Thrombus formation in the left ventricle after large myocardial infarction – assessment with cardiac magnetic resonance imaging
DOI: https://doi.org/10.4414/smw.2015.14122
Daniel
Sürder, Valentin
Gisler, Roberto
Corti, Tiziano
Moccetti, Catherine
Klersy, Michel
Zuber, Stephan
Windecker, Aris
Moschovitis, Sebastian
Kozerke, Thomas Felix
Lüscher, Paul
Erne, Robert
Manka
Summary
INTRODUCTION: Left ventricular thrombus (LVT) formation may worsen the post-infarct outcome as a result of thromboembolic events. It also complicates the use of modern antiplatelet regimens, which are not compatible with long-term oral anticoagulation. The knowledge of the incidence of LVT may therefore be of importance to guide antiplatelet and antithrombotic therapy after acute myocardial infarction (AMI).
METHODS: In 177 patients with large, mainly anterior AMI, standard cardiac magnetic resonance imaging (CMR) including cine and late gadolinium enhancement (LGE) imaging was performed shortly after AMI as per protocol. CMR images were analysed at an independent core laboratory blinded to the clinical data. Transthoracic echocardiography (TTE) was not mandatory for the trial, but was performed in 64% of the cases following standard of care. In a logistic model, 3 out of 61 parameters were used in a multivariable model to predict LVT.
RESULTS: LVT was detected by use of CMR in 6.2% (95% confidence interval [CI] 3.1%–10.8%). LGE sequences were best to detect LVT, which may be missed in cine sequences. We identified body mass index (odds ratio 1.18; p = 0.01), baseline platelet count (odds ratio 1.01, p = 0.01) and infarct size as assessed by use of CMR (odds ratio 1.03, p = 0.02) as best predictors for LVT. The agreement between TTE and CMR for the detection of LVT is substantial (kappa = 0.70).
DISCUSSION: In the current analysis, the incidence of LVT shortly after AMI is relatively low, even in a patient population at high risk. An optimal modality for LVT detection is LGE-CMR but TTE has an acceptable accuracy when LGE-CMR is not available.
Introduction
Acute myocardial infarction (AMI) still represents a major cause of mortality and morbidity [1]. Early mortality decreased dramatically because of widespread use of out clinic automatic defibrillators, and improvement in acute cardiac care leading to shorter times between first medical contact and primary percutaneous coronary intervention (PCI) [2]. Nevertheless, morbidity due to post-infarct complications is still an important issue.

Figure 1
Left ventricular thrombus (4.03 cm3) in the short axis (A), vertical long axis (B) and horizontal long axis (C) delayed enhancement cardiac magnetic resonance imaging (DE-CMR) sequences. This thrombus is as well detectable in the CMR-cine sequences, here shown in the vertical axis (D).
Among other factors, left ventricular thrombus (LVT) formation may worsen the post-infarct outcome owing to thromboembolic events [3, 4]. Most left ventricular (LV) thrombi are “mural”, covering the left ventricular endocardial wall with thrombotic material. In the prethrombolytic era, the incidence of LVT after AMI was reported to lie between 17% [5] and 21% [6], with a peak incidence in anterior-located infarcts of up to 46% [5], depending mostly on infarct size. More recently, in the era of rapid reperfusion therapy, the incidence of LVT markedly decreased to 6.2% [7], when assessed by use of transthoracic echocardiography (TTE), and up to 8.8% [8], when entirely assessed with late gadolinium enhancement (LGE) cardiac magnetic resonance imaging (CMR). Although LVT with only thin layers of thrombotic material may be difficult to detect by looking at anatomical criteria (e.g. TTE; cine CMR), tissue characterisation criteria (i.e. LGE-CMR) are very powerful to detect these types of thrombi.
In the prethrombolytic and thrombolytic eras, prophylactic long-term oral anticoagulation with a vitamin K antagonist was often recommended [9–11] to prevent LVT formation. To date, in the era of primary PCI and drug-eluting stents (DES), the European guidelines [12] consider long-term anticoagulation in the case of extensive, mainly anterior AMI whereas they clearly recommend it exclusively in the case of documented mural LVT.
Recent guidelines from the American Heart Association [13], consider long-term anticoagulation therapy as a class IIb indication for patients with a general risk of thromboembolic events, without, however, explicitly mentioning LVT formation as a specific clinical situation.
This is of particular importance as combining oral anticoagulation and double antiplatelet therapy (DAPT) into a triple therapy increases bleeding risk and limits the choice of newer P2Y12 inhibitors, such as prasugrel or ticagrelor, which are prohibited in combination with vitamin K antagonists as well as to newer oral anticoagulants such as dabigatran, rivaroxaban, edoxaban or apixaban [13].
Short-term anticoagulation regimens after AMI, on the other hand, are not precisely addressed in current guidelines [12, 13]. In absence of data from randomised trials, it is not clear if therapeutic parenteral anticoagulation using either heparin or low molecular weight heparin (LMWH) directly after primary PCI may efficiently prevent LVT formation and for how long such therapy should be maintained.
Furthermore, patient- or infarct-related characteristics to qualify for long-term oral anticoagulation to prevent LVT formation are not well considered.
The aim of the present study is therefore three-fold: first, to assess prospectively the incidence of mural LVT in a patient population that would be considered at risk for LVT in accordance with current guidelines; second, to determine the optimal imaging modality to detect reliably LV thrombus formation; and third; to look at patient- and infarct-related factors predisposing to mural LVT formation in the modern era of primary PCI.
Methods
The present analysis is a substudy of the SWISS-AMI-trial [14, 15]. This randomised, multicentre trial, aiming to test the effect of intracoronary bone-marrow-derived mononuclear cell (BM-MNC) administration, was performed in four Swiss cardiovascular centres between 2006 and 2012, and included a total of 200 patients, mainly with anterior AMI, successfully reperfused by means of primary PCI and with a left ventricular ejection fraction (LVEF) less than 45%. As per protocol, all patients were studied with LGE-CMR at baseline. The main inclusion criteria were a successful primary PCI (or rescue PCI after initial thrombolysis) within 24 hours after the onset of chest pain and a visual LVEF equal or lower than 45%, as assessed by use of left ventricular angiography the day of AMI or, alternatively, with TTE the day following the AMI. The study was approved by the local ethics committee as well as by the competent federal authorities.
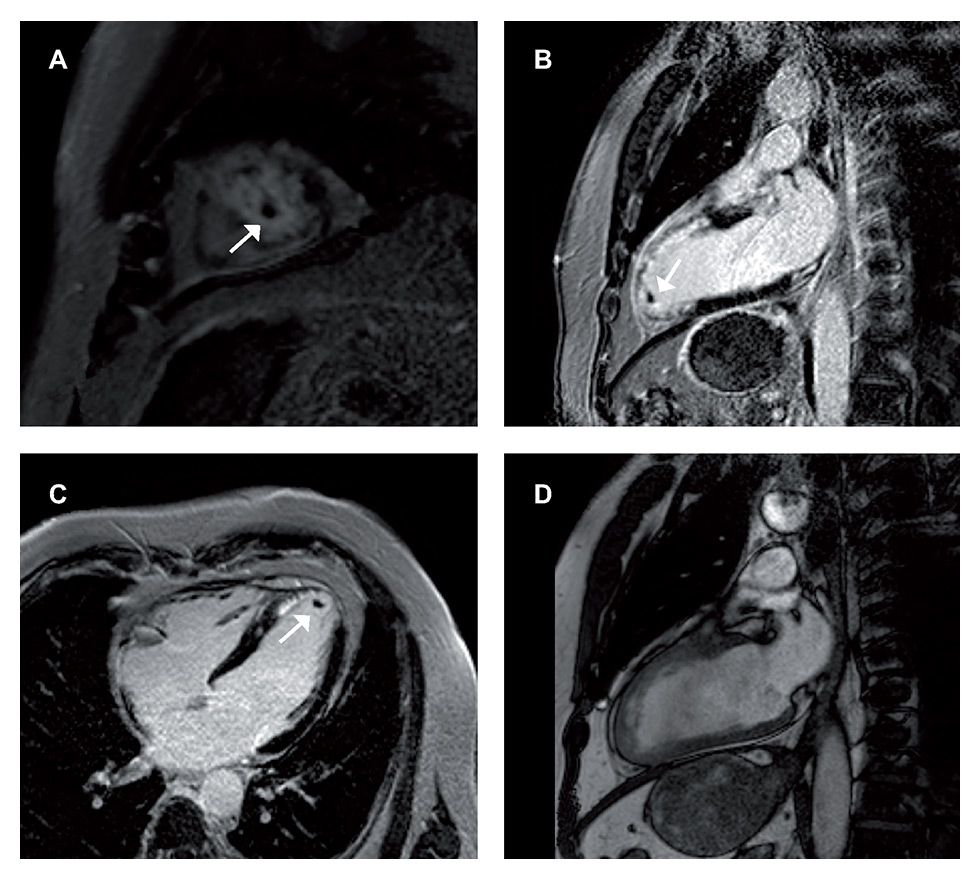
Figure 2
Left ventricular thrombus (0.166 cm3) shown in the short axis (A), vertical long axis (B) and horizontal long axis (C) delayed enhancement cardiac magnetic resonance imaging (DE-CMR) sequences. This thrombus is not detectable in the CMR-cine sequences, here shown the vertical axis (D).
Cardiac magnetic resonance imaging
CMR was performed at a median of 6 days (4 to 8) after the AMI using 1.5-Tesla clinical MR system (Siemens Sonata 1.5 Tesla; Philips Achiever 1.5 Tesla) with a dedicated cardiac phased-array receiver coil for signal reception, following a standard imaging protocol. It included cardiac function (cine) and LGE imaging. Cine imaging of the LV was performed by standard ECG-triggered steady-state free precession sequence during repetitive breath-holds in three long-axis orientations and in contiguous short-axis orientation covering the entire LV. LGE imaging was performed 20 minutes after administration of a bolus of a conventional extracellular gadolinium-chelates contrast medium at a dose of 0.20 mmol per kilogram of bodyweight by using an inversion-recovery (IR) fast gradient echo imaging sequence [16–18]. After determination of the inversion time nulling for normal myocardium, LGE imaging was performed in identical locations to those where cine data were acquired. CMR data analysis including research for mural LVT was entirely performed in an experimented core laboratory (University Hospital Zurich/CH) using a dedicated cardiac analysis software (GTVolume, Gyrotools Ltd, Zurich/CH). First, LV end-diastolic (LV-EDV) and end-systolic (LV-ESV) volumes, LVEF and LV mass were analysed. Scar mass and tissue with microvascular obstruction (MVO) were assessed in grams and as a percentage of LV mass and of scar mass, respectively. The core laboratory was completely blinded to all clinical data of the included patients. They were blinded as to whether LVT was found at TTE or not.
The presence or absence of LVT was assessed by analysing visually LGE images (short axis, vertical long axis, horizontal long axis) of each patient as well as by analysing the corresponding cine sequences. LGE imaging has the advantage over cine sequences in that gadolinium may improve the contrast between thrombus and myocardium. Furthermore, image quality is not compromised by arrhythmias as images are acquired in end-systole. Thrombus size was assessed by planimetry in short axis and vertical long axis. Dedicated additional techniques, such as sequential infrared imaging and a long-inversion time, as described later by Weinsaft [19] and co-workers, had not yet been sufficiently studied when the trial started in 2006.
Transthoracic echocardiography
TTE was not part of the original study protocol. However, it was often used for clinical purposes, particularly in order to screen for eligibility for the SWISS AMI trial as one of the key inclusion criteria was a visual LVEF of <45%. Of note, no study-specific protocol was applied; assessment of LV function and the research for mural LVT was performed according to the routine protocol of the participating centre, and the use of contrast medium was neither encouraged nor prohibited. In 15 patients (13.2%) it was effectively utilised to evaluate the absence or presence of LVT. Operators or readers of TTE were not blinded to the results of CMR per protocol; however, in only a minority of cases (14 out of 113) were CMR results available at the time of TTE.
Statistical analysis
Descriptive statistics of continuous variables are presented as mean and standard deviation or median and 25th and 75th percentiles, if skewed. Nominal variables are summarised in terms of frequencies and percentages.
The agreement between echo and the reference test CMR to identify thrombi was assessed with the kappa statistic. Also, sensitivity, specificity and predictive values (with 95% confidence intervals [CI]) were computed [20].
Predictors of LVT at CMR were assessed by means of either logistic regression or exact logistic regression models; odds ratios (OR) and 95% CIs were computed for 61 parameters in a univariable analysis. Multivariable modelling was limited by the low number of patients with LVT; for this reason we only included the best predictor among each of cardiovascular risk factors, biomarkers and CMR- or infarct-related parameters. The receiver operating characteristic (ROC) area und the curve were computed for each model to measure discrimination and the shrinkage coefficient to measure overfitting (for both, the closer to 1, the better) [21].
Continuous variables with skewed distribution were log transformed. All p-values are two–sided and values ≤0.05 are considered significant. Stata 13 (StataCorp, College Station, TX, USA) was used for computation.
|
Table 1:Baseline patient characteristics. |
|
|
CMR
(n = 177)
|
CMR and TTE
(n = 113)
|
| Age – years |
56.8 (10.2) |
56.0 (9.6) |
| BMI – kg/m2
|
27.3 (3.9) |
27.3 (3.6) |
| Male gender – % |
85.3 |
87.6 |
| Hypertension – % |
42.1 |
40.2 |
| Hyperlipidemia – % |
41.5 |
43.7 |
| Diabetes – % |
10.8 |
8.9 |
| Smoking (active/previous) – % |
58.5 |
64.3 |
| Family history of CAD – % |
30.1 |
33.9 |
| 1 / 2 / 3 vessel disease – % |
57.9 / 27.3 / 14.8 |
59.8 / 27.7 / 12.5 |
| Previous PCI before AMI – % |
2.3 |
3.6 |
|
Infarct treatment and parameters
|
| Primary PCI – % |
97.2 |
97.3 |
| Concomitant PCI other
than infarct related artery – % |
14.3 |
16.2 |
| Infarct vessel LAD / LCX / RCA – % |
93.2 / 2.3 / 4.5 |
95.5 / 0.9 / 3.6 |
| Pain to revascularization time – h * |
4.51 (2.75–8.00) |
4.00 (2.75–7.25) |
| Stent diameter – mm |
3.27 (0.34) |
3.26 (0.34) |
| Drug eluting stent – % |
77.4 |
73.5 |
| TIMI 0 flow before PCI – % |
89.6 |
86.2 |
| TIMI 3 flow after PCI – % |
94.2 |
92.7 |
| Use of GP IIb/IIIa inhib. / bivalirudin – % |
76.7 |
78.6 |
| Maximal creatine kinase – U/l* |
3891 (2206–5805) |
4280 (2598–6169) |
| Maximal creatine kinase-MB – U/l |
305 (191–493) |
327 (185–489) |
| Baseline NT-proBNP – ng/l |
1421 (811–2555) |
1678 (877–2605) |
| Total cholesterol – mmol/l |
4.56 (1.27) |
4.64 (1.24) |
| LDL-cholesterol – mmol/l |
2.85 (1.19) |
2.92 (1.17) |
| Creatinine – mmol/l |
83.4 (19.7) |
81.9 (16.2) |
| CRP – mg/l |
6.9 (3–24) |
11 (4–26) |
| ASAT – U/l |
60 (34–155) |
66 (34–180) |
| LDH – U/l |
773 (455–1274) |
863 (559–1478) |
| WBC – (x109/l) |
9.83 (3.94) |
9.8 (4.2) |
| Haemoglobin – g/dl |
13.72 (1.70) |
13.7 (1.8) |
| Haematocrit – % |
39.75 (4.73) |
39.7 (4.9) |
| Platelets – (x109/l) |
263.1 (80.1) |
270.3 (82.8) |
|
Infarct treatment at CMR
|
| Infarct size – g |
42.6 (24.5) |
45.4 (26.1) |
| % infarction |
28.5 (12.2) |
29.2 (12.9) |
| Microvascular obstruction – g |
0.64 (0–2.69) |
0.92 (0–3.77) |
| LVEDV – ml |
154.7 (38.1) |
160.4 (38.8) |
| LVESV – ml |
97.6 (32.6) |
103.9 (34.1) |
| LVEF – % |
37.7 (9.5) |
36.0 (9.4) |
|
Medication before AMI– % |
| Aspirin |
5.1 |
5.4 |
| Clopidogrel |
1.1 |
1.8 |
| ACE-inihibitor |
6.3 |
5.4 |
| ATII receptor blocker |
8.6 |
8.9 |
| Beta-blocker |
5.1 |
3.6 |
| Diuretic |
3.4 |
3.6 |
| Statin |
5.7 |
6.2 |
| Oral antidiabetic drug |
4.0 |
5.4 |
|
Medication at discharge– % |
| Aspirin |
98.3 |
97.3 |
| Clopidogrel |
69.1 |
66.1 |
| Prasugrel |
30.9 |
33.9 |
| ACE-inihibitor |
88.6 |
85.7 |
| ATII receptor blocker |
8.6 |
8.9 |
| Beta-blocker |
90.3 |
91.1 |
| Diuretic |
30.3 |
33.9 |
| Statin |
98.9 |
99.1 |
| Oral antidiabetic drug |
6.3 |
7.1 |
| ACE = angiotensin converting-enzyme; AMI = acute myocardial infarction; ASAT = aspartate aminotransferase; ATII = angiotensin II receptor; BMI = body mass index; CAD = coronary artery disease; CMR = cardiac magnetic resonance imaging; CRP = C-reactive protein; LAD = left anterior descending artery; LCX = left circumflex artery; LDH = lactate dehydrogenase; LDL = low-density lipoprotein; LVEDV = left ventricular end-diastolic volume; LVEF = left ventricular ejection fraction; LVESV = left ventricular end-systolic volume; NT-proBNP = N-terminal of prohormone brain natriuretic peptide; PCI = percutaneous coronary intervention RCA = right coronary artery; TTE = transthoracic echocardiography; WBC = white blood cell count;
Continuous variables are presented as mean and standard deviation, or (*) median and 25th–75th percentiles if skewed. Nominal variables are summarised in terms of percentages. |
|
Table 2:Characteristics of the left ventricular thrombi and left ventricular thrombus detection. |
|
Thrombus
|
LE-CMR sequences
|
CMR-cine sequences
|
TTE
|
Time delay
|
| No. |
Dimension (cm3) |
sax |
vla |
hla |
3D |
sax |
vla |
hla |
| 1 |
0.166 |
+ |
+ |
+ |
ND |
– |
– |
– |
ND |
ND |
| 2 |
5.724 |
+ |
+ |
+ |
ND |
+ |
+ |
+ |
+ * |
–1 |
| 3 |
4.836 |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
–15 |
| 4 |
5.062 |
+ |
+ |
+ |
ND |
+ |
+ |
+ |
+ |
1 |
| 5 |
2.188 |
+ |
+ |
+ |
+ |
– |
– |
+ |
+ |
1 |
| 6 |
14.3 |
+ |
+ |
+ |
ND |
+ |
+ |
+ |
– |
3 |
| 7 |
4.03 |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ * |
–1 |
| 8 |
0.027 |
+ |
+ |
– |
+ |
– |
– |
– |
+ |
10 |
| 9 |
0.427 |
+ |
+ |
+ |
+ |
– |
– |
– |
– |
6 |
| 10 |
0.768 |
+ |
+ |
– |
+ |
+ |
– |
– |
+ |
–2 |
| 11 |
8.067 |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
| 3D = three dimensional; CMR = cardiac magnetic resonance imaging; hla: horizontal long axis; LE = late enhancement; ND = not done; No. = number; sax = short axis; vla = vertical long axis; TTE: trans-thoracic echocardiography
Time delay is the time delay between TTE and CMR (if positive TTE before CMR, if negative TTE after CMR).
* TTE was also performed using contrast medium. |
Results
A total of 200 patients were included in the SWISS AMI study between October 2006 and January 2012 in four Swiss participating centres. Excluding early drop-outs and other cases in which for some reason no CMR has been performed at baseline, 177 patients were included in the present study. In 113 cases (63.8%), besides baseline CMR imaging, TTE was also performed in the first days after AMI.
The patients’ mean age was 56.8 (10.2) years and 85% were of male gender. Overall, 93% of the patients had anterior ST-elevation myocardial infarction (STEMI) due to occlusion of the left anterior descending coronary artery, and the time from onset of chest pain to reperfusion therapy was 6.5 (5.8) hours. Overall, 77% of the patients were treated with glycoprotein IIb/IIIa inhibitors or bivalirudin and 77% received a DES with a mean diameter of 3.3 (0.3) mm. Maximal creatine kinase (CK) plasma levels were 4,291 (2,499) U/l, and baseline LVEF, as assessed by CMR was 37.7% (9.5%).
The entire recorded patient characteristics are shown in table 1.
Thrombus detection
A total of 11 (prevalence: 6.2%, 95% CI 3.1%–10.8%) mural LV thrombi were detected, with a mean volume of 4.1 (4.3) cm3 (table 2). Although all thrombi were detected in the short axis and vertical long axis of the LGE sequences, in two cases with a small thrombus burden (<1 cm3), LVT was not seen in the horizontal long axis of the LGE sequences. LVT was more frequently missed in the cine sequences: in three cases, owing to a small volume (<0.5 cm3), LVT was not detectable at all and in two cases even larger LV thrombi were detected in only one out of three cine sequences.
TTE was performed in 113 (63.8%) of the included patients within the first days after AMI. It was available in 10 of the 11 LVT cases and was performed at a median of 1 day (–0.75 to 2.5 days) after CMR. In two cases LVT was not visible at TTE. In one case this may be explained by the small size of the LVT (0.43 cm3) but in the second case the missed LVT was the largest of all detected LV thrombi (14.3 cm3). In four cases TTE images were positive or suspicious for LVT, without confirmation by CMR. Of note, the TTE positive for LVT were performed in all cases before CMR (5 days, 2 days, 1 day and 1 day earlier) and subsequent CMR imaging was therefore performed under full anticoagulation therapy, which was immediately started after receiving the TTE results or maintained if already/still active. Contrast medium (CM) was used in 15 patients (13.3%). In 10 patients the use of CM permitted LVT to be ruled out, which was confirmed by CMR in all cases. Among the five patients who were tested “positive” for LVT by TTE using CM, this was, confirmed only in two cases.
Whether CM was used or not, the diagnostic value of TTE was fairly good (supplemental table), with an area under the ROC curve of 0.88 (95% CI 0.75–1), a sensitivity of 80% (95% CI 44.4%–97.5%) and a specificity of 96.1% (95% CI 90.4%–98.9%). The agreement between TTE and CMR for the detection of LVT is substantial (kappa statistic = 0.70; 7.1% positive agreement, 86.1% negative agreement).
Predictors for LVT formation
Searching for predictors of LVT, a total of 61 parameters have been addressed in a univariable analysis (table 3). In a logistic model we identified BMI (odds ratio 1.18; p = 0.01), baseline platelet count (odds ratio 1.01, p = 0.01) and infarct size as assessed by CMR (odds ratio 1.03, p = 0.02) as best predictors for LVT. Taken together in a multivariable model, these three parameters solidly describe the risk of LVT formation (chi-square –26.63, p = 0.002), with a good discrimination ability (area under the ROC curve = 0.83) and little overfitting (shrinkage 0.80).
Clinical events
As defined in the study protocol, the incidence of stroke (and other clinical events such as death, myocardial infarction or coronary revascularisation) has been assessed in all included patients. The 11 patients with documented thrombus formation were entirely treated with oral anticoagulation on top of DAPT in addition to therapeutic LWMH or unfractionated heparin until a therapeutic INR (between 2 and 3) was reached. No stroke has been reported in the first 4 months, whereas 2 (0.6%) strokes occurred in the patients without documented LVT formation.
|
Table 3:Predictors of LVT formation. |
|
|
Presence of Thrombus
|
Absence of Thrombus
|
OR (95%CI)
|
p
|
|
Gender – no (%) |
| Male |
10 (6.6) |
141 (93.3) |
– |
0.71 |
| Female |
1 (3.8) |
25 (96.2) |
0.56 (0.01–4.30) |
|
Infarct vessel – no (%) |
| LAD |
11 (6.7) |
153 (93.3) |
– |
0.74 |
| LCX |
|
4 (100) |
2.71 (0–23.2) |
| RCA |
|
8 (100) |
1.30 (0–9.36) |
|
Coronary artery disease– no (%) |
| 1 vessel disease |
9 (8.8) |
93 (91.2) |
– |
0.18 |
| 2 vessel disease |
2 (4.2) |
46 (95.8) |
0.45 (0.05–2.3) |
| 3 vessel disease |
|
26 (100) |
0.29 (0–1.96) |
| Previous PCI before AMI |
1 (25) |
3 (75) |
5.30 (0.09–73.34) |
0.23 |
| No previous PCI before AMI |
10 (5.8) |
162 (94.2) |
– |
|
Infarct treatment and circumstances of AMI– no (%) |
| Primary PCI |
11 (6.4) |
161 (93.6) |
– |
1.00 |
| Thrombolysis |
|
5 (100) |
2.2 (0–17.9) |
| Concomitant PCI other than infarct related artery |
1 (4) |
24 (96) |
– |
0.71 |
| No Concomitant PCI other than infarct related artery |
10 (6.7) |
140 (93.3) |
0.58 (0.01–4.46) |
| TIMI flow before PCI = 0 |
8 (5.2) |
147 (94.8) |
– |
1.00 |
| TIMI flow before PCI = 1 |
1 (7.7) |
12 (92.3) |
1.53 (0.32–13.17) |
| TIMI flow before PCI = 2 |
|
4 (100) |
3.61 (0–31.87) |
| TIMI flow before PCI = 3 |
|
1 (100) |
18.5 (0–721) |
| TIMI flow after PCI = 2 |
1 (10) |
9 (90) |
– |
1.00 |
| TIMI flow after PCI = 3 |
8 (4.9) |
155 (95.1) |
0.47 (0.52–22.87) |
| Use of Glycoprotein IIb/IIIa
Inhibitors / bivalirudin |
8 |
127 |
– |
1.00 |
| No use of Glycoprotein
Ib/IIIa Inhibitors / bivalirudin |
3 (7.3) |
38 (92.7) |
0.80 (0.18–4.90) |
| Early heart failure after AMI |
4 (13.8) |
25 (86.2) |
– |
0.09 |
| No heart failure after AMI |
7 (4.8) |
140 (95.2) |
3.17 (0.63–13.60) |
| Initial VF during AMI |
3 (9.1) |
30 (90.9) |
– |
0.69 |
| No VF during AMI |
8 (5.6) |
135 (94.4) |
1.68 (0.27–7.55) |
| Time from initial chest pain to revascularization (h) – * |
4.4 (3.0–18.0) |
4.5 (2.7–77) |
1.56 (0.66–3.67) |
0.32 |
|
Cardiovascular risk factors– no (%) |
| Arterial hypertension |
6 (8.1) |
68 (91.9) |
– |
0.53 |
| No Arterial hypertension |
5 (4.9) |
97 (95.1) |
1.71 (0.42–7.37 |
| Hyperlipidemia |
6 (8.2) |
67 (91.8) |
– |
0.53 |
| No Hyperlipidemia |
5 (4.8) |
98 (95.1) |
1.75 (0.42–7.56) |
| Familiar history for CVD |
3 (5.7) |
50 (94.3) |
– |
1.00 |
| No Familiar history for CVD |
8 (6.5) |
115 (93.5) |
0.86 (0.14–3.79) |
| Smoking (former or active) |
8 (7.8) |
95 (92.2) |
– |
0.37 |
| No smoking (former or active) |
3 (4.1) |
70 (95.9) |
1.96 (0.45–11.87) |
| Diabetes mellitus |
3 (15.8) |
16 (84.2) |
– |
0.10 |
| No diabetes mellitus |
8 (5.1) |
149 (94.9) |
3.46 (0.54–16.38) |
| BMI – mean (SD) |
30.4 (5.3) |
27.1 (3.7) |
1.18 (1.04–1.34) |
0.01 |
| Age (years) – mean (SD) |
57.0 (8.5) |
56.8 (10.3) |
1.00 (0.94–1.06) |
0.95 |
|
Baseline biological markers– mean (SD) or *median (25th-75th) |
| Maximal CK – U/l* |
4363 (1815–6480) |
3882 (2212–5794) |
0.96 (0.39–2.41) |
0.93 |
| Maximal CK-MB – U/l* |
493 (218–660) |
301 (184–489) |
1.86 (0.78–4.45) |
0.14 |
| nt-pro BNP – ng/l* |
2350 (1661–3002) |
1325 811-2496) |
1.26 (0.54–2.94) |
0.58 |
| Hemoglobin – g/dl |
14.1 (1.2) |
13.7 (1.7) |
1.16 (0.77–1.76) |
0.47 |
| Hematocrite – % |
40.5 (4.2) |
39.7 (4.8) |
1.04 (0.90–1.20) |
0.60 |
| Platelets – [x109/l] |
334 (108) |
258 (76) |
1.01 (1.00–1.02) |
0.01 |
| Creatinine – mcmol/l |
88.9 (26.3) |
83.1 (19.4) |
1.01 (0.98–1.05) |
0.44 |
| White blood cells – [x109/l]* |
12.8 (10–14.1) |
8.7 (6.6–12.2) |
5.81 (0.84–40.46) |
0.07 |
| C-reactice protein – mg/l* |
9.5 (4–24) |
6.85 (3–24) |
1.04 (0.63–1.72) |
0.89 |
|
CMR characteristics of the LV – mean (SD)or *median (25th–75th) |
| Delay between AMI and CMR – days* |
8 (4–10) |
6 (4–8) |
2.00 (0.58–6.91) |
0.27 |
| LVEF – % |
35.2 (6.3) |
37.8 (9.7) |
0.97 (0.91–1.04) |
0.36 |
| LVEDV – ml |
174 (24) |
153 (39) |
1.01 (1.00–1.03) |
0.10 |
| LVESV – ml |
113 (22) |
97 (33) |
1.01 (1.00–1.03) |
0.12 |
| Infarct size – g |
60.0 (23.7) |
41.4 (24.2) |
1.03 (1.00–1.05) |
0.02 |
| Myocardial scar – % |
34.0 (9.5) |
28.1 (12.3) |
1.04 (0.99–1.09) |
0.13 |
| LV mass diastolic |
147 (36) |
124 (41) |
1.01 (1.00–1.03) |
0.08 |
| Presence of MVO – no (%) |
6 (6.1) |
92 (93.9) |
– |
1.00 |
| Absence of MVO – no (%) |
3 (5.2) |
55 (94.8) |
1.19 (0.24–7.68) |
|
Medication before AMI– no (%) |
| Aspirin |
1 (11.1) |
8 (88.9) |
– |
1.00 |
| No Aspirin |
10 (6.0) |
156 (94.0) |
1.94 (0.40–17.22) |
| Clopidogrel |
|
2 (100) |
– |
1.00 |
| No Clopidogrel |
11 (6.4) |
162 (93.6) |
6.22 (0–82.33) |
| ACE-inhibitor |
|
11 (100) |
– |
0.63 |
| No ACE-inhibitor |
11 (6.7) |
153 (93.3) |
0.94 (0–6.45) |
| ATII receptor blocker |
|
15 (100) |
– |
0.60 |
| No ATII receptor blocker |
11 (6.9) |
149 (93.1) |
0.7 (0–4.44) |
| Beta-blocker |
|
9 (100) |
– |
0.65 |
| No Beta-blocker |
11 (6.6) |
155 (93.4) |
1.17 (0-8.24) |
| Statin |
|
10 (100) |
– |
0.64 |
| No statin |
11 (6.7) |
154 (93.3) |
1.04 (0–7.24) |
|
Medication acutely after AMI– no (%) |
| Aspirin |
11 (6.4) |
161 (93.6) |
– |
1.00 |
| No Aspirin |
|
3 (100) |
0.26 (0.03–Inf) |
| Clopidogrel |
8 (6.6) |
113 (93.4) |
– |
1.00 |
| No Clopidogrel |
3 (5.6) |
51 (94.4) |
1.20 (0.27–7.32) |
| Prasugrel |
3 (5.6) |
51 (94.4) |
– |
1.00 |
| No Prasugrel |
8 (6.6) |
113 (93.4) |
0.83 (0.14–3.65) |
| Continuous variables are presented as mean and standard deviation or (*) median and 25th–75th percentiles, if skewed and log-transformed for regression analysis.
Nominal variables are summarized in terms of percentages.
Abbreviations: OR: odds ration; LAD: left anterior descending coronary artery; LCX: left circumflex artery; RCA right coronary artery; PCI: Percutaneous coronary intervention; AMI: acute myocardial infarction; TIMI: Thrombolysis in Myocardial Infarction; VF: ventricular fibrillation; h: hours; CVD: cardiovascular disease; BMI: body mass index; CK: creatine kinase; CK-MB: creatine kinase – muscle brain; nt-pro BNP: nt-pro terminal brain natriuretic peptide; LVEF: left ventricular ejection fraction; LVEDV: left ventricular end-diastolic volume; LVESV: left ventricular end-systolic volume; MVO: microvascular obstruction; ACE: angiotensin-converting–enzyme; AT: angiotensin. |
|
Supplementary table:Sensitivity, specificity and predictive values of TTE, compared to CMR. |
|
|
Thrombus at TTE
|
|
|
Thrombus at CMR
|
Absence |
Presence |
Total
|
| Presence |
2 (1.8%) |
8 (7.1%) |
10
|
| Absence |
99 (87.6%) |
4 (3.5%) |
103
|
|
Total
|
101
|
12
|
113
|
| Kappa = 0.70 (substantial agreement); sensitivity = 80% (95% CI 44%–97%); specificity = 96.1% (95% CI 90%–99%); positive predictive value = 66.7% (95% CI 35%–90%); negative predictive value = 98% (95% CI 93%–100%) with 8.8% prevalence
CI = confidence interval |
Discussion
Prevention, detection and management of mural LVT after AMI is challenging and has often been a matter of discussion [20]. Most of the evidence concerning mural LVT formation derives from studies of the pre-fibrinolysis and fibrinolysis eras. Currently, American and European guidelines for the management of STEMI [12, 13] do not precisely consider the length of therapeutic anticoagulation with unfractionated heparin or LMWH after primary PCI. According to guidelines, triple therapy with a vitamin K antagonist (up to 3 months), aspirin, and a P2Y12 receptor inhibitor after STEMI should be restricted to specific clinical situations in which the risk of systemic thromboembolism is considered to exceed that of bleeding. It is, furthermore, recommended to consider patient preferences, as patients may weigh these outcomes differently [13]. As risk factors for LVT, guidelines mention antero-apical akinesia or dyskinesia without specifying the imaging modality used to look for this and the optimal time point to consider LVT formation after AMI.
In the current study, most of the patients would have been considered at risk for LVT in accordance with guidelines as they had large, mainly anterior infarction with a mean LVEF of 37.4%. Nevertheless, the incidence of LVT in our study (6.2%) did not differ much from a recently published similar series [7, 8], which reported an incidence of 6.2%–8.8%. Best predictors for LVT formation are a higher BMI, elevated platelet counts and a large scar size on CMR.
The potential predictive value of large infarct size on LVT formation appears logical and has already been described by others [8, 19]. Obesity, expressed as elevated BMI, has also been discussed as a potential predictor for a higher incidence of left atrial thrombus in patients with atrial fibrillation [21]. Among the possible explanations for this phenomenon are high fibrinogen levels [22] as well as chronic inflammation directly promoted by excretion of proinflammatory proteins from the adipose tissue [23] leading to prothrombosis [24, 25]. The precise mechanism of the effect of obesity on LVT formation remains unclear.
A higher platelet count is commonly known to be a risk factor for intravascular thrombus formation [28]; however, it has never been shown to be associated with LVT formation after AMI.
As for the protocol of the original SWISS AMI study, all patients underwent CMR, according to a standard protocol including LGE imaging, within a median of 6 days (4–8) after AMI. This technique robustly detects LVT [8], even without dedicated additional techniques, such as sequential infrared imaging and a long inversion time, as described later by Weinsaft [19] and co-workers in 2006, when the current trial was already started. Similar to the results of earlier studies [8, 19], our data confirm that LVT may be missed with TTE and even CMR with cine imaging only. This underscores the impact of LGE-CMR in the early management of AMI.
Given the low incidence of LVT in this patient population at high risk for LVT, the potential risk of systematic prophylactic long-term oral anticoagulation combined with DAPT may outweigh the potential benefit as it clearly increases the risk of bleeding [29, 30] and as it is, furthermore, not compatible with modern antiplatelet regimens including prasugrel or ticagrelor. It should, therefore, be considered only in patients with clearly documented LVT, at best by use of LGE-CMR. Whether prophylactic long-term oral anticoagulation might be indicated in special situations or with patients characteristics indicating risk (i.e. in the case of elevated BMI, higher platelet counts and large infarct size) cannot be answered by the present study. Prospective, randomised controlled trials (such as Piek J et al.; Clinical trials.gov id:NCT01556659) comparing in a randomised manner DAPT with or without additional oral anticoagulation may be helpful in the future.
Study limitations
First, when starting the study, our knowledge of dedicated CMR sequences to detect LVT were limited as alternative sequences [19] were published later.
Second, the original trial was not designed to evaluate the incidence of LVT formation, nor to address the sensitivity and specificity of TTE to detect LVT, as CMR and TTE were generally not performed on the same day and TTE was performed “out of protocol”. Therefore, the possibility that the time delay between the two examinations may be responsible for false negative or false positive results cannot be completely excluded. Especially given the higher sensitivity of TTE to detect LVT (sensitivity = 80%) in our study compared with earlier results [8, 31].
Third, the long duration between enrolment of the first and the last patients may lead to imbalances in the management of STEMI, as during the study period new antiplatelet agents were introduced (such as bivalirudin and prasugrel).
Fourth, in the original study protocol the length and the type of therapeutic anticoagulation regimen was not specifically defined.
Fifth, because of the large number of statistical tests (particularly the search for predictors for LVT out of 61 parameters), there may be an increased overall probability of a type I error. Thus, the results of this study have to be therefore strictly considered as “hypothesis generating”.
In conclusion, the incidence of mural LVT shortly after AMI is relatively low, even in a patient population, which may be considered at higher risk for thrombus formation, which may be actually explained by the rapid access to and the constant improvement in revascularisation techniques for patients with AMI. Nevertheless, a systematic research of LVT shall be considered in all patients after AMI. An optimal modality for LVT detection is LGE-CMR but echocardiography has an acceptable sensitivity and specificity when CMR is not available. If for a subgroup of patients with high BMI and large scar size and high platelet levels, prophylactic long-term anticoagulation may be indicated, remains unclear.
Authors’ contribution: DS and VG contributed equally to the article.
References
1 Nichols M, Townsend N, Scarborough P, Rayner M. Trends in age-specific coronary heart disease mortality in the European union over three decades: 1980–2009. Eur Heart J. 2013;34(39):3017–27.
2 Fothergill RT, Watson LR, Virdi GK, Moore FP, Whitbread M. Survival of resuscitated cardiac arrest patients with st-elevation myocardial infarction (STEMI) conveyed directly to a heart attack centre by ambulance clinicians. Resuscitation. 2013, Sep 19.
3 Stratton JR, Resnick AD. Increased embolic risk in patients with left ventricular thrombi. Circulation. 1987;75(5):1004–11.
4 Vaitkus PT, Barnathan ES. Embolic potential, prevention and management of mural thrombus complicating anterior myocardial infarction: A meta-analysis. J Am Coll Cardiol. 1993;22(4):1004–9.
5 Asinger RW, Mikell FL, Elsperger J, Hodges M. Incidence of left-ventricular thrombosis after acute transmural myocardial infarction. Serial evaluation by two-dimensional echocardiography. N Engl J Med. 1981;305(6):297–302.
6 Jugdutt BI, Sivaram CA. Prospective two-dimensional echocardiographic evaluation of left ventricular thrombus and embolism after acute myocardial infarction. J Am Coll Cardiol. 1989;13(3):554–64.
7 Osherov AB, Borovik-Raz M, Aronson D, Agmon Y, Kapeliovich M, Kerner A, et al. Incidence of early left ventricular thrombus after acute anterior wall myocardial infarction in the primary coronary intervention era. Am Heart J. 2009;157(6):1074–80.
8 Delewi R, Nijveldt R, Hirsch A, Marcu CB, Robbers L, Hassell ME, et al. Left ventricular thrombus formation after acute myocardial infarction as assessed by cardiovascular magnetic resonance imaging. Eur J Radiol. 2012;81(12):3900–4.
9 Keating EC, Gross SA, Schlamowitz RA, Glassman J, Mazur JH, Pitt WA, Miller D. Mural thrombi in myocardial infarctions. Prospective evaluation by two-dimensional echocardiography. Am J Med. 1983;74(6):989–95.
10 Weinreich DJ, Burke JF, Pauletto FJ. Left ventricular mural thrombi complicating acute myocardial infarction. Long-term follow-up with serial echocardiography. Ann Intern Med. 1984;100(6):789–94.
11 Cregler LL. Antithrombotic therapy in left ventricular thrombosis and systemic embolism. Am Heart J. 1992;123(4 Pt 2):1110–4.
12 Steg PG, James SK, Atar D, Badano LP, Blömstrom-Lundqvist C, Borger MA, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with st-segment elevation. Eur Heart J. 2012;33(20):2569–619.
13 O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA guideline for the management of st-elevation myocardial infarction: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2013;127(4):e362–425.
14 Surder D, Schwitter J, Moccetti T, Astori G, Rufibach K, Plein S, et al. Cell-based therapy for myocardial repair in patients with acute myocardial infarction: Rationale and study design of the swiss multicenter intracoronary stem cells study in acute myocardial infarction (SWISS-AMI). Am Heart J. 2010;160(1):58–64.
15 Sürder D, Manka R, Lo Cicero V, Moccetti T, Rufibach K, Soncin S, et al. Intracoronary injection of bone marrow derived mononuclear cells, early or late after acute myocardial infarction: Effects on global left ventricular function four months results of the SWISS-AMI trial. Circulation. 2013, Apr 17.
16 Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343(20):1445–53.
17 Knuesel PR, Nanz D, Wyss C, Buechi M, Kaufmann PA, von Schulthess GK, et al. Characterization of dysfunctional myocardium by positron emission tomography and magnetic resonance: Relation to functional outcome after revascularization. Circulation. 2003;108(9):1095–100.
18 Goetti R, Kozerke S, Donati OF, Sürder D, Stolzmann P, Kaufmann PA, et al. Acute, subacute, and chronic myocardial infarction: Quantitative comparison of 2D and 3D late gadolinium enhancement MR imaging. Radiology. 2011;259(3):704–11.
19 Weinsaft JW, Kim HW, Shah DJ, Klem I, Crowley AL, Brosnan R, et al. Detection of left ventricular thrombus by delayed-enhancement cardiovascular magnetic resonance prevalence and markers in patients with systolic dysfunction. J Am Coll Cardiol. 2008;52(2):148–57.
20 Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74
21 Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic values: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–87.
22 Yalcinkaya E, Bugan B, Celik M, Yasar S, Demir M, Management of left ventricular thrombosis in patients with apical aneurysm. Swiss Med Wkly. 2013;143:w13866.
23 Tang RB, Liu XH, Kalifa J, Li ZA, Dong JZ, Yang Y, et al. Body mass index and risk of left atrial thrombus in patients with atrial fibrillation. Am J Cardiol. 2009;104(12):1699–703.
24 Solá E, Vayá A, Corella D, Santaolaria ML, España F, Estellés A, Hernández-Mijares A. Erythrocyte hyperaggregation in obesity: Determining factors and weight loss influence. Obesity (Silver Spring) 2007;15(8):2128–34.
25 Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96(9):939–49.
26 Bowles LK, Cooper JA, Howarth DJ, Miller GJ, MacCallum PK. Associations of haemostatic variables with body mass index: A community-based study. Blood Coagul Fibrinolysis. 2003;14(6):569–73.
27 Darvall KA, Sam RC, Silverman SH, et al. Obesity and thrombosis. Eur J Vasc Endovasc Surg. 2007;33(2):223–33.
28 Tefferi A. Polycythemia vera and essential thrombocythemia: 2013 update on diagnosis, risk-stratification, and management. Am J Hematol. 2013;88(6):507–16.
29 Dewilde WJ, Oirbans T, Verheugt FW, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: An open-label, randomised, controlled trial. Lancet. 2013;381(9872):1107–15.
30 Vonbach P, Reich R, Möll F, Krähenbühl S, Ballmer PE, Meier CR. Risk factor for gastrointestinal bleeding: a hospital-based case-control study. Swiss Med Wkly. 2007;137(49):705–10.
31 Srichai MB, Junor C, Rodriguez LL, Stillman AE, Grimm RA, Lieber ML, et al. Clinical, imaging, and pathological characteristics of left ventricular thrombus: A comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J. 2006;152(1):75–84.

