Salient features of the coronary collateral circulation and its clinical relevance
DOI: https://doi.org/10.4414/smw.2015.14154
Michael
Stoller, Christian
Seiler
Summary
The coronary collateral circulation provides an alternative source of blood supply to myocardium jeopardised by ischaemia. Collaterals enlarge with obstructive coronary artery disease to allow bulk flow, but blood flow deliverable by the native, pre-formed collateral extent can already be sizeable. Genetic determinants contribute significantly to the wide variability observed in both native collateral extent and its capacity to enlarge, and the severity of the coronary stenosis is the most significant environmental determinant for collateral enlargement. The protective effect of a well-developed coronary collateral circulation translates into relevant improvements in all-cause and cardiac mortality in the acute and chronic phases of coronary artery disease, as well as into a reduction of future adverse cardiovascular events.
Introduction
Coronary collaterals are cross-connections between coronary arteries, which are present regardless of coronary artery disease (CAD) [1]. As natural bypasses, coronary collaterals provide an alternative source of blood flow to a myocardial area when normal antegrade supply is compromised.
In the presence of arterial stenoses, the process of collateral remodelling leads to a gradual calibre increase of the collateral vessels, allowing the effective delivery of bulk flow to jeopardised tissue [2]. Conversely, the collateral network in the absence of obstructive coronary artery disease represents the native collateral circulation, pre-existing in the absence of coronary narrowing. Contrary to popular belief, blood flow deliverable by native collaterals can be sizeable [3]. The relevance of the coronary collateral circulation becomes eminent in CAD, which remains a leading cause of death globally [4]. In acute coronary occlusion, the extent of collateral pathways is a significant determinant of the severity of myocardial infarction [5–8]. In the chronic phase of CAD, the collateral circulation variably compensates for the flow-limiting features of atherosclerosis, i.e., stenotic lesions.
The purpose of this article is to give a concise review of the salient features of the coronary collateral circulation and provide an overview of its assessment and clinical relevance.
Coronary collateral dynamics
The dynamics of the coronary collateral circulation are governed by the physical laws of pressure and flow [9]. In the native collateral network, there is an oscillatory movement of blood, as it is fed from opposing directions [1, 10]. Thus, there is typically little to no net blood flow, as blood moves to-and-fro along the length of the collateral circulation, which prevents haemostatic thrombosis. Effective flow across collaterals to jeopardised myocardium is induced when normal antegrade blood supply is compromised – the collaterals are recruited (fig. 1).

Figure 1
Lifecycle of the collateral circulation. The native collateral extent in the adult is determined by its formation in embryonic collaterogenesis (top right). Blood moves to and fro in native collaterals, while effective flow is recruited by acute obstruction. With continued obstruction, there is collateral enlargement, which occurs independent of ischaemia. Neocollateral formation has been suggested in chronic obstructive disease, whereas experimental studies have shown collateral rarefaction with aging.
(Data from Faber JE, et al. A brief etymology of the collateral circulation. Arterioscler Thromb Vasc Biol. 2014;34:1854–9 [1], with permission.)
Regulation of collateral growth independent of ischaemia
Alterations in blood flow and blood pressure are the key regulators of the adaptive process in recruited collaterals, known as remodelling. A haemodynamically significant coronary stenosis reduces coronary perfusion pressure distal to the narrowed segment as a result of energy losses. Autoregulation maintains basal flow distal to the stenosis by microvascular vasodilation up to a critical reduction in vessel lumen area, beyond which resting coronary blood flow is also reduced [11]. Concurrently, the pressure drop across the stenosis leads to a pressure gradient along the pre-existing collaterals connecting to the constricted vessel, leading to their recruitment: effective flow is induced from the donor artery across the collateral network to the recipient coronary artery. Heightened flow velocity leads to a proportional increase in fluid shear stress (FSS), whereas the increased intravascular pressure leads to a proportional increase in circumferential wall stress (CWS) [3, 12]. With a prolonged stimulus, the collateral network reacts by increasing the lumen diameter to normalise FSS and by increasing the wall thickness to normalise CWS [13].
Range of adaptation of collaterals
The term arteriogenesis was introduced to differentiate growth of pre-existing collaterals from angiogenesis, which is triggered by ischaemia [12, 14]. Blood flow can increase maximally 10- to 20-fold by arteriogenesis, but only 1.5- to 1.7 fold by angiogenesis, which refers to the sprouting of new, minute, high-resistance, low-flow capillaries [13]. To generate the same lumen area as the artery by angiogenesis would in essence mean replacing the organ with capillaries – with the end result of a haemangioma [13, 15]. Thus, arteriogenesis has the capacity to compensate for an occluded artery, whereas angiogenesis does not [12, 16].
Even though mature collaterals are able to deliver bulk flow to jeopardised tissue, collateral remodelling stops short of completely reconstituting the function of an occluded artery [17, 18]. Primarily responsible for this circumstance is the premature normalisation of the dominant driving force for continued outward remodelling – flow-induced fluid shear stress. Even a small increase in luminal diameter leads to a much larger fall in FSS, a consequence of FSS being inversely related to the third power of the vessel radius. In physiological circumstances, normal maximal blood flow is, therefore, not achievable even in healthy collateral-dependent tissues [19]. Conversely, a complete functional reconstitution, even surpassing normal maximal function, is achieved when FSS is artificially increased [17, 18, 20, 21].
Collateral remodelling and its relation to the phases of coronary artery disease
Collateral remodelling is a mitotic process which takes two to three days before a relevant increase beyond the acutely recruitable blood flow begins to occur [2, 3, 16, 22]. The proliferation of endothelial and smooth muscle cells with perivascular gathering of bone marrow derived cells (notably macrophages and T cells) is followed by controlled destruction of the collateral vessel to build a new scaffold for the much larger vessel [3]. In the process of many remodelling collateral connections, a larger diameter in some collaterals presents an advantage in the ensuing competition for flow. As a consequence, only a few collaterals ultimately reach maturation, while many others are closed by an overshoot of intimal proliferation – a process known as pruning [2, 23].
In patients with chronic total occlusion (CTO), collateral function improves over a time period of 12 weeks after occlusion [24], which is in line with observations in experimental studies [25]. The collateral remodelling in these patients typically relates to chronic stable CAD in a setting of a more or less gradual progression of coronary obstruction, which allows sufficient time for the growing collaterals to preserve myocardial viability.
In patients with acute coronary occlusion, the timeframe for insufficient collaterals to remodel (further) is generally too short. Plaque rupture is the most common cause of acute coronary syndromes, which, when followed by atherothrombosis, leads to rapid and insidious thrombotic occlusion [26]. In this context, it is of note that most of the coronary lesions ultimately responsible for major adverse cardiovascular events (MACE) have been shown to be angiographically mild [27]. Therefore, the culprit lesion itself is unlikely to cause relevant collateral remodelling [27–29]. In the absence of stenosis-induced enlargement, the protective effect from acute ischaemia is entirely dependent on the extent of the native collateral network. Therefore, the notion of the native collateral circulation is pertinent not only in the healthy state, but also in coronary artery disease (CAD).
Notably, myocardial viability is not a prerequisite for the remodelling of coronary collaterals [31, 32]. Thus, well-developed collaterals can be observed to grow only after its dependent myocardium has become entirely necrotic, again consistent with the notion that collateral growth can occur independently of ischaemia.
Genetic and environmental determinants of the coronary collateral circulation
The wide interindividual variability in coronary collaterals is evident not only with regard to the preformed extent, but also with regard to its capacity to remodel. Specifically, the extent of native collaterals relates to the formation and maturation of collaterals in early life [30], whereas collateral remodelling takes place generally much later (most often under the influence of coronary obstruction). In experimental studies, the use of different animal models permits these aspects to be studied also in healthy tissues, which allows genetic factors to be separated from environmental influences. Conversely, the protective role of collaterals becomes eminent chiefly in CAD, the risk of which increases with the number and severity of cardiovascular risk factors. Accordingly, the impact of the risk factors predisposing to atherosclerosis has to be considered, too [31].
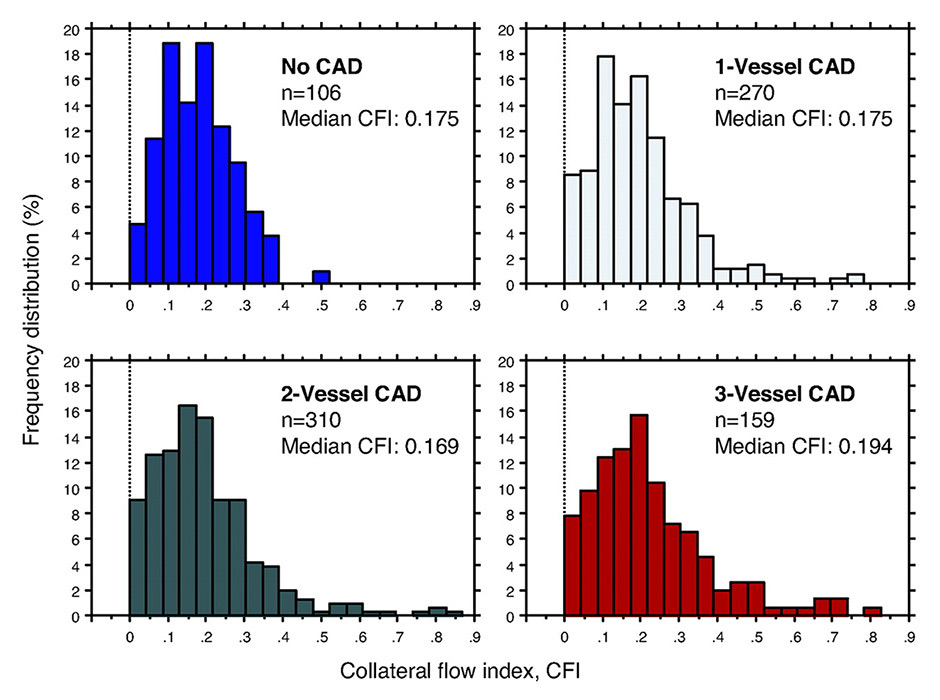
Figure 2
Frequency distribution (vertical axes in percent) of collateral flow index (CFI, horizontal axes) in patients without coronary artery disease (CAD; top left) and with increasing severity of CAD. Sufficient collaterals by CFI are present in a fourth of patients with angiographically normal coronary arteries, whereas this proportion increases to one third in patients with CAD.
(Data from Meier P, et al. Beneficial effect of recruitable collaterals: a 10-year follow-up study in patients with stable coronary artery disease undergoing quantitative collateral measurements. Circulation. 2007;116:975–83 [40], with permission.)
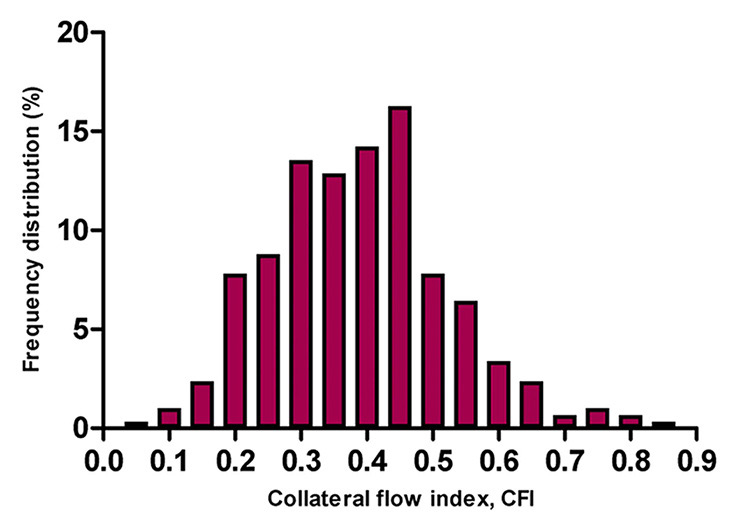
Figure 3
Frequency distribution (vertical axis in percent) of collateral flow index (CFI, horizontal axis) in 295 patients with chronic total occlusion. In this population mean CFI was 0.39 ± 0.14.
(Adapted from van der Hoeven NW, et al. Clinical parameters associated with collateral development in patients with chronic total coronary occlusion. Heart. 2013;99:1100–5 [38], with permission from BMJ Publishing Group Ltd.)
Severity of coronary obstruction
The presence of a haemodynamically significant stenosis is the sine qua non for the calibre increase of collaterals in remodelling. The severity of the flow impediment dictates the pressure drop, which in turn determines the amount of shear stress in recruited collaterals, with higher shear stress leading to more pronounced remodelling. Consequently, in patients with CAD, the severity of the underlying arterial obstruction has been found to be the only independent predictor for good collateral function [32, 33]. Furthermore, collateral flow was found to be higher in CTO than in nonocclusive obstructions [24, 34–38].
The effect of continued remodelling is well illustrated by the frequency distributions of collateral function with increasing severity of the underlying CAD (fig. 2) [32, 39, 40]. Although sufficient collaterals are already present in a significant minority of patients with angiographically normal coronary arteries [39], with CAD there is a rightward shift of the near-Gaussian distribution, which increases from one- to three-vessel disease and is most pronounced in CTO (fig. 3) [40]. However, there is still considerable interindividual variability in the level of collateral function for a given stenosis severity, which highlights the modulation of the arteriogenic response by other factors [36].
Determinants of coronary collateral supply in the absence of remodelling
The structural extent of native collaterals varies significantly between individuals, with respect to both number and average diameter [41]. Likewise, in healthy human subjects, invasively determined collateral function varies by 10-fold in the absence of angiographically detectable CAD [32, 40].
A plausible explanation for this marked variation is the lack of selective pressure in the genetic background underlying native collateral extent, which relates to genes that control formation and maturation of collaterals [30, 42, 43]. In other words, redundancies that compensate for significant changes in expression of collateralogenic genes have not evolved because the protective effects are needed only well after the peak reproductive years [44]. In experimental studies, significant advances have been made with regard to the genetic architecture underlying variation in the extent of the collateral circulation [43]. A substantial amount of the variability appears to arise from naturally occurring polymorphisms in genetic loci directing the formation of the collateral circulation [30, 43, 45]. In particular, a quantitative trait locus predominantly affecting native collateral number and diameter has been identified in mice [43]. In the same context, several genes showed differential expression for well versus poorly developed collateral function in patients with no angiographically significant CAD [46].
Although the genetic determinants appear to have a major influence on the extent of native collaterals, clinical variables have also been identified. In patients free of coronary stenosis, multivariable regression analysis with inclusion of 39 clinical test variables notably showed that bradycardia (not related to beta-blockers) and absence of arterial hypertension were independent positive predictors for collateral function [47].
Determinants of arteriogenic response
As mentioned previously, differences in the genetic background for collateral formation and maturation contribute significantly to the considerable interindividual variation in the pre-existing collateral extent [48]. By the same token, another genetic pathway has been found to significantly determine the remodelling capacity of the native collateral extent [43]. Similarly, marked variability has also been observed in the arteriogenic response, that is, the extent of remodelling. This is well illustrated by experimental studies where, although the arteriogenic stimulus in the form of total vascular occlusion is kept as a constant, the arteriogenic response has been found to vary substantially even in same strains. In humans, gene expression analysis has shown that a distinct genetic profile in monocytes relates to quantitatively determined collateral function in obstructive CAD [46].
Apart from the marked variability in both native collateral extent and capacity to remodel, another consistent finding is that a poor arteriogenic response is also associated with poor native collateral extent [17, 46, 47]. Indeed, animals with poor pre-existing collaterals reach significantly lower levels of collateral function after arterial occlusion compared with animals with good pre-existing collaterals both within, as well as between, strains. Although this observation may be consistent with a genetic link influencing both maturation and remodelling of collaterals, such an association has not been found. Then again, on a conceptual basis, such an assumption appears superfluous, given the plausibility that the pre-existing collateral extent presents a limiting factor for its enlargement in remodelling. In other words, a poor native collateral circulation itself appears to be a limiting factor for its later positive remodelling, in that the extent of flow recovery reaches levels inferior to that from a rich native collateral network [48]. Thus, the pre-existing collateral extent determines critically the degree of protection from ischaemic injury not only in the absence, but also in the presence of collateral remodelling, in that it also governs the capacity for outward remodelling in the latter [16, 48–51].
Influence of traditional cardiovascular risk factors
In the majority of cases, coronary atherosclerosis coincides with one or more cardiovascular risk factors. Given that endothelial dysfunction is a well-established response to cardiovascular risk factors, it is conceivable that the collateral circulation might equally be affected. Notably though, collateral arteries and arterioles do not seem prone to atherosclerosis [6].
Dyslipidaemia has been shown to hamper arteriogenesis in hyperlipidaemic experimental models [52, 53]. Contrarily, no evidence exists for such an effect in humans: dyslipidaemia has not been found to be an independent predictor for quantitatively determined collateral function in patients with or without CAD [32]. Furthermore, in a large clinical study in patients with CAD, no effect of statins on coronary collateral function was found [54].
Arterial hypertension is reflected in the tortuosity and enlarged calibre of arteries, secondary to an adaptive response to increased shear stress [55]. Two clinical studies in patients with obstructive CAD found conflicting results in this regard, but both were limited in that occlusion of the collateral-receiving artery was not uniformly present or performed for appropriate angiographic assessment [56, 57]. In patients with CTO with quantitatively assessed collateral function, hypertension was a predictor for better developed collaterals in a multivariate analysis. In the human heart without CAD, the contrary has been found to be true: better collateral function in the absenceof arterial hypertension [47].
Increasing age is a well-known risk factor for atherosclerosis. Structural alterations with advancing age are accompanied by a decline in vascular function [58]. However, its impact on the collateral circulation is equivocal. Experimental studies have documented a decline in structural extent of pre-existing collaterals, which is compounded by reduced remodelling [51]. A possible mechanism has been suggested to be the unique haemodynamic environment with oscillatory shear stress that could predispose the collateral circulation to accelerated aging compared with the general circulation. Alternatively, or in addition, impaired endothelial nitric oxide signalling, deemed to be an important factor for maintenance of collateral density, as well as remodelling in obstructive disease, has been implicated in the increased propensity of endothelial cells to undergo apoptosis and thereby cause collateral rarefaction [51, 59]. Clinically, a causal link between increased mortality of elderly individuals in acute myocardial infarction and reduced collateral extent could be supported by the inverse relation between age and the level of angiographic collateral extent seen in these patients [60, 61]. However, the clinical association of higher mortality in ischaemic cardiovascular disease and poorly developed collaterals has not been shown to be independent. In a univariate analysis of a large database including quantitatively determined collateral function, only a trend towards the described inverse relation was seen [37]. Furthermore, in a pooled analysis of patients with a CTO and quantitatively determined collateral function, an association between higher age and poorly developed collaterals could not be confirmed [38]. In essence, although experimental data are consistent with an age-associated decline in collateral extent and remodelling, on balance, age is probably not a major determinant of collateral function in humans.
No differences have been found in quantitatively determined collateral function among matched patients with versus without diabetes in angiographically normal or diseased coronary arteries [62]. Furthermore, in a patient cohort with CTO, a multivariate analysis confirmed these findings [38]. Earlier controversies in this context are likely attributable to the above-mentioned methodical flaw in angiographic assessment [63–65].
Assessment of the coronary collateral circulation
An accurate assessment of the coronary collateral circulation requires normal antegrade and collateral blood flow to be distinguished. In the absence of a coronary stenosis there is essentially no measurable net blood flow across pre-formed, but not recruited collaterals. Conversely, in the presence of nonocclusive disease, the contribution of normal antegrade blood flow cannot be differentiated from the recruited blood flow via the anastomotic pathways. Therefore, for exclusive characterisation of recruitable collateral blood flow, (transient) coronary occlusion is required, regardless of the method employed [66, 67]. Accordingly, noninvasive imaging techniques are limited to the remodeled collateral circulation supplying chronic total coronary occlusions [66]. Conversely, invasive techniques, i.e., during coronary catheterisation, permit an assessment of both the native and remodelled collateral circulation.

Figure 4
Immersion radiography of an ex vivo arteriogram of a normal human heart. Tissue shadows are almost eliminated by immersion of the specimen in saline. Blood vessels are radiologically demonstrated to 20 µm, a spatial resolution sufficient to reproduce the dense collateral network, over which retrograde filling of the branch of the left circumflex coronary artery doubly ligated (arrow) before contrast injection occurs. In contrast, coronary angiography does not detect the majority of anastomotic vessels due to its inferior spatial resolution.
(Data from Fulton WF. Immersion radiography of injected specimens. Br J Radiol. 1963;36:685–8 [121], by permission of the Oxford University Press.)
Different modalities allow evaluation of the ischaemia-abating function of coronary collaterals. Abnormalities in distribution and quantity of myocardial blood flow are the earliest pathophysiological events in myocardial ischaemia [67]. Imaging techniques that measure perfusion are therefore most distinctive in collateral assessment, given that sufficient collaterals may prevent even stress-induced abnormalities occurring later in the ischaemic cascade [68]. Only a critical reduction in perfusion leads first to an impairment of diastolic, then of systolic function, followed by electrocardiographic (ECG) changes and ultimately by symptomatic expression (angina pectoris).
Commonly employed imaging techniques that delineate coronary anatomy allow only a very limited structural assessment of coronary collaterals, given that most collaterals are below their spatial resolution and therefore go undetected.
Noninvasive assessment
Clinically, myocardial perfusion and wall motion function are measurable notably by (myocardial contrast) echocardiography (MCE) [69], positron emission tomography (PET) [66, 70], single-photon emission computed tomography (SPECT) [71], cardiac magnetic resonance imaging (CMR) [72], and cardiac computed tomography (CT). Preservation of perfusion and, consequently, wall motion function [73] vary as a function of the underlying collateral supply and the extent and degree of coronary lesions. Similarly, for a given lesion severity, there is a continuum from a completely normal ECG under stress conditions to transient, chronic and acute repolarisation abnormalities [74]. It follows from the above considerations that collateral assessment by noninvasive techniques is most distinctive in the presence of total coronary occlusion. In nonocclusive CAD, the quality of collaterals can be gauged when comparing the extent of anatomical CAD against the observed functional consequences, i.e. the ischaemic burden. With acute coronary occlusion, a noninvasive estimate of the myocardium at risk is typically practicable only with the ECG [74].
Noninvasive coronary angiography is feasible with coronary CT angiography (CCTA) and coronary MR angiography. Compared with invasive coronary angiography, the spatial and temporal resolution achievable by these techniques remain considerably lower [75], essentially rendering them unsuitable for structural evaluation of coronary collaterals [76–78].
Angiographic assessment
The qualitative angiographic methods include the often-employed Rentrop score and the more recently introduced collateral connection (CC) grading [79, 80]. The limitation inherent to collateral assessment with angiography lies in the restricted spatial resolution, which means that the majority of the anastomotic vessels remain undetected (fig. 4) [41, 81]. In addition, angiography assesses only structural qualities, from which conclusion of functional properties is notoriously imprecise, even more so in view of the above constraints [82].
For proper angiographic assessment, occlusion of the collateral-receiving artery either spontaneously, or by balloon inflation, is mandatory, even when some angiographic contrast filling of collaterals is already spontaneously present [66]. Furthermore, proper assessment by the angiographic method usually requires a double intubation technique to allow injection of contrast agent in the presumable (ipsi- and/or contralateral) donor artery and placement of an occluding balloon in the recipient artery. However, in clinical practice and even in clinical studies, balloon occlusion is rarely performed when occlusion is not spontaneously present, which lowers the sensitivity of the already blunt method. Obviously, lesser stenoses are ipso facto associated with absence of angiographic visibility of collaterals, whereas collaterals are preferentially visible with more severe stenoses. Thus, a systematic bias is introduced, where the extent of CAD confounds the grade of collateral supply. To exaggerate the point, the angiographic visibility of collaterals, when natural or transient artificial occlusion is not present, is more an indicator of the severity of the stenosis, than of the quality of the collaterals [33, 83, 84].
With the Rentrop score, absent contrast filling of collateral connections or up to the side-branch of the recipient artery denote insufficient collaterals (score of 0 and 1, respectively), while contrast filling of the epicardial main branch of the recipient artery, either partial (score of 2), or complete (score of 3) indicate sufficient collaterals. Although the Rentrop score shows (albeit moderate) correlation with functional collateral measures, it has relevant shortcomings. A major limitation is the inability of the Rentrop score to assess adequately the functional significance, i.e. the protective effect, especially of recruitable, but not spontaneously present collateral vessels [33]. Furthermore, in CTO, the Rentrop grading lacks further differentiation, as collaterals are predominantly of Rentrop grade 3 in this setting [80].
The CC score was evaluated in patients with CTO and its applicability in nonocclusive lesions has so far not been established [80]. Collaterals are evaluated according to the presence of a continuous connection between donor and recipient artery: CC0, no continuous connection between donor and recipient artery; CC1, continuous, threadlike connection; and CC2, continuous, small side branch–like size of the collateral throughout its course [80]. Regarding the clinical relevance of the CC grading, in patients without prior Q-wave myocardial infarction, the regional wall motion was best preserved with grade CC2 collaterals [80]. Furthermore, the CC score was closely associated with invasively determined parameters of collateral haemodynamics [80].
Instead of angiographic grading, assessment of collaterals by means of the semiquantitative washout collaterometry relies on the time to clearance of contrast medium trapped by balloon occlusion [85]. It correlates well with invasively determined collateral function and distinguishes accurately between sufficient and insufficient collaterals [86].
Functional assessment
In contrast to the ordinal angiographic scores, functional assessment allows quantification of collateral pathways on a continuous scale. Apart from noninvasive imaging techniques in the setting of a CTO, direct myocardial perfusion can be measured only during invasively performed temporary coronary occlusion with quantitative myocardial contrast echocardiography (MCE) [69].
Invasively determined, quantitative measures of collateral function rely on determination of distal coronary pressure or flow velocity signals [87]. To this purpose, a coronary guide wire, equipped with a pressure and/or Doppler sensor near its tip, is positioned distally in the coronary of interest. The normal antegrade flow is thereafter temporarily blocked, either during therapeutic coronary angioplasty or diagnostic balloon occlusion at low inflation pressure, the acute and long-term safety of which has been confirmed [88]. Distal coronary occlusive pressure (Poccl) is then set in relation to aortic pressure measured via the angioplasty guide catheter, both corrected for the back pressure, represented by central venous pressure (CVP), to derive the pressure-derived collateral flow index (CFI, fig. 5) [87]. Similarly, distal flow velocity during coronary occlusion is compared with resting flow velocity when normal antegrade flow has been restored, to derive velocity-derived CFI [87]. Pressure signals can be reliably obtained and are robust to influences such as vessel anatomy, whereas Doppler signals are prone to wall-motion artifacts and registration of optimal signals is often not achievable [86, 87].
Pressure-derived CFI has been shown to correlate closely to myocardial perfusion during balloon occlusion as assessed by perfusion imaging and quantitative myocardial contrast echocardiography (MCE) [89]. The limitation that beyond a critical level of left ventricular filling pressure, Poccl is determined solely by this transmural force and no longer by collateral driving pressure, applies in acute myocardial infarction, but infrequently in the setting of stable CAD [90]. Currently, pressure-derived CFI measurement is the gold-standard for collateral assessment.
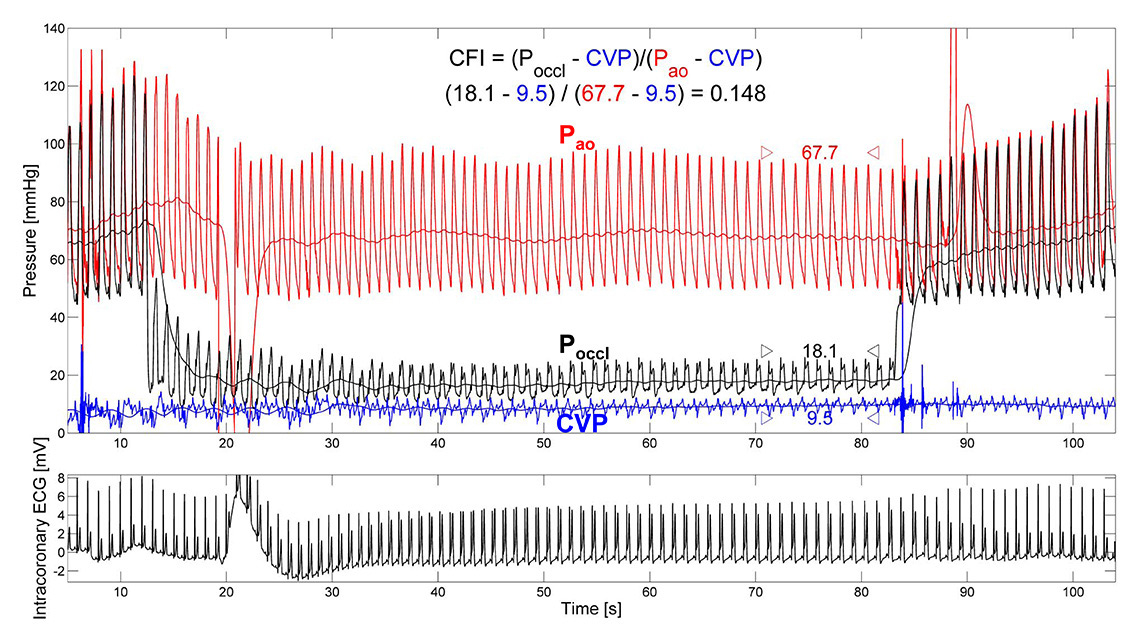
Figure 5
Functional collateral assessment by use of pressure-derived collateral flow index (CFI) during a 1-minute coronary balloon occlusion. Distal occlusive pressure (Poccl, mm Hg;) is set in relation to the effective perfusion pressure, aortic pressure (Pao, mm Hg;), after correction for the back pressure, central venous pressure (CVP, mm Hg;). Marked ST-segment elevation in the intracoronary ECG demonstrates collateral function insufficient to prevent myocardial ischaemia at a CFI = 0.148.
(Reproduced from Seiler C, et al. Prognostic relevance of coronary collateral function: confounded or causal relationship? Heart. 2013;99(19):1408-14 [5], with permission from BMJ Publication Group Ltd.)
In a strict sense, sufficient collaterals have been defined as those preventing signs of myocardial ischaemia in the very sensitive intracoronary ECG during coronary (balloon) occlusion [91]. In this context, a pressure-derived CFI ≥0.217 best detects sufficient collateral supply [92]. In other words, a collateral supply amounting to more than 22% of normal antegrade flow is sufficient to prevent myocardial ischemia under resting conditions, a finding supported by a study performed with single photon emission tomography among patients with acute myocardial infarction before revascularisation [93].
Clinical relevance of the coronary collateral circulation
The clinical relevance of the coronary collateral circulation relates to the beneficial effect of well-developed collaterals in preserving viability and function of dependent myocardium in the context of the acute and chronic manifestations of CAD. On the other hand, there are also drawbacks, which manifest preferentially in the presence of a well-developed collateral network.
Coronary steal
The microvascular resistance in the coronary territory distal to a coronary obstruction is reduced to keep resting myocardial blood flow constant. Therefore, in obstructive coronary disease, an uneven distribution of microvascular resistances develops, which forms the basis of coronary steal. The dilatory reserve in an affected territory decreases with increasing severity of the coronary stenosis and becomes exhausted at a critical point when demand is increased, such as occurs with exertion. Consequently, the flow provided to the obstructed region by antegrade (if any) and collateral inflow becomes maximal. In this situation collateral inflow becomes entirely pressure-dependent, as microvascular resistance cannot be further reduced. Any further reduction in the microvascular resistance in the collateral-donor territory will consequently reduce the pressure gradient driving the collateral inflow. Collateral inflow and with it the net flow is reduced beyond a critical level of hyperaemia, and myocardial ischaemia precipitated. Coronary flow to a myocardial region during hyperaemia that decreases below its resting level consequently constitutes coronary steal [94–96].
Experimental and clinical studies have shown that coronary steal is facilitated by a coronary stenosis in the collateral donor artery and well-developed collaterals. A prevalence of coronary steal in 10%–20% of patients with nonocclusive CAD [97] and a third to a half in patients with a CTO demonstrates the relevance of this phenomenon [98–100]. Clinically, coronary steal can be suspected when ischaemic symptoms can be worsened or provoked by vasodilators, such as nifedipine [65, 66].
Regression of collateral flow and risk of restenosis after percutaneous coronary intervention
Percutaneous coronary intervention (PCI) of a coronary stenosis removes its associated resistance to antegrade coronary flow. Concurrently, the pressure gradient across the collateral network driving effective collateral flow is also diminished. In line with the experimental findings [101–103], clinical studies have accordingly shown a regression of collateral function over time after revascularisation [35, 104–106]. Moreover, it has been shown that recanalisation of occlusive lesions was associated with a greater decrease in collateral function immediately after revascularisation compared with nonocclusive lesions [104]. Consistent with prior findings, collateral function was significantly higher with occlusive than nonocclusive CAD. Furthermore, in patients with nonocclusive lesions, a collateral recruitment could be seen with the second balloon inflation (performed immediately after stent implantation), while patients with a CTO showed acute collateral derecruitment [104, 106]. In the months following revascularisation, a further decrease in collateral function could be observed in patients without reocclusion [35, 106]. Notably, at 6 months follow-up, only 4% of patients with subtotal or total occlusions at baseline showed sufficient collaterals [35], whereas this was observed in 18% of patients exclusively with a CTO after a mean of 5 months [106].
Incidentally, 10% of patients showed reocclusion after PCI of a CTO; in these patients collateral function was not different at follow-up when compared with the baseline value. Also in the context of prior studies [107, 108], an association between well-developed collateral function and a risk for restenosis or reocclusion is conceivable: the sizeable collateral flow especially after PCI for a CTO could compete with restored antegrade flow and predispose to stent restenosis in a process similar to atherosclerosis [100, 101]. In view of rather inconsistent findings in prior studies, the impact of the collateral circulation on the risk for restenosis has recently been determined in a meta-analysis, integrating a total of seven studies with angiographic or functional collateral assessment [109]. Across studies, good collaterals were predictive for restenosis, with a relative risk increase of 40% (95% confidence interval 1.09‒1.80, p = 0.009).
Protective effect of the coronary collateral circulation
The partly contradictory results regarding the protective effect of coronary collaterals are chiefly related to the collateral circulation being both a marker of CAD severity and a predictor of future cardiac events [5]. In other words, the positive correlation between the angiographic presence of coronary collaterals and an unfavourable prognosis in patients with ischaemic heart disease is confounded by the extent of CAD severity (explaining both) [110]. Therefore, an investigation aimed at the prognostic impact of collaterals has to correct for the severity of the underling coronary disease. On a conceptual basis, the protective effect of the collateral circulation can hardly be refuted. A major determinant of long-term survival in CAD is left ventricular ejection fraction, the preservation of which is associated with the coronary collateral circulation both in acute and chronic CAD [81, 82].
With acute ischaemia, the outcome is critically dependent on the extent of myocardial infarction, which increases with coronary artery occlusion time and with the area at risk for infarction, but decreases with increasing collateral supply [7, 111, 112]. Therefore, the beneficial effect of better developed collaterals is self-evident in acute coronary syndrome [7]. Moreover, not only is the recovery of left ventricular function after reperfused acute myocardial infarction significantly determined by the extent of collateral supply, but it is also less dependent on time to reperfusion in patients with sufficient collaterals [113, 114]. Furthermore, in patients with acute myocardial infarction, poor collaterals are related to the early occurrence of cardiogenic shock, which portends a particularly high mortality [115]. In the context of the arrhythmogenic potential of myocardial ischaemia, a clinical study has shown a protective effect of the collateral circulation on ischaemia-induced QT prolongation, whereas experimental studies primarily investigated the susceptibility to ventricular fibrillation in acute coronary occlusion [116, 117].
With chronic ischaemia, a (further) decline in left ventricular function results from hibernating and stunned myocardium [118]. Here, the not infrequently encountered case of completely normal ventricular function in the presence of a CTO exemplifies the protective effect of the coronary collateral network (fig. 6) [24, 72]. Furthermore, it has been shown that regional LV function is directly related to the amount of collateral flow during both acute and chronic coronary occlusion [24, 119]. Concerning postinfarction sequelae, the relevant protective role of collaterals has been shown to result in a reduction of postinfarct ventricular dilatation and less ventricular aneurysm formation [7].
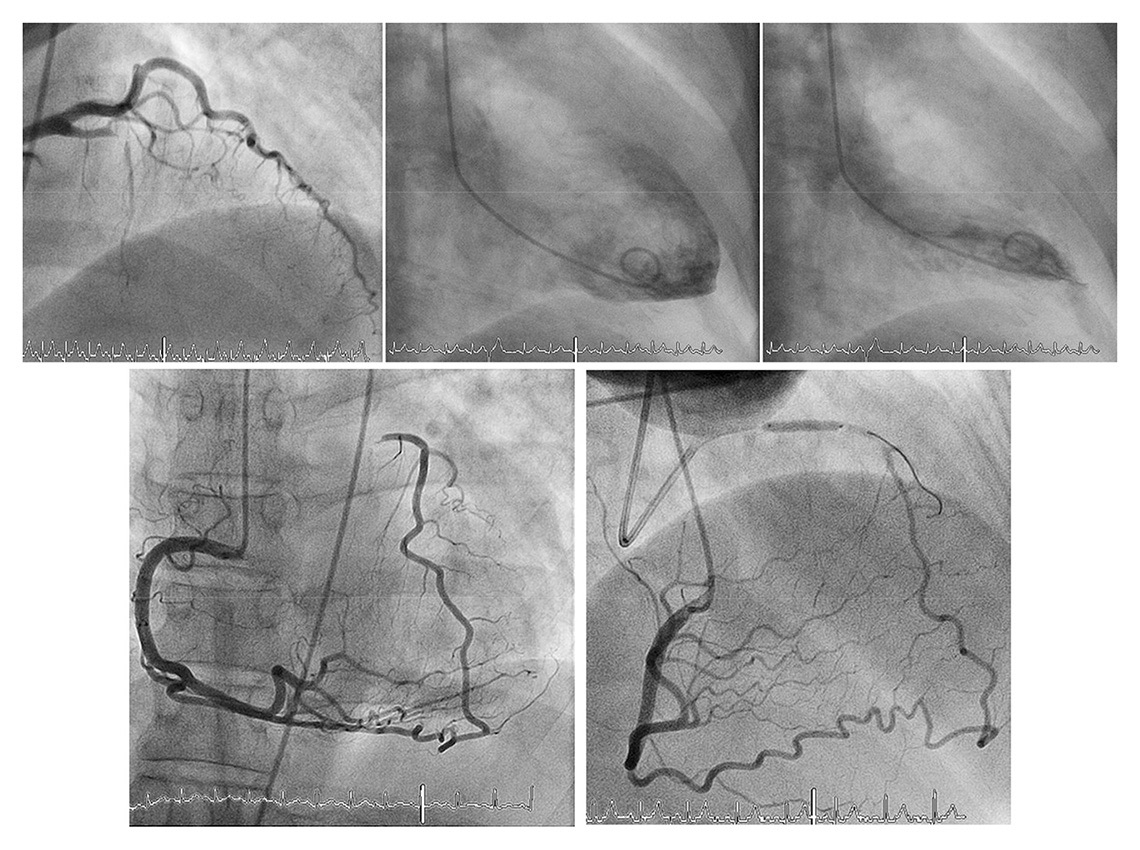
Figure 6
Completely normal left ventricular angiogram (top middle and right) due to extensive collateral supply of the proximally occluded left anterior descending artery via apical branch and septal collateral by the right coronary artery (bottom). Functional assessment is performed by balloon occlusion after recanalization of the chronic total occlusion (bottom right).
(Data from Seiler C. Assessment and impact of the human coronary collateral circulation on myocardial ischemia and outcome. Circ Cardiovasc Interv. 2013;6:719–28 [86], with permission.)
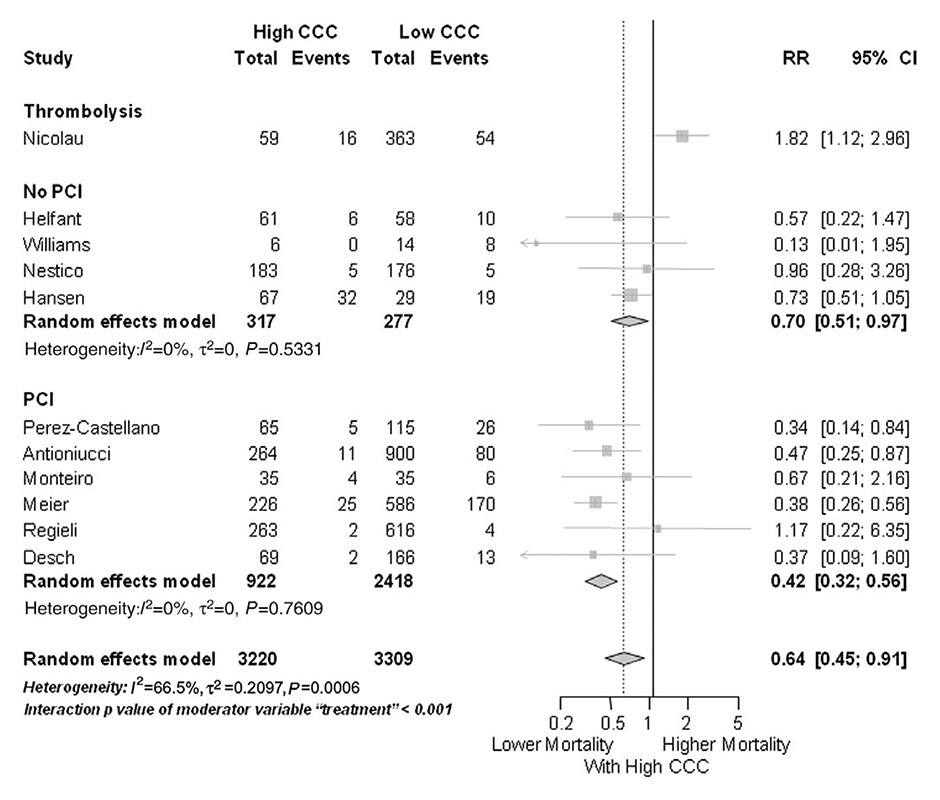
Figure 7
Forest plot of risk ratios for mortality risk, stratified by type of intervention (PCI, no PCI, and thrombolysis). CAD, coronary artery disease; CCC, coronary collateral circulation; CI, confidence interval; MI, myocardial infarction. Horizontal bars indicate 95% confidence intervals.
(Data from Meier P, et al. Beneficial effect of recruitable collaterals: a 10-year follow-up study in patients with stable coronary artery disease undergoing quantitative collateral measurements. Circulation. 2007;116:975–83 [40], by permission of Oxford University Press.)
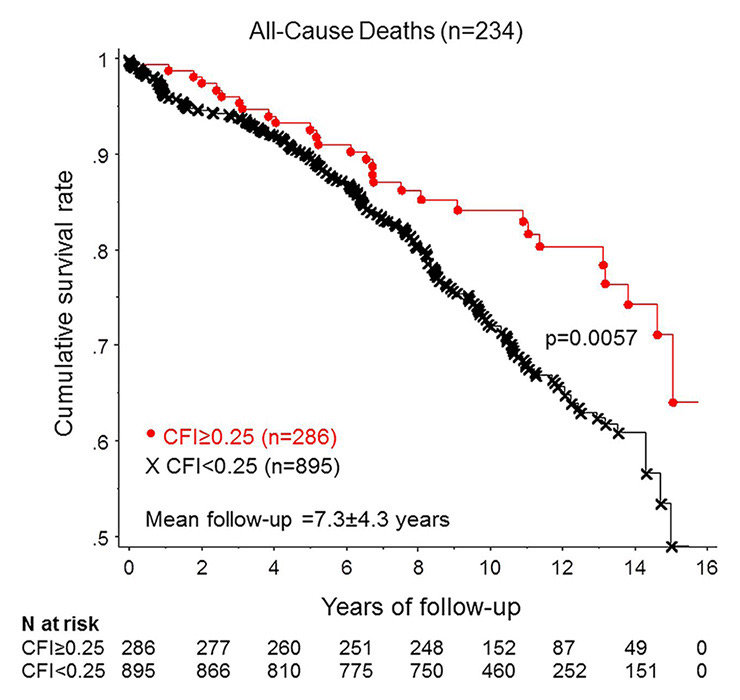
Figure 8
Cumulative survival rates in patients with low and with high collateral flow index (CFI).
(Data from Seiler C, et al. Prognostic relevance of coronary collateral function: confounded or causal relationship? Heart. 2013;99(19):1408-14 [5], with permission from BMJ Publication Group Ltd.)
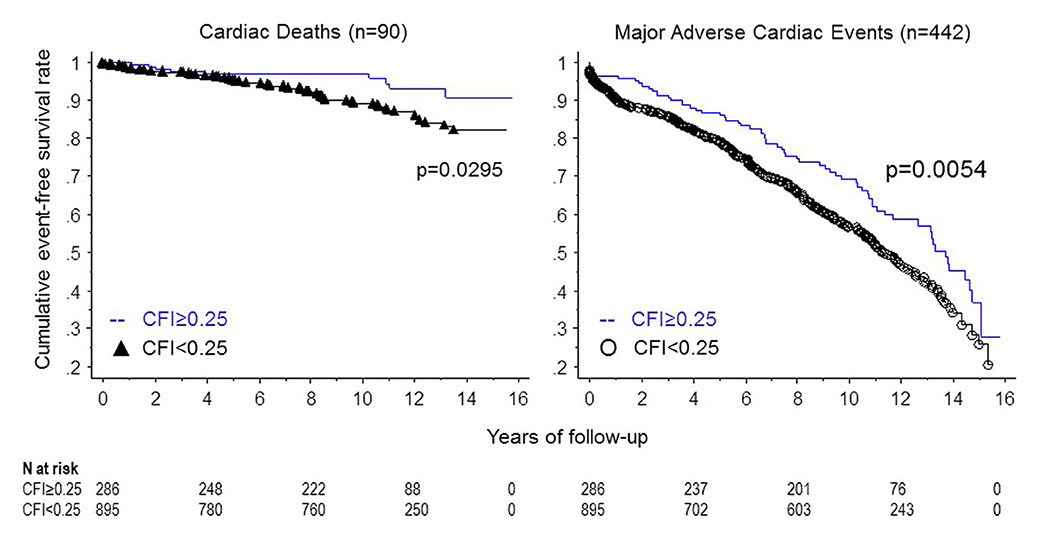
Figure 9
Cumulative survival rates related to cardiac mortality (left panel) and to major adverse cardiac events (right panel) in patients with low and with high collateral flow index (CFI).
(Data from Seiler C, et al. Prognostic relevance of coronary collateral function: confounded or causal relationship? Heart. 2013;99(19):1408-14 [5], with permission from BMJ Publication Group Ltd.)
Regarding the impact of the coronary collateral circulation on mortality, the majority of studies have relied on angiographic assessment. A recent meta-analysis included 12 angiographic studies, as well as a large study with quantitatively determined collateral assessment [120]. The pooled study population consisted of more than 6,500 patients with stable CAD, or subacute and acute myocardial infarction. With high versus low coronary collateral circulation a significant, 36% reduced mortality was demonstrated (fig. 7) [120]. Similarly, the latest follow-up (mean 7.3 ± 4.3 years) of a large prospective cohort with chronic stable CAD and quantitatively assessed collateral function again showed a reduction in all-cause mortality (fig. 8), and specifically, relevant and significant reductions in cardiac mortality and MACE (fig. 9) with a well-functioning coronary collateral circulation [5]. Notably, the prediction of all-cause mortality by a well-functioning coronary circulation was independent.
Conclusions
The coronary collateral circulation is a network of preformed interarterial connections present irrespective of coronary artery disease. Genetic determinants contribute considerably to the wide variation in the extent of the native collateral circulation. Stenosis severity is the single most important environmental determinant for its enlargement in CAD. Accurate collateral assessment requires an invasive approach, with pressure-derived CFI measurements currently being the gold standard. In patients with CAD, a well-functioning coronary collateral circulation is independently associated with a reduction in infarct size, left ventricular dysfunction and cardiovascular events, which translates into a relevant improvement in survival.
References
1 Faber JE, Chilian WM, Deindl E, van Royen N, Simons M. A brief etymology of the collateral circulation. Arterioscler Thromb Vasc Biol. 2014;34:1854–9.
2 Schaper W. Collateral circulation: past and present. Basic Res Cardiol. 2009;104:5–21.
3 Schaper W and Ito WD. Molecular mechanisms of coronary collateral vessel growth. Circ Res. 1996;79:911–9.
4 Global status report on noncommunicable diseases 2010. Geneva, World Health Organization, 2011.
5 Seiler C, Engler R, Berner L, Stoller M, Meier P, Steck H, Traupe T. Prognostic relevance of coronary collateral function: confounded or causal relationship? Heart. 2013;99(19):1408-14.
6 Seiler C, Stoller M, Pitt B, Meier P. The human coronary collateral circulation: development and clinical importance. Eur Heart J. 2013.
7 Habib GB, Heibig J, Forman SA, Brown BG, Roberts R, Terrin ML, Bolli R. Influence of coronary collateral vessels on myocardial infarct size in humans. Results of phase I thrombolysis in myocardial infarction (TIMI) trial. The TIMI Investigators. Circulation. 1991;83:739–46.
8 Liebeskind DS, Cotsonis GA, Saver JL, Lynn MJ, Turan TN, Cloft HJ, Chimowitz MI. Collaterals dramatically alter stroke risk in intracranial atherosclerosis. Ann Neurol. 69:963–74.
9 Hoffman JI and Spaan JA. Pressure-flow relations in coronary circulation. Physiol Rev. 1990;70:331–90.
10 Toriumi H, Tatarishvili J, Tomita M, Tomita Y, Unekawa M and Suzuki N. Dually supplied T-junctions in arteriolo-arteriolar anastomosis in mice: key to local hemodynamic homeostasis in normal and ischemic states? Stroke. 2009;40:3378–83.
11 Gould KL. Pressure-flow characteristics of coronary stenoses in unsedated dogs at rest and during coronary vasodilation. Circ Res. 1978;43:242–53.
12 Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–95.
13 Schaper W, Scholz D. Factors regulating arteriogenesis. Arterioscler Thromb Vasc Biol. 2003;23:1143–51.
14 Helisch A, Schaper W. Arteriogenesis: the development and growth of collateral arteries. Microcirculation. 2003;10:83–97.
15 Carmeliet P. VEGF gene therapy: stimulating angiogenesis or angioma-genesis? Nat Med. 2000;6:1102–3.
16 Scholz D, Ziegelhoeffer T, Helisch A, Wagner S, Friedrich C, Podzuweit T, Schaper W. Contribution of arteriogenesis and angiogenesis to postocclusive hindlimb perfusion in mice. J Mol Cell Cardiol. 2002;34:775–87.
17 Eitenmuller I, Volger O, Kluge A, Troidl K, Barancik M, Cai WJ, et al. The range of adaptation by collateral vessels after femoral artery occlusion. Circ Res. 2006;99:656–62.
18 Heil M, Schaper W. Influence of mechanical, cellular, and molecular factors on collateral artery growth (arteriogenesis). Circ Res. 2004;95:449-58.
19 Hoefer IE, van Royen N, Buschmann IR, Piek JJ, Schaper W. Time course of arteriogenesis following femoral artery occlusion in the rabbit. Cardiovasc Res. 2001;49:609–17.
20 Pipp F, Boehm S, Cai WJ, Adili F, Ziegler B, Karanovic G, et al. Elevated fluid shear stress enhances postocclusive collateral artery growth and gene expression in the pig hind limb. Arterioscler Thromb Vasc Biol. 2004;24:1664–8.
21 Vogel R, Traupe T, Steiger VS, Seiler C. Physical coronary arteriogenesis: a human “model” of collateral growth promotion. Trends Cardiovasc Med. 2010;20:129–33.
22 van Royen N, Piek JJ, Schaper W, Fulton WF. A critical review of clinical arteriogenesis research. J Am Coll Cardiol. 2009;55:17–25.
23 Hacking WJ, VanBavel E, Spaan JA. Shear stress is not sufficient to control growth of vascular networks: a model study. Am J Physiol. 1996;270:H364–75.
24 Werner GS, Ferrari M, Betge S, Gastmann O, Richartz BM, Figulla HR. Collateral function in chronic total coronary occlusions is related to regional myocardial function and duration of occlusion. Circulation. 2001;104:2784–90.
25 Schaper W. Pathophysiology of coronary circulation. Prog Cardiovasc Dis. 1971;14:275–96.
26 Xie Y, Mintz GS, Yang J, Doi H, Iniguez A, Dangas GD, et al. Clinical outcome of nonculprit plaque ruptures in patients with acute coronary syndrome in the PROSPECT study. JACC Cardiovasc Imaging. 2014;7:397–405.
27 Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–35.
28 Glaser R, Selzer F, Faxon DP, Laskey WK, Cohen HA, Slater J, et al. Clinical progression of incidental, asymptomatic lesions discovered during culprit vessel coronary intervention. Circulation. 2005;111:143–9.
29 Ambrose JA, Tannenbaum MA, Alexopoulos D, Hjemdahl-Monsen CE, Leavy J, Weiss M, et al. Angiographic progression of coronary artery disease and the development of myocardial infarction. J Am Coll Cardiol. 1988;12:56–62.
30 Chalothorn D, Faber JE. Formation and maturation of the native cerebral collateral circulation. J Mol Cell Cardiol. 49:251–9.
31 Kinnaird T, Stabile E, Zbinden S, Burnett MS, Epstein SE. Cardiovascular risk factors impair native collateral development and may impair efficacy of therapeutic interventions. Cardiovasc Res. 2008;78:257–64.
32 Pohl T, Seiler C, Billinger M, Herren E, Wustmann K, Mehta H, et al. Frequency distribution of collateral flow and factors influencing collateral channel development. Functional collateral channel measurement in 450 patients with coronary artery disease. J Am Coll Cardiol. 2001;38:1872–8.
33 Piek JJ, van Liebergen RA, Koch KT, Peters RJ, David GK. Clinical, angiographic and hemodynamic predictors of recruitable collateral flow assessed during balloon angioplasty coronary occlusion. J Am Coll Cardiol. 1997;29:275–82.
34 Sachdeva R, Agrawal M, Flynn SE, Werner GS, Uretsky BF. The myocardium supplied by a chronic total occlusion is a persistently ischemic zone. Catheter Cardiovasc Interv. 2014;83:9–16.
35 Perera D, Kanaganayagam GS, Saha M, Rashid R, Marber MS, Redwood SR. Coronary collaterals remain recruitable after percutaneous intervention. Circulation. 2007;115:2015–21.
36 Teunissen PF, Horrevoets AJ, van Royen N. The coronary collateral circulation: genetic and environmental determinants in experimental models and humans. J Mol Cell Cardiol. 2012;52:897–904.
37 Seiler C. Collateral circulation of the heart. London: Springer 2009.
38 van der Hoeven NW, Teunissen PF, Werner GS, Delewi R, Schirmer SH, Traupe T, et al. Clinical parameters associated with collateral development in patients with chronic total coronary occlusion. Heart. 2013;99:1100–5.
39 Wustmann K, Zbinden S, Windecker S, Meier B, Seiler C. Is there functional collateral flow during vascular occlusion in angiographically normal coronary arteries? Circulation. 2003;107:2213–20.
40 Meier P, Gloekler S, Zbinden R, Beckh S, de Marchi SF, Zbinden S, et al. Beneficial effect of recruitable collaterals: a 10-year follow-up study in patients with stable coronary artery disease undergoing quantitative collateral measurements. Circulation. 2007;116:975–83.
41 Fulton WF. Arterial Anastomoses in the Coronary Circulation. I. Anatomical Features in Normal and Diseased Hearts Demonstrated by Stereoarteriography. Scott Med J. 1963;8:420–34.
42 Zhang H, Prabhakar P, Sealock R, Faber JE. Wide genetic variation in the native pial collateral circulation is a major determinant of variation in severity of stroke. J Cereb Blood Flow Metab. 2010;30:923–34.
43 Wang S, Zhang H, Dai X, Sealock R, Faber JE. Genetic architecture underlying variation in extent and remodeling of the collateral circulation. Circ Res. 2010;107:558–68.
44 Faber JE, Dai X, Lucitti J. Genetic and environmental mechanisms controlling formation and maintenance of the native collateral circulation. in: Arteriogenesis – Molecular Regulation, Pathophysiology and Therapeutics I E Deindl, W Schaper (eds), Shaker Verlag, Ch 1, pp. 1–22. 2011.
45 Chalothorn D, Faber JE. Strain-dependent variation in collateral circulatory function in mouse hindlimb. Physiol Genomics. 42:469–79.
46 Meier P, Antonov J, Zbinden R, Kuhn A, Zbinden S, Gloekler S, et al. Non-invasive gene-expression-based detection of well-developed collateral function in individuals with and without coronary artery disease. Heart. 2009;95:900–8.
47 de Marchi SF, Gloekler S, Meier P, Traupe T, Steck H, Cook S, et al. Determinants of Preformed Collateral Vessels in the Human Heart without Coronary Artery Disease. Cardiology. 118:198–206.
48 Helisch A, Wagner S, Khan N, Drinane M, Wolfram S, Heil M, et al. Impact of mouse strain differences in innate hindlimb collateral vasculature. Arterioscler Thromb Vasc Biol. 2006;26:520–6.
49 Zbinden S, Clavijo LC, Kantor B, Morsli H, Cortes GA, Andrews JA, et al. Interanimal variability in preexisting collaterals is a major factor determining outcome in experimental angiogenesis trials. Am J Physiol Heart Circ Physiol. 2007;292:H1891–7.
50 Dokun AO, Keum S, Hazarika S, Li Y, Lamonte GM, Wheeler F, et al. A quantitative trait locus (LSq-1) on mouse chromosome 7 is linked to the absence of tissue loss after surgical hindlimb ischemia. Circulation. 2008;117:1207–15.
51 Faber JE, Zhang H, Lassance-Soares RM, Prabhakar P, Najafi AH, Burnett MS, Epstein SE. Aging causes collateral rarefaction and increased severity of ischemic injury in multiple tissues. Arterioscler Thromb Vasc Biol. 2011;31:1748–56.
52 van Royen N, Hoefer I, Buschmann I, Kostin S, Voskuil M, Bode C, et al. Effects of local MCP-1 protein therapy on the development of the collateral circulation and atherosclerosis in Watanabe hyperlipidemic rabbits. Cardiovasc Res. 2003;57:178–85.
53 Boodhwani M, Nakai Y, Mieno S, Voisine P, Bianchi C, Araujo EG, et al. Hypercholesterolemia impairs the myocardial angiogenic response in a swine model of chronic ischemia: role of endostatin and oxidative stress. Ann Thorac Surg. 2006;81:634–41.
54 Zbinden S, Brunner N, Wustmann K, Billinger M, Meier B, Seiler C. Effect of statin treatment on coronary collateral flow in patients with coronary artery disease. Heart. 2004;90:448–9.
55 Pries AR, Reglin B, Secomb TW. Remodeling of blood vessels: responses of diameter and wall thickness to hemodynamic and metabolic stimuli. Hypertension. 2005;46:725–31.
56 Koerselman J, de Jaegere PP, Verhaar MC, van der Graaf Y, Grobbee DE. High blood pressure is inversely related with the presence and extent of coronary collaterals. J Hum Hypertens. 2005;19:809–17.
57 Kyriakides ZS, Kremastinos DT, Michelakakis NA, Matsakas EP, Demovelis T, Toutouzas PK. Coronary collateral circulation in coronary artery disease and systemic hypertension. Am J Cardiol. 1991;67:687–90.
58 Wang M, Monticone RE, Lakatta EG. Arterial aging: a journey into subclinical arterial disease. Curr Opin Nephrol Hypertens. 2010;19:201–7.
59 Dai X, Faber JE. Endothelial nitric oxide synthase deficiency causes collateral vessel rarefaction and impairs activation of a cell cycle gene network during arteriogenesis. Circ Res. 106:1870–81.
60 Nakae I, Fujita M, Miwa K, Hasegawa K, Kihara Y, Nohara R, et al. Age-dependent impairment of coronary collateral development in humans. Heart Vessels. 2000;15:176–80.
61 Kurotobi T, Sato H, Kinjo K, Nakatani D, Mizuno H, Shimizu M, et al. Reduced collateral circulation to the infarct-related artery in elderly patients with acute myocardial infarction. J Am Coll Cardiol. 2004;44:28–34.
62 Zbinden R, Zbinden S, Billinger M, Windecker S, Meier B, Seiler C. Influence of diabetes mellitus on coronary collateral flow: an answer to an old controversy. Heart. 2005;91:1289–93.
63 Melidonis A, Tournis S, Kouvaras G, Baltaretsou E, Hadanis S, Hajissavas I, et al. Comparison of coronary collateral circulation in diabetic and nondiabetic patients suffering from coronary artery disease. Clin Cardiol. 1999;22:465–71.
64 Sodha NR, Clements RT, Boodhwani M, Xu SH, Laham RJ, Bianchi C, Sellke FW. Endostatin and angiostatin are increased in diabetic patients with coronary artery disease and associated with impaired coronary collateral formation. Am J Physiol Heart Circ Physiol. 2009;296:H428–34.
65 Abaci A, Oguzhan A, Kahraman S, Eryol NK, Unal S, Arinc H, Ergin A. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation. 1999;99:2239–42.
66 Demer LL, Gould KL, Goldstein RA, Kirkeeide RL. Noninvasive assessment of coronary collaterals in man by PET perfusion imaging. J Nucl Med. 1990;31:259–70.
67 Nesto RW, Kowalchuk GJ. The ischemic cascade: temporal sequence of hemodynamic, electrocardiographic and symptomatic expressions of ischemia. Am J Cardiol. 1987;59:23C–30C.
68 Bache RJ, Schwartz JS. Myocardial blood flow during exercise after gradual coronary occlusion in the dog. Am J Physiol. 1983;245:H131–8.
69 Vogel R, Indermuhle A, Reinhardt J, Meier P, Siegrist PT, Namdar M, et al. The quantification of absolute myocardial perfusion in humans by contrast echocardiography: algorithm and validation. J Am Coll Cardiol. 2005;45:754–62.
70 McFalls EO, Araujo LI, Lammertsma A, Rhodes CG, Bloomfield P, Pupita G, et al. Vasodilator reserve in collateral-dependent myocardium as measured by positron emission tomography. Eur Heart J. 1993;14:336–43.
71 Aboul-Enein F, Kar S, Hayes SW, Sciammarella M, Abidov A, Makkar R, et al. Influence of angiographic collateral circulation on myocardial perfusion in patients with chronic total occlusion of a single coronary artery and no prior myocardial infarction. J Nucl Med. 2004;45:950–5.
72 Choi JH, Chang SA, Choi JO, Song YB, Hahn JY, Choi SH, et al. Frequency of myocardial infarction and its relationship to angiographic collateral flow in territories supplied by chronically occluded coronary arteries. Circulation. 2013;127:703–9.
73 Heyndrickx GR, Millard RW, McRitchie RJ, Maroko PR, Vatner SF. Regional myocardial functional and electrophysiological alterations after brief coronary artery occlusion in conscious dogs. J Clin Invest. 1975;56:978–85.
74 Christian TF, Gibbons RJ, Clements IP, Berger PB, Selvester RH, Wagner GS. Estimates of myocardium at risk and collateral flow in acute myocardial infarction using electrocardiographic indexes with comparison to radionuclide and angiographic measures. J Am Coll Cardiol. 1995;26:388–93.
75 Stefanini GG, Windecker S. Can coronary computed tomography angiography replace invasive angiography? Coronary computed tomography angiography cannot replace invasive angiography. Circulation. 2015;131:418–25; discussion 426.
76 Choi JH, Kim EK, Kim SM, Song YB, Hahn JY, Choi SH, et al. Noninvasive evaluation of coronary collateral arterial flow by coronary computed tomographic angiography. Circ Cardiovasc Imaging. 2014;7:482–90.
77 Zhang J, Li Y, Li M, Pan J, Lu Z. Collateral vessel opacification with CT in patients with coronary total occlusion and its relationship with downstream myocardial infarction. Radiology. 2014;271:703–10.
78 Rieber J, Sheth TN, Mooyaart EA, Shapiro MD, Butler J, Ferencik M, et al. Assessment of the presence and extent of coronary collateralization by coronary computed tomographic angiography in patients with total occlusions. Int J Cardiovasc Imaging. 2009;25:331–7.
79 Rentrop KP, Cohen M, Blanke H, Phillips RA. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol. 1985;5:587–92.
80 Werner GS, Ferrari M, Heinke S, Kuethe F, Surber R, Richartz BM and Figulla HR. Angiographic assessment of collateral connections in comparison with invasively determined collateral function in chronic coronary occlusions. Circulation. 2003;107:1972–7.
81 Fulton WF. Arterial Anastomoses in the Coronary Circulation. Ii. Distribution, Enumeration and Measurement of Coronary Arterial Anastomoses in Health and Disease. Scott Med J. 1963;8:466–74.
82 Rockstroh J, Brown BG. Coronary collateral size, flow capacity, and growth: estimates from the angiogram in patients with obstructive coronary disease. Circulation. 2002;105:168–73.
83 Seiler C. The human coronary collateral circulation. Heart. 2003;89:1352–7.
84 van Liebergen RA, Piek JJ, Koch KT, de Winter RJ, Schotborgh CE, Lie KI. Quantification of collateral flow in humans: a comparison of angiographic, electrocardiographic and hemodynamic variables. J Am Coll Cardiol. 1999;33:670–7.
85 Seiler C, Billinger M, Fleisch M, Meier B. Washout collaterometry: a new method of assessing collaterals using angiographic contrast clearance during coronary occlusion. Heart. 2001;86:540–6.
86 Seiler C. Assessment and impact of the human coronary collateral circulation on myocardial ischemia and outcome. Circ Cardiovasc Interv. 2013;6:719–28.
87 Seiler C, Fleisch M, Garachemani A, Meier B. Coronary collateral quantitation in patients with coronary artery disease using intravascular flow velocity or pressure measurements. J Am Coll Cardiol. 1998;32:1272–9.
88 Gloekler S, Traupe T, Meier P, Steck H, de Marchi SF, Seiler C. Safety of diagnostic balloon occlusion in normal coronary arteries. Am J Cardiol. 2010;105:1716–22.
89 Matsuo H, Watanabe S, Kadosaki T, Yamaki T, Tanaka S, Miyata S, et al. Validation of collateral fractional flow reserve by myocardial perfusion imaging. Circulation. 2002;105:1060–5.
90 de Marchi SF, Oswald P, Windecker S, Meier B, Seiler C. Reciprocal relationship between left ventricular filling pressure and the recruitable human coronary collateral circulation. Eur Heart J. 2005;26:558–66.
91 Friedman PL, Shook TL, Kirshenbaum JM, Selwyn AP, Ganz P. Value of the intracoronary electrocardiogram to monitor myocardial ischemia during percutaneous transluminal coronary angioplasty. Circulation. 1986;74:330–9.
92 de Marchi SF, Streuli S, Haefeli P, Gloekler S, Traupe T, Warncke C, et al. Determinants of prognostically relevant intracoronary electrocardiogram ST-segment shift during coronary balloon occlusion. Am J Cardiol. 2012;110:1234–9.
93 Christian TF, Berger PB, O’Connor MK, Hodge DO, Gibbons RJ. Threshold values for preserved viability with a noninvasive measurement of collateral blood flow during acute myocardial infarction treated by direct coronary angioplasty. Circulation. 1999;100:2392–5.
94 Rowe GG. Inequalities of myocardial perfusion in coronary artery disease (“coronary steal”). Circulation. 1970;42:193–4.
95 Stoller M, Seiler C. Pathophysiology of coronary collaterals. Curr Cardiol Rev. 2014;10:38–56.
96 Werner GS, Figulla HR. Direct assessment of coronary steal and associated changes of collateral hemodynamics in chronic total coronary occlusions. Circulation. 2002;106:435–40.
97 Seiler C, Fleisch M, Meier B. Direct intracoronary evidence of collateral steal in humans. Circulation. 1997;96:4261–7.
98 Werner GS, Fritzenwanger M, Prochnau D, Schwarz G, Ferrari M, Aarnoudse W, et al. Determinants of coronary steal in chronic total coronary occlusions donor artery, collateral, and microvascular resistance. J Am Coll Cardiol. 2006;48:51–8.
99 Werner GS, Surber R, Ferrari M, Fritzenwanger M, Figulla HR. The functional reserve of collaterals supplying long-term chronic total coronary occlusions in patients without prior myocardial infarction. Eur Heart J. 2006;27:2406–12.
100 Akinboboye OO, Idris O, Chou RL, Sciacca RR, Cannon PJ, Bergmann SR. Absolute quantitation of coronary steal induced by intravenous dipyridamole. J Am Coll Cardiol. 2001;37:109–16.
101 Khouri EM, Gregg DE, McGranahan GM, Jr. Regression and reappearance of coronary collaterals. Am J Physiol. 1971;220:655–61.
102 Watanabe N, Yonekura S, Williams AG, Jr., Scheel KW, Downey HF. Regression and recovery of well-developed coronary collateral function in canine hearts after aorta-coronary bypass. J Thorac Cardiovasc Surg. 1989;97:286–96.
103 Fujita M, McKown DP, McKown MD, Franklin D. Coronary collateral regression in conscious dogs. Angiology. 1990;41:621–30.
104 Pohl T, Hochstrasser P, Billinger M, Fleisch M, Meier B, Seiler C. Influence on collateral flow of recanalising chronic total coronary occlusions: a case-control study. Heart. 2001;86:438–43.
105 Werner GS, Richartz BM, Gastmann O, Ferrari M, Figulla HR. Immediate changes of collateral function after successful recanalization of chronic total coronary occlusions. Circulation. 2000;102:2959–65.
106 Werner GS, Emig U, Mutschke O, Schwarz G, Bahrmann P, Figulla HR. Regression of collateral function after recanalization of chronic total coronary occlusions: a serial assessment by intracoronary pressure and Doppler recordings. Circulation. 2003;108:2877–82.
107 Lee CW, Hong MK, Choi SW, Kim JH, Kim JJ, Park SW and Park SJ. Influence of coronary collateral flow on restenosis following primary angioplasty for acute myocardial infarction. Catheter Cardiovasc Interv. 2002;55:477–81.
108 Jensen LO, Thayssen P, Lassen JF, Hansen HS, Kelbaek H, Junker A, et al. Recruitable collateral blood flow index predicts coronary instent restenosis after percutaneous coronary intervention. Eur Heart J. 2007;28:1820–6.
109 Meier P, Indermuehle A, Pitt B, Traupe T, de Marchi SF, Crake T, et al. Coronary collaterals and risk for restenosis after percutaneous coronary interventions: a meta-analysis. BMC medicine. 2012;10:62.
110 Koerselman J, de Jaegere PP, Verhaar MC, Grobbee DE, van der Graaf Y. Prognostic significance of coronary collaterals in patients with coronary heart disease having percutaneous transluminal coronary angioplasty. Am J Cardiol. 2005;96:390–4.
111 Seiler C. The human coronary collateral circulation. Eur J Clin Invest. 2010;40:465–76.
112 Reimer KA, Ideker RE, Jennings RB. Effect of coronary occlusion site on ischaemic bed size and collateral blood flow in dogs. Cardiovasc Res. 1981;15:668–74.
113 Lee CW, Park SW, Cho GY, Hong MK, Kim JJ, Kang DH, et al. Pressure-derived fractional collateral blood flow: a primary determinant of left ventricular recovery after reperfused acute myocardial infarction. J Am Coll Cardiol. 2000;35:949–55.
114 Rogers WJ, Hood WP, Jr., Mantle JA, Baxley WA, Kirklin JK, Zorn GL and Nath HP. Return of left ventricular function after reperfusion in patients with myocardial infarction: importance of subtotal stenoses or intact collaterals. Circulation. 1984;69:338–49.
115 Waldecker B, Waas W, Haberbosch W, Voss R, Wiecha J, Tillmanns H. Prevalence and significance of coronary collateral circulation in patients with acute myocardial infarct. Z Kardiol. 2002;91:243–8.
116 Meier P, Gloekler S, de Marchi SF, Zbinden R, Delacretaz E, Seiler C. An indicator of sudden cardiac death during brief coronary occlusion: electrocardiogram QT time and the role of collaterals. Eur Heart J. 31:1197–204.
117 Garza DA, White FC, Hall RE, Bloor CM. Effect of coronary collateral development on ventricular fibrillation threshold. Basic Res Cardiol. 1974;69:371–8.
118 Chareonthaitawee P, Gersh BJ, Araoz PA, Gibbons RJ. Revascularization in severe left ventricular dysfunction: the role of viability testing. J Am Coll Cardiol. 2005;46:567–74.
119 Seiler C, Pohl T, Lipp E, Hutter D, Meier B. Regional left ventricular function during transient coronary occlusion: relation with coronary collateral flow. Heart. 2002;88:35–42.
120 Meier P, Hemingway H, Lansky AJ, Knapp G, Pitt B, Seiler C. The impact of the coronary collateral circulation on mortality: a meta-analysis. Eur Heart J. 2011.
121 Fulton WF. Immersion Radiography of Injected Specimens. Br J Radiol. 1963;36:685–8.








