(F)Utility of invasive haemodynamic measurements to guide percutaneous intervention in chronic coronary artery disease
DOI: https://doi.org/10.4414/smw.2015.14143
Summary
Percutaneous coronary intervention (PCI) in chronic stable coronary artery disease has not been shown to reduce the incidence of myocardial infarction or death. There is, however, evidence that the outcome from PCI is dependent on the amount of myocardial ischaemia. This review provides an overview of coronary circulatory pathophysiology and focuses on fractional flow reserve from a semantical, conceptual and practical point of view.
Glossary
Coronary autoregulation Conservation of coronary blood supply to the myocardium under resting conditions (1 ml/min/g) over a wide range of coronary perfusion pressures
Coronary flow Blood volume flow rate in a coronary artery or one of its branches in ml/min
Coronary flow reserve Maximal, i.e., hyperaemic coronary blood flow divided by blood flow at identical measurement site at rest
Coronary flow velocity reserve Maximal, i.e., hyperaemic coronary blood flow velocity divided by blood flow velocity at identical measurement site at rest
Coronary microcirculation Coronary arteriolar and capillary circulation; coronary arterioles are the regulators of microvascular resistance due to their small vascular calibre and their vasomotor capabilities
Fractional flow reserve (FFR) The ratio of mean (time-averaged) coronary pressure distal to an atherosclerotic stenosis during hyperaemia divided by the simultaneously obtained mean aortic pressure
Hyperaemia State of maximal tissue blood flow
Hyperaemic microvascular resistance (HMR) Mean (time-averaged) coronary pressure distal to a stenosis divided by average peak Doppler flow velocity as obtained at the same site
Index of microcirculatory resistance (IMR) Mean (time-averaged) coronary pressure distal to a stenosis multiplied by mean transit time
Instantaneous wave-free ratio (iFR) Mean (time-averaged) coronary pressure as obtained without hyperaemia during the diastolic, so-called wave-free period dived by the simultaneous mean aortic pressure. The wave-free period extends from 80 ms after the dicrotic notch in the phasic aortic pressure curve until the end of diastole at the beginning of the ECG QRS complex
Microcirculatory resistance Resistance to blood flow as regulated by the vasomotor state of the microcirculation at the arteriolar level
Myocardial perfusion Coronary blood flow rate per unit of myocardial mass
Transit time The time necessary for a bolus of “cold” saline in the coronary circulation to travel a given distance
Introduction
Whereas in patients with acute coronary syndrome the beneficial effect on outcomes of percutaneous coronary intervention (PCI) is undisputed [1], PCI in chronic stable coronary artery disease (CAD) has not been demonstrated to reduce the incidence of myocardial infarction or death [2, 3]. In a very recent meta-analysis including more than 93,000 patients [4], it has, however, been concluded that there may be “evidence for improved survival with new generation drug eluting stents but no other percutaneous revascularisation technology compared with medical treatment”. There is, at present, even more evidence that the outcome from PCI is dependent on the amount of myocardial ischaemia [5, 6]. In this context and because only a minority of patients with stable CAD undergo noninvasive stress testing for ischaemia detection prior to elective PCI, an array of invasive coronary haemodynamic measurements for functional stenosis severity assessment has been developed [7]. Owing to the technical robustness of coronary pressure measurements, pressure-derived haemodynamic parameters are employed more frequently than coronary flow velocity or temporal flow parameters. At present, the most often used invasive coronary haemodynamic parameter is pressure-derived fractional flow reserve (FFR), which is calculated as the ratio of mean, i.e., time-averaged, coronary pressure obtained distal to one or more stenotic lesions of interest (Pd in mm Hg) divided by the simultaneous mean aortic pressure (Pao in mm Hg) during hyperaemia (fig. 1) [8]. An FFR ≤0.80 as described by the authors of the most recently published study on the topic is “a drop in maximal blood flow of 20% or more caused by stenosis” [9]. The open-label FFR vs Angiography for Multivessel Evaluation 2 (FAME2) trial randomly allocated patients with clinically overt chronic CAD and FFR ≤0.80 to PCI plus medical therapy or to medical therapy alone [9]. The rate of the composite of death from any cause, myocardial infarction, or urgent revascularisation within 2 years was 8.1% in the PCI group and 19.5% in the medical therapy group (p <0.0001). Not entirely surprisingly, this reduction in adverse events was driven by a higher rate of urgent revascularisation in the medical group.
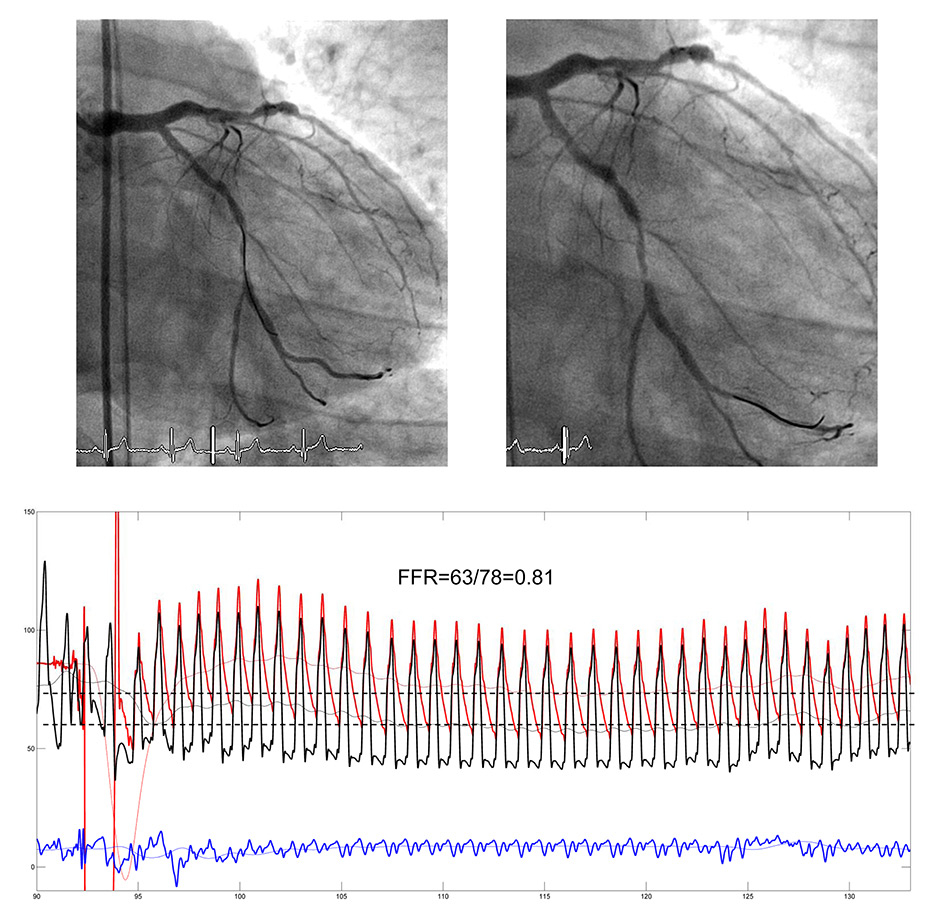
Figure 1
Angiogram of the left coronary artery with serial stenoses of the left circumflex artery (upper panels). The pressure sensor wire (location of the sensor at the end of the opaque tip) is first positioned proximal to the tightest stenosis (left upper panel), and then distal to it (right upper panel). The pressure tracings (lower panel) are recorded with the pressure sensor distal to the stenoses (wire tip in the marginal branch). The following phasic and mean pressures are recorded simultaneously: Aortic pressure, Pao (mean) in red, coronary pressure distal to the stenoses in black (Pd), central venous pressure in blue (CVP). Fractional flow reserve (FFR) is taken as the steady state minimum ratio of Pd/Pao (broken lines) over several cardiac cycles.
The purpose of this review is to provide an overview of the coronary circulatory pathophysiology relevant for understanding the different and briefly described invasive measurements for ischaemia detection. Secondly, it focuses on FFR from a semantical, conceptual, and practical standpoint of view.
Coronary flow-derived haemodynamic measurements for ischaemia detection
The coronary arterial tree is structurally designed so that its vascular calibres and branching features allow minimum viscous energy loss during the transport of blood to the myocardium [10]. This biological design principle prevalent in fluid and gas conductive systems of a variety of species and organs covers widely varying flow rates and the concept of maximum limited and adaptive vascular wall, i.e., endothelial shear stress, which is the necessary condition for a functional coronary vasomotion [10]. Coronary microcirculatory function as resistance-to-flow regulator is key to myocardial oxygen supply in response to metabolic demand, which can be matched only by flow changes and not by varying oxygen release from haemoglobin as in other organs. The principle of coronary flow autoregulation is tightly related to the role of the microcirculatory resistance, and it is defined as the maintenance of resting myocardial perfusion (1 ml/min/g) under perfusion pressure challenges over a wide range of 50–150 mm Hg (fig. 2) [11, 12]. Autoregulation of coronary flow can be inhibited by inducing constant minimal microcirculatory resistance during maximal myocardial hyperaemia. This is achieved in response to a brief and systematically applied episode of maximal ischaemia, by physical exercise, the cold pressor test, and various pharmacological substances such as acetylcholine, adenosine, dipyridamole, dobutamine and papaverine. During complete inactivation of the coronary microcirculatory vasomotor function, coronary flow or perfusion changes are directly reflected by coronary perfusion pressure changes (fig. 2) [11]. This implies that Ohm’s physical law can be reasonably applied to the coronary circulation:
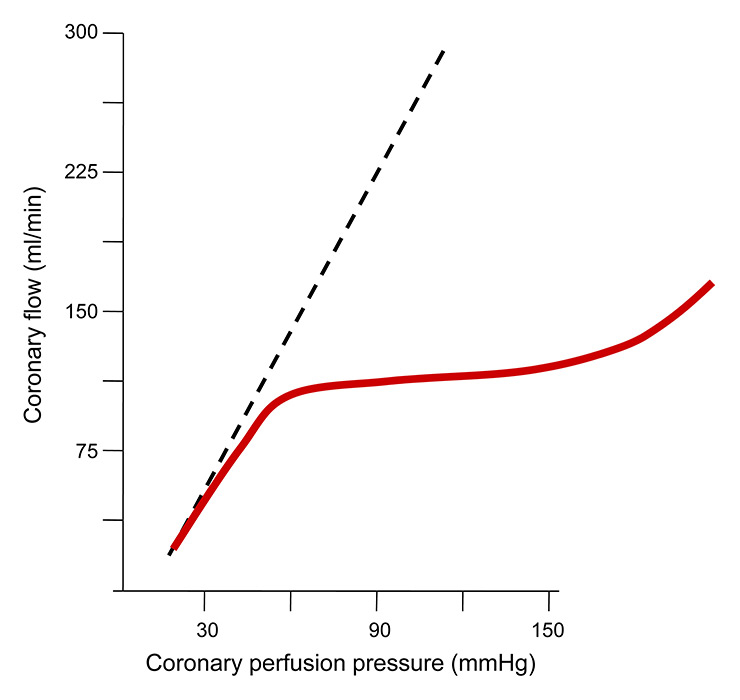
Figure 2
Schematic illustration of autoregulation of coronary blood flow, which is constant at around 1 ml/min over a wide range of perfusion pressures (red line). During the state of maximum microcirculatory vasodilation (broken line), i.e., minimum resistance, coronary flow varies directly with varying perfusion pressures.
ΔP = R×Q
where ΔP is the pressure drop between Pao and central venous pressure (CVP in mm Hg), R is microcirculatory vascular resistance (in dyne•s•cm–5), Q is coronary volume flow rate (in ml/min). Indeed, it is the basis of practically all clinical coronary invasive measurements for the assessment of the haemodynamic severity of stenotic lesion(s). This is the case despite several theoretical shortcomings of Ohm’s law in the presence of a fluid such as blood instead of water, i.e., the viscosity of water remains constant despite augmenting shear rate at the inner vascular surface, whereas it increases in blood. This non-Newtonian behaviour of blood has an over-proportional influence of Q increases on endothelial activation and on R. However, theoretical caveats such as these or as the influence of pressure recovery downstream of a turbulent instead of laminar flow field on FFR measurements are minute in comparison to those discussed below.
Based on Ohm’s law, the following candidates for haemodynamic stenosis severity assessment exist, whereby in the clinical setting it is most challenging and practically not feasible to obtain invasively flow rate Q or even perfusion, i.e., flow rate per regional myocardial mass: practically obtainable surrogates of Q, i.e., Doppler-derived coronary flow velocity or the transit time of an intracoronary bolus of saline at room temperature over a defined distance, and coronary pressure. From these directly obtainable parameters, coronary haemodynamic indices are calculated during hypaeremia (table 1), i.e., Doppler- and transit-time-derived coronary flow reserve (CFR), hyperaemic microvascular resistance (HMR), and index of microvascular resistance (IMR) [7]. Coronary hyperaemia is mostly induced by the very short acting adenosine at a dose of 140 μg/min/kg through a central vein (infusion duration of 4‒5 minutes), or at an intracoronary bolus of 40–60 μg for the left coronary artery and 20–30 μg for the right coronary artery [13]. Under the condition of maximal microcirculatory dilatation, experimentally and clinically defined abnormal values of all the coronary haemodynamic parameters (table 1) then indicate a relevant coronary artery stenosis to be treated with PCI. In this context, several sources of error using flow-derived parameters and the induction of hyperaemia have to be considered.
|
Table 1: Invasive haemodynamic parameters for coronary stenosis severity assessment. |
|
Parameter
|
Method
|
Definition
|
Abnormal value
|
Limitations
|
| CFR |
Doppler |
Vhyperaemia ÷ Vbaseline
|
<2.0 |
Composite epicardial and microvascular resistance
Dependence on haemodynamic factors
Doppler: prone to artifacts |
| |
Thermodilution |
Tbaseline ÷ Thyperaemia
|
<2.0 |
| |
|
|
|
| HMR |
Doppler |
Pd ÷ Vhyperaemia
|
>3.25 mm Hg / cm s |
V: Surrogate of flow |
| IMR |
Thermodilution |
Thyperaemia × Pd
|
>32–35 U |
T: surrogate of flow |
| |
|
|
|
|
| iFR |
Pressure |
Pd ÷ Pao
|
<0.90 |
Not stenosis specific |
| FFR |
Pressure |
Hyperaemic Pd ÷ Pao * |
≤0.80 |
Not stenosis specific
Variable influence of collateral function
Strongly dependent of maximal hyperaemia |
| FFR = fractional flow reserve; HMR = hyperaemic microvascular resistance; iFR = instantaneous wave-free ratio; IMR = index of microcirculatory resistance; Pao = mean aortic pressure; Pd = distal mean coronary pressure; T = mean transit time
*: mean pressures obtained during the entire cardiac cycle |
Critique of flow-derived parameters and coronary hyperaemia induction
According to Ohm’s law, the above described coronary resistance indices also contain a pressure term aside from a flow surrogate. This section focuses on critical aspects of parameters other than coronary pressure, which itself can be obtained very reliably.
Since during clinical invasive coronary examination, Q cannot be directly measured, a surrogate is taken using 12-MHz Doppler sensor angioplasty guidewires. Coronary flow velocity V linearly reflects Q under the condition of a maximally dilated epicardial coronary artery calibre (by nitroglycerin), because Q = V×A, where A is the vascular cross-sectional area. V in this equation is, however and strictly speaking, the spatially averaged coronary flow velocity, which is variably different from the Doppler sensor-obtained peak velocity depending on the cross-sectional flow velocity profile [14]. In the case of a parabolic profile, V is about half the Doppler-obtained peak flow velocity. More importantly, coronary Doppler flow velocity profiles may be technically difficult to obtain, depending on the site of measurement in the coronary tree, on its tortuosity, on the absence or presence of hyperaemia (more difficult at rest). Altering the longitudinal position of the Doppler sensor for optimising signal quality changes coronary flow velocity by a factor of 1.25 at every bifurcation (decreasing downstream) [15]. These aspects introduce considerable measurement variability and thus error in the calculation of derived parameters. For example, if during measurement of CFR, resting coronary velocity as the variable in the denominator of the ratio is underestimated in the context of overly difficult data acquisition, CFR may be severely overestimated.
Coronary transit time as a surrogate for Q is determined on the basis of the indicator-dilution principle, which states that Q = volume of indicator injected divided by mean transit time of the indicator [16]. Theoretically, the indicator volume is the ratio between the known quantity of indicator particles and the measured concentration of particles in a downstream sample. In the case of coronary transit time measurements, two thermo-sensors take the up- and downstream temperature of an approximately 4 ml coronary bolus of “cold” saline. The distance between the two thermistors is variable interindividually in the context of coronary anatomic variation, but it should be (kept) constant intraindividually during assessment of a particular single or serial stenosis. The latter is crucial for CFR measurement, since varying positions of the sensor wire introduce a directly proportional error in CFR. Practically, the risk of altering the angioplasty guidewire tip position (= position of the distal thermistor) is high due to the bolus injection, which has to occur fast and may result in a pushback of the coronary guiding catheter and, thus, pullback of the sensor wire. Immediately after versus before the bolus injection, the sensor tip has, thus, possibly changed position, which directly affects transit time. The effect of such a displacement is bigger on CFR than on IMR, because it may influence CFR twice (resting and hyperaemic transit time) and IMR only during hyperaemia (see also table 1).
CFR does not reflect exclusively the haemodynamic stenosis severity but is also influenced by the vasodilatory capacity of the microcirculation. Thus in the case of, e.g., a microcirculatory dysfunction due to hypertensive heart disease with left ventricular hypertrophy, CFR is impaired irrespective of coronary stenosis severity [17].
Coronary hyperaemia induction
Aside from the above mentioned adenosine, other pharmacological substances and physical manoeuvres are employed for hyperaemia stimulation, such as papaverine, regadenoson, dipyridamole, dobutamine, acetylcholine and nicorandil; brief total ischaemia, physical exercise (treadmill or handgrip), cold pressor, hyperventilation/apnoea [13, 18, 19]. The induction of coronary hyperaemia in response to the most frequently used adenosine may not be maximal, and thus variable. Casella and coworkers demonstrated that only intracoronary dosages of adenosine as high as 150 μg resulted in similar FFR values as the reference intravenous application of 140 μg/kg/min [20]. The finding of de Luca et al. that intracoronary dosages of adenosine between 60 and up to 720 μg were associated with continuously decreasing FFR values indicates that the intravenous dose of 140 μg/kg/min cannot be taken either as the reference for maximal hyperaemia [21]. Furthermore, the case of a patient with a severe stenotic lesion undergoing an FFR measurement at 140 μg/kg/min of intravenous adenosine illustrates that the subsequent 1-minute coronary occlusion is a stronger hyperaemic stimulus, since the resulting FFR is lower than the adenosine-induced (fig. 3).
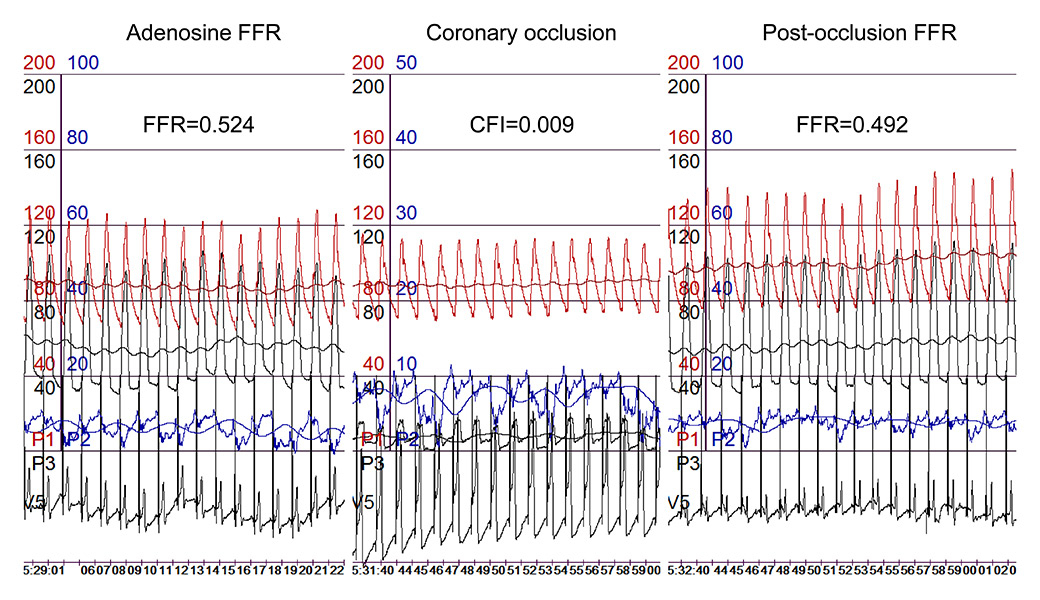
Figure 3
Simultaneous recordings of phasic and mean aortic (Pao for mean pressure in red), distal coronary (Pd for mean pressure in black), occlusive coronary (Poccl in black), and central venous pressure (CVP in blue), as well as an intracoronary ECG lead. During coronary occlusion, marked ECG ST-segment elevations are visible.
CFI = collateral flow index; FFR = fractional flow reserve.
Coronary pressure-derived parameters for ischaemia detection
Under the condition of maximal coronary microcirculatory vasodilation, i.e., minimal coronary microcirculatory resistance, the physiological principle of a constant coronary flow at rest despite perfusion pressure challenges (autoregulation) is abolished [11]. Consequently, coronary pressure changes directly reflect coronary flow alterations. Since the measurement of the reference parameter volume flow rate Q is difficult in the invasive clinical setting, the robust measurement of coronary pressure P as surrogate of Q is convenient. In this context, achieving maximal coronary hyperaemia is of paramount importance, because without it coronary resistance in Ohm’s law is not constant and thus, P does not linearly reflect Q.
As an alternative to pharmacological hyperaemia induction, minimal and constant coronary resistance has been shown to be present at rest during the diastolic, so called wave-free, period of every cardiac cycle [22]. The ratio of mean coronary pressure distal to a stenosis (Pd) to the mean aortic pressure (Pao) as obtained during the wave-free period at rest (instantaneous wave-free ratio, iFR; see table 1) has been found to be similar and significantly correlated to that during intravenous adenosine [22]. Accordingly, iFR has been proposed as a “drug-free index of stenosis severity comparable to fractional flow reserve” [22], the concept of which would be, indeed, appealing. More recently, Berry et al. challenged the view that iFR is independent of hyperaemia, i.e., iFR at rest was 0.82 ± 0.16 versus 0.64 ± 0.18 with hyperaemia (p <0.001) [23]. The accuracy of resting iFR in that study was similar to that of resting Pd/Pao in detecting hyperaemic FFR <0.80, and both were inferior to hyperaemic iFR [23]. Jeremias at al. confirmed that iFR and resting Pd/Pao detected hyperaemic FFR <0.80 with similar overall accuracy of around 80%, and they concluded rightly that clinical outcome studies would be required to determine whether nonhyperaemic parameters such as iFR might avoid the need for hyperaemia [24].
Fractional flow reserve for ischaemia detection
Currently, the pressure-derived, hyperaemic ratio of Pd/Pao during adenosine infusion at 140 μg/kg/min (fractional flow reserve, FFR) is regarded as standard for the invasive coronary stenosis severity assessment [8, 13]. It is based on the above described theoretical framework involving Ohm’s law and the need for maximal hyperaemia for inhibition of coronary autoregulation. FFR measurement of intermediate coronary stenoses is recommended by guidelines when demonstration of ischaemia by noninvasive testing is unavailable [25]. Notwithstanding, visual angiographic stenosis assessment of percent diameter reduction as the decisional basis for or against revascularisation continues to be the more predominant method by far [26]. Technically and in the context of the above mentioned robust coronary pressure recordings, Pd and simultaneous Pao are simple to acquire, and the induction of hyperaemia using intravenous adenosine is safe. It is likely that the low acceptance rate of FFR in the clinical routine is related rather to the small effort required in addition to angiography than to its theoretical shortcomings, which are now outlined.
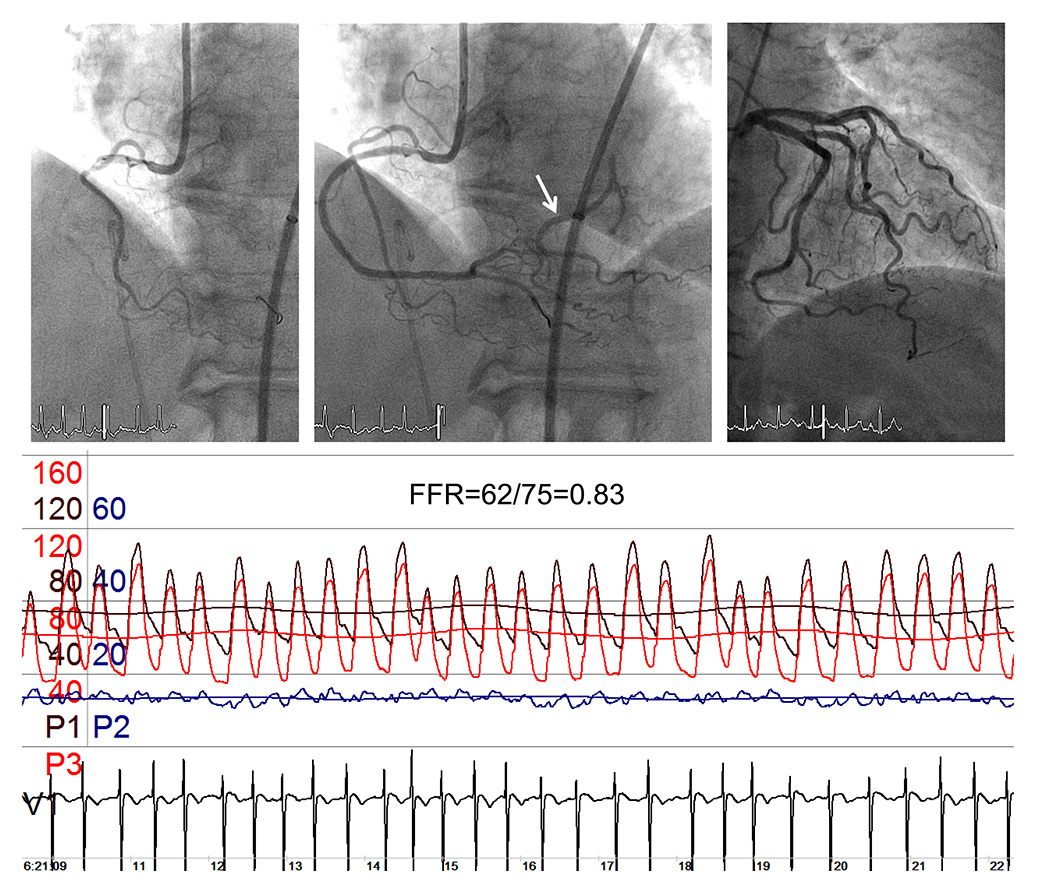
Figure 4
Coronary angiograms of the balloon-occluded right coronary artery (RCA, left upper panel), of the patent RCA with an ostial stenosis (mid upper panel) and showing part of the distal left circumflex coronary artery (arrow); the upper right panel depicts a left coronary angiogram with an identical trace of the distal left circumflex artery as shown in the mid upper panel. The pressure and ECG tracings are recorded during ostial RCA balloon occlusion: mean (Pao) and phasic aortic pressure in black, mean (Poccl) and phasic coronary occlusive pressure in red, central venous pressure (CVP) in blue, intracoronary ECG. FFR = fractional flow reserve as Poccl/Pao.

Figure 5
Relation between the fractional flow reserve (FFR) difference (post-ischaemic minus adenosine-induced FFR, x-axis) and collateral flow index as obtained in the same vessel (y-axis).
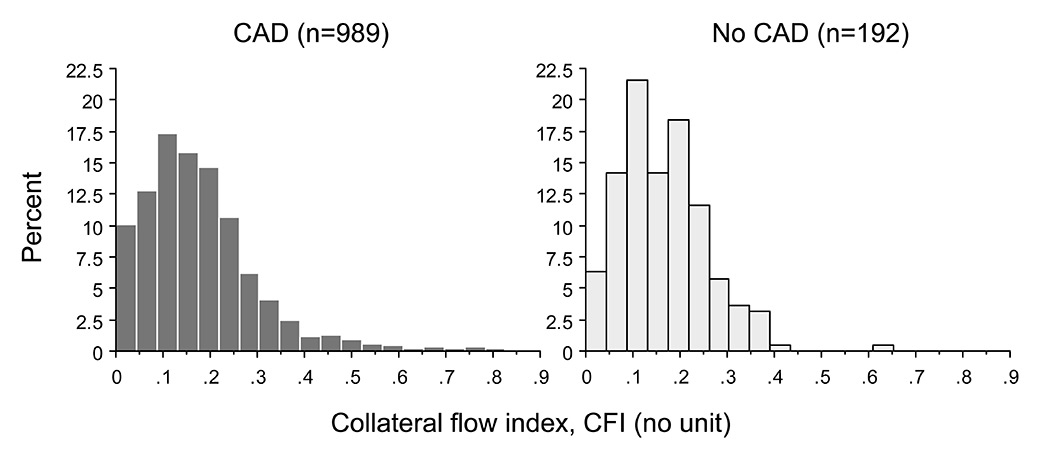
Figure 6
Frequency distribution of collateral flow index in patients with (dark gray columns) and without (light gray columns) coronary artery disease (CAD).
In two of the studies importantly contributing to the guideline recommendation mentioned [25], FFR is defined as “maximal blood flow in a stenotic artery to normal maximal flow” (FAME 1, [27]), and an FFR ≤0.80 is described as “a drop in maximal blood flow of 20% or more caused by stenosis”, respectively [9]. The two FFR definitions are consistent, and they both point to the relevant variables “blood flow”, “maximal blood flow” and “stenosis”. According to Ohm’s law, coronary pressure Pd can be taken for flow Q only under the necessary condition of a constant and minimal microcirculatory resistance R: ΔP = R×Q. As outlined above, it is questionable whether R is constant and minimal using standard hyperaemia induction with intravenous adenosine (see above; fig. 3). Therefore, the term “flow” and the adjective “maximal” are subject to error. Furthermore, but of minor importance, ΔP indicates that central venous pressure (CVP) as the coronary backpressure should be, ideally, recorded and used for the calculation of FFR ([Pd-CVP]/ ([Pao-CVP]).
The case shown in figure 4 of a balloon-occluded ostial stenosis of the right coronary artery with simultaneously obtained FFR = 0.83 indicates that also the term “stenosis” in the above description of FFR is a misnomer (fig. 4). FFR in the situation of a total coronary occlusion would be expected to be below the “ischaemic” detection threshold of 0.80, and it is only the angiographically depicted collateral supply from the left circumflex coronary artery, which can explain a “compensation” of a low FFR caused by the occlusion. Indeed, FFR as recorded in the catheterization lab for stenosis assessment is not a stenosis-specific but a myocardial haemodynamic parameter [8]:
FFR = FFRS+CFI
where FFRS is the stenosis-specific FFR and CFI (collateral flow index) is a parameter of collateral function as determined during a 1-minute coronary balloon occlusion [28]. The above description of FFR implies that the contribution of functional anastomoses between the coronary territories (collateral circulation) is negligible. The case shown in figure 4 contradicts the assumption of nonfunctional anastomoses. Furthermore, immediately postocclusive hyperaemia would be different from adenosine-induced hyperaemia depending on the function of collaterals to the occluded vessel. Figure 3 depicts a case without functional collaterals, showing a lower postischaemic than adenosine-induced hyperaemia FFR (fig. 3). The difference in FFR between the two types of hyperaemia induction is directly related to CFI as obtained during a systematic 1-minute proximal coronary balloon occlusion (fig. 5); CFI is calculated as (Poccl-CVP)/(Pao-CVP), where Poccl is the coronary pressure distal to the occluding balloon (see also fig. 4). Indeed, it is generally agreed that a majority of patients with and even without functionally relevant CAD have functional collaterals (fig. 6) [29], and that collaterals providing sufficient flow to prevent ECG signs of myocardial ischaemia during a brief coronary occlusion are prognostically relevant (fig. 7) [30].
The direct and stenosis-mitigating influence of coronary collateral function on FFR leave it open as to whether the survival benefit of patients deferred for PCI on the basis of FFR >0.80 (FAME1 trial, [27]) is due to the insignificance of the stenosis or to the protective effect of the collateral circulation [31]. This uncertainty does, however, not contradict the recommendation of FFR measurement as invasive ischaemia test in the absence of noninvasive testing. Its purpose is sorting out the coronary stenosis in which no PCI has to be performed. Conversely, in haemodynamically relevant stenosis (FFR ≤0.80) PCI should be proven superior to medical therapy among patients with chronic CAD. This was the aim of the initially cited FAME2 study [9]; its only positive finding of a 4.0% rate during 2 years in urgent revascularisation in the PCI group compared with 16.3% in the medical group (p <0.0001) cannot be interpreted as PCI efficiency, but as a self-fulfilling prophecy keeping in mind that the study was open-label in designed and the FFR threshold for ischaemia ≤0.80 was well accepted beforehand. In this setting, the physician of a patient in the medical group with chest discomfort was overly motivated for urgent referral for PCI. In other words, there was a bias by study design in favour of urgent revascularisation in the medical group by omitting PCI at study inclusion.
Conclusion
In patients with chronic stable coronary artery disease, flow-derived invasive measurements for stenosis severity assessment such as coronary flow reserve or resistance indices are, technically, prone to error, and thus not ready for clinical use. Purely pressure-derived haemodynamic indices yield more dependable source data. Despite many conceptual flaws, pressure-derived fractional flow reserve (FFR) as obtained during intravenous adenosine-induced myocardial hyperaemia can be used for invasive decision-making on percutaneous coronary intervention or medical treatment in intermediate stenosis. FFR is not purely stenosis-specific, as erroneously suggested, but provides a composite myocardial measure of the haemodynamic stenosis severity and the stenosis- or ischaemia-mitigating effect of the coronary collateral circulation.
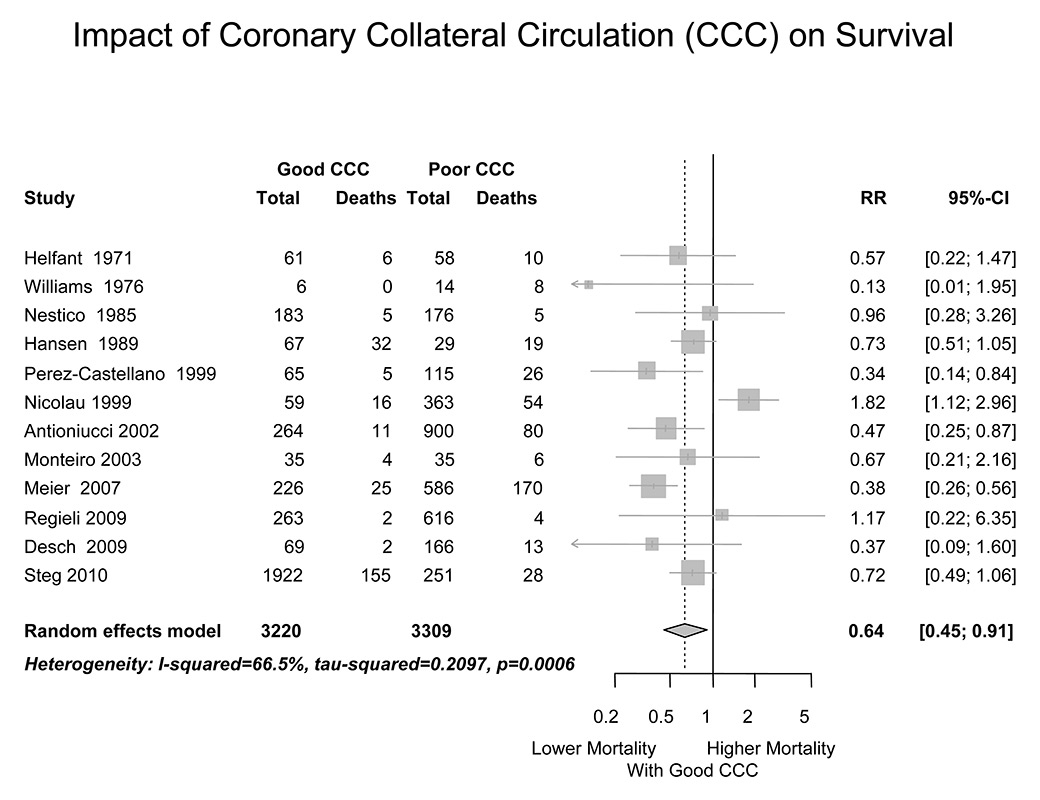
Figure 7
Forest plot of the effect of a “good” coronary collateral circulation on all-cause mortality of patients with coronary artery disease. In all studies except for one, “good” collaterals were defined angiographically as extensive filling of the main coronary branch of interest via anastomoses from an adjacent vessel.
References
1 Mehta SR, Cannon CP, Fox KA, Wallentin L, Boden WE, Spacek R, et al. Routine vs selective invasive strategies in patients with acute coronary syndromes: A collaborative meta-analysis of randomized trials. JAMA. 2005;293:2908–17.
2 Katritsis DG, Ioannidis JP. Percutaneous coronary intervention versus conservative therapy in nonacute coronary artery disease: A meta-analysis. Circulation. 2005;111:2906–12.
3 Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, et al. Optimal medical therapy with or without pci for stable coronary disease. N Engl J Med. 2007;356:1503–16.
4 Windecker S, Stortecky S, Stefanini GG, da Costa BR, Rutjes AW, Di Nisio M, et al. Revascularisation versus medical treatment in patients with stable coronary artery disease: Network meta-analysis. BMJ. 2014;348:epub ahead of print
5 Hachamovitch R, Rozanski A, Shaw LJ, Stone GW, Thomson LE, Friedman JD, et al. Impact of ischaemia and scar on the therapeutic benefit derived from myocardial revascularization vs. Medical therapy among patients undergoing stress-rest myocardial perfusion scintigraphy. Eur Heart J. 32:1012–24.
6 Shaw LJ, Berman DS, Maron DJ, Mancini GB, Hayes SW, Hartigan PM, et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: Results from the clinical outcomes utilizing revascularization and aggressive drug evaluation (courage) trial nuclear substudy. Circulation. 2008;117:1283–91.
7 Amier RP, Teunissen PF, Marques KM, Knaapen P, van Royen N. Invasive measurement of coronary microvascular resistance in patients with acute myocardial infarction treated by primary pci. Heart. 2014;100:13–20.
8 Pijls NHJ, van Son JAM, Kirkeeide RL, de Bruyne B, Gould KL. Experimental basis of determining maximum coronary, myocardial, and collateral blood flow by pressure measurements for assessing functional stenosis severity before and after percutaneous coronary angioplasty. Circulation. 1993;86:1354–67.
9 De Bruyne B, Fearon WF, Pijls NH, Barbato E, Tonino P, Piroth Z, et al., Investigators. FT. Fractional flow reserve-guided pci for stable coronary artery disease. N Engl J Med. 2014;371:1208–17.
10 Seiler C, Kirkeeide RL, Gould KL. Basic structure-function relations of the epicardial coronary vascular tree. Basis of quantitative coronary arteriography for diffuse coronary artery disease. Circulation. 1992;85:1987–2003.
11 Westerhof N, Boer C, Lamberts RR, Sipkema P. Cross-talk between cardiac muscle and coronary vasculature. Physiol Rev. 2006;86:1263–308.
12 de Marchi SF, Gloekler S, Rimoldi SF, Rölli P, Steck H, Seiler C. Microvascular response to metabolic and pressure challenge in the human coronary circulation. Am J Physiol Heart Circ Physiol. 2011;301:H434–441.
13 Kern MJ, Lerman A, Bech JW, De Bruyne B, Eeckhout E, Fearon WF, et al., American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization CoCC. Physiological assessment of coronary artery disease in the cardiac catheterization laboratory: A scientific statement from the american heart association committee on diagnostic and interventional cardiac catheterization, council on clinical cardiology. Circulation. 2006;114:1321–41.
14 Doucette JW, Corl PD, Payne HM, Flynn AE, Goto M, Nassi M, Segal J. Validation of a doppler guide wire for intravascular measurement of coronary artery flow velocity. Circulation. 1992;85:1899–911.
15 Seiler C. Coronary velocity pressure tracings. Eur Heart J. 1997;18:697–9.
16 De Bruyne B, Pijls NH, Smith L, Wievegg M, Heyndrickx GR. Coronary thermodilution to assess flow reserve: Experimental validation. Circulation. 2001;104:2003–6.
17 Indermühle A, Vogel R, Meier P, Wirth S, Stoop R, Mohaupt MG, Seiler C. The relative myocardial blood volume differentiates between hypertensive heart disease and athlete’s heart in humans. Eur Heart J. 2006;27:1571–8.
18 de Marchi SF, Schwerzmann M, Billinger M, Windecker S, Meier B, Seiler C. Sympathetic stimulation using the cold pressor test increases coronary collateral flow. Swiss Med Wkly. 2001;131:351–6.
19 Lim WH, Koo BK, Nam CW, Doh JH, Park JJ, Yang HM, et al. Variability of fractional flow reserve according to the methods of hyperemia induction. Catheter Cardiovasc Interv. 2014:epub ahead of print
20 Casella G, Leibig M, Schiele TM, Schrepf R, Seelig V, Stempfle HU, et al. Are high doses of intracoronary adenosine an alternative to standard intravenous adenosine for the assessment of fractional flow reserve? Am Heart J. 2004;148:590–5.
21 De Luca G, Venegoni L, Iorio S, Giuliani L, Marino P. Effects of increasing doses of intracoronary adenosine on the assessment of fractional flow reserve. JACC Cardiovasc Interv. 2011;4:1079–84.
22 Sen S, Escaned J, Malik IS, Mikhail GW, Foale RA, Mila R, et al. Development and validation of a new adenosine-independent index of stenosis severity from coronary wave-intensity analysis: Results of the advise (adenosine vasodilator independent stenosis evaluation) study. J Am Coll Cardiol. 2012;59:1392–402.
23 Berry C, van ’t Veer M, Witt N, Kala P, Bocek O, Pyxaras SA, et al. Verify (verification of instantaneous wave-free ratio and fractional flow reserve for the assessment of coronary artery stenosis severity in everyday practice): A multicenter study in consecutive patients. J Am Coll Cardiol. 2013;61:1421–7.
24 Jeremias A, Maehara A, Généreux P, Asrress KN, Berry C, De Bruyne B, et al. Multicenter core laboratory comparison of the instantaneous wave-free ratio and resting pd/pa with fractional flow reserve: The resolve study. J Am Coll Cardiol. 2014;63:1253–61.
25 Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, et al. 2014 esc/eacts guidelines on myocardial revascularization: The task force on myocardial revascularization of the european society of cardiology (esc) and the european association for cardio-thoracic surgery (eacts) developed with the special contribution of the european association of percutaneous cardiovascular interventions (eapci). Eur Heart J. 2014;35:2541–619.
26 Toth GG, Toth B, Johnson NP, De Vroey F, Di Serafino L, Pyxaras S, et al. Revascularization decisions in patients with stable angina and intermediate lesions: Results of the international survey on interventional strategy. Circ Cardiovasc Interv. 2014:[Epub ahead of print]
27 Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’ t Veer M, et al., Investigators FS. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–24.
28 Seiler C, Fleisch M, Garachemani A, Meier B. Coronary collateral quantitation in patients with coronary artery disease using intravascular flow velocity or pressure measurements. J Am Coll Cardiol. 1998;32:1272–9.
29 Meier P, Gloekler S, Zbinden R, Beckh S, de Marchi S, Zbinden S, et al. Beneficial effect of recruitable collaterals: A 10–year follow-up study in patients with stable coronary artery disease undergoing quantitative collateral measurements. Circulation. 2007;116:975–83.
30 Meier P, Hemingway H, Lansky AJ, Knapp G, Pitt B, Seiler C. The impact of the coronary collateral circulation on mortality: A meta-analysis. Eur Heart J. 2012;33:614–21.
31 Johnson NP, Tóth GG, Lai D, Zhu H, Açar G, Agostoni P, et al. Prognostic value of fractional flow reserve: Linking physiologic severity to clinical outcomes. J Am Coll Cardiol. 2014;64:1641–54.






