Endovascular treatment of pulmonary arteriovenous malformations in hereditary haemorrhagic telangiectasia
DOI: https://doi.org/10.4414/smw.2015.14151
Malik
Babaker, Stephane
Breault, Catherine
Beigelman, Romain
Lazor, Nicole
Aebischer, Salah Dine
Qanadli
Summary
PRINCIPLES: To assess the efficiency and complication rates of vaso-occlusion of pulmonary arteriovenous malformations (PAVMs) in Rendu-Osler-Weber disease (hereditary haemorrhagic telangectasia; HHT).
METHODS: Seventy-two patients were investigated in our institution for HHT between March 2000 and November 2011. Sixteen presented PAVMs (22.2%), and 11 (68.8%) were treated with vaso-occlusion for a total of 18 procedures. Procedures included coils, plugs and combined approaches. Immediate success and recurrence rate, complication were recorded, as well as persistent and new PAVMs during clinical and computed tomography (CT) follow-up.
RESULTS: Eighteen procedures were performed and a total of 37 PAVMs were treated, 19 with coils, 16 with plugs and 2 with combined treatment. Mean CT follow-up time was 41 months (1‒164). No major complication was observed. One distal translocation was treated during the same intervention. Two PAVMs persisted after treatment (5.7%), both treated by means of plug embolisation. One new PAVM was observed during follow-up CT. PAVMs with an afferent artery of less than 3mm or asymptomatic PAVMs were not treated.
CONCLUSION: Recent studies have demonstrated that vaso-occlusion has become the gold standard treatment for PAVM. This study is in accordance with previous results and shows a minimal complication rate and little recurrence, whether by coils, plugs, or combined treatments.
Introduction
Pulmonary arteriovenous malformations (PAVM) are a rare disorder in the general population, with an estimated prevalence at autopsy of 3:15'000, although no population-based studies are available to determine its real incidence [1]. The vast majority of PAVMs (80%‒95%) are diagnosed in patients presenting with hereditary haemorrhagic telangiectasia (HHT) or Rendu-Osler-Weber disease (fig. 1) [2]. HHT is a rare genetic autosomal-dominant disease caused by mutations in five to six genes, the most studied and known being ENG (on chromosome 9), ALK1 (on chromosome 9) and SMAD4 ((on chromosome 18). All three genes are involved in the cascade resulting in the transforming growth factor-beta (TGF-β) [3]. Genetic testing has been made available for these three known genes, allowing identification of approximately 80% of affected patients. Penetrance is age-related and is almost complete by the age of 40 years old [4]. Current clinical diagnostic criteria, called Curaçao criteria, include epistaxis, muco-cutaneous telangiectasia, visceral AVMs (pulmonary, hepatic, cerebral, spinal AVMs, gastrointestinal telangiectasia) and family history [5, 6]. HHT is a rare disease. A large epidemiological study in France by Bideau and Plauchu showed a prevalence of 1/8,345 in France [7]. At the time of writing, no such study is available in Switzerland, but two out of three French regions with higher prevalences (1/3,375 in the Ain department and 1/5,062 in Jura) are located near Swiss borders, with patients from these regions being followed in our institution, for geographical proximity reasons.
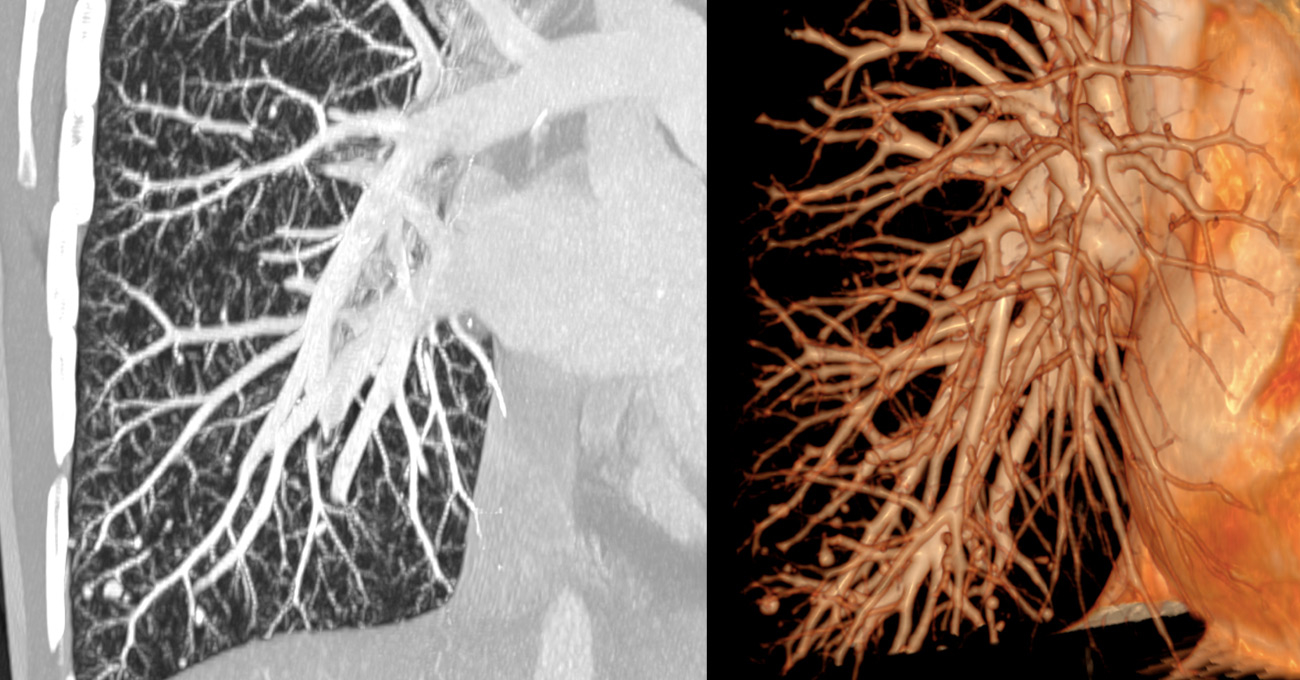
Figure 1
Maximun intensity imaging and 3D reconstructions of pulmonary computed tomography angiography showing numerous pulmonary arteriovenous malformations in a female 50 years old female patient known to have hereditary haemorrhagic telangectasia.
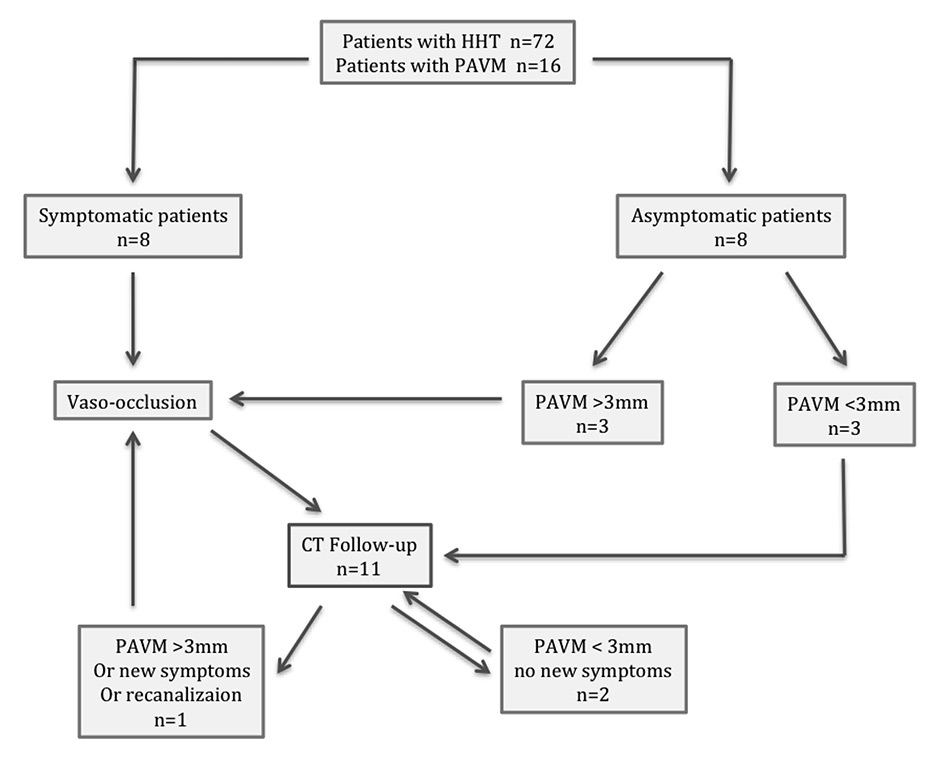
Figure 2
Treatment for symptomatic or >3 mm pulmonary arteriovenous malformations and follow-up.
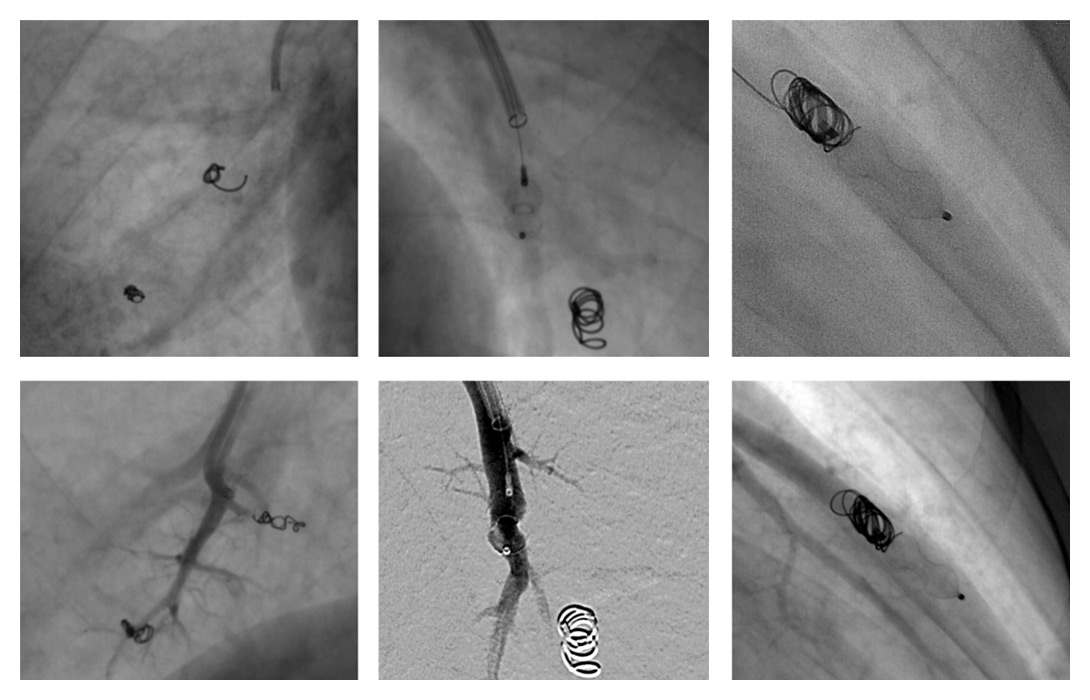
Figure 3
Examples of pulmonary arteriovenous malformation endovascular vaso-occlusions with vascular coils, plugs and combined treatment. Beneath each case is an angiographic control showing no vascular opacification beyond the occlusive device.
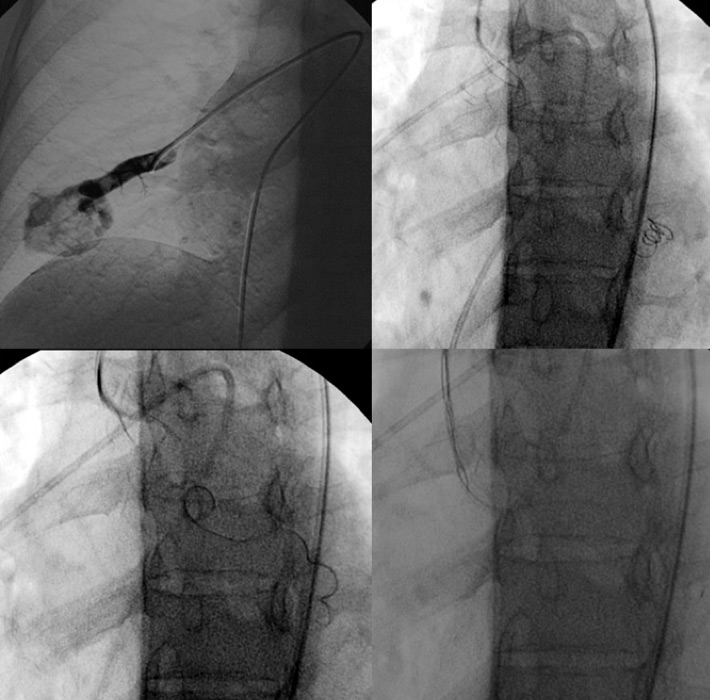
Figure 4
Embolisation attempt of a large pulmonary arteriovenous malformation (PAVM) with a feeding artery of 12 mm diameter with a 10/5 mm 10.035 Tornado® coil. Owing to the size of the AVM, distal translocation of the coil in the left ventricle occurred. A snare-assisted removal was then performed before a second and successful attempt at occluding this PAVM.
The prevalence of PAVM in patients with HHT is estimated to be between 30% and 50% [8]. A PAVM results in an arteriovenous shunt bypassing the capillary bed and depriving the blood flow of oxygenation and a filtering mechanism. Thus complications of PAVMs include hypoxaemia from the right to left shunt, stroke, transient ischaemic attack, brain abscess, migraines, decompression illness, haemoptysis and haemothorax [8–11]. Percutaneous transcatheter vaso-occlusion (PTV) has been demonstrated to be the gold standard treatment of PAVM. It has been shown to be effective in improving arterial oxygenation and in reducing right-to-left anatomic shunt fraction [12]. Advantages over surgical interventions include being less invasive and easy to repeat. Surgery is no longer considered in first intention. Disadvantages include collateralisation and revascularisation over time [3]. It has been advocated that, regardless of symptoms, any PAVM with a feeding artery of ≥3 mm in diameter should be considered for PTV [3, 13]. Embolic devices consist of vascular coils and/or vascular plugs [14]. In this context, the purpose of this study was to report our experience and assess the efficiency and complications of endovascular treatment of PAVMs in HHT in our institution.
Materials and methods
Clinical and radiological data of 72 consecutive patients (38 females, 34 males, mean age 58 years) investigated in our institution for HHT between March 2000 and November 2011 were reviewed. Anamnestic data, genetic investigations and clinical follow-up were gathered by the pneumonology department of our institution. Thoracic computed tomography angiography (CTA) was prescribed on the basis of clinical suspicion [3]. When a PAVM was diagnosed, assessment of the number and size of PAVMs, and feasibility of treatment were assessed. Criteria for treatment were: afferent artery diameter >3 mm, history of paradoxical embolism with recognisable responsible vessel, and hypoxaemia with a left-to-right shunt. Based on CT findings, endovascular treatment was proposed to the patient. Standard vaso-occlusion procedures are performed with local anaesthesia. A standard 6F or 8F 65 cm long introducer sheath (6F or 8F 6 Fr. x 25-5/8" (65 cm) Super Arrow-Flex®, Teleflex, Arrow intl, PA, USA) was positioned in the main pulmonary artery, then a selective 5F catheter was used to reach the pulmonary artery branch and a microcatheter was used in order to be superselective (fig. 4). Available endovascular treatment devices consisted of vascular coils and vascular plugs. No balloon was used. Vascular platinum coils (Tornado® Embolization Coil, Cook Medical, Bloomington, IN, USA) are the core of PAVM vaso-occlusion [3, 15]. They consist of linear platinum coils with polyester fibres over the surface that, once out of the catheter, take a spherical or a spiral shape and occlude the vessel. Amplatzer Vascular Plugs (Amplatzer™, St Jude Medical, Little Canada, Minnesota, USA) are self-expandable, cylindrical devices made from a Nitinol wire mesh secured on both ends with platinum marker bands. A stainless steel micro screw is welded to one of the platinum marker bands. The devices were pushed by a 135 cm pusher wire. In our study, type I and type IV Amplatzer plugs were used, with a diameter range between 4 and 8 mm. Hyperion vascular plugs (Hyperion™, Comed B.V., Bolsward, The Netherlands) are self-expandable wire mesh devices with different shapes (fig. 5), pushed by a 110 cm long loader. After the confirmation of the right position, the plug is deployed into the feeding artery. When recanalisation was feared, because of the size or of the complexity of the PAVM, a combined plug and coil vaso-occlusion was made, in accordance with previous studies [16] (fig. 4). Angiographic control was made to confirm the occlusion of the feeding artery and the absence of distal translocation. Follow-up CT was advised 6–12 months after the treatment and every 3 years thereafter [6]. CT protocol consisted of helicoidal CT acquisition with Iodine contrast media injection in pulmonary arterial time. Statistical anaylsis was made using Microsoft® Excel (Microsoft, Redmond, USA). All patients benefitted from a clinical follow-up.
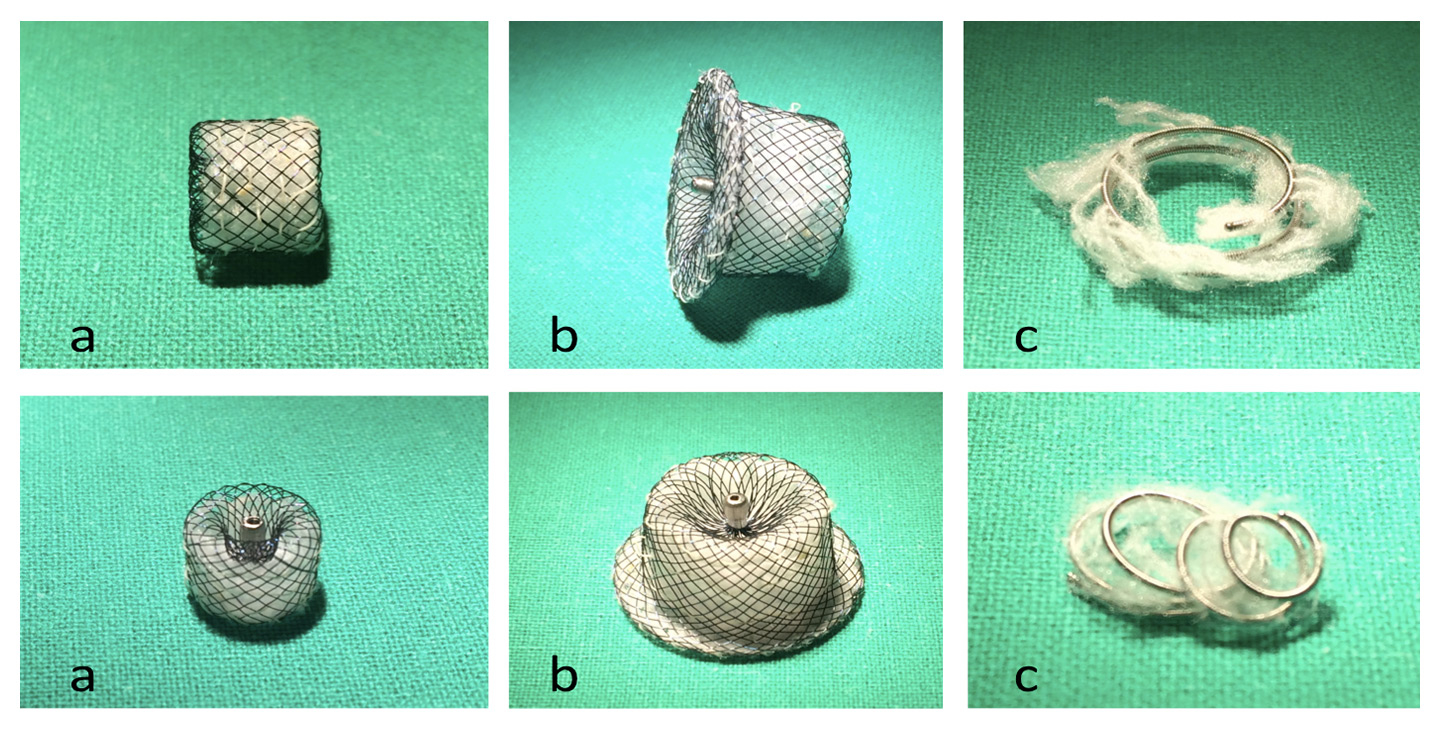
Figure 5
Examples of Hyperion™ vascular plugs (a, b) and a Tornado® Embolization Coil (c)
|
Table 1: Patient demographics and number of procedures. |
| Number of patients with HHT |
72 |
| Mean age |
58 y (20–93) |
| Patients with PAVMs |
16 (22.2%) |
| Treated patients with PAVMs |
11 (68.7%) |
| Male:female ratio |
1:1.67 |
| Total PAVMs |
54 |
| Total treated PAVMs |
37 |
| Mean PAVMs per patient |
3.9 (1–12) |
| Total procedures |
18 (1–4) |
| Total recurrence rate per treated PAVM |
5.4% |
| Mean Follow-up time |
41 m (1–164) |
| New PAVM per year |
0.29/y |
| HHT = hereditary haemorrhagic telangiectasia; PAVM = pulmonary arteriovenous malformation |
Results
A total of 16 patients out of 72 followed-up for HHT (22%, 10 women, 6 men, m:f 1:1.67) presented a total of 54 PAVMs (1‒12, mean 3.9 PAVMs per patient,). Mean follow-up CT time was 41 months (1‒164). Based on the above-mentioned criteria for treatment, 11 patients were treated with vaso-occlusion for a total of 37 PAVMs throughout 18 procedures including 2 second treatments for 2 PAVMs (table 1). Eight patients were symptomatic and three presented asymptomatic PAVMs with feeding arteries larger than 3 mm (fig. 2). Mean procedures per patient was 1.64. Total treated PAVMs per total PAVM was 37/54 (68.5%). Nineteen PAVMs were treated with coils, 16 with plugs and 2 with combined treatment (table 2, fig. 3). Thirty-four (91.9%) PAVMs were successfully treated on the first intervention. Total recurrence rate per patient was 18.2% (2/11). Total recurrence rate per procedure was 12.5% (2/16). Total recurrence rate per treated PAVM was 5.4% (2/37). Two PAVMs (5.4%) treated by means of plugs needed a second procedure (at 10 days and 3 months), both treated by use of Amplatzer plugs. One case of a voluminous PAVM presented a large afferent artery of 12 mm diameter. A 15 mm long coil was initially used, but migrated into the main pulmonary artery and required a snare-assisted coil retrieval. Then a smaller sized 10/5 mm Tornado® coil was released, but it migrated distally into the left atrium, the left ventricle and finally the mitral valve. A snare-assisted coil retrieval was again successfully attempted (fig. 3). No clinical manifestation was observed. A second intervention a month later allowed for a coil-assisted successful vaso-occlusion, using multiple coils between 5 and 10 mm. Eighteen (94.7%) plug-treated PAVMs were successfully treated on the first intervention compared with 14 (87.5%) for coil vaso-occlusion, but Fisher’s test gave a p = 0.58 value, considered not significant. One new PAVM was observed during follow-up CT (0.29 per year) and was not treated because of its size. After the first intervention, mean procedure number per patient during follow-up was 0.63 (6 interventions for 11 patients). No major complication was observed. No surgery was needed in our series.
|
Table 2: Results of embolotherapy by method. Recurrent pulmonary ateriovenous malformations (PAVMs) occurred in patients treated with vascular plugs at 10 days and 3 months. |
|
Embolotherapy method
|
Number of treated PAVMs
|
Number of procedures
|
Number of devices used
|
Size range
|
Distal translocation
|
Recurrent PAVM
|
| Coil |
19 |
9 |
20 |
3/7 – 5/10 |
1 |
|
| Plug |
16 |
7 |
18 |
4–8 mm |
|
2 |
| Combined treatment |
2 |
2 |
2 |
|
|
|
| Total |
37 |
18 |
|
|
|
|
Discussion
HHT is a heterogenous, autosomal dominant disorder characterised by recurrent epistaxis, mucocutaneous telangiectasia, visceral, pulmonary and cerebral arteriovenous malformations. Its incidence in the general population is estimated to be 1:5,000 to 1:8,000 with a penetrance approaching 97% by age 45 years. PAVMs are a rare condition in the general population with an approximated incidence estimated at 3:100,000. Treatment is adapted to the number, to the size and to the diffusion of PAVMs [17]. Since about 90% of PAVMs occur in patients with HHT, diagnosis and clinical follow-up of affected patients is critical for detecting and treating this condition. Therefore, a close interaction between the referring clinician, a referral centre and the interventional radiologist is beneficial for optimising diagnosis, treatment and follow-up. Vaso-occlusion is now recognised as the standard of care for patients with HHT and a PAVM with a feeding artery of >3 mm [18]. Immediate vaso-occlusion has been proven to correlate with improved life expectancy and quality-adjusted survival rates [3]. The incidence of PAVMs in our group followed for HHT (22%) was a little lower than previous estimates. Female-to-male ratio was consistent with previous studies. In our group, 50% of patients presented with multiple PAVMs at the time of initial diagnosis (35%–65% in the literature). Imaging diagnosis was made with pulmonary CTA, considered to be the gold standard diagnostic tool as it allows easy screening, high resolution and definition of the type of PAVMs [4, 17]. Angiographic magnetic resonance was ruled out as it was less efficient than CTA. Endovascular treatment is made by means of transcatheter vaso-occlusion. PAVMs, even asymptomatic, with a feeding vessel 3 mm in diameter or more should be treated. Historically, vaso-occlusion treatments mainly consisted of detachable occlusion balloons. They were removed from US markets in 2002 [19], and were prone to deflate [20]. Onyx and glue vaso-occlusions are not indicated as a result of the relatively large diameter size of afferent arteries of treatable PAVMs [21]. The device of choice then consisted of vascular coils. Advantages include a wide array of sizes and the great flexibility of the device. Disadvantages include distal migration, which is attenuated by the use of detachable coils. Vascular plugs, such as Amplatzer or Hyperion plugs may also be used, especially for PAVMs with larger feeding vessels [22]. First generation plugs did not present a favourable morphology and were not adapted to pulmonary artery anatomy, but current generation and recent studies show favourable outcomes [23]. A combined coil and plug approach has been described in the case of complex or large afferent artery cases [16]. In our series, the choice between coils and plugs depended on multiple factors, such as the size of the afferent artery, but also the specific anatomy of the PAVM and its feeding vessel, so that the choice of which device to use is still depending on each specific situation. Only one case presented with distal coil translocation in the context of a large feeding artery (12 mm); this diameter proved difficult to treat with either coil or plug vaso-occlusion. Larger studies are needed to assess the efficiency of both devices in these specific situations. Recurring PAVMs needing a second treatment only occurred with vascular plugs, but because of the small number of recanalisations (n = 2, 5.4% of treated PAVMs), an association with the vaso-occlusion device could not be determined. New vascular plugs such as Hyperion plugs look promising, but further studies are needed to assess their efficiency.
PAVMs are frequent in HHT and their potential complications call for a rigorous detection, diagnosis and treatment, which can be achieved by specialised clinical assessment and follow-up and close collaboration with the interventional radiologist. Our series correlates with previous studies showing the overall excellent success rates of endovascular treatment of PAVMs which are compliant with current treatment criteria. Whereas plugs and coils give excellent final results, statistical comparison didnot show a significant difference in outcome; moreover, the variety of factors determining which device to use did not allow for a feasible comparison study. Further studies are needed to assess the efficiency of each device with defined criteria.
References
1 Gossage JR, Kanj G. Pulmonary arteriovenous malformations. A state of the art review. Am J Respir Crit Care Med. 1998;158:643–61.
2 Bayrak-Toydemir P, Mao R, Lewin S, McDonald J. Hereditary hemorrhagic telangiectasia: an overview of diagnosis and management in the molecular era for clinicians. Genet Med. 2004;6:175–91.
3 Trerotola SO, Pyeritz RE. PAVM embolization: an update. AJR Am J Roentgenol. 2010;195:837–45.
4 Lacombe P, Lacout A, Marcy PY, Binsse S, Sellier J, Bensalah M, et al. Diagnosis and treatment of pulmonary arteriovenous malformations in hereditary hemorrhagic telangiectasia: An overview. Diagn Interv Imaging. 2013;94:835–48.
5 Shovlin CL, Guttmacher AE, Buscarini E, Faughnan ME, Hyland RH, Westermann CJ. et al. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am J Med Genet. 2000;91:66–7.
6 Faughnan ME, Palda VA, Garcia-Tsao G, Geisthoff UW, McDonald J, Proctor DD, et al. International guidelines for the diagnosis and management of hereditary haemorrhagic telangiectasia. J Med Genet. 2011;48:73–87.
7 Bideau A, Plauchu H, Brunet G, Robert J. Epidemiological investigation of Rendu-Osler disease in France: its geographical distribution and prevalence. Popul. 1989;44:3–22.
8 Cottin V, Dupuis-Girod S, Lesca G, Cordier JF. Pulmonary vascular manifestations of hereditary hemorrhagic telangiectasia (rendu-osler disease). Respiration. 2007;74:361–78.
9 Drouet A, Le Moigne F, Donat A, Guilloton L, Felten D. Brain abscess as the first clinical manifestation of isolated pulmonary arteriovenous malformation without Rendu-Osler disease. Rev Neurol. (Paris) 2011;167:29–34.
10 Dupuis-Girod S, Giraud S, Decullier E, et al. Hemorrhagic hereditary telangiectasia (Rendu-Osler disease) and infectious diseases: an underestimated association. Clin Infect Dis. 2007;44:841–5.
11 Luthra S, Antippa P, Tatoulis J. Pulmonary arteriovenous aneurysm as manifestation of Osler-Weber-Rendu syndrome. Heart Lung Circ. 2008;17:336–9.
12 Gupta P, Mordin C, Curtis J, Hughes JM, Shovlin CL, Jackson JE. Pulmonary arteriovenous malformations: effect of embolization on right-to-left shunt, hypoxemia, and exercise tolerance in 66 patients. AJR Am J Roentgeno. 2002;179:347–55.
13 Andersen PE, Kjeldsen AD, Oxhoj H, Vase P, White RI Jr. Embolotherapy for pulmonary arteriovenous malformations in patients with hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Acta Radiol. 1998;39:723–6.
14 Abdel Aal AK, Hamed MF, Biosca RF, Saddekni S, Raghuram K. Occlusion time for Amplatzer vascular plug in the management of pulmonary arteriovenous malformations AJR Am J Roentgeno. 2009;192:793–9.
15 Pollak JS, Saluja S, Thabet A, Henderson KJ, Denbow N, White RI, Jr. Clinical and anatomic outcomes after embolotherapy of pulmonary arteriovenous malformations. J Vasc Interv Radiol 2006;17:35–44; quiz 5.
16 Trerotola SO, Pyeritz RE. Does use of coils in addition to amplatzer vascular plugs prevent recanalization? AJR American journal of roentgenology. 2010;195:766–71.
17 Qanadli SD, Mesurolle B, Mignon F, Barre O, Bruckert F, Dubourg O, et al. [Bronchial and pulmonary vaso-occlusions]. Rev Mal Respir. 1999;16:719–29. French.
18 White RI Jr., Lynch-Nyhan A, Terry P, Buescher PC, Farmlett EJ, Charnas L, et al. Pulmonary arteriovenous malformations: techniques and long-term outcome of embolotherapy. Radiology. 1988;169:663–9.
19 White RI Jr. Pulmonary arteriovenous malformations: how do I embolize? Tech Vasc Interv Radiol. 2007;10:283–90.
20 Saluja S, Sitko I, Lee DW, Pollak J, White RI, Jr. Embolotherapy of pulmonary arteriovenous malformations with detachable balloons: long-term durability and efficacy. J Vasc Interv Radiol. 1999;10:883–9.
21 Lubarsky M, Ray C, Funaki B. Embolization agents-which one should be used when? Part 2: small-vessel embolization. Semin Intervent Radiol. 2010;27:99–104.
22 Hu S, Davis C. Endovascular embolization with a vascular plug corrects a PAVM. JAAPA 2009;22:27–8, 31, 5.
23 Kucukay F, Ozdemir M, Senol E, Okten S, Ereren M, Karan A. Large Pulmonary Arteriovenous Malformations: Long-Term Results of Embolization with AMPLATZER Vascular Plugs. J Vasc Interv Radiol. 2014.




