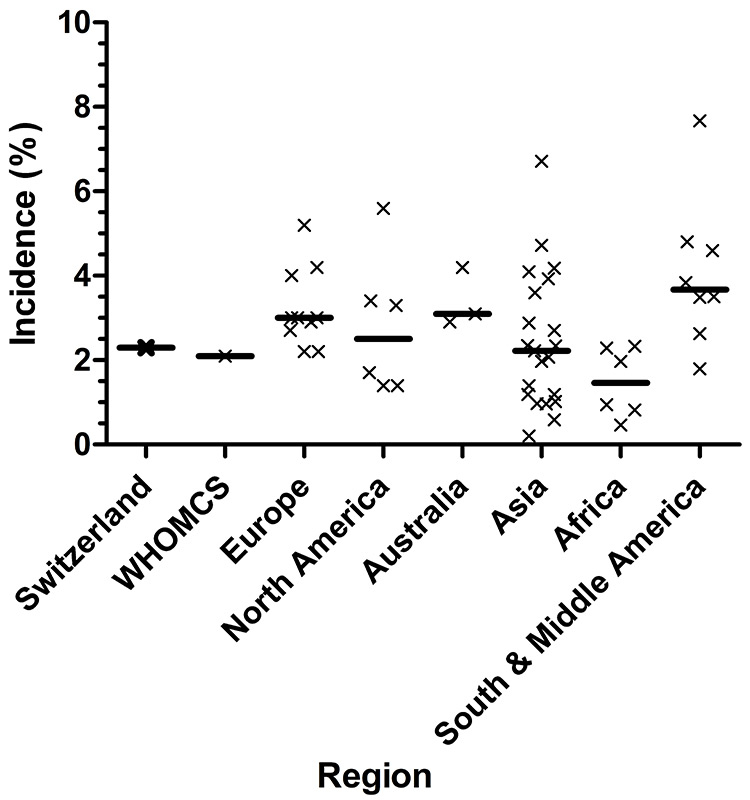
Figure 2
Observed incidence of preeclampsia in the Swiss PRADO study compared with worldwide and regional incidences.
X denotes a point incidence observed at a specific period of time in a specific country. A bold horizontal line indicates the median of all observed incidences within a regional group. Data are taken from references [18, 26, 27, 33, 43–57] and are specified in more detail in table 3. Summary data from WHOMCS (World Health Organization Multicountry Survey on Maternal and Newborn Health) originate from reference [26].
Our cohort had a mean maternal age of 31.0 years, a proportion of 70.1% of vaginal deliveries, and a mean birth weight of 3,282 g; 91.7% of study participants were of Caucasian descent. This compares with national characteristics of a mean maternal age at delivery of 30.8‒31.4 years during the years of 2007‒2011 [30], a proportion of 69.9% vaginal deliveries in 2004 [31], a mean birth weight of 3,296 g in 2011 [32], and a proportion of 91.8% of mothers originating from Europe, North America or Oceania in 2011 [30]. These data suggest that our cohort can be considered to be representative of Switzerland.
There are many different definitions of preeclampsia. The unified definition proposed by the ISSHP is mentioned above in the introduction. In essence, it is blood pressure ≥140/90 in the second half of gestation that returns to normal postpartum, and proteinuria of ≥300 mg/day or 1+ on a dipstick or a ratio of ≥30 mg of protein per mmol of creatinine [4]. The WHO definition, on the other hand, considers only diastolic blood pressure of ≥90 mmHg to be significant [5]. Proteinuria is defined by the WHO as ≥300 mg per litre, ≥300 mg in a 24-hour urine collection or ≥1+ on dipstick, although it is recommended that the dipstick test be verified by another test. In addition, the WHO definition encompasses postpartum preeclampsia [5]. There are also other definitions of preeclampsia. Our study employed the following blood pressure and proteinuria criteria for preeclampsia: blood pressure >140/90 mm Hg or a rise of >30 mm Hg in systolic and >20 mm Hg in diastolic, proteinuria ≥300 mg/24 h. It is evident that unless a uniform definition of preeclampsia is available, incidences of preeclampsia differ to some extent because of the different definitions of preeclampsia employed.
There have been studies carried out to find the incidence of preeclampsia in many countries. A secondary analysis of the WHO Multicountry Survey on Maternal and Newborn Health (the survey itself was carried out between 2004 and 2008) contained data from 29 developing countries [26]. Numbers range from 0.2% in Vietnam to 7.67% in Nicaragua, with an incidence of 2.16% in total. Another study measured the incidence of preeclampsia in eight regions of developed countries [33]. There the range seems to be smaller – from 1.4% in Alberta, Canada to 4.0% in Norway. All the studies included in that paper were carried out between 1997 and 2007. Other population-based studies from Europe show a similar incidence range: from 2.2% in the Netherlands (2000–2008) to 4.5% in Norway (1998–2002) [27, 28]. This present study shows that Switzerland is at the lower end of the preeclampsia incidence range in Europe. Nevertheless, with a total of 82,731 births registered in Switzerland during 2013 [30], the observed preeclampsia incidence of 2.31% (95% CI 1.62%–3.28%) extrapolates to 1,911 preeclampsia cases (range 1,340–2,713) occurring per year in Switzerland. This is in accordance with 2,005 cases reported for the year 2004 derived from a retrospective register-survey published by the national office of statistics [31]. From a public health perspective, these numbers will be important when it comes to judging the cost-effectiveness of interventions allowing for early recognition and treatment of women with a high risk for development of preeclampsia.
The reported incidence range of early-onset preeclampsia (defined as developing before or during the 34th gestational week) elsewhere in the world is 0.2%–1.4% [34, 35]. The incidence of HELLP syndrome is quoted as 0.17%–0.85% of all pregnancies [36], while three other studies found it to be slightly lower than that [37–39]. In the PRADO collective, HELLP syndrome with an incidence of 0.19% (95% CI 0.07%–0.56%) and early-onset preeclampsia with an incidence of 0.13% (95% CI 0.04%–0.46%) occur as frequently as in these other collectives. Early-onset preeclampsia, however, is at the lower range of frequencies observed elsewhere. Studies investigating the incidence of preeclampsia in different regions of the world are summarised in figure 2. Further details on the different reported incidences are given in table 3. Variations in blood pressure, body weight, parity, age, and glucose tolerance are associated with the incidence of preeclampsia. Further, the incidence of preeclampsia is also dependent on the quality of antenatal care [33]. Although the present study was not designed to identify factors leading to a low incidence of preeclampsia, it can be hypothesised that the distribution and composition of risk factors leading to preeclampsia is supposedly favourable in Switzerland, when compared with other countries.
Commonly quoted risk factors for developing preeclampsia are nulliparity, advanced maternal age, obesity, multiple gestation, prior preeclampsia and preexisting diseases like diabetes and chronic hypertension [1, 16–18]. Further, parity >3 represents another risk factor for preeclampsia, which, owing to the low median number of children per woman, is often ignored in the Western world [40]. As a result of the relatively rare occurrence of preeclampsia in our study, not all associations could be confirmed owing to statistical type II error. However, we found the occurrence of preeclampsia to be significantly correlated with prior preeclampsia, high BMI, and both systolic and diastolic blood pressure.
Adverse effects of preeclampsia for the mother include pulmonary oedema, cerebral haemorrhage, disseminated intravascular coagulation and renal failure [5, 41]. A history of preeclampsia is associated with a higher risk of renal and cardiovascular diseases later in life [42]. The baby is at risk of intrauterine growth restriction, premature birth, low birth weight, hypertension, cardiovascular disease, and diabetes later in life [1, 5, 16, 19, 41]. We could reproduce the correlation of preeclampsia with preterm delivery.
The main limitation of this study was its relatively small sample of preeclampsia cases. As the occurrence of preeclampsia is a rare event, a small sample size hinders the detection of all risk factors and outcomes. However, some strongly correlated factors were found, and the main focus of the study was to determine the incidence of preeclampsia, not the risk factors for its development. In addition, we proposed a standard definition for diagnosing preeclampsia but did not get a detailed report on how it actually was diagnosed (i.e., exact specification of blood pressure and proteinuria at diagnosis). Thirdly, follow-up data were available for about 83% of the cases, which might introduce bias. However, when comparing the women with and without follow-up, it can be seen that the two groups do not differ in a meaningful manner. Two of nearly 40 statistical tests in this comparison were significant, leading to an inherent statistical type I error. In our view the comparability of the women with and without follow-up validates our approach of restricting the analysis to women with follow-up. Lastly, since multiple gestation was an exclusion criterion, our results may not be valid for women benefitting from artificial reproductive technologies (ART). We consider the potential for bias small as only about 2% of deliveries in Switzerland occur following ART. In sum, we do not believe that these limitations invalidate our findings.
To our knowledge, the present study is the first to describe the incidence of preeclampsia in Switzerland by means of a prospective study. Our reported incidence, 2.31%, is in line with the mean incidence reported worldwide. Among European countries, the preeclampsia incidence observed in our study is at the lower end of observed frequencies. Nevertheless, extrapolation to a national level indicates that a sizeable number of 1,340–2,713 preeclampsia cases per year are currently occurring in Switzerland.
| Table 3: Incidence of preeclampsia elsewhere than Switzerland. | |||||
| Country/Region | Study period | Enrolment | Population size | Preeclampsia incidence | Ref. |
| Europe | |||||
| UK (Scotland) | 1997–2006 | Prospective | 531,662 | 2.2% | [33] |
| The Netherlands | 2000–2008 | Prospective | 1,457,576 | 2.2% | [27] |
| Denmark | 1997–2006 | Prospective | 645,993 | 2.7% | [33] |
| Sweden | 1997–2006 | Prospective | 913,779 | 2.9% | [33] |
| Denmark | 1997–2003 | Retrospective | 70,924 | 3.0% | [43] |
| Norway | 1967–2008 | Register-based | 2,416,501 | 3.0% | [44] |
| Sweden | 1987–2004 | Prospective | 763,795 | 3.0% | [45] |
| Norway | 1999-2006 | Prospective | 456,353 | 4.0% | [33] |
| Norway | 1999-2004 | Register-based | 315,085 | 4.2% | [46] |
| Sweden (Uppsala) | 1987-1993 | Register-based | 10,659 | 5.2% | [47] |
| North America | |||||
| Canada (Alberta) | 2002–2007 | Prospective | 256,137 | 1.4% | [33] |
| USA (New York) | 1999–2003 | Register-based | 77,358 | 1.4% | [48] |
| Canada (Alberta) | 1991–1996 | Retrospective | 87,798 | 1.7% | [49] |
| USA (Massachusetts) | 1998–2007 | Prospective | 762,723 | 3.3% | [33] |
| USA | 1980–2010 | Retrospective | 120 million | 3.4% | [50] |
| Canada (Newfoundland) | 1996–1997 | Retrospective | 5,172 | 5.6% | [51] |
| Australia | |||||
| Western Australia | 2000–2005 | Prospective | 149,624 | 2.9% | [33] |
| New South Wales | 1998–2006 | Prospective | 732,288 | 3.1% | [33] |
| New South Wales | 2000–2002 | Register-based | 250,173 | 4.2% | [52] |
| Asia | |||||
| Vietnam | 2010–2011 | Prospective | 15,421 | 0.20% | [26] |
| Nepal | 2010–2011 | Prospective | 11,293 | 0.59% | [26] |
| Sri Lanka | 2010–2011 | Prospective | 18,108 | 0.96% | [26] |
| Afghanistan | 2010–2011 | Prospective | 25,913 | 0.97% | [26] |
| Lebanon | 2010–2011 | Prospective | 4,042 | 1.02% | [26] |
| Japan | 2010–2011 | Prospective | 3,534 | 1.19% | [26] |
| Pakistan | 2010–2011 | Prospective | 13,122 | 1.19% | [26] |
| Taiwan | 1990–1998 | Register-based | 29,735 | 1.4% | [53] |
| India | 2010–2011 | Prospective | 31,168 | 1.97% | [26] |
| China | 2010–2011 | Prospective | 13,273 | 2.07% | [26] |
| Thailand | 2010–2011 | Prospective | 8,942 | 2.22% | [26] |
| Cambodia | 2010–2011 | Prospective | 4,691 | 2.34% | [26] |
| Palestinian Territory | 2010-2011 | Prospective | 980 | 2.35% | [26] |
| Japan | 2001–2005 | Register-based | 241,292 | 2.7% | [54] |
| China | 2011 | Retrospective | 112,386 | 2.88% | [55] |
| Philippines | 2010–2011 | Prospective | 10,762 | 3.60% | [26] |
| Qatar | 2010–2011 | Prospective | 3,950 | 3.93% | [26] |
| Israel (Negev) | 1988–2007 | Retrospective | 201,955 | 4.1% | [56] |
| India (Mumbai) | 1993–1996 | Retrospective | 29,562 | 4.18% | [57] |
| Jordan | 2010–2011 | Prospective | 1,166 | 4.72% | [26] |
| Mongolia | 2010–2011 | Prospective | 7,343 | 6.71% | [26] |
| Africa | |||||
| Niger | 2010–2011 | Prospective | 10,963 | 0.46% | [26] |
| DR Congo | 2010–2011 | Prospective | 8,700 | 0.82% | [26] |
| Uganda | 2010–2011 | Prospective | 10,828 | 0.95% | [26] |
| Kenya | 2010–2011 | Prospective | 20,280 | 1.97% | [26] |
| Angola | 2010–2011 | Prospective | 10,414 | 2.29% | [26] |
| Nigeria | 2010–2011 | Prospective | 12,585 | 2.33% | [26] |
| South & Middle America | |||||
| Paraguay | 2010–2011 | Prospective | 3,607 | 1.80% | [26] |
| Argentina | 2010–2011 | Prospective | 9,797 | 2.63% | [26] |
| Ecuador | 2010–2011 | Prospective | 10,224 | 3.49% | [26] |
| Peru | 2010–2011 | Prospective | 15,181 | 3.5% | [26] |
| Brazil | 2010–2011 | Prospective | 7,052 | 4.60% | [26] |
| Latin America / Caribbean | 1985–1997 | Register-based | 878,680 | 4.8% | [18] |
| Nicaragua | 2010–2011 | Prospective | 6,472 | 7.67% | [26] |
| Mexico | 2010–2011 | Prospective | 13,273 | 3.84% | [26] |
Acknowledgements:The PRADO study team is indebted to the obstetricians involved in the PRADO study. Without their close and meticulous collaboration, this study would not have been possible.
1 Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–99.
2 Harlow FH, Brown MA. The diversity of diagnoses of preeclampsia. Hypertension in pregnancy 2001; 20:57-67.
3 Chappell S, Morgan L. Searching for genetic clues to the causes of pre-eclampsia. Clinical science. 2006;110:443–58.
4 Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertens Pregnancy. 2001;20:IX–XIV.
5 The hypertensive disorders of pregnancy. Report of a WHO study group. World Health Organ Tech Rep Ser. 1987;758:1–114.
6 Baumann MU, Bersinger NA, Surbek DV. Serum markers for predicting pre-eclampsia. Mol Aspects Med. 2007;28:227–44.
7 Carty DM, Delles C, Dominiczak AF. Novel biomarkers for predicting preeclampsia. Trends Cardiovasc Med. 2008;18:186–94.
8 Moslemi Zadeh N, Naghshvar F, Peyvandi S, Gheshlaghi P, Ehetshami S. PP13 and PAPP-A in the First and Second Trimesters: Predictive Factors for Preeclampsia? ISRN Obstet Gynecol. 2012;2012:263871.
9 Baumann MU, Bersinger NA, Mohaupt MG, Raio L, Gerber S, Surbek DV. First-trimester serum levels of soluble endoglin and soluble fms-like tyrosine kinase-1 as first-trimester markers for late-onset preeclampsia. Am J Obstet Gynecol. 2008;199:266e261–6.
10 Cnossen JS, Morris RK, ter Riet G, et al. Use of uterine artery Doppler ultrasonography to predict pre-eclampsia and intrauterine growth restriction: a systematic review and bivariable meta-analysis. Can Med Assoc J. 2008;178:701–11.
11 Espinoza J, Romero R, Nien JK, et al. Identification of patients at risk for early onset and/or severe preeclampsia with the use of uterine artery Doppler velocimetry and placental growth factor. Am J Obstet Gynecol. 2007;196:326 e321–13.
12 Baumann M, Korner M, Huang X, Wenger F, Surbek D, Albrecht C. Placental ABCA1 and ABCG1 expression in gestational disease: Pre-eclampsia affects ABCA1 levels in syncytiotrophoblasts. Placenta. 2013;34:1079–86.
13 Gong YH, Jia J, Lu DH, Dai L, Bai Y, Zhou R. Outcome and risk factors of early onset severe preeclampsia. Chin Med J (Engl). 2012;125:2623–7.
14 Madazli R, Yuksel MA, Imamoglu M, et al. Comparison of clinical and perinatal outcomes in early- and late-onset preeclampsia. Arch Gynecol Ostet. 2014;290:53–7.
15 Vatten LJ, Skjaerven R. Is pre-eclampsia more than one disease? BJOG. 2004;111:298–302.
16 Eiland E, Nzerue C, Faulkner M. Preeclampsia 2012. JPregnancy 2012;2012:586578.
17 Al-Jameil N, Aziz Khan F, Fareed Khan M, Tabassum H. A brief overview of preeclampsia. J Clin Med Res. 2014;6:1–7.
18 Conde-Agudelo A, Belizan JM. Risk factors for pre-eclampsia in a large cohort of Latin American and Caribbean women. BJOG. 2000;107:75–83.
19 Dekker G, Sibai B. Primary, secondary, and tertiary prevention of pre-eclampsia. Lancet. 2001;357:209–15.
20 Cnattingius S, Mills JL, Yuen J, Eriksson O, Salonen H. The paradoxical effect of smoking in preeclamptic pregnancies: smoking reduces the incidence but increases the rates of perinatal mortality, abruptio placentae, and intrauterine growth restriction. Am J Obstet Gynecol. 1997;177:156–61.
21 Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005;330:565.
22 Assis TR, Viana FP, Rassi S. Study on the major maternal risk factors in hypertensive syndromes. Arq Bras Cardiol. 2008;91:11–7.
23 Savitz DA, Danilack VA, Engel SM, Elston B, Lipkind HS. Descriptive epidemiology of chronic hypertension, gestational hypertension, and preeclampsia in New York State, 1995-2004. Matern Child Health J. 2014;18:829–38.
24 Khalil A, Rezende J, Akolekar R, Syngelaki A, Nicolaides KH. Maternal racial origin and adverse pregnancy outcome: a cohort study. Ultrasound Obstet Gynecol. 2013;41:278–85.
25 Altman D, Carroli G, Duley L, et al. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359:1877–90.
26 Abalos E, Cuesta C, Carroli G, et al. Pre-eclampsia, eclampsia and adverse maternal and perinatal outcomes: a secondary analysis of the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG. 2014;121Suppl1:14–24.
27 von Schmidt auf Altenstadt JF, Hukkelhoven CW, van Roosmalen J, Bloemenkamp KW. Pre-eclampsia increases the risk of postpartum haemorrhage: a nationwide cohort study in the Netherlands. PloS one. 2013;8:e81959.
28 Dahlstrom BL, Engh ME, Bukholm G, Oian P. Changes in the prevalence of pre-eclampsia in Akershus County and the rest of Norway during the past 35 years. Acta Obstet Gynecol Scand. 2006;85:916–21.
29 Lamminpaa R, Vehvilainen-Julkunen K, Gissler M, Heinonen S. Preeclampsia complicated by advanced maternal age: a registry-based study on primiparous women in Finland 1997–2008. BMC Pregnacy Childbrith. 2012;12:47.
30 Statistik; Bf, Bevölkerungsbewegung – Detaillierte Daten; Geburt und Fruchtbarkeit. http://www.bfs.admin.ch/bfs/portal/de/index/themen/01/06/blank/data/01.html: Bundesamt für Statistik, 2015.
31 Schwab P, Zwimpfer A. Statistik der Schweiz. Gebären in Schweizer Spitälern. Spitalaufenthalte während Schwangerschaft und Entbindung. Neuchatel: Bundesamt für Statistk. 2007.
32 Statistik; Bf, Fortpflanzung, Gesundheit der Neugeborenen – Daten, Indikatoren. http://www.bfs.admin.ch/bfs/portal/de/index/themen/14/02/03/key/04.html: Bundesamt für Statistik. 2015.
33 Roberts CL, Ford JB, Algert CS, et al. Population-based trends in pregnancy hypertension and pre-eclampsia: an international comparative study. BMJ open. 2011;1:e000101.
34 Schneuer FJ, Nassar N, Khambalia AZ, et al. First trimester screening of maternal placental protein 13 for predicting preeclampsia and small for gestational age: in-house study and systematic review. Placenta. 2012;33:735–40.
35 Caradeux J, Serra R, Nien JK, et al. First trimester prediction of early onset preeclampsia using demographic, clinical, and sonographic data: a cohort study. Prenat Diagn. 2013;33:732–6.
36 Mihu D, Costin N, Mihu CM, Seicean A, Ciortea R. HELLP syndrome – a multisystemic disorder. J Gastrointestin Liver Dis. 2007;16:419–24.
37 Abraham KA, Connolly G, Farrell J, Walshe JJ. The HELLP syndrome, a prospective study. Renal failure. 2001;23:705–13.
38 Magann EF, Perry KG, Jr., Chauhan SP, Graves GR, Blake PG, Martin JN, Jr. Neonatal salvage by week's gestation in pregnancies complicated by HELLP syndrome. J Soc Gynecol Investlg. 1994;1:206–9.
39 Oliveira N, Doyle LE, Atlas RO, Jenkins CB, Blitzer MG, Baschat AA. External validity of first-trimester algorithms in the prediction of pre-eclampsia disease severity. Ultrasound Obstet Gynecol. 2014;44:286–92.
40 Abu-Heija AT, Chalabi HE. Great grand multiparity: is it a risk? J Obstet Gynecol. 1998;18:136–8.
41 Zhang J, Meikle S, Trumble A. Severe maternal morbidity associated with hypertensive disorders in pregnancy in the United States. Hypertens Pregnancy. 2003; 22:203-212.
42 Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974.
43 Catov JM, Ness RB, Kip KE, Olsen J. Risk of early or severe pre-eclampsia related to pre-existing conditions. Int J Epidemiol. 2007;36:412–9.
44 Klungsoyr K, Morken NH, Irgens L, Vollset SE, Skjaerven R. Secular trends in the epidemiology of pre-eclampsia throughout 40 years in Norway: prevalence, risk factors and perinatal survival. Paediatr Perinat Epidemiol. 2012;26:190–8.
45 Hernandez-Diaz S, Toh S, Cnattingius S. Risk of pre-eclampsia in first and subsequent pregnancies: prospective cohort study. BMJ. 2009;338:b2255.
46 Eskild A, Vatten LJ. Abnormal bleeding associated with preeclampsia: a population study of 315,085 pregnancies. Acta Obstet Gynecol Scand. 2009;88:154–8.
47 Ros HS, Cnattingius S, Lipworth L. Comparison of risk factors for preeclampsia and gestational hypertension in a population-based cohort study. Am J Epidemiol. 1998;147:1062–70.
48 Lawler J, Osman M, Shelton JA, Yeh J. Population-based analysis of hypertensive disorders in pregnancy. HypertensPregnancy. 2007;26:67–76.
49 Xiong X, Demianczuk NN, Saunders LD, Wang FL, Fraser WD. Impact of preeclampsia and gestational hypertension on birth weight by gestational age. Am J Epidemiol. 2002;155:203–9.
50 Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980–2010: age-period-cohort analysis. BMJ. 2013;347:f6564.
51 Dawson LM, Parfrey PS, Hefferton D, et al. Familial risk of preeclampsia in Newfoundland: a population-based study. J Am Soc Nephrol. 2002;13:1901–6.
52 Roberts CL, Algert CS, Morris JM, Ford JB, Henderson-Smart DJ. Hypertensive disorders in pregnancy: a population-based study. Med J Aust. 2005;182:332–5.
53 Lee CJ, Hsieh TT, Chiu TH, Chen KC, Lo LM, Hung TH. Risk factors for pre-eclampsia in an Asian population. Int J Gynaecol Obstet. 2000;70:327–33.
54 Shiozaki A, Matsuda Y, Satoh S, Saito S. Comparison of risk factors for gestational hypertension and preeclampsia in Japanese singleton pregnancies. J Obstet Gynaecol Res. 2013;39:492–9.
55 Ye C, Ruan Y, Zou L, et al. The 2011 survey on hypertensive disorders of pregnancy (HDP) in China: prevalence, risk factors, complications, pregnancy and perinatal outcomes. PloS one. 2014;9:e100180.
56 Shental O, Friger M, Sheiner E. Ethnic differences in the monthly variation of preeclampsia among Bedouin and Jewish parturients in the Negev. HypertensPregnancy. 2010;29:342–9.
57 Subramaniam V. Seasonal variation in the incidence of preeclampsia and eclampsia in tropical climatic conditions. BMC Womens Health. 2007;7:18.
Disclosures: This work was funded by an in-house grant of the Labormedizinisches Zentrum Dr. Risch.
Authors’ contribution: MTP and MB contributed equally