Figure 1
Scheme for the application of a live vaccine when the extent of the pharmacological immunosuppression is unclear.
DOI: https://doi.org/10.4414/smw.2015.14159
In Europe, an estimated 2% of the adult population suffers from an autoimmune inflammatory rheumatic disease (AIIRD) [10]. With a population of around 740 million, this amounts to approximately 15 million individuals in Europe. Patients with AIIRD are at an increased risk of infections, and vaccinations as an important means for prevention are indicated in this vulnerable population. The use of immunosuppressive drugs has increased in recent years in these patients [11]. As a result of different immunosuppressive medications the immunogenicity of vaccines may be reduced. Furthermore, the administration of live vaccines bears the potential risk of invasive infection with the attenuated vaccine strain, and should generally be avoided under immunosuppressive therapy; in most international guidelines, live vaccines are contraindicated under immunosuppressive therapy with a systemic effect [1, 2, 4, 12].
In Switzerland, vaccination recommendations for AIIRD patients were published in 2010 by the Swiss Society of Rheumatology [6]. In recent years, new disease-modifying medications, and biological agents, in particular, have been approved for the treatment of AIIRD patients. The growing experience with vaccinations under different immunosuppressive regimens allows the previous recommendations to be updated.
The formulation of new recommendations was initiated by the Swiss Federal Office of Public Health (FOPH) and prepared by a working group of the Federal Commission for Vaccination Issues. The main principles were published in the bulletin of the FOPH in February 2014 [13]. Here we present the background information that forms the basis of the article published by the FOPH, as well as detailed practical recommendations. The recommendations are based on an extensive literature search. As scientific data on many aspects are still scarce, the majority of recommendations given in this article are based on clinical experience and expert opinion of a diverse group of specialists in the fields of rheumatology, immunology, infectiology, travel medicine and vaccination. Specific advice is given regarding timing of vaccinations in relation to different medications, as well as certain circumstances and approaches under which live vaccines may be considered during immunosuppressive therapy.
These new recommendations were initiated by the Swiss Federal Office of Public Health and prepared by a working group of the Federal Commission for Vaccination Issues. This panel was composed of members with a wide breadth of specialties (immunology, infectious diseases, vaccinology, travel medicine, rheumatology). Drafts were distributed to panel members for comments and were discussed in four in-person meetings. The recommendations were reviewed by the Swiss Society of Rheumatology and by the Swiss Society for Allergology and Immunology. National and international experts in the field of vaccination recommendations in immunocompromised persons were consulted in the process and were composed of immunologists, rheumatologists, infectiologists and travel medicine experts.
The GRADE approach was applied to rate the quality of evidence (QoE) as well as the strength of recommendation (SoR) [14]. Quality of evidence has four categories: “high quality”, “moderate quality”, “low quality” and “very low quality”. When no published evidence could be found, it was stated as “grade of evidence not possible“. The strength of recommendation was categorised into “strong” and “weak”.
Published evidence was searched for in electronic databases (Cochrane, Medline, PubMed, Embase). Unpublished (grey) literature (unpublished reports, conference abstracts) was retrieved through a targeted website search of relevant organisations (including international vaccination recommendations such as recommendations by: the United States Centers for Disease Control and Prevention [1], the European League Against Rheumatism (EULAR) [2], the Infectious Diseases Society of America (IDSA) [3] several European countries [4–8] and others [9]) and international conferences dealing with vaccination, infectious diseases and rheumatology. Additional articles were identified through reference lists of selected papers. Literature published up to 29 January 2014 was reviewed and analysed.
The search terms from box 1 were used in combination. Only literature on adult AIIRD were included, with the exception of literature on live vaccine, such as measles, mumps, rubella and varicella, in which case the majority of data were available for paediatric patients. Mostly English and German articles were included. A summary of all relevant papers was produced. Only data from meta-analyses, systematic reviews, randomised trials, and observational studies were taken into account. Case reports were not included in this review with the exception of two case series on live vaccination under immunosuppression therapy and of adverse reactions to pneumococcal polysaccharide vaccine in patients with Behçet’s disease by Hugle et al. [15], which was considered to report important information.
Articles were screened for information on increased risk of infection, vaccine-preventable infections, safety of vaccinations, immunogenicity of vaccination, timing of vaccination in relation to disease activity, timing of vaccination in relation to medication, effects of additional vaccine doses.
Box 1:Search terms ‒ autoimmune inflammatory rheumatic diseases, infections and vaccines considered in the literature search
Autoimmune inflammatory rheumatic diseases: “rheumatology”, “rheumatic”, “AIIRD”, “autoimmune”, “immunocompromised”, “autoimmune inflammatory rheumatic disease”, “rheumatoid arthritis”, “lupus erythematosus”, “spondylarthritis”, “spondyloarthritis”, “vasculitis”, “connective tissue disease”, “ scleroderma”, “systemic sclerosis”, “Behҫet’s disease”, “Wegener granulomatosis”, “Churg-Strauss syndrome”, “dermatomyositis”, “polymyositis”, “polyarteriitis nodosa” “Takayasu arteritis”, “giant cell arteritis”, “psoriatic arthritis”
Infections: “infection”, “risk of infection”, “tetanus”, “diphtheria”, “poliomyelitis”, “polio”, “pertussis”, “hepatitis A”, “hepatitis B”, “haemophilus influenza b”, “yellow fever”, “mumps”, “measles”, “rubella”, “varicella”, “herpes zoster” “rabies”, “tick borne encephalitis”, “TBE”, “Japanese encephalitis”, “cholera”, “human papillomavirus”, “HPV”, “typhoid fever”, “meningococcal” “pneumococcal”, “influenza”, “H1N1”, “tuberculosis”
Vaccines: “vaccination”, “vaccine”, “vaccination guideline”, “inactivated vaccin*”, “live vaccin*”, “conjugate vaccin*”, “polysaccharide vaccin*”, “tetanus vaccin*”, “diphtheria vaccin*”, “poliomyelitis vaccin*”, “polio vaccin*”, “pertussis vaccin*”, “hepatitis A vaccin*”, “hepatitis B vaccin*”, “haemophilus influenza b vaccin*”, “yellow fever vaccin*”, “mumps vaccin*”, “measles vaccin* ”, “rubella vaccin*”, “varicella vaccin*”, “herpes zoster vaccin*” “rabies vaccin*”, “tick borne encephalitis vaccin*”, “TBE vaccin*”, “Japanese encephalitis vaccin*”, “cholera vaccin*”, “human papillomavirus vaccin*”, “HPV vaccin*”, “typhoid fever vaccin*”, “meningococcal vaccin*” “pneumococc*vaccin*”, “influenza vaccin*”, “H1N1 vaccin*”, “tuberculosis vaccin*”, “BCG”
BCG = Bacille Calmette-Guérin; HPV = human papillomavirus; TBE = tick-borne encephalitis
Infections are a substantial cause of morbidity and mortality in persons with autoimmune inflammatory rheumatic diseases [16]. The risk of infection may be increased by the disease itself [17–19], but also by the use of immunosuppressive and immunomodulatory drugs [20–27, 188]. In the following chapters, the term “immunosuppressive therapy” will include both “immunosuppressive” and “immunomodulatory” therapy.
In patients with AIIRD, the risk of acquiring a confirmed infection can be 1.7 times higher than in the general population [28]. Not only is the risk of infection higher in AIIRD patients, but also the course of infection can be more severe [29, 30]. In particular, the risk of an infection requiring hospitalisation has been shown to be 1.8 times higher in patients with AIIRD compared with healthy persons [28].
Patients with AIIRD have an increased risk for influenza infection [31–33], invasive pneumococcal disease [34, 35], and for herpes zoster [36–40]. Several studies have demonstrated that the risk of tuberculosis infection is higher in rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) patients compared to the general population [41–45]. Also the risk of human papillomavirus (HPV) infection [46] and the incidence of cervical dysplasia are increased in SLE patients, while clearance of the virus is decreased compared with the general population [47]. Hepatitis B reactivation can be severe and can sometimes have a high mortality in patients under immunosuppressive therapy (esp. during the tapering process [48]).
Sixteen key recommendations have been formulated (box 2) and will be elaborated in the following sections.
Box 2: Key recommendations
1. Benefits of vaccination greatly outweigh the risks of infection and vaccinations do not cause autoimmune inflammatory rheumatic diseases, nor their exacerbations.
2. There are no specific contraindications for vaccination with inactivated and live vaccines in patients with autoimmune inflammatory rheumatic disease without immunosuppressive treatment.
3. The vaccination status of the patients should be assessed and documented at the earliest time point after diagnosis and recommended vaccinations should be administered as soon as possible. If possible, vaccinate before initiation of immunosuppressive therapy. Live attenuated vaccines should be given at least four weeks before initiation of immunosuppressive treatment.
4. The immune response to a booster vaccine administered during immunosuppressive treatment is considered to be less affected than the response to a primary vaccine dose.
5. In already treated AIIRD patients, vaccines should ideally be administered when immunosuppressive therapy is lowest.
6. It is generally safe to administer inactivated vaccines to patients with AIIRD under immunosuppressive treatment; the immunogenicity may be reduced.
7. The administration of live vaccines to immunosuppressed patients bears the risk of replication of the attenuated micro-organism and invasive infections. Live vaccines with a high potential of replication (e.g. yellow fever vaccine) should generally be avoided in patients with autoimmune inflammatory rheumatic disease under treatment with a systemic immunosuppressive effect; specific exceptions apply. Live vaccines with a low risk of replication (typhoid oral vaccine, varicella / herpes zoster vaccine) may be used with caution in selected patients under immunosuppressive therapy.
8. Depending on the drug, different intervals after interrupting immunosuppressive treatment are advised before immune reconstitution has been established.
9. General recommendations for basic vaccinations also apply to patients with AIIRD.
10. Specific vaccinations are recommended for AIIRD patients as they may require more comprehensive protection: These include the annual influenza vaccination and the pneumococcal vaccination. The use of the 13-valent pneumococcal conjugate vaccine (Prevenar®) should be preferred to the 23-valent polysaccharide vaccine. Vaccination against hepatitis B is encouraged in all AIIRD patients and vaccination against human papillomavirus in female patients with systemic lupus erythematosus aged 11–26 years. Herpes zoster vaccination will be recommended in AIIRD patients aged >50 years when the vaccine becomes available in Switzerland.
11. Four to six weeks after a completed primary course of vaccination, serology testing should be performed if the corresponding serological test is available.
12. In a patient undergoing immunosuppressive therapy and in whom immunity towards measles, rubella and varicella is unknown, a specific serological test should be performed. The same approach should be followed in a person under immunosuppressive therapy who received the yellow fever vaccination in the past and intends to travel to a yellow fever endemic area.
13. As the immunocompromised person may not be protected against diseases despite previous vaccination (e.g. against mumps, measles, rubella, varicella, influenza), insist on checking the vaccination status of their household and other close contacts, and vaccinate if indicated.
14. If the immunocompromised person is not protected against measles and/or varicella and has contact with an infected person, consider immunoglobulins/antivirals.
15. Always use conjugate vaccines in preference to polysaccharide vaccines because they induce higher affinity antibody responses, longer lasting immune responses and memory responses.
16. Vaccination should preferentially be administered during stable disease.
A causal relationship between vaccination and autoimmune diseases has been debated at great length in the literature [49, 50]. There is no reliable evidence that autoimmune diseases are caused by vaccination. Nevertheless, specific vaccines have been associated with autoimmune reactions, for example Guillain Barré syndrome (GBS) after 1976 swine influenza A (H1N1) subtype A/NJ/76 vaccination in the United States [51]. An increase in GBS cases after influenza A (H1N1) 2009 monovalent vaccination was reported (around 1 excess case in 1 million vaccinated doses) and an association with the vaccine has not been ruled out [26, 52–60]. An increase of Guillain Barré syndrome was also reported in patients having received brain-derived rabies vaccine, which is not in use in Europe [61, 62].
An association between mumps/measles/rubella (MMR) vaccine and immune thrombocytopenic purpura (ITP) was also reported (around 1 case per 22,300–50,000 doses) [63–65], although a second MMR dose was not associated with an increased risk of ITP [66, 67].
Some have also suspected vaccine adjuvants of triggering autoimmune diseases (autoimmune/inflammatory syndrome induced by adjuvants (ASIA) [68–70]). An association has not been demonstrated to date [71–73] with the exception of AS03 adjuvanted H1N1 influenza vaccine, which has been suspected to be associated with narcolepsy in Finland, Sweden, Norway, Ireland, and the United Kingdom [74, 75]. The association was most pronounced in children.
In conclusion, few vaccines were found to be associated with autoimmune reactions and they are suspected to be linked to specific antigens. Importantly, the risk of GBS and other immune diseases is increased by infection. For example, the risk of GBS within 90 days after influenza-like illness was found to be 7-fold increased (risk ratio 7.35, 95% CI 4.36–12.38) [76] and the risk of GBS after influenza infection is 4–7 times higher than after influenza vaccination [77].
In conclusion, it is important to consider the overall risks and benefits of vaccination, as defined by epidemiological studies. Clinicians, policy makers and those eligible for vaccination should be assured that the benefits of most vaccines greatly outweigh the risks of infection.
Similarly, exacerbations of rheumatic diseases after vaccination have been extensively discussed in the literature and there are several case reports of worsening disease symptoms after vaccination. However, the majority of published data support the conclusion that immunisation with inactivated vaccines is safe and does not increase disease activity in AIIRD patients, measured by clinical and biological means [78–81]. Several studies in patients with rheumatoid arthritis, connective tissue disease, spondyloarthritis, and systemic vasculitis showed that the administration of influenza, pneumococcal, tetanus toxoid, Haemophilus influenzae type b, hepatitis A, hepatitis B and human papillomavirus vaccine was safe and did not increase disease activity [79, 82, 83]. It should be noted that most vaccination studies in AIIRD patients were performed in patients with stable disease (please see section 16).
A patient with untreated AIIRD, i.e. who is not under systemic immunosuppressive therapy, should be vaccinated like any other person, although the clinical assessment of the individual patient has to be taken into account. Therapy with hydroxychloroquine or sulfasalazine is not considered to be immunosuppressive or influence vaccine immune responses, although a possible interference of hydroxychloroquine in the case of intradermal vaccination has been observed [84]. Of note, prophylactic paracetamol administration has also been shown to reduce antibody responses after vaccination in children [85].
Furthermore, additional vaccinations for patients with chronic diseases are recommended as a result of the increased risk of infections or their complications.
Of note, in a recent case series, severe local reactions and severe systemic inflammatory responses to 23-valent polysaccharide pneumococcal vaccine (23-PPV) were observed in patients with Behçet’s disease under treatment with abatacept/prednisolone, etanercept, azathioprine or sole anti-inflammatory drugs [15]. No such adverse reactions were seen in patients with other autoimmune or autoinflammatory diseases receiving 23-PPV vaccine. It was postulated that the presumed auto-inflammatory mechanism underlying Behçet’s disease could be responsible for the severe adverse events after pneumococcal vaccination. Owing to this potential streptococcal hypersensitivity reaction, caution is warranted when giving pneumococcal vaccination to patients with Behçet’s disease.
Ideally, the vaccination status of the patients should be assessed and documented at the earliest time point after diagnosis, and recommended vaccinations should be administered as soon as possible. If possible, vaccines should be administered before initiation of immunosuppressive therapy. Furthermore, if live attenuated vaccines are administered, the start of immunosuppressive therapy must be delayed for at least 4 weeks. Inactivated vaccines should ideally also be given at least 4 weeks before starting high-dose systemic corticosteroids or immunosuppressive medication.
There are three main reasons for assessing the vaccination status of patients with AIIRDs as soon as possible: (i) some AIIRDs per se confer an increased risk of infection and vaccinations should be updated as soon as possible after diagnosis; many patients do not immediately receive immunosuppressive treatment and thus vaccines can be administered at a time point when (ii) the immunogenicity of vaccination is not compromised by the immunosuppression and (iii) live vaccines can be safely administered.
Immunosuppressive drugs interfere with the activation and clonal expansion of T and B cells. As a result, the immune response to a primary vaccine dose can be impaired. The T and B cell immune response to a vaccine booster dose is generally better preserved as more memory B and T cells (induced by the first vaccine dose) are present [86, 87]. Thus, a booster vaccination in a person under immunosuppressive therapy will usually generate protective antibody levels, although antibody responses may be lower and protection shorter than in persons without immunosuppressive treatment. It has been shown that for a booster tetanus vaccine dose immune responses were preserved under various immunosuppressive therapies [88, 89].
The immunogenicity of vaccines may be reduced by an immunosuppressive therapy. The effect can be expected to be dependent on the total taken dose. To achieve the best protection, patients under immunosuppressive therapies should be vaccinated when the dosage is lowest.
Furthermore, live vaccines bear the risk of a potential replication of the attenuated virus and are thus generally contraindicated under immunosuppressive therapies. Under specific circumstances live vaccines may be administered (sections 7 and 8).
The majority of published data show that the administration of inactivated vaccines to AIIRD patients under immunosuppressive therapy is safe [16, 90]. Their administration was not associated with a higher risk of vaccine reactions, nor with a worsening or reactivation of the underlying disease (see section 1).
The immunogenicity of vaccinations during the use of nonbiological disease-modifying antirheumatic drugs (DMARDs), corticosteroids and/or biological agents has been studied in patients with rheumatoid arthritis, spondyloarthritis, connective tissue disease, and vasculitis. Data exist on the immunogenicity of seasonal influenza, pandemic influenza (H1N1), pneumococcal polysaccharide, pneumococcal conjugate, hepatitis A, hepatitis B, tetanus toxoid, Haemophilus influenzae b and human papillomavirus vaccination [91]. Most studies showed slightly reduced, but sufficient immune responses following vaccination of patients with AIIRD under therapy with corticosteroids, non-biological DMARDs (with the exception of methotrexate) and tumour necrosis factor (TNF) blockers (table 1).
| Table 1: Published evidence on immunogenicity of inactivated vaccines in different rheumatic disease groups and for various immunosuppressive agents. | ||||||||||||
| No medication | Corticosteroids | MTX | Other DMARDs | MTX + TNFi | TNFi | RTX | Abatacept | Tocilizumab | ||||
| Seasonal influenza | RA | Good immunogenicity [88, 98, 99, 101, 114–118]. | Good to moderate immunogenicity [98, 99, 113–116, 118, 119], with reduced level of humoral immune response [110, 117]. | Good immunogenicity [88, 98, 99, 101, 113-116, 118]. | Reduced immunogenicity (MTX + certolizumab) (47.4%) [97]. | Good [99–101, 115, 120] to moderate immunogenicity [98]. Immunogenicity dependent on time point of vaccination in relation to infliximab treatment. Reduced immunogenicity in [116, 119], immunogenicity reduced under certolizumab [97]. | Reduced to blunted immune response, in particular if vaccine given close to RTX administration [113, 118, 121]. | Reduced immunogenicity [106]. | Good immunogenicity [108, 110]. | |||
| CTD | Good [115, 122] to moderate immunogenicity [123]. Reduced immunogenicity in [124, 125] (no neg. effect by corticosteroids). Reduced immunogenicity in [126]. | Good immunogenicity [115]. Reduced immunogenicity [125] (neg. effect of MTX). | AZA had a negative effect on humoral vaccine responses [124]. | Moderate [123] to good immunogenicity [115]. | ||||||||
| SpA | Good immunogenicity [116] (n = 1). | Good immunogenicity [116] (n = 1). | Good immunogenicity [116] (n = 1). | Moderate [116] to good immunogenicity [98]. | ||||||||
| VAS | Good immunogenicity in WG patients [127]. | Good immunogenicity in WG patients [127, 128]. | ||||||||||
| Pandemic Influenza | RA | Reduced immunogenicity [103, 129–133]; no neg. effect by corticosteroids). | Reduced immunogenicity [103, 129, 134, 135]. Reduced immunogenicity in [132] (no neg. effect by MTX). A 2nd vaccine dose achieved good vaccine responses [129]. | Reduced immunogenicity under AZA, leflunomide, MMF, CYC. A 2nd vaccine dose achieved good vaccine responses [129]. | Reduced immunogenicity [132, 133]. Reduced immunogenicity [129, 131] (no neg. effect by TNFi). | Reduced immunogenicity [103, 129]. Recent RTX treatment had a negative effect [129]. Severely reduced immunogenicity in [136]. | Severely reduced immunogenicity [102, 103]. | Good immunogenicity [103]. | ||||
| CTD | Reduced immunogenicity [134], for seroconversion and GMT fold increase, international immunogenicity criteria were met. | Reduced immunogenicity [123, 129, 130, 134] (no neg. effect by corticosteroids). A 2nd vaccine dose achieved good vaccine responses [129, 135]. | Immunogenicity was decreased [103, 129, 134, 135]. A 2nd vaccine dose achieved good vaccine responses [129, 135]. | Reasonable [123] to decreased immunogenicity [103, 129, 130, 134, 135]. A 2nd vaccine dose achieved good responses [129, 135]. Under chloroquine, good immunogenicity [134]. | Overall reduced immunogenicity [103, 129], but not demonstrated for TNFi. | Reduced immunogenicity [103, 129]. In [129], recent RTX treatment (<12 weeks) had a negative effect on immunogenicity. | Reduced immunogenicity [103]. | |||||
| SpA | Good immunogenicity [103]. | Good [103, 133] and reduced immunogenicity [129, 130] (no neg. effect by corticosteroids). A 2nd vaccine dose achieved good vaccine responses [129]. | Good [103, 133] and reduced immunogenicity [129] (neg. effect by cDMARDs). A 2nd vaccine dose achieved good vaccine responses [129]. | Reduced immunogenicity [103], but not for TNFi. Reduced immunogenicity [133] for adalimumab and infliximab, but not for etanercept. | Reduced immunogenicity [129] (neg. effect by RTX within the past 12 weeks). A 2nd vaccine dose achieved good vaccine responses. | Reduced immunogenicity [103]. | ||||||
| VAS | Good immunogenicity [130]. Vaccination more immunogenic in Takayasu arteriitis than in WG and BD patients. | Reduced immunogenicity [103, 129], but insufficient evidence for a neg. influence by corticosteroids. A 2nd vaccine dose achieved good vaccine responses [129]. | Reduced immunogenicity under MTX [103, 129]. A 2nd vaccine dose achieved good vaccine responses | Only weak evidence for a neg. influence by cDMARDS other than MTX. Reduced immunogenicity in [129] (neg. effect by leflunomide, AZA, MMF, CYC). A 2nd vaccine dose achieved good vaccine responses. | Reduced immunogenicity [103], but insufficient evidence for a negative influence by TNFi. | Reduced immunogenicity [103]. Reduced immunogenicity (neg. effect by RTX); a 2nd vaccine dose achieved good vaccine responses [129]. | Reduced immunogenicity [103]. | |||||
| Pneumococcal poly-saccharide | RA | Good immunogenicity [94, 99, 137]. Reduced immunogenicity in [95, 96, 138] (no neg. effect by corticosteroids). | Reduced [93–95] to good immunogenicity [137, 138]. | Reduced immunogenicity compared with MTX or TNFi alone [96, 97]. | Good [94, 95] and moderately reduced immunogenicity [97, 138]. | Reduced immunogenicity under combination of RTX + MTX [89]. | Reduced immunogenicity in patients and healthy persons [104, 105]. | Good immunogenicity [108, 111]. TCZ in addition to MTX did not significantly reduce immune responses further [109]. | ||||
| CTD | Moderate immunogenicity [139]. | Good to moderate immunogenicity [137, 139] (no neg. effect by corticosteroids). In [140, 141], trend to lower immunogenicity with corticosteroids [140]. | Good to moderate immunogenicity [137, 140] (no evidence for a neg. effect by MTX). | Good [141] to moderate immunogenicity [137, 139] (no neg. effect by cDMARDs). Trend for lower immunogenicity under cDMARDs [140]. | ||||||||
| SpA | Good to moderate immunogenicity [138, 142] (no neg. effect by corticosteroids). | Good to moderate immunogenicity [138] (no neg. effect by MTX). Reduced immunogenicity [142]. | Moderately reduced [143] to reduced immunogenicity [138]. Good immunogenicity under etanercept [142]. | |||||||||
| VAS | ||||||||||||
| Pneumococcal conjugate | RA | Good immunogenicity [93]. | Reduced immunogenicity [93, 144]. | Good immunogenicity [93, 144]. | Severely reduced immunogenicity (10% pos. antibody responses), effect more enhanced under RTX/MTX combination (0% positive antibody responses) [145]. | Severely reduced immunogenicity (17.6% developed positive antibody responses) [145]. | ||||||
| CTD | ||||||||||||
| SpA | Reduced immunogenicity [144]. | Good [144] and severely reduced immunogenicity [147]. | ||||||||||
| VAS | ||||||||||||
| Tetanus | RA | Good immunogenicity [148]. | Good immunogenicity [88]. | Reduced immunogenicity [89]. | Reduced immunogenicity [89] and good immune response [88]. | Reduced immunogenicity under MTX, RTX did not reduce antibody responses further [89]. | Immune responses reduced in healthy subjects [104]. | TCZ in addition to MTX did not significantly reduce immune responses further [109]. | ||||
| CTD | Good [148] and reduced [149] immunogenicity. | Trend to decreased antibody responses for corticosteroids [140]. | Moderate immunogenicity, [140] (no evidence for a neg. effect by MTX). | Trend to decreased antibody responses for cDMARDs [140]. | ||||||||
| SpA | no data | |||||||||||
| VAS | no data | |||||||||||
| Diphtheria | no data | |||||||||||
| Polio | no data | |||||||||||
| Pertussis | no data | |||||||||||
| Hepatitis A | RA | 72% of travellers with several IMIDs developed a protective response (number of vaccine doses not specified) [150]. | 57% of travellers with several immune-compromising conditions developed protective responses [150]. ≥20 mIU/ml: 6%; ≥10 mIU/ml: 6% protected after 1 dose [92]. In a third study (underlying disease not specified): seroprotection rates 62% after 1 dose, 98% after 2 doses [151]. | Good immunogenicity in IMID patients taking mercaptopurine derivatives (e.g. AZA). Not immunogenic under tacrolimus (n = 2) [150]. In [151], (underlying disease not specified): sero-protection rates: 62% after 1 dose, 98% after 2 doses for AZA, and 67% after 1 dose and 100% after 2 vaccine doses for other immunosuppressive therapies. | ≥20 mIU/ml: 5% protection after 1 dose after 1 month; ≥10 mIU/ml: 15% protection after 1 dose [92]. | 20% of travellers with IMIDs did not develop a protective response [150] ≥20 mIU/ml: 20% protection after 1 dose after 1 month; ≥10 mIU/ml: 73% protected after 1 vaccine dose [92]. In patients under TNFi therapy (underlying disease not specified): seroprotection rates were 46% after 1 dose; 79% after 2 doses [151]. | Seroprotection rates were 67% after 1 dose and 100% after 2 vaccine doses (underlying disease not specified) for other immunosuppressive therapies (incl. cytostatics, leflunomide, interferon, tacrolimus, cyclosporine, natalizumab, tocilizumab(n = 1)) [151]. | |||||
| CTD | no data | |||||||||||
| SpA | no data | |||||||||||
| VAS | no data | |||||||||||
| Hepatitis B | RA | Moderate immunogenicity (68% antibody levels >10 IU/l) after 3 doses [152] (patients under treatment with NSAIDs, prednisone, HCQ, MTX, AZA, gold, sulfasalazine) | Moderate immunogenicity (68% antibody levels >10 IU/l) after 3 doses [152] (patients under treatment with NSAIDs, prednisone, HCQ, MTX, AZA, gold, sulfasalazine) | Moderate immunogenicity (68% antibody levels >10 IU/l) after 3 doses [152] (patients under treatment with NSAIDs, prednisone, HCQ, MTX, AZA, gold, sulfasalazine). | ||||||||
| CTD | Good immunogenicity; seroconversion in 93% [153]. 2/28 (the remaining 7%) patients seroconverted after a 4th dose. | |||||||||||
| SpA | Severely reduced immune responses [143, 147], only 4/20 (22.2%) raised a robust response [143]. | |||||||||||
| VAS | Good immunogenicity; seroconversion in 93% in patients with BD [154]. Patients were treated with colchicine. | |||||||||||
| Haemophilus influenzaeb | RA | no data | ||||||||||
| CTD | Good immunogenicity [140], trend to decreased antibody responses for steroids. | Good immunogenicity [140], trend to decreased antibody responses for AZA and CYC. | ||||||||||
| SpA | no data | |||||||||||
| VAS | no data | |||||||||||
| HPV | RA | no data | ||||||||||
| CTD | Reasonably immunogenic, prednisolone had a negative effect on HPV 16 titres [83]. | Reasonably immunogenic, MMF had a negative effect on HPV6, HPV16 and HPV18 titres [83]. | ||||||||||
| SpA | no data | |||||||||||
| VAS | no data | |||||||||||
| Tick-borne encephalitis | no data | |||||||||||
| Rabies | no data | |||||||||||
| Japanese encephalitis | no data | |||||||||||
| Meningococcal vaccine (polysaccharide or conjugate) | no data | |||||||||||
| Typhoid fever (inactivated or oral live) | no data | |||||||||||
| Cholera | no data | |||||||||||
| AZA = azathioprine; BD = Behςet’s Disease; cDMARDs = classical disease-modifying antirheumatic drugs; CTD = connective tissue disease; CYC = cyclophosphamide; GMT = geometric mean titre; HC = healthy control; Hib =Haemophilus influenzae type b; HPV = Human Papilloma Virus; HCQ = hydroxychloroquine; IMID = immune-mediated inflammatory disease; MMF = mycophenolate mofetil; MTX = methotrexate; NSAIDs = nonsteroidal anti-inflammatory drugs; RA = rheumatoid arthritis; RTX = rituximab; SOT = solid organ transplant; SpA = spondyloarthritis; TCZ = tocilizumab; TNF = tumour necrosis factor; TNFi = tumour necrosis factor inhibitor; VAS = vasculitis; WG = Wegener’s granulomatosis.; Other DMARDs include all classical DMARDs apart from methotrexate (azathioprine, cyclophosphamide, cyclosporine, gold, hydroxychloroquine, leflunomide, mycophenolate mofetil, sulfasalazine, tacrolimus). | ||||||||||||
However, it was shown in several studies that methotrexate blunted the response to influenza, pneumococcal vaccination, tetanus and hepatitis A vaccination [89, 92–95]. A combination therapy of TNFα blocking agents and methotrexate appeared to decrease serological responses even further [92, 96, 97].
One study suggests that the immunogenicity of inactivated vaccines may be preserved when administered on the same day as infusion of the TNF inhibitor infliximab (IFX), but may be hampered when administered several weeks afterwards, when the full immunosuppressive effect has developed. This effect was observed in rheumatoid arthritis patients, but patients with ankylosing spondylitis developed good antibody responses independent of the time of vaccination in relation to IFX infusion [98]. For now, inactivated vaccinations should be applied regardless of timing of IFX, as most studies have shown sufficient immunogenicity of inactivated vaccinations under sole IFX treatment [94, 99–101]. More data will be necessary for a reliable recommendation regarding the timing of vaccination and infliximab infusions.
Abatacept is a fusion protein composed of the extracellular domain of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and the IgG1 Fc, which blocks the interaction between the surface molecules CD80/86 on an activated antigen-presenting cell and CD28 on T cells and inhibits T cell activation. It has been shown to reduce significantly the humoral immune response to influenza, pneumococcal and tetanus toxoid vaccination [102, 103]. In healthy subjects receiving abatacept, this effect was most severe when the vaccination was administered 2 weeks after a single dose of abatacept treatment. If vaccination was given before abatacept, the effect was minor [104–106]. In contrast, tocilizumab (a humanised monoclonal antibody that binds soluble as well as membrane-bound interleukin-6 receptors and thereby blocks the proinflammatory effects of interleukin-6) does not seem to affect the immunogenicity of influenza, pneumococcal conjugate, pneumococcal polysaccharide or tetanus vaccination [107–111].
Most impressively, humoral immune responses to influenza and pneumococcal vaccination were nearly absent in the 6 months after rituximab administration due to the transient loss of immunocompetent B cells [112, 113]. (Rituximab [RTX] is a chimeric monoclonal antibody directed against CD20, a molecule expressed specifically on B cells.) Also, administering rituximab in the weeks after immunisation may compromise vaccine responses. Immunogenicity seems to be partly restored 6‒8 months after RTX therapy [113].
As a general rule, the immunogenicity of vaccinations may be reduced under immunosuppressive therapy. Nonetheless, vaccination of AIIRD patients under immunosuppressive treatment with inactivated vaccines is generally recommended. As vaccines may not be sufficiently immunogenic under immunosuppressive treatment, serologic testing is indicated, especially after a primary vaccination course (see section 11). Some further relevant aspects on timing of vaccination under different immunosuppressive agents can be found in section 8.
Scientific data on live vaccinations are scarce in AIIRD patients and are mostly available on re-vaccinations (table 2). In general, live vaccines should be avoided during systemic immunosuppressive therapy, but some exceptions apply (table 3). An individual patient approach may be necessary, taking into account the underlying disease, the medication, the replication capacity of the vaccine and the risk of infection. If a live vaccine is indicated under immunosuppressive treatment, a specialist (e.g. vaccination / travel medicine specialist / immunologist) should be consulted.
The administration of live vaccines to immunodeficient patients bears the risk of replication of the attenuated vaccine micro-organism and clinically manifest infection. Several case reports on severe side effects after the administration of a live vaccine to patients under immunosuppressive treatment have been published [155, 156].
Some live vaccines (e.g. yellow fever vaccine) have a greater replication capacity than others (e.g. varicella) [157–160]. When deciding on whether a person under immunosuppressive treatment can receive a live vaccine, the replication capacity, the relative risks of different live vaccines as well as the availability of an antiviral agent, immunoglobulin or antibiotic treatment must be taken into account (table 4).
When evaluating the administration of a live vaccine to an immunocompromised patient, the risk of exposure has to be taken into account. In Switzerland, for instance, wild type varicella, mumps, measles and rubella viruses are present, and exposure cannot be safely avoided. Contrary to this, exposure to yellow fever is a travel-associated risk, which can usually be avoided.
If an AIIRD patient is under immunosuppressive therapy and immunity towards measles, rubella, varicella and yellow fever is unknown (for yellow fever only if a previous vaccination was performed), specific serological tests should be performed. If the patient is not immune against measles, rubella and varicella and cannot be vaccinated owing to the immunosuppressive therapy, the vaccination status of their household and other close contacts should be checked. Please see sections 12, 13 and 14 for detailed recommendations.
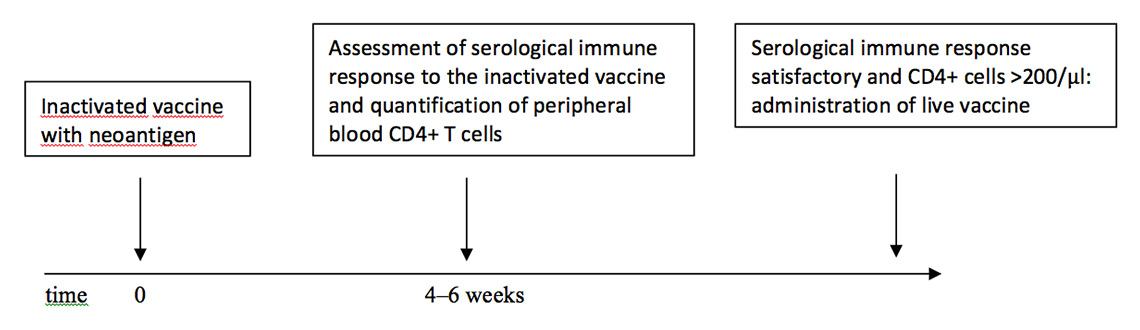
If a live vaccine is indicated but the extent of the pharmacological immunosuppression is unclear, the specialist can adopt the following approach: administer an inactivated vaccine containing a neoantigen (e.g. hepatitis A vaccine) and measure the humoral immune response after 4–6 weeks, together with the number of CD4+ cells. If the humoral immune response to the inactivated vaccine is satisfactory and the CD4+ cells are over a specific threshold (200 cells/µl), a live vaccine may be administered (fig. 1).
| Table 2: Published studies on live vaccines in patients with rheumatic diseases. | |||
| MMR | Yellow fever | Varicella | Herpes zoster |
| MMR revaccination was safe and immunogenic in a prospective nested case-control study in 15 children with JIA, partly under treatment with MTX alone or in combination with etanercept [161]. MMR revaccination was safe in a retrospective cohort study in children with JRD (n = 207), partly treated with MTX (n = 49) [161, 162]. In one randomised controlled trial, MMR revaccination was safe and immunogenic in 63 children with JIA including 29 taking MTX, 1 leflunomide, 6 TNFi (not infliximab) and 3 anakinra. The biologicals were interrupted for 5 half-lives before vaccination [163]. In a prospective observational study, MMR vaccination was given to 28 patients with JRD. Out of these, 19 were under therapy with MTX. Out of these 19, five additionally received prednisone and two were on TNFi. In 2/28, MMR was a first-timevaccination. In one of them (therapy with MTX and corticosteroids) fever and a skin rash appeared 20 days after vaccination. It was concluded that the rash was part of the disease activity (systemic JIA) rather than a side effect of vaccination (personal communication). In all others, vaccination was safe [164]. | Safety of YF revaccination was demonstrated in two case series [165, 166] (Scheinberg et al.: 19 patients all under infliximab treatment, MTX, no corticosteroids, de Mota et al.: 70 patients, concomitant treatment MTX, corticosteroids, nonbiological DMARDS). Immunogenicity was explored in [165] in patients treated with infliximab/MTX. YF revaccination was immunogenic, but a trend to lower antibody responses was observed compared with healthy persons. In a prospective cohort study, 19 patients under long-term low dose or short-term high-dose corticosteroid treatment were vaccinated for the first timeagainst YF, and 15 were revaccinated. Apart from more local side effects, vaccination was safe and immunogenic [167]. | VZV first-time vaccination was safe in [168]; in this prospective study, 20 children with JRD (20 varicella-naive and 5 with positive varicella titres) under MTX treatment, corticosteroids and other DMARDs were vaccinated once. Seroconversion rates were slightly reduced compared with controls. In a prospective study, 50 patients with JRD and without VZV history (all under medication with MTX [dosage range 10–27 mg/m2/week], 23 also on prednisone, 5 on TNFi) were vaccinated against VZV, 32 of them received 2 doses. First-timevaccination was safe. Seroconversion rates were 50% after 1 dose, 87% after 2 doses. All 4 who did not seroconvert received TNFi. 2 who were vaccinated once and were seronegative after 1 y developed varicella [169]. In a prospective controlled study, 26 SLE patients with VZV history received VZV vaccination. It was well tolerated and antibody production was appropriate; IFNγ-producing cells were lower than in HCs. No HZ cases occurred in the vaccinated group, but 4 cases occurred in the unvaccinated group [170]. | HZ vaccine was safe and immunogenic in two large retrospective cohort studies ([i] n = 463,541 IMID patients, out of these 18,683 patients were vaccinated; [ii] n = 44,115 patients where 551 received HZ vaccination). Patients included had different autoimmune diseases under treatment with nonbiological DMARDs (MTX, HCQ, sulfasalazine, AZA, leflunomide, ciclosporin, 6-mercaptopurine), and oral glucocorticoids, as well as biologicals (incl. TNFi, abatacept, RTX) [171, 172]. It was not specified whether a history of previous varicella infection or vaccination was taken before HZ vaccine administration. One prospective, controlled study was performed in 10 SLE patients under treatment with prednisone ≤10 mg daily, HCQ ≤6.5 mg/kg daily, MTX ≤20 mg/weekly, AZA ≤150 mg daily, (no other medications were allowed, incl. biological agents). All were seropositive for VZV.Vaccination was safe, but slightly less immunogenic than in control subjects [173]. |
| AZA = azathioprine; HCQ = hydroxychloroquine; HC = healthy control; HZ = herpes zoster; IFN = interferon; IMID = immune-mediated inflammatory disease; JIA = juvenile idiopathic arthritis; JRD = juvenile rheumatic disease; MMR = mumps; measles; rubella; MTX = methotrexate; RTX = rituximab; SLE = systemic lupus erythematosus; TNF = tumour necrosis factor; TNFi = tumour necrosis factor inhibitor; VZV = varicella zoster virus; YF = yellow fever. | |||
| Table 3:Live vaccines during immunosuppressive therapy. | ||
| Therapeutic agent | Herpes zoster / varicella vaccination | Mumps, measles, rubella (MMR), yellow fever vaccination |
| Low-dose systemic or topical corticosteroids: • Short- or long-term daily or alternate-day therapy with <20 mg of prednisone or equivalent • Glucocorticosteroid replacement therapy in adrenal insufficiency / topical steroids (airways, skin, ears, or eyes) • Intra-articular, bursal, or tendon injection of steroids Sulfasalazine Hydroxychloroquine | No restrictions* | No restrictions* |
| Methotrexate | ≤0.4 mg/kg/week (≤20 mg/week): vaccination possible* | ≤0.4 mg/kg/week (≤20 mg/week): vaccination possible*† |
| >0.4 mg/kg/week (>20 mg/week): contraindication | >0.4 mg/kg/week (>20 mg/week): contraindication | |
| Azathioprine¶ | ≤3.0 mg/kg/day: vaccination possible* | Contraindication |
| >3.0 mg/kg/day: contraindication | ||
| 6-Mercaptopurine§ | ≤1.5 mg/kg day: vaccination possible* | Contraindication |
| >1.5 mg/kg/day: contraindication | ||
| Abatacept Adalimumab Anakinra Certolizumab Cyclosporine A Cyclophosphamide Etanercept Golimumab High-dose systemic steroids (≥20 mg per day of prednisone or equivalent for >2 weeks) Infliximab Leflunomide Mycophenolate mofetil Rituximab Tacrolimus Tocilizumab Ustekinumab | Contraindication | Contraindication |
| In general, live vaccines should be avoided in AIIRD patients under systemic immunosuppressive therapy. Some exceptions apply. * This recommendation is provided for patients taking a single immunosuppressant, not for combination therapy. In the case of a combination therapy the immunosuppressive effect can be enhanced and live vaccines may be contraindicated. † Live vaccination generally contraindicated if methotrexate dosage >0.4 mg/kg/week or >20 mg/week. Only varicella and herpes zoster: vaccination possible if ≤0.4 mg/kg/week or ≤20 mg/week to prevent varicella infection or reactivation. MMR, yellow fever: in clinically stable cases, these live vaccines can be given during low dosage therapy: methotrexate ≤0.4 mg/kg/week or ≤20 mg/week [174] (QoE: grade of evidence not possible, SoR: weak). In the vaccination clinic of Service de Médecine Tropicale et Humanitaire, Hôpitaux Universitaires de Genève and the Swiss Tropical and Public Health Institute, the administration of live vaccines (including yellow fever) under low dose MTX has been clinical practice since 2006 and no severe side effects have been reported; the recommendation will require future follow-up. Of note, during a yellow fever vaccination campaign in Peru, a >20 times risk of YEL-AVD (yellow -fever-vaccine-associated viscerotropic disease) was found to be associated with one particular vaccine lot. Among 42,742 vaccinees who received a vaccine from this lot, 5 developed a YEL-AVD. Amongst these was a 49-year-old female with a past history of rheumatoid arthritis and systemic lupus erythematosus as well as treatment with methotrexate and dexamethasone starting 4 days after vaccination (dosage unknown) [175]. ¶ Only varicella and herpes zoster: vaccination possible if azathioprine dosage ≤3.0 mg/kg/day to prevent varicella infection or reactivation, above this threshold or other live vaccines: contraindicated. § Only varicella and herpes zoster: vaccination possible if mercaptopurine dosage ≤1.5 mg/kg/day to prevent varicella infection or re-activation, above this threshold or other live vaccines: contraindicated. | ||
| Table 4:Replication capacity and complication risk of live vaccines. | ||
| Systemic replication capacity* | Theoretical risk of complication† | |
| Yellow fever | ++++ | ++ |
| Mumps, measles, rubella | ++ | + |
| Varicella and herpes zoster | + | (+) |
| Oral typhoid vaccine | (+) | – |
| The replication capacity, relative risks of different live vaccines as well as the availability of an antiviral agent, immunoglobulin or antibiotic treatment must be considered when deciding on whether a person under immunosuppressive treatment can be vaccinated. * ++++ very strong systemic replication capacity, +++ strong systemic replication capacity, ++ moderate systemic replication capacity, + weak systemic replication capacity, (+) very weak systemic replication capacity † ++++ very strong risk of complication, +++ strong risk of complication, ++ moderate risk of complication, + weak risk of complication, (+) very weak risk of complication | ||
From safety aspects, inactivated vaccines can be given at any timepoint during immunosuppressive therapy.

Figure 1
Scheme for the application of a live vaccine when the extent of the pharmacological immunosuppression is unclear.
For several medications, the timing of vaccination in relation to medication appears to have an effect on immunogenicity (see also section 6) and timing of inactivated vaccination in relation to immunosuppressive treatment should be considered (table 5).
For safety reasons, it is advisable to wait for a certain time period after cessation or interruption of an immunosuppressive agent before administrating a live vaccine.
The duration of the immunosuppressive effect depends on (i) the half-life of the active drug component and (ii) recovery from the immunological effect (e.g. B and/or T cell depletion). For most immunosuppressive medications this timepoint has not been clearly defined [87].
There are some general recommendations summarised in table 6.
| Table 5: Recommended time period between interruption of immunosuppressive therapy and administration of inactivated vaccines. | |
| Medication | Inactivated vaccine |
| Corticosteroids Low-dose systemic or topical corticosteroids • Short- or long-term daily or alternate-day therapy with <20 mg of prednisone or equivalent • Glucocorticosteroid replacement therapy in adrenal insufficiency / topical steroids (airways, skin, ears, or eyes) • Intra-articular, bursal, or tendon injection of steroids High-dose systemic steroids (≥20 mg per day of prednisone or equivalent for >2 weeks) Adalimumab Anakinra Azathioprine Certolizumab Ciclosporin Cyclophosphamide Etanercept Golimumab Hydroxychloroquine Infliximab Leflunomide 6-Mercaptopurine Methotrexate Mycophenolate mofetil Sulfasalazine Tacrolimus Tocilizumab Ustekinumab | No time lag necessary |
| Abatacept | If possible, vaccinate shortly before abatacept administration* |
| Rituximab | Wait at least 6 months for revaccination and 12 months for primary vaccination, if possible |
| It is generally safe to administer inactivated vaccines to patients under immunosuppressive therapy; the immunogenicity may be reduced. For most immunosuppressants, no specific time lag between interrupting the immunosuppressive agent and administration of an inactivated vaccine has to be respected. As vaccines may not be sufficiently immunogenic under immunosuppressive treatment, serological testing 4‒6 weeks after vaccination may be indicated, especially after a primary vaccination course. If at all possible, for immunogenicity reasons it may be advisable to respect the same time intervals for inactivated vaccines as those recommended for live vaccines (table 6). If these time intervals can be respected, no reduced immunogenicity has to be expected. Regarding B-cell depleting therapy, immune responses to inactivated vaccines may be insufficient when immunisation is performed within 6 months after RTX. Inactivated vaccines should therefore be given several weeks before initiating RTX, or at least 6 months afterwards. This recommendation is not made for safety reasons, but for immunogenicity reasons only. The recommendation is based upon the half-life of rituximab, on immunogenicity studies of inactivated vaccines as well as on CD19+ cell measurements under rituximab therapy [113, 176]. If a vaccine is indicated within 6 months after rituximab (e.g. influenza vaccine during the influenza season), it may be given. The immunogenicity and the duration of protection may be reduced significantly. CD19+ B cells can be measured before vaccination as the best proxy available for the quantity of B cells in the periphery. In addition, immunoglobulin levels can be measured. * For abatacept, it may be advisable to vaccinate shortly before abatacept application as immune responses have been shown to be severely reduced when vaccinations were given 2 weeks after abatacept [104]. | |
| Table 6:Recommended time period between interruption of immunosuppressive therapy and administration of live vaccines. | |
| Medication | Mumps, measles, rubella (MMR) vaccine, varicella vaccine, yellow fever vaccine |
| Low-dose systemic or topical corticosteroids • Short- or long-term daily or alternate-day therapy with <20 mg of prednisone or equivalentglucocorticosteroid replacement therapy in adrenal insufficiency / topical steroids (airways, skin, ears, or eyes) • Intra-articular, bursal, or tendon injection of steroids Sulfasalazine Hydroxychloroquine | No pausing or time lag necessary |
| High-dose systemic steroids (≥20 mg per day of prednisone or equivalent for >2 weeks) | Wait at least 1 month |
| Etanercept | Wait at least 1 month* |
| Methotrexate | ≤0.4 mg/kg/week (≤20 mg/week)† |
| >0.4 mg/ kg/ week (>20 mg/ week): Wait at least 1–3 months¶ | |
| Abatacept Adalimumab Anakinra§ Azathioprine Certolizumab Cyclosporine Cyclophosphamide Golimumab Infliximab 6-Mercaptopurine Mycophenolate mofetil Tacrolimus Tocilizumab Ustekinumab | Wait at least 3 months* |
| Rituximab | Wait at least 12 months‡ |
| Leflunomide | Wait at least 2 years** |
| * As currently no data are available, these recommendations are mostly based on expert opinion and medication half-lives. † Only varicella and herpes zoster: no time lag if methotrexate ≤0.4 mg/kg/week or ≤20 mg/week to prevent varicella infection or reactivation. Please consult table 3 for further exceptions. ¶ According to medication half-life, 1 month is sufficient. If there is sufficient time and the clinical situation permits, wait for 3 months after interruption of methotrexate. § Owing to the short half-life (4–6 hours) of anakinra, live vaccines might be given earlier than 3 months after cessation of the therapy. But so far, only data on the safe and immunogenic administration of a second MMR vaccination in three cases after cessation of anakinra for 5 half-lives have been reported [163]. ‡ As currently no data are available, this recommendation is based on expert opinion, the half-life of rituximab and on immunogenicity studies of inactivated vaccines. The reasoning for this recommendation is that if an inactivated vaccine is capable of inducing a humoral immune response after a certain time period after rituximab administration, the immune competence will also be sufficiently restored to be able to deal with a live vaccine.CD19+ B cells should be measured at the best proxy available for B cells in the periphery. Immunoglobulin levels should be measured and vaccinations may only be administered if they are at a normal level ** For safety reasons, live vaccines are contraindicated for at least 2 years after leflunomide therapy. But there is a specific wash-out option with inactivated carbon or cholestyramine: According to Sanofi Pasteur a schedule similar to the one recommended for pregnancies under leflunomide can be followed before administration of a live vaccine: After cessation of leflunomide therapy: “washout with 8 g cholestyramine 3 times daily over 11 days or 50 g activated carbon 4 times daily over 11 days. Independent of the washout method, the determination of the plasma level of leflunomide is necessary in two tests that are at least 14 days apart. After the first test with a plasma level below 0.02 mg/l it is necessary to wait for another 1.5 months before fertilisation is possible”. | |
Generally recommended vaccinations include immunisations against tetanus, diphtheria, polio, pertussis, hepatitis B, Haemophilus influenzab, mumps, measles, rubella and varicella. All these vaccinations are also recommended for AIIRD patients in accordance with general Swiss vaccination recommendations [177].
It appears reasonable to give tetanus/diphtheria booster vaccinations every 10 years in immunocompromised persons, although this has not been studied. Regarding the live vaccines mumps, measles and rubella, the general Swiss vaccination guidelines recommend the MMR vaccinations (up to two doses 1 month apart) for unvaccinated persons, or persons who were only vaccinated once and were born after 1963.
Adults <40 years without a history of varicella infection should be vaccinated against varicella. In the case of a negative or uncertain history of varicella infection, specific serum antibodies can be measured [177].
MMR and varicella vaccination may not be administered to persons taking medication with an immunosuppressive effect (table 3); in these persons the respective serological tests should be performed in the case of a negative/uncertain disease or vaccination history.
In immunocompromised adults exposed to an increased risk (e.g. work in kindergarten or hospital), immunity against measles and rubella should also be checked in those born before 1964, and in those ≥40 years also the immunity to varicella.
Please consult sections 12, 13 and 14 for further recommendations.
Vaccinations against hepatitis A, meningococci, tick-borne encephalitis and specific travel vaccinations should be performed according to general Swiss vaccination recommendations [177, 178]. Also in this case, as vaccines may not be sufficiently immunogenic under immunosuppressive treatment, serological testing is indicated, especially after a primary vaccination course (see section 11). It has been shown that one dose of a monovalent hepatitis A vaccination may not provide protection in patients under therapy with methotrexate, TNF blocking agents or combination therapy with methotrexate and TNF inhibitors [92, 179]. More than one dose before departure may be required for protection against hepatitis A [92]. Specific recommendations apply to live vaccines (see sections 7, 8 and 12).
Owing to an increased risk of influenza and pneumococcal infections [31, 32, 34, 35] and associated complications, the pneumococcal and annual seasonal influenza vaccinationsare recommended in AIIRD patients. Use of the 13-valent pneumococcal conjugate vaccine (Prevenar®) once is recommended [180]. In opposition to EULAR and CDC recommendations [90, 181], the use of the polysaccharide vaccine is no longer recommended (please see section 15 for further details).
It could be demonstrated that the primary immune response to a pneumococcal conjugate vaccine in AIIRD patients under immunosuppressive therapy is similar to primary immune responses to the polysaccharide vaccine under therapy with non-biological DMARDs and TNFα blocking therapy [93]. Treatment with methotrexate or methotrexate in combination with TNFα blocking therapy appears to hamper the immune response to both conjugate and polysaccharide pneumococcal vaccines [94–96, 144].
Because of an increased risk of herpes zoster in AIIRD patients,herpes zoster vaccinationis recommended in those aged >50 years. At the moment, herpes zoster vaccine is not available in Switzerland. If it becomes available, the Advisory Committee on Immunisation Practices (ACIP) recommendations may be followed [182].
Hepatitis B infections can be more severe in AIIRD patients under immunosuppressive therapy [48] and thus vaccination against hepatitis B is encouraged in this patient group.
Vaccination against HPV is recommended in female patients aged 11–14 years and should especially be encouraged in patients with systemic lupus erythematosus. Vaccination can be recommended up to the age of 26 years, according to FOPH recommendations [46, 47, 177].
In immunosuppressed persons, the vaccine response should be assessed 4–6 weeks after a primary course of a vaccination. In booster vaccinations, serology is not necessary as better immune responses can be expected. The most common method for verification is the measurement of antibodies to the antigens contained in the vaccine after a complete course of vaccination (e.g. after the third vaccination against tick-borne encephalitis). For certain recommended vaccinations (diphtheria, tetanus, Haemophilus influenzae type b, hepatitis B, pneumococccal disease, tick-borne encephalitis, rabies) the protective correlates are known and the available serological tests are capable of measuring the vaccine-induced antibody responses (please refer to table 1 in the vaccination recommendations published by the Swiss Federal office of Public Health for stem cell recipients [183]). Other available serological tests are capable of measuring immune responses after a natural infection, but are less sensitive in evaluating vaccine responses (hepatitis A, measles, and rubella). However, a serological check is also recommended after these vaccinations. A positive result indicates a good immune response. A negative result after measles or rubella vaccination does not necessarily indicate vaccine failure. Also, the generally used serological methods for varicella are often not sensitive enough. More sensitive tests can be performed at “Hôpitaux Universitaires de Genève” (address at the end of the article). If serological thresholds are not reached with a generally recommended course of vaccination, additional doses may be necessary for achieving protection. In such a case, it is recommended to check the serology again 4–6 weeks after the additional vaccine dose. It has been shown that in AIIRD patients who had not responded to one H1N1 vaccine dose, better antibody titres and seroprotection rates could be achieved after a second dose [129]. Similarly, in one study, SLE patients who had not seroconverted after three doses of hepatitis B vaccine, developed positive anti-HBs antibodies after a fourth vaccine dose [153].
For pertussis and mumps vaccines, it has not been possible to define a protective threshold for vaccine antibodies. Thus, serology after these vaccines is not useful to assess whether a person is protected. For other vaccines, such as meningococci and human papillomavirus, no serology is routinely available to date. Please consult section 12 for recommendations concerning serology after yellow fever vaccination.
If a vaccine serology is not available or reliable, an alternative approach may be considered to decide whether a vaccine could prove immunogenic in a patient under immunosuppressive treatment: measuring basic immunology parameters by flow-cytometry in the peripheral blood (number of circulating CD4+, CD8+ and CD19+ cells) as well as total serum immunoglobulins, and consulting an expert.
If it is unclear whether an AIIRD patient under treatment with immunosuppressants is protected against measles or rubella (in the case of measles, independent of history and vaccination status), it is advisable to test serologically for immunity. The serology sample should be sent to a “general laboratory”. If the results are equivocal, the test should be repeated in a reference laboratory (Laboratoire de virologie, Hôpitaux Universitaires de Genève).
If it is unclear whether an AIIRD patient under treatment with immunsupressants is protected against varicella(independent of history and vaccination status), it is advisable to test serologically for immunity. The serology sample should be sent to a “general laboratory”. If the results are equivocal the test should be repeated in a reference laboratory. In this case, serum can be sent to “Laboratoire de vaccinologie des Hôpitaux Universitaires de Genève” where a more sensitive test can be performed.
If an AIIRD patient under immunosuppressive therapy intends to travel to a yellow fever endemic region and has been vaccinated against yellow fever in the past, neutralising antibody levels should be measured. The immunity against yellow fever should be checked irrespective of time point of vaccination, i.e., immunity should be checked if the vaccination was given ≤10 years ago as the immunosuppressive treatment may have reduced immunity. The immunity should also be checked if the vaccination was administered >10 years ago, as protection may last longer than 10 years and may still be present.
To attain protection for the immunocompromised person, the vaccination status of household members and close contacts should also be checked. Vaccinations should be supplemented as appropriate, especially those against mumps, measles, rubella, varicella and influenza.
If the immunocompromised person is not protected against measles and has contact with an infected person, intravenous immunoglobulins (IVIg 400 mg/kg body weight) should be considered. In the case of exposure to chicken pox or disseminated herpes zoster, an unimmunised, seronegative AIIRD patient under immunosuppression should receive varicella zoster immunoglobulin within 10 days. After the receipt of immunoglobulins, the patient should be observed for one month. In the case of signs of varicella infection, antiviral treatment (e.g. aciclovir) should be given promptly without awaiting confirmatory laboratory results [182, 184].
Conjugate vaccines should be preferred to polysaccharide vaccines for several reasons: the former induce higher affinity antibodies, longer-lasting antibody responses and memory responses, and booster vaccinations induce even higher antibody levels. Contrarily, after the administration of a polysaccharide vaccine, secondary vaccination may elicit only poor immune responses owing to a lack of memory cells. The administration of a first dose of plain polysaccharide vaccine may even blunt the immune response to subsequent doses (also to a subsequent dose of a conjugate vaccine), such that the antibody levels following a second vaccination, and possibly the magnitude of clinical protection, may be lower than following a first vaccination [180, 185].
So far, there are no data on meningococcal vaccination in adult AIIRD patients. However, it can be concluded from immunological concepts [186] that the meningococcal conjugate vaccine should also be preferred over the polysaccharide vaccine.
Only a few studies have compared immunogenicity and safety of vaccinations between AIIRD patients with stable and unstable disease. Most vaccination studies in AIIRD patients were performed in patients with stable disease.
In several studies on influenza, pandemic influenza and pneumococcal vaccine in patients with rheumatoid arthritis, spondyloarthritis, vasculitis, or connective tissue diseases, no increase in side effects or disease flares, nor decreased vaccine immunogenicity was seen when also patients with moderate or severe disease activity were included [122, 130, 131, 135, 137].
In studies on seasonal influenza, pneumococcal, tetanus toxoid and Haemophilus influenzae type b vaccines in SLE patients, and one study on hepatitis B vaccination in rheumatoid arthritis patients, the immunogenicity of the vaccines seemed to be reduced in patients with increased disease activity. This effect might also be attributed to the fact that patients with higher disease activity also received more immunosuppressive therapies [126, 140, 146, 152]. However, the numbers of patients in these studies were too small to draw a definite conclusion.
Vaccination should therefore be preferentially administered during stable disease, more because of concerns of reduced immunogenicity (due to a higher level of immunosuppressive therapy) than of vaccine-induced flares in patients with active disease. This approach will also avoid the confusion of flare-ups of the underlying disease with vaccine-induced side effects.
The recommendations for vaccination in patients with autoimmune inflammatory rheumatic diseases presented in this article have been formulated after an evaluation of the currently available data in the literature and after several rounds of consultations of experts in the fields of rheumatology, immunology, infectious diseases, vaccinology and travel medicine.
Most of the data available refer to influenza and pneumococcal vaccination; for other inactivated vaccines available evidence is still scarce. However, based on the data one can conclude that inactivated vaccines are (i) safe in AIIRD patients with or without immunosuppressive therapy and that (ii) under most immunosuppressive therapies vaccines induce good to moderate immune responses. Severely reduced immunogenicity can be found under methotrexate, methotrexate plus TNF inhibitor combination therapy, abatacept and, especially, rituximab. However, it is unclear whether the humoral immune responses (most commonly used as surrogates of protection in vaccination studies) correlate with protection. Only very few studies used actual “effectiveness” of vaccinations as an endpoint [31, 32, 187].
Few studies on live vaccination under immunosuppression could be identified, most of them examined revaccination under immunosuppressive therapy. Thus recommendations on live vaccinations were kept rather conservative.
We have attempted to give less restrictive recommendations regarding live vaccines under low-dose methotrexate therapy, backed up by the growing clinical experience with live vaccinations under this medication. Additionally, in this document, advice for specialists is given on specific approaches, which can guide the decision process of administering a live vaccine in unclear situations (section 7).
The reader of these recommendations should keep in mind that the majority of recommendations given in this article are based on clinical experience and expert opinion as scientific data are still scarce on many aspects.
More evidence regarding the immunogenicity and safety of vaccinations in AIIRD patients under various therapies is urgently needed. Vaccination recommendations need to be updated on a regular basis, as more scientific data regarding vaccination efficacy and safety, emergent infectious threats, new vaccines as well as new immunosuppressive therapies will become available.
Measles, rubella
Laboratoire de virologie
Médecine génétique et de laboratoire
– Maladies infectieuses
Université de Genève
Rue Gabrielle-Perret-Gentil 4
1211 Genève 14
Switzerland
Varicella and pneumococcal disease
Département de Pédiatrie
Centre de Vaccinologie et d'Immunologie Néonatale
Université de Genève, C.M.U.
1 rue Michel-Servet
1211 Genève 4
Switzerland
Yellow fever
Zentrum für Labormedizin
Frohbergstrasse 3
9001 St.Gallen
Switzerland
Blood samples for yellow fever neutralisation assays will be forwarded to the Robert Koch Institut, Berlin, Germany
Clinical Microbiology Laboratory
(Contact person: L. G. Visser)
Leiden University Medical Centre Albinusdreef 2
Leiden 2333 ZA
The Netherlands
Phone: +31 71 526 2613
Fax: +31 71 526 6758
https://www.lumc.nl/sub/2010/att/14050105241357/14050105321157.pdf
Acknowledgments:We would like to thank François Spertini, Christoph Berger, Martine Bouvier-Gallacchi, Catherine Bourquin, Pierre Landry for sharing their experiences and inputs and for their invaluable input during the development of these recommendations.
1 Centers for Disease Control and Prevention. Immunocompromised Travelers – Chapter 8 – 2014 Yellow Book | Travelers’ Health | CDC. Centers for Disease Control and Prevention; 2014.
2 van Assen S, Agmon-Levin N, Elkayam O, Cervera R, Doran MF, Dougados M, et al. EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2011;70:414–22.
3 Rubin LG, Levin MJ, Ljungman P, Davies EG, Avery R, Tomblyn M, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58:e44–e100.
4 Papadopoulou D, Sipsas NV. Comparison of national clinical practice guidelines and recommendations on vaccination of adult patients with autoimmune rheumatic diseases. Rheumatol Int. 2014;34:151–63.
5 Goeb V, Ardizzone M, Arnaud L, Avouac J, Baillet A, Belot A, et al. Recommendations for using TNFalpha antagonists and French Clinical Practice Guidelines endorsed by the French National Authority for Health. Joint, bone, spine: revue du rhumatisme 2013;80:574–81.
6 Schweizerische Gesellschaft für R. Impfempfehlungen für Patienten mit entzündlich-rheumatischen Erkrankungen. 2010.
7 Gompel FV, Peetermans W, Laethem YV, Callens S, Malfroot A, Levy J, et al. Advisory Report Superior Health Council (n° 8561) regarding the vaccination of immunocompromised children and adults with a chronic illness. German.
8 Repo H, Peltomaa R, Finnish Rheumatology A. Vaccination of adults with inflammatory rheumatic diseases. 2012.
9 Bombardier C, Hazlewood GS, Akhavan P, Schieir O, Dooley A, Haraoui B, et al. Canadian Rheumatology Association recommendations for the pharmacological management of rheumatoid arthritis with traditional and biologic disease-modifying antirheumatic drugs: part II safety. J Rheumatol. 2012;39:1583–602.
10 Zink A, Minden K, List SM. Entzündlich-rheumatische Erkrankungen. Berlin, Germany 2010. German.
11 Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59:762–84.
12 Public Health Agency of C. Recommended Immunizations. 2013.
13 Bundesamt für Gesundheit, Eidenössische Kommission für Impffragen. Impfprinzipien und Empfehlungen für Personen mit autoimmun-entzündlichen rheumatischen Erkrankungen. 2014:159–61. German.
14 Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical research ed) 2008;336:924–6.
15 Hugle T, Bircher A, Walker UA. Streptococcal hypersensitivity reloaded: severe inflammatory syndrome in Behcet’s disease following 23-valent polysaccharide Streptococcus pneumoniae vaccine. Rheumatology (Oxford). 2012;51:761–2.
16 Bijl M, Kallenberg CG, van Assen S. Vaccination of the immune-compromised patients with focus on patients with autoimmune-inflammatory diseases. Neth J Med. 2011;69:5–13.
17 Fauci ASLCA. Harrison’s Rheumatology. Philadelphia, USA: Lippincott Williams and Wilins; 2006.
18 Koetz K, Bryl E, Spickschen K, O’Fallon WM, Goronzy JJ, Weyand CM. T cell homeostasis in patients with rheumatoid arthritis. Proc Natl Acad Sci U S A. 2000;97:9203–8.
19 Wagner UG, Koetz K, Weyand CM, Goronzy JJ. Perturbation of the T cell repertoire in rheumatoid arthritis. Proc Natl Acad Sci U S A. 1998;95:14447–52.
20 Gluck T, Kiefmann B, Grohmann M, Falk W, Straub RH, Scholmerich J. Immune status and risk for infection in patients receiving chronic immunosuppressive therapy. J Rheumatol. 2005;32:1473–80.
21 Bernatsky S, Hudson M, Suissa S. Anti-rheumatic drug use and risk of serious infections in rheumatoid arthritis. Rheumatology (Oxford). 2007;46:1157–60.
22 Grijalva CG, Kaltenbach L, Arbogast PG, Mitchel EF, Jr., Griffin MR. Initiation of rheumatoid arthritis treatments and the risk of serious infections. Rheumatology (Oxford). 2010;49:82–90.
23 Strangfeld A, Eveslage M, Schneider M, Bergerhausen HJ, Klopsch T, Zink A, et al. Treatment benefit or survival of the fittest: what drives the time-dependent decrease in serious infection rates under TNF inhibition and what does this imply for the individual patient? Ann Rheum Dis. 2011;70:1914–20.
24 Dixon WG, Kezouh A, Bernatsky S, Suissa S. The influence of systemic glucocorticoid therapy upon the risk of non-serious infection in older patients with rheumatoid arthritis: a nested case-control study. Ann Rheum Dis. 2011;70:956–60.
25 Greenberg JD, Reed G, Kremer JM, Tindall E, Kavanaugh A, Zheng C, et al. Association of methotrexate and tumour necrosis factor antagonists with risk of infectious outcomes including opportunistic infections in the CORRONA registry. Ann Rheum Dis. 2010;69:380–6.
26 Greene SK, Rett M, Weintraub ES, Li L, Yin R, Amato AA, et al. Risk of confirmed Guillain-Barre syndrome following receipt of monovalent inactivated influenza A (H1N1) and seasonal influenza vaccines in the Vaccine Safety Datalink Project, 2009–2010. Am J Epidemiol. 2012;175:1100–9.
27 Galloway JB, Hyrich KL, Mercer LK, Dixon WG, Ustianowski AP, Helbert M, et al. Risk of septic arthritis in patients with rheumatoid arthritis and the effect of anti-TNF therapy: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2011;70:1810–4.
28 Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46:2287–93.
29 Kinder A, Stephens S, Mortimer N, Sheldon P. Severe herpes zoster after infliximab infusion. Postgrad Med J. 2004;80:26–.
30 Takahashi E, Kurosaka D, Yoshida K, Yanagimachi M, Kingetsu I, Yamada A. Onset of modified measles after etanercept treatment in rheumatoid arthritis. Nihon Rinsho Meneki Gakkai Kaishi. 2010;33:37–41.
31 Hak E, Nordin J, Wei F, Mullooly J, Poblete S, Strikas R, et al. Influence of high-risk medical conditions on the effectiveness of influenza vaccination among elderly members of 3 large managed-care organizations. Clin Infect Dis. 2002;35:370–7.
32 Nichol KL, Wuorenma J, von Sternberg T. Benefits of influenza vaccination for low-, intermediate-, and high-risk senior citizens. Arch Intern Med. 1998;158:1769–76.
33 Van Kerkhove MD, Vandemaele KA, Shinde V, Jaramillo-Gutierrez G, Koukounari A, Donnelly CA, et al. Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Med. 2011;8:e1001053.
34 Naveau C, Houssiau FA. Pneumococcal sepsis in patients with systemic lupus erythematosus. Lupus. 2005;14:903–6.
35 Yee AM, Ng SC, Sobel RE, Salmon JE. Fc gammaRIIA polymorphism as a risk factor for invasive pneumococcal infections in systemic lupus erythematosus. Arthritis Rheum. 1997;40:1180–2.
36 Chakravarty E, Michaud K, Katz R, Wolfe F. Increased incidence of herpes zoster among patients with systemic lupus erythematosus. Lupus. 2013;22:238–44.
37 Wolfe F, Michaud K, Chakravarty EF. Rates and predictors of herpes zoster in patients with rheumatoid arthritis and non-inflammatory musculoskeletal disorders. Rheumatology (Oxford). 2006;45:1370–5.
38 Strangfeld A, Listing J, Herzer P, Liebhaber A, Rockwitz K, Richter C, et al. Risk of herpes zoster in patients with rheumatoid arthritis treated with anti-TNF-alpha agents. JAMA. 2009;301:737–44.
39 Smitten AL, Choi HK, Hochberg MC, Suissa S, Simon TA, Testa MA, et al. The risk of herpes zoster in patients with rheumatoid arthritis in the United States and the United Kingdom. Arthritis Rheum. 2007;57:1431–8.
40 Manzi S, Kuller LH, Kutzer J, Pazin GJ, Sinacore J, Medsger TA, et al. Herpes zoster in systemic lupus erythematosus. J Rheumatol. 1995;22:1254–8.
41 Seong SS, Choi CB, Woo JH, Bae KW, Joung CL, Uhm WS, et al. Incidence of tuberculosis in Korean patients with rheumatoid arthritis (RA): effects of RA itself and of tumor necrosis factor blockers. J Rheumatol. 2007;34:706–11.
42 Brassard P, Lowe AM, Bernatsky S, Kezouh A, Suissa S. Rheumatoid arthritis, its treatments, and the risk of tuberculosis in Quebec, Canada. Arthritis Rheum. 2009;61:300–4.
43 Askling J, Fored CM, Brandt L, Baecklund E, Bertilsson L, Coster L, et al. Risk and case characteristics of tuberculosis in rheumatoid arthritis associated with tumor necrosis factor antagonists in Sweden. Arthritis Rheum. 2005;52:1986–92.
44 Tam LS, Li EK, Wong SM, Szeto CC. Risk factors and clinical features for tuberculosis among patients with systemic lupus erythematosus in Hong Kong. Scand J Rheumatol. 2002;31:296–300.
45 Yun JE, Lee SW, Kim TH, Jun JB, Jung S, Bae SC, et al. The incidence and clinical characteristics of Mycobacterium tuberculosis infection among systemic lupus erythematosus and rheumatoid arthritis patients in Korea. Clin Exp Rheumatol. 2002;20:127–32.
46 Santana IU, Gomes Ado N, Lyrio LD, Rios Grassi MF, Santiago MB. Systemic lupus erythematosus, human papillomavirus infection, cervical pre-malignant and malignant lesions: a systematic review. Clin Rheumatol. 2011;30:665–72.
47 Tam L-S, Chan PKS, Ho SC, Yu MMY, Yim S-F, Cheung T-H, et al. Natural history of cervical papilloma virus infection in systemic lupus erythematosus – a prospective cohort study. J Rheumatol. 2010;37:330–40.
48 Tanaka E, Urata Y. Risk of hepatitis B reactivation in patients treated with tumor necrosis factor-alpha inhibitors. Hepatol Res. 2012;42:333–9.
49 Wraith DC, Goldman M, Lambert P-H. Vaccination and autoimmune disease: what is the evidence? Lancet. 2003;362:1659–66.
50 Salemi S, D’Amelio R. Could Autoimmunity Be Induced by Vaccination? Int Rev Immunol. 2010;29:247–69.
51 Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, Keenlyside RA, Ziegler DW, Retailliau HF, et al. Guillain-Barre syndrome following vaccination in the National Influenza Immunization Program, United States, 1976–1977. Am J Epidemiol. 1979;110:105–23.
52 DeStefano F, Vellozzi C, Schonberger LB, Chen RT. Safety of adjuvanted pandemic influenza A (H1N1) 2009 vaccines. BMJ. (Clinical research ed) 2011;343:d4159-d.
53 Dieleman J, Romio S, Johansen K, Weibel D, Bonhoeffer J, Sturkenboom M. Guillain-Barre syndrome and adjuvanted pandemic influenza A (H1N1) 2009 vaccine: multinational case-control study in Europe. BMJ. (Clinical research ed) 2011;343:d3908-d.
54 Salmon DA, Proschan M, Forshee R, Gargiullo P, Bleser W, Burwen DR, et al. Association between Guillain-Barré syndrome and influenza A (H1N1) 2009 monovalent inactivated vaccines in the USA: a meta-analysis. The Lancet. 2013;381:1461–8.
55 Tokars JI, Lewis P, DeStefano F, Wise M, Viray M, Morgan O, et al. The risk of Guillain-Barré syndrome associated with influenza A (H1N1) 2009 monovalent vaccine and 2009–2010 seasonal influenza vaccines: results from self-controlled analyses. Pharmacoepidemiol Drug Saf. 2012;21:546–52.
56 Vellozzi C, Broder KR, Haber P, Guh A, Nguyen M, Cano M, et al. Adverse events following influenza A (H1N1) 2009 monovalent vaccines reported to the Vaccine Adverse Event Reporting System, United States, October 1, 2009-January 31, 2010. Vaccine. 2010;28:7248–55.
57 Vellozzi C, Iqbal S, Broder K. Guillain-Barre syndrome, influenza, and influenza vaccination: the epidemiologic evidence. Clin Infect Dis. 2014;58:1149–55.
58 Williams SE, Pahud BA, Vellozzi C, Donofrio PD, Dekker CL, Halsey N, et al. Causality assessment of serious neurologic adverse events following 2009 H1N1 vaccination. Vaccine. 2011;29:8302–8.
59 Wise ME, Viray M, Sejvar JJ, Lewis P, Baughman AL, Connor W, et al. Guillain-Barre syndrome during the 2009-2010 H1N1 influenza vaccination campaign: population-based surveillance among 45 million Americans. Am J Epidemiol. 2012;175:1110–9.
60 Yih WK, Lee GM, Lieu TA, Ball R, Kulldorff M, Rett M, et al. Surveillance for adverse events following receipt of pandemic 2009 H1N1 vaccine in the Post-Licensure Rapid Immunization Safety Monitoring (PRISM) System, 2009–2010. Am J Epidemiol. 2012;175:1120–8.
61 Courrier A, Simonnet PH, Lopez D, Scherer CL, Stenbach G, Rumilly P, et al. Peripheral neuropathy following fetal bovine cell rabies vaccine. Lancet. 1986;327:1273–.
62 Siddiqui A, Usmani RI, Anwer S, Afsar S. Guillain-Barre syndrome occurring after rabies vaccination J Pak Med Assoc. 2005;55:87–8.
63 Andrews N, Stowe J, Miller E, Svanstr√∂m H, Johansen K, Bonhoeffer J, et al. A collaborative approach to investigating the risk of thrombocytopenic purpura after measles-mumps-rubella vaccination in England and Denmark. Vaccine. 2012;30:3042–6.
64 Miller E, Waight P, Farrington CP, Andrews N, Stowe J, Taylor B. Idiopathic thrombocytopenic purpura and MMR vaccine. Arch Dis Child. 2001;84:227–9.
65 Nieminen U, Peltola H, Syrjälä MT, Mäkipernaa A, Kekomäki R. Acute thrombocytopenic purpura following measles, mumps and rubella vaccination. A report on 23 patients. Acta Paediatr. 1993;82:267–70.
66 Stowe J, Kafatos G, Andrews N, Miller E. Idiopathic thrombocytopenic purpura and the second dose of MMR. Arch Dis Child. 2008;93:182–3.
67 Mantadakis E, Farmaki E, Buchanan GR. Thrombocytopenic purpura after measles-mumps-rubella vaccination: a systematic review of the literature and guidance for management. J Pediatr. 2010;156:623–8.
68 Cruz-Tapias P, Agmon-Levin N, Israeli E, Anaya JM, Shoenfeld Y. Autoimmune (auto-inflammatory) syndrome induced by adjuvants (ASIA) – animal models as a proof of concept. Curr Med Chem. 2013;20:4030–6.
69 Perricone C, Colafrancesco S, Mazor RD, Soriano A, Agmon-Levin N, Shoenfeld Y. Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) 2013: Unveiling the pathogenic, clinical and diagnostic aspects. J Autoimm. 2013;47:1–16.
70 Agmon-Levin N, Arango MT, Kivity S, Katzav A, Gilburd B, Blank M, et al. Immunization with hepatitis B vaccine accelerates SLE-like disease in a murine model. J Autoimmun. 2014;54:21–32.
71 Israeli E, Agmon-Levin N, Blank M, Shoenfeld Y. Adjuvants and autoimmunity. Lupus. 2009;18:1217–25.
72 WHO | Questions and Answers about macrophagic myofasciitis (MMF). World Health Organization.
73 CDC – Veterans Health – Gulf War Studies – Defining Gulf War Illness.
74 Nohynek H, Jokinen J, Partinen M, Vaarala O, Kirjavainen T, Sundman J, et al. AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PloS one 2012;7:e33536-e.
75 Partinen M, Kornum BR, Plazzi G, Jennum P, Julkunen I, Vaarala O. Narcolepsy as an autoimmune disease: the role of H1N1 infection and vaccination. Lancet Neurol. 2014;13:600–13.
76 Stowe J, Andrews N, Wise L, Miller E. Investigation of the temporal association of Guillain-Barre syndrome with influenza vaccine and influenzalike illness using the United Kingdom General Practice Research Database. Am J Epidemiol. 2009;169:382–8.
77 Poland GA, Jacobsen SJ. Influenza vaccine, Guillain-Barré syndrome, and chasing zero. Vaccine. 2012;30:5801–3.
78 Rahier J-F, Moutschen M, Van Gompel A, Van Ranst M, Louis E, Segaert S, et al. Vaccinations in patients with immune-mediated inflammatory diseases. Rheumatology (Oxford). 2010;49:1815–27.
79 van Assen S, Elkayam O, Agmon-Levin N, Cervera R, Doran MF, Dougados M, et al. Vaccination in adult patients with auto-immune inflammatory rheumatic diseases: a systematic literature review for the European League Against Rheumatism evidence-based recommendations for vaccination in adult patients with auto-immune inflammatory rheuma. Autoimmun Rev. 2011;10:341–52.
80 Bengtsson C, Kapetanovic MC, Källberg H, Sverdrup B, Nordmark B, Klareskog L, et al. Common vaccinations among adults do not increase the risk of developing rheumatoid arthritis: results from the Swedish EIRA study. Ann Rheum Dis. 2010;69:1831–3.
81 Salemi S, D’Amelio R. Are anti-infectious vaccinations safe and effective in patients with autoimmunity? Int Rev Immunol. 2010;29:270–314.
82 Bijl M, Agmon-Levin N, Dayer JM, Israeli E, Gatto M, Shoenfeld Y. Vaccination of patients with auto-immune inflammatory rheumatic diseases requires careful benefit-risk assessment. Autoimmun Rev. 2012;11:572–6.
83 Mok CC, Ho LY, Fong LS, To CH. Immunogenicity and safety of a quadrivalent human papillomavirus vaccine in patients with systemic lupus erythematosus: a case-control study. Ann Rheum Dis. 2012.
84 Pappaioanou M, Fishbein DB, Dreesen DW, Schwartz IK, Campbell GH, Sumner JW, et al. Antibody response to preexposure human diploid-cell rabies vaccine given concurrently with chloroquine. N Engl J Med. 1986;314:280–4.
85 Prymula R, Siegrist C-A, Chlibek R, Zemlickova H, Vackova M, Smetana J, et al. Effect of prophylactic paracetamol administration at time of vaccination on febrile reactions and antibody responses in children: two open-label, randomised controlled trials. Lancet. 2009;374:1339–50.
86 Visser LG. TNF-alpha Antagonists and Immunization. Curr Infect Dis Rep. 2011;13:243–7.
87 Visser LG. The immunosuppressed traveler. Infect Dis Clin North Am. 2012;26:609–24.
88 Denman EJ, Denman AM, Greenwood BM, Gall D, Heath RB. Failure of cytotoxic drugs to suppress immune responses of patients with rheumatoid arthritis. Ann Rheum Dis. 1970;29:220–31.
89 Bingham 3rd CO, Looney RJ, Deodhar A, Halsey N, Greenwald M, Codding C, et al. Immunization responses in rheumatoid arthritis patients treated with rituximab: results from a controlled clinical trial. Arthritis Rheum. 2010;62:64–74.
90 van Assen S, Bijl M. Immunization of patients with autoimmune inflammatory rheumatic diseases (the EULAR recommendations). Lupus. 2012;21:162–7.
91 Eidgenössische Kommission für Impffragen, Bühler S, Hatz C. Vaccination in patients with autoimmune inflammatory rheumatic diseases (AIIRD) – Background document Eidgenössische Kommission für Impffragen; 2014.
92 Askling HH, Rombo L, van Vollenhoven R, Hallén I, Thörner Å, Nordin M, et al. Hepatitis A vaccine for immunosuppressed patients with rheumatoid arthritis: A prospective, open-label, multi-centre study. Travel Med Infect Dis. 2014;12:134–42.
93 Kapetanovic MC, Roseman C, Jonsson G, Truedsson L. Heptavalent pneumococcal conjugate vaccine elicits similar antibody response as standard 23-valent polysaccharide vaccine in adult patients with RA treated with immunomodulating drugs. Clin Rheumatol. 2011;30:1555–61.
94 Kapetanovic MC, Saxne T, Sjoholm A, Truedsson L, Jonsson G, Geborek P. Influence of methotrexate, TNF blockers and prednisolone on antibody responses to pneumococcal polysaccharide vaccine in patients with rheumatoid arthritis. Rheumatology (Oxford). 2006;45:106–11.
95 Visvanathan S, Keenan GF, Baker DG, Levinson AI, Wagner CL. Response to pneumococcal vaccine in patients with early rheumatoid arthritis receiving infliximab plus methotrexate or methotrexate alone. J Rheumatol. 2007;34:952–7.
96 Gelinck LB, van der Bijl AE, Visser LG, Huizinga TW, van Hogezand RA, Rijkers GT, et al. Synergistic immunosuppressive effect of anti-TNF combined with methotrexate on antibody responses to the 23 valent pneumococcal polysaccharide vaccine. Vaccine. 2008;26:3528–33.
97 Kivitz AJ, Schechtman J, Texter M, Fichtner A, de Longueville M, Chartash EK. Vaccine responses in patients with rheumatoid arthritis treated with certolizumab pegol: results from a single-blind randomized phase IV trial. J Rheumatol. 2014;41:648–57.
98 Elkayam O, Bashkin A, Mandelboim M, Litinsky I, Comaheshter D, Levartovsky D, et al. The effect of infliximab and timing of vaccination on the humoral response to influenza vaccination in patients with rheumatoid arthritis and ankylosing spondylitis. Semin Arthritis Rheum. 2010;39:442–7.
99 Kaine JL, Kivitz AJ, Birbara C, Luo AY. Immune responses following administration of influenza and pneumococcal vaccines to patients with rheumatoid arthritis receiving adalimumab. J Rheumatol. 2007;34:272–9.
100 Kubota T, Nii T, Nanki T, Kohsaka H, Harigai M, Komano Y, et al. Anti-tumor necrosis factor therapy does not diminish the immune response to influenza vaccine in Japanese patients with rheumatoid arthritis. Mod Rheumatol. 2007;17:531–3.
101 Fomin I, Caspi D, Levy V, Varsano N, Shalev Y, Paran D, et al. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNF alpha blockers. Ann Rheum Dis. 2006;65:191–4.
102 Ribeiro AC, Laurindo IM, Guedes LK, Saad CG, Moraes JC, Silva CvA, et al. Abatacept severely reduces the immune response to pandemic 2009 influenza A/H1N1 vaccination in patients with rheumatoid arthritis. Arthritis care & research 2012;65:476–80.
103 Adler S, Krivine A, Weix J, Rozenberg F, Launay O, Huesler J, et al. Protective effect of A/H1N1 vaccination in immune-mediated disease – a prospectively controlled vaccination study. Rheumatology (Oxford). 2012;51:695–700.
104 Tay L, Leon F, Vratsanos G, Raymond R, Corbo M. Vaccination response to tetanus toxoid and 23-valent pneumococcal vaccines following administration of a single dose of abatacept: a randomized, open-label, parallel group study in healthy subjects. Arthritis Res Therapy. 2007;9:R38.
105 Schiff M, Kaell A, Vratsanos G, Bahrt K. Response to pneumococcal vaccine in rheumatoid arthritis patients with an inadequate response to anti-TNF therapy treated with abatacept in the ARRIVE trial. Ann Rheum Dis. 2007;66(S11):437.
106 Schiff M, Saewert M, Bahrt K, Genovese MC. Response to influenza vaccine in rheumatoid arthritis patients with an inadequate response to anti-TNF therapy treated with abatacept in the ARRIVE trial. Arthritis Rheum. 2007;56:S392.
107 Kapetanovic MC, Roseman C, Jönsson G, et al. Rituximab and Methotrexate but Not TNF-Blockers Are Associated with Impaired Antibody Response Following Pneumococcal Vaccination Using 7-Valent Conjugate Vaccine (Prevenar®) In Patients with Established Rheumatoid Arthritis. Ann Rheum Dis. London, UK2011.
108 Tsuru T, Terao K, Murakami M, Matsutani T, Suzaki M, Amamoto T, et al. Immune response to influenza vaccine and pneumococcal polysaccharide vaccine under IL-6 signal inhibition therapy with tocilizumab. Modern rheumatology / The Japan Rheumatism Association 2013;24:511–6.
109 Bingham CO, Rizzo W, Kivitz A, Hassanali A, Upmanyu R, Klearman M. Humoral immune response to vaccines in patients with rheumatoid arthritis treated with tocilizumab: results of a randomised controlled trial (VISARA). Ann Rheum Dis. 2014;doi: 10.1136/annrheumdis-2013-204427.
110 Mori S, Ueki Y, Hirakata N, Oribe M, Hidaka T, Oishi K. Impact of tocilizumab therapy on antibody response to influenza vaccine in patients with rheumatoid arthritis. Ann Rheum Dis. 2012.
111 Mori S, Ueki Y, Akeda Y, Hirakata N, Oribe M, Shiohira Y, et al. Pneumococcal polysaccharide vaccination in rheumatoid arthritis patients receiving tocilizumab therapy. Ann Rheum Dis. 2013;72:1362–6.
112 Hua C, Barnetche T, Combe B, Morel J. Effect of methotrexate, anti-tumor necrosis factor α, and rituximab on the immune response to influenza and pneumococcal vaccines in patients with rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res. 2013;66:1016–26.
113 van Assen S, Holvast A, Benne CA, Posthumus MD, van Leeuwen MA, Voskuyl AE, et al. Humoral responses after influenza vaccination are severely reduced in patients with rheumatoid arthritis treated with rituximab. Arthritis Rheum. 2010;62:75–81.
114 Chalmers A, Scheifele D, Patterson C, Williams D, Weber J, Shuckett R, et al. Immunization of patients with rheumatoid arthritis against influenza: a study of vaccine safety and immunogenicity. J Rheumatol. 1994;21:1203–6.
115 Del Porto F, Lagana B, Biselli R, Donatelli I, Campitelli L, Nisini R, et al. Influenza vaccine administration in patients with systemic lupus erythematosus and rheumatoid arthritis. Safety and immunogenicity. Vaccine. 2006;24:3217–23.
116 Gelinck LB, van der Bijl AE, Beyer WE, Visser LG, Huizinga TW, van Hogezand RA, et al. The effect of anti-tumour necrosis factor alpha treatment on the antibody response to influenza vaccination. Ann Rheum Dis. 2008;67:713–6.
117 Kobie JJ, Zheng B, Bryk P, Barnes M, Ritchlin CT, Tabechian DA, et al. Decreased influenza-specific B cell responses in rheumatoid arthritis patients treated with anti-tumor necrosis factor. Arthritis Res Ther. 2011;13:R209-R.
118 Oren S, Mandelboim M, Braun-Moscovici Y, Paran D, Ablin J, Litinsky I, et al. Vaccination against influenza in patients with rheumatoid arthritis: the effect of rituximab on the humoral response. Ann Rheum Dis. 2008;67:937–41.
119 Kapetanovic MC, Saxne T, Nilsson JA, Geborek P. Influenza vaccination as model for testing immune modulation induced by anti-TNF and methotrexate therapy in rheumatoid arthritis patients. Rheumatology (Oxford). 2007;46:608–11.
120 Nii T, Kubota T, Nanki T, Komano Y, Harigai M, Kohsaka H, et al. Reevaluation of antibody titers 1 year after influenza vaccination in patients with rheumatoid arthritis receiving TNF blockers. Mod Rheumatol. 2009;19:216–8.
121 Gelinck LB, Teng YK, Rimmelzwaan GF, van den Bemt BJ, Kroon FP, van Laar JM. Poor serological responses upon influenza vaccination in patients with rheumatoid arthritis treated with rituximab. Ann Rheum Dis. 2007;66:1402–3.
122 Mercado U, Acosta H, Avendano L. Influenza vaccination of patients with systemic lupus erythematosus. Rev Invest Clin. 2004;56:16–20.
123 Kostianovsky A, Charles P, Alves JF, Goulet M, Pagnoux C, Le Guern V, et al. Immunogenicity and safety of seasonal and 2009 pandemic A/H1N1 influenza vaccines for patients with autoimmune diseases: a prospective, monocentre trial on 199 patients. Clin Exp Rheumatol. 2012;30:S83–9.
124 Holvast A, Huckriede A, Wilschut J, Horst G, De Vries JJ, Benne CA, et al. Safety and efficacy of influenza vaccination in systemic lupus erythematosus patients with quiescent disease. Ann Rheum Dis. 2006;65:913–8.
125 Wiesik-Szewczyk E, Romanowska M, Mielnik P, Chwalinska-Sadowska H, Brydak LB, Olesinska M, et al. Anti-influenza vaccination in systemic lupus erythematosus patients: an analysis of specific humoral response and vaccination safety. Clin Rheumatol. 2010;29:605–13.
126 Crowe SR, Merrill JT, Vista ES, Dedeke AB, Thompson DM, Stewart S, et al. Influenza vaccination responses in human systemic lupus erythematosus: impact of clinical and demographic features. Arthritis Rheum. 2011;63:2396–406.
127 Holvast A, Stegeman CA, Benne CA, Huckriede A, Wilschut JC, Palache AM, et al. Wegener’s granulomatosis patients show an adequate antibody response to influenza vaccination. Ann Rheum Dis. 2009;68:873–8.
128 Zycinska K, Romanowska M, Nowak I, Rybicka K, Wardyn KA, Brydak LB. Antibody response to inactivated subunit influenza vaccine in patients with Wegener's granulomatosis. J Physiol Pharmacol. 2007;58(Suppl 5):819–28.
129 Gabay C, Bel M, Combescure C, Ribi C, Meier S, Posfay-Barbe K, et al. Impact of synthetic and biologic disease-modifying antirheumatic drugs on antibody responses to the AS03-adjuvanted pandemic influenza vaccine: a prospective, open-label, parallel-cohort, single-center study. Arthritis Rheum. 2011;63:1486–96.
130 Saad CG, Borba EF, Aikawa NE, Silva CA, Pereira RM, Calich AL, et al. Immunogenicity and safety of he 2009 non-adjuvanted influenza A/H1N1 vaccine in a large cohort of autoimmune rheumatic diseases. Ann Rheum Dis. 2011;70:1068–73.
131 Ribeiro AC, Guedes LK, Moraes JC, Saad CG, Aikawa NE, Calich AL, et al. Reduced seroprotection after pandemic H1N1 influenza adjuvant-free vaccination in patients with rheumatoid arthritis: implications for clinical practice. Ann Rheum Dis. 2011;70:2144–7.
132 Iwamoto M, Homma S, Onishi S, Kamata Y, Nagatani K, Yamagata Z, et al. Low level of seroconversion after a novel influenza A/H1N1/2009 vaccination in Japanese patients with rheumatoid arthritis in the 2009 season. Rheumatol Int. 2011;32:3691–4.
133 França ILA, Ribeiro ACM, Aikawa NE, Saad CGßS, Moraes JCB, Goldstein-Schainberg Cu, et al. TNF blockers show distinct patterns of immune response to the pandemic influenza A H1N1 vaccine in inflammatory arthritis patients. Rheumatology (Oxford). 2012;51:2091–8.
134 Borba EF, Saad CGS, Pasoto SG, Calich ALG, Aikawa NE, Ribeiro ACM, et al. Influenza A/H1N1 vaccination of patients with SLE: can antimalarial drugs restore diminished response under immunosuppressive therapy? Rheumatology (Oxford,). 2012;51:1061–9.
135 Mathian A, Devilliers H, Krivine A, Costedoat-Chalumeau N, Haroche J, Huong DB-LT, et al. Factors influencing the efficacy of two injections of a pandemic 2009 influenza A (H1N1) nonadjuvanted vaccine in systemic lupus erythematosus. Arthritis Rheum. 2011;63:3502–11.
136 Kapetanovic MC, Kristensen L-E, Saxne T, Aktas T, Mörner A, Geborek P. Impact of anti-rheumatic treatment on immunogenicity of pandemic H1N1 influenza vaccine in patients with arthritis. Arthritis Res Ther. 2014;16:R2-R.
137 Elkayam O, Paran D, Caspi D, Litinsky I, Yaron M, Charboneau D, et al. Immunogenicity and safety of pneumococcal vaccination in patients with rheumatoid arthritis or systemic lupus erythematosus. Clin Infect Dis. 2002;34:147–53.
138 Elkayam O, Caspi D, Reitblatt T, Charboneau D, Rubins JB. The effect of tumor necrosis factor blockade on the response to pneumococcal vaccination in patients with rheumatoid arthritis and ankylosing spondylitis. Semin Arthritis Rheum. 2004;33:283–8.
139 Jarrett MP, Schiffman G, Barland P, Grayzel AI. Impaired response to pneumococcal vaccine in systemic lupus erythematosus. Arthritis Rheum. 1980;23:1287–93.
140 Battafarano DF, Battafarano NJ, Larsen L, Dyer PD, Older SA, Muehlbauer S, et al. Antigen-specific antibody responses in lupus patients following immunization. Arthritis Rheum. 1998;41:1828–34.
141 Lipnick RN, Karsh J, Stahl NI, Blackwelder WC, Schiffman G, Klippel JH. Pneumococcal immunization in patients with systemic lupus erythematosus treated with immunosuppressives. J Rheumatol. 1985;12:1118–21.
142 Mease PJ, Ritchlin CT, Martin RW, Gottlieb AB, Baumgartner SW, Burge DJ, et al. Pneumococcal vaccine response in psoriatic arthritis patients during treatment with etanercept. J Rheumatol. 2004;31:1356–61.
143 Franco Salinas G, De Rycke L, Barendregt B, Paramarta JE, Hreggvidstdottir H, Cantaert T, et al. Anti-TNF treatment blocks the induction of T cell-dependent humoral responses. Ann Rheum Dis. 2013;72:1037–43.
144 Kapetanovic MC, Roseman C, Jonsson G, Truedsson L, Saxne T, Geborek P. Antibody response is reduced following vaccination with 7-valent conjugate pneumococcal vaccine in adult methotrexate-treated patients with established arthritis, but not those treated with tumor necrosis factor inhibitors. Arthritis Rheum. 2011;63:3723–32.
145 Kapetanovic MC, Saxne T, Jönsson G, Truedsson L, Geborek P. Rituximab and abatacept but not tocilizumab impair antibody response to pneumococcal conjugate vaccine in patients with rheumatoid arthritis. Arthritis Res Ther. 2013;15:R171-R.
146 Kapetanovic MC, Saxne T, Jönsson G, Truedsson L, Geborek P. Rituximab and abatacept but not tocilizum... Arthritis Res Ther. 2013 – PubMed – NCBI. 2013. •••Please provide full reference•••
147 Salinas GF, De Rycke L, et al. TNF Alpha Impairs Humoral T Cell Dependent Antibody Responses. ACR/ARHP Annual Scientific Meeting. Philadelphia 2009.
148 Devey ME, Bleasdale K, Isenberg DA. Antibody affinity and IgG subclass of responses to tetanus toxoid in patients with rheumatoid arthritis and systemic lupus erythematosus. Clin Exp Immunol. 1987;68:562–9.
149 Nies K, Boyer R, Stevens R, Louie J. Anti-tetanus toxoid antibody synthesis after booster immunization in systemic lupus erythematosus. Comparison of the in vitro and in vivo responses. Arthritis Rheum. 1980;23:1343–50.
150 Ter Warbeek H, Dukers-Muijrers N, Hoebe C. A Study on the Effectiveness of Hepatitis A Vaccination in Travelers with Immunosuppressive Medication. CISTM11. Budapest, Hungary 2009.
151 van den Bijllaardt W, Siers HM, Timmerman-Kok C, Pessers FG, Natrop G, van Baars JF, et al. Seroprotection after hepatitis a vaccination in patients with drug-induced immunosuppression. J Travel Med. 20:278–82.
152 Elkayam O, Yaron M, Caspi D. Safety and efficacy of vaccination against hepatitis B in patients with rheumatoid arthritis. Ann Rheum Dis. 2002;61:623–5.
153 Kuruma KA, Borba EF, Lopes MH, de Carvalho JF, Bonfa E. Safety and efficacy of hepatitis B vaccine in systemic lupus erythematosus. Lupus. 2007;16:350–4.
154 Erkek E, Ayaslioglu E, Erkek AB, Kurtipek GS, Bagci Y. Response to vaccination against hepatitis B in patients with Behcet’s disease. J Gastroenterol Hepatol. 2005;20:1508–11.
155 Levitsky J, Te HS, Faust TW, Cohen SM. Varicella infection following varicella vaccination in a liver transplant recipient. Am J Transplant. 2002;2:880–2.
156 Kraft JN, Shaw JC. Varicella infection caused by Oka strain vaccine in a heart transplant recipient. Arch Dermatol. 2006;142:943–5.
157 Plotkin SA, Orenstein WA, Offit PA. Vaccines. 6th ed: Elsevier Saunders; 2012.
158 Schalk JAC, de Vries CGJCA, Jongen PMJM. Potency estimation of measles, mumps and rubella trivalent vaccines with quantitative PCR infectivity assay. Biologicals. 2005;33:71–9.
159 Schalk JAC, van den Elzen C, Ovelgönne H, Baas C, Jongen PMJM. Estimation of the number of infectious measles viruses in live virus vaccines using quantitative real-time PCR. J Virol Methods. 2004;117:179–87.
160 Valsamakis A, Kaneshima H, Griffin DE. Strains of measles vaccine differ in their ability to replicate in an damage human thymus. J Infect Dis. 2001;183:498–502.
161 Borte S, Liebert UG, Borte M, Sack U. Efficacy of measles, mumps and rubella revaccination in children with juvenile idiopathic arthritis treated with methotrexate and etanercept. Rheumatology (Oxford). 2009;48:144–8.
162 Heijstek MW, Pileggi GCS, Zonneveld-Huijssoon E, Armbrust W, Hoppenreijs EPAH, Uiterwaal CSPM, et al. Safety of measles, mumps and rubella vaccination in juvenile idiopathic arthritis. Ann Rheum Dis. 2007;66:1384–7.
163 Heijstek MW, Gorter S, Vries LDD, Smits GP, Gageldonk PGV, Berbers GAM, et al. Effects of the Live Attenuated Measles-Mumps-Rubella Booster Vaccination With Juvenile Idiopathic Arthritis. JAMA. 2013;309.
164 Pileggi GS, Terreri MTRA, Barbosa CP, Ferriani VPL. Measles, mumps and rubella vaccination safety in patients with juvenile rheumatic diseases taking immunosuppressive drugs. Ann Rheum Dis. Berlin, Germany. 2012. p. 430.
165 Scheinberg M, Guedes-Barbosa LS, Mangueira C, Rosseto EA, Mota L, Oliveira AC, et al. Yellow fever revaccination during infliximab therapy. Arthritis Care Res (Hoboken). 2010;62:896–8.
166 Mota LM, Oliveira AC, Lima RA, Santos-Neto LL, Tauil PL. Vaccination against yellow fever among patients on immunosuppressors with diagnoses of rheumatic diseases. Rev Soc Bras Med Trop. 2009;42:23–7.
167 Kernéis S, Launay O, Ancelle T, Iordache L, Naneix-Laroche Vr, Méchaï F, et al. Safety and immunogenicity of yellow fever 17D vaccine in adults receiving systemic corticosteroid therapy: An observational cohort study. Arthritis Care Res (Hoboken). 2013 Sep;65(9):1522-8.
168 Pileggi GS, de Souza CBS, Ferriani VnPL. Safety and immunogenicity of varicella vaccine in patients with juvenile rheumatic diseases receiving methotrexate and corticosteroids. Arthritis Care Res. 2010;62:1034–9.
169 Pileggi GS, de Souza CBS, Ferriani VPL. Safety and efficacy of varicella vaccine in patients with juvenile rheumatic diseases: A five years experience. Ann Rheum Dis. 2012. p. 430.
170 Barbosa CMPL, Terreri MTRA, Rosário PO, de Moraes-Pinto MI, Silva CAA, Hilário MOE. Immune response and tolerability of varicella vaccine in children and adolescents with systemic lupus erythematosus previously exposed to varicella-zoster virus. Clin Exp Rheumatol. 30:791–8.
171 Zhang J, Xie F, Delzell E, Chen L, Winthrop KL, Lewis JD, et al. Association between vaccination for herpes zoster and risk of herpes zoster infection among older patients with selected immune-mediated diseases. JAMA. 2012;308:43–9.
172 Zhang J, Delzell E, Xie F, Baddley JW, Spettell C, McMahan RM, et al. The use, safety, and effectiveness of herpes zoster vaccination in individuals with inflammatory and autoimmune diseases: a longitudinal observational study. Arthritis Res Ther. 2011;13:R174.
173 Guthridge JM, Cogman A, Merrill JT, Macwana S, Bean KM, Powe T, et al. Herpes zoster vaccination in SLE: a pilot study of immunogenicity. J Rheumatol. 2013;40:1875–80.
174 Eperon G, Vaudaux B. Vaccination chez le voyageur immunosupprimé. Rev Med Suisse. 9:970–8 2013:970–8.
175 World Health Organization. Safety of Yellow Fever Vaccination. 2008.
176 Mease P, van Vollenhoven R, Durez P, Sheeran T, Dougados M, Gomez Reino JJ, et al. Analysis of infection risk in patients with limited return of peripheral B cells after a period of 2 years or more following any rituximab treatment course in RA clinical trials. Annals of the Rheumatic Diseases. Berlin, Germany 2012. p. 381.
177 Bundesamt für Gesundheit. Schweizerischer Impfplan 2014. German.
178 Bundesamt für Gesundheit. Reisemedizin Impfungen und Malariaschutz bei Auslandreisen. Empfehlungen Stand März 2014. German.
179 Bühler S, Visser LG. Hepatitis A vaccination in patients with rheumatic diseases and drug-induced immunosuppression. Travel Med Infect Dis. 2014;12:115–7.
180 Bundesamt für Gesundheit. Pneumokokkenimpfung: Empfehlungen zur Verhinderung von invasiven Pneumokokkenerkrankungen bei Risikogruppen. German.
181 Centers for Disease Control and Prevention. Use of 13-Valent Pneumococcal Conjugate Vaccine and 23-Valent Pneumococcal Polysaccharide Vaccine Among Adults Aged ≥65 Years: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report (MMWR)2014.
182 Prevention of Herpes Zoster Recommendations of the Advisory Committee on Immunization Practices (ACIP).
183 Bundesamt für Gesundheit und Eidgenössische Kommission für Impffragen. Empfehlungen zur Impfung von Empfängerinnen und Empfängern von Blut-Stammzellen. 2012. German.
184 Marin M, Güris D, Chaves SS, Schmid S, Seward JF. Prevention of Varicella Recommendations of the Advisory Committee on Immunization Practices (ACIP). 2007.
185 Siegrist CA. Vaccine Immunology. Vaccines. 6th ed: Elsevier; 2012.
186 Broker M, Veitch K. Quadrivalent meningococcal vaccines: hyporesponsiveness as an important consideration when choosing between the use of conjugate vaccine or polysaccharide vaccine. Travel Med Infect Dis. 2010;8:47–50.
187 Milanovic M, Stojanovich L, Djokovic A, Kontic M, Gvozdenovic E. Influenza vaccination in autoimmune rheumatic disease patients. Tohoku J Exp Med. 2013;229:29–34.
188 Singh JA, Wells GA, Christensen R, Tanjong Ghogomu E, Maxwell L, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011;(2):CD008794.
Disclosures: No financial support and no other potential conflict of interest relevant to this article was reported.