Acute kidney injury KDIGO stage 2 to 3 in HIV-positive patients treated with cART – a case series over 11 years in a cohort of 1,153 patients
DOI: https://doi.org/10.4414/smw.2015.14135
Mario
Kurz, Felix
Burkhalter, Michael
Dickenmann, Helmut
Hopfer, Michael
Mayr, Luigia
Elzi, Manuel
Battegay
Summary
OBJECTIVES: We aimed to explore acute kidney injury (AKI) Kidney Disease Improving Global Guidelines (KDIGO) stage 2 to 3 in a cohort of antiretroviral treated HIV-infected individuals.
METHODS: HIV-infected individuals of the Swiss HIV Cohort Study (Basel site), treated with combination antiretroviral therapy (cART) 2002–2013, were included. AKI was defined and classified according to the KDIGO Clinical Practice Guidelines for AKI. Data were prospectively collected and reports of kidney biopsies obtained from records.
RESULTS: Among 1,153 cART-treated patients, 13 experienced AKI KDIGO stage 2 to 3 (1 patient stage 2, 12 patients stage 3; median age 46 years; 9 male; median CD4 count 366 cells/μl), corresponding to an incidence rate of AKI of 0.77 (95% confidence interval 0.45–1.33) per 1000 patient-years. Baseline estimated glomerular filtration rate (eGFR) was 87 ml/min (interquartile range 66–100). Ten patients were treated with tenofovir (TDF). Nine patients (69%) had ≥1 cardiovascular risk factor, only two patients had known pre-existing kidney disease. Three patients needed chronic and two temporary dialysis. AKI was associated with TDF therapy in 6 of 13 (46%) patients (mean TDF exposure time before AKI 41 months). Impaired renal function was partially reversible in all patients. In three patients with biopsy-proven pre-existing kidney disease (AA amyloidosis, calcineurin inhibitor-induced nephropathy and minimal change glomerulopathy), TDF potentially added to AKI.
CONCLUSIONS: AKI KDIGO stage 2 to 3 demonstrates complex associations at the individual level and can occur without early signs. Although treatment with TDF and presence of cardiovascular risk factors were found frequently, predicting AKI seems very difficult.
Introduction
Acute kidney injury (AKI) has been associated with increased morbidity and mortality in HIV-infected patients [1, 2]. The leading causes of AKI in the era before combination antiretroviral therapy (cART) were mainly volume depletion, sepsis and drug-related toxicity due to treatment of opportunistic infections [3, 4]. Nowadays, prerenal azotaemia, acute tubular necrosis, acute interstitial nephritis, urinary obstruction or drug-related nephrotoxicity account for the majority of cases of AKI [5, 6]. Prior renal impairment, lower CD4 cell counts, an AIDS-defining disease, co-infection with hepatitis C virus and liver disease have been reported as risk factors for AKI in HIV-infected individuals [5]. Since cART has dramatically increased life expectancy, aging has become an additional risk factor for AKI as a result of atherosclerosis, metabolic disease, and long-term use of nephrotoxic drugs, in particular tenofovir (TDF) and protease inhibitors [7]. The use of TDF, lopinavir and atazanavir boosted with ritonavir are independent predictors of chronic renal impairment in HIV-infected persons [8]. However, severe acute renal toxicity due to TDF therapy is considered to be uncommon [7, 9].
The aim of this study was to explore AKI Kidney Disease Improving Global Guidelines (KDIGO) stage 2 to 3 in a cohort of treated HIV-infected individuals at a tertiary-care hospital.
Methods
Study design and population
We describe a case-series. The study population consisted of all HIV-infected patients participating in the Swiss HIV Cohort Study (SHCS) at the study site of Basel, Switzerland, who were treated with cART between 2002 and 2013. We excluded patients with chronic kidney failure requiring permanent dialysis.
The SHCS, a large prospective cohort study of adult HIV-infected persons is described in detail elsewhere [11]. Briefly, HIV-infected individuals aged 18 years or older are followed up in seven outpatient clinics of Swiss hospitals and by associated physicians. Basic sociodemographic characteristics, data on the clinical course, hepatitis co-infection, cART, comedication, cardiovascular risk factors, and immunological and virological parameters as well as laboratory values including the serum creatinine are collected at study enrolment and semi-annually thereafter on standardised data collection forms. Clinical data including creatinine values were extracted from the prospective SHCS database, whereas information on pre-existing kidney disease, risk factors for kidney disease [13] and the use of dialysis as well as pathology reports of kidney biopsies were taken by chart review. All cases included in this study were seen and assessed by at least one staff nephrologist.
Definitions
Representative baseline creatinine values were obtained from available laboratory data before onset of acute kidney injury (AKI). AKI was defined as an increase in serum creatinine of ≥26.5 µmol/l within 48 hours, which was known or presumed to have occurred within the prior seven days [10]. Patients were classified as KDIGO stage 2 AKI if serum creatinine was 2.0–2.9 times baseline or as stage 3 AKI if serum creatinine was >3 times baseline or >353.6 µmol/l, respectively. Estimated glomerular filtration rate (eGFR) was calculated using the Cockroft-Gault formula [12, 14].
We evaluated the causality of the association between TDF-containing cART and onset of AKI according to the following criteria: temporal relation, reversibility of impaired kidney function after discontinuation of the drug, presence of tubulointerstitial proteinuria [15, 16] and acute tubular necrosis in the kidney biopsy [17]. The temporal association was recorded if the patient was receiving a TDF-contaning cART when developing AKI. Reversibility of impaired kidney function was defined as at least two-fold increase in eGFR 6 months after severe AKI. Tubulointerstitial proteinuria was defined as urine concentration of either α-1-microglobulin >1.36 mg/mmol creatinine or retinol-binding protein >0.08 mg/mmol creatinine. A probable association was assumed if a patient met three out of the four criteria.
Dialysis was considered either as temporary if the duration was less than 1 month or as permanent if duration was more than 1 month.
cART was defined as an antiretroviral regimen containing at least three drugs, i.e. two nucleoside/nucleotide reverse-transcriptase inhibitors (NRTIs) in combination with either a non-nucleoside reverse-transcriptase inhibitor (NNRTI) or a ritonavir boosted protease inhibitor (PI/r).
Results
At the SHCS site of Basel 1,153 patients were treated with cART from April 2002 to May 2013. Of these, 936 (81%) received a TDF-containing regimen (67% male, 33% female), 630 (54.6%) patients were treated with protease inhibitors, 523 (45.4%) patients with non-nucleoside reverse transcriptase inhibitors. Among 1,153 patients receiving cART, 13 developed AKI KDIGO stage 2 to 3 (1 patient with AKI KDIGO stage 2, 12 patients with stage 3) after a median time of 8 years (interquartile range [IQR] 2–10) of antiretroviral treatment, corresponding to an incidence rate of AKI of 0.8 (95% confidence interval 0.5–1.3) per 1,000 person-years. If only patients receiving a tenofovir-containing regimen were considered, the incidence rate of AKI was 2.8 (95% confidence interval 1.5–5.3) per 1,000 person-years. Baseline characteristics of the study population are presented in table 1. The median age at diagnosis of AKI was 46 years (IQR 40‒58), nine patients were male (69%). The median CD4 count at diagnosis of AKI was 366 cells/μl (IQR 197‒491), nine patients had previously been diagnosed with an AIDS-defining condition. Ten out of 13 patients were treated with a TDF-containing regimen (table 1 and fig. 1, patients 1‒9 and patient 12). Nine patients (69%) had at least one established renal risk factor such as diabetes mellitus, arterial hypertension or arteriosclerosis. However, only two patients had a known pre-existing kidney disease, one a calcineurin inhibitor-induced renal disorder (patient 8) and the other patient’s known kidney disease remained unknown regarding its cause (patient 13). Eleven patients (85%) underwent a kidney biopsy at the time of AKI. In two patients a renal biopsy was not performed either because the clinical picture was rather clear owing to overt Fanconi syndrome (patient no. 4) or because of anticoagulation (patient 6). Five patients were in need of dialysis (three chronic, two temporary).
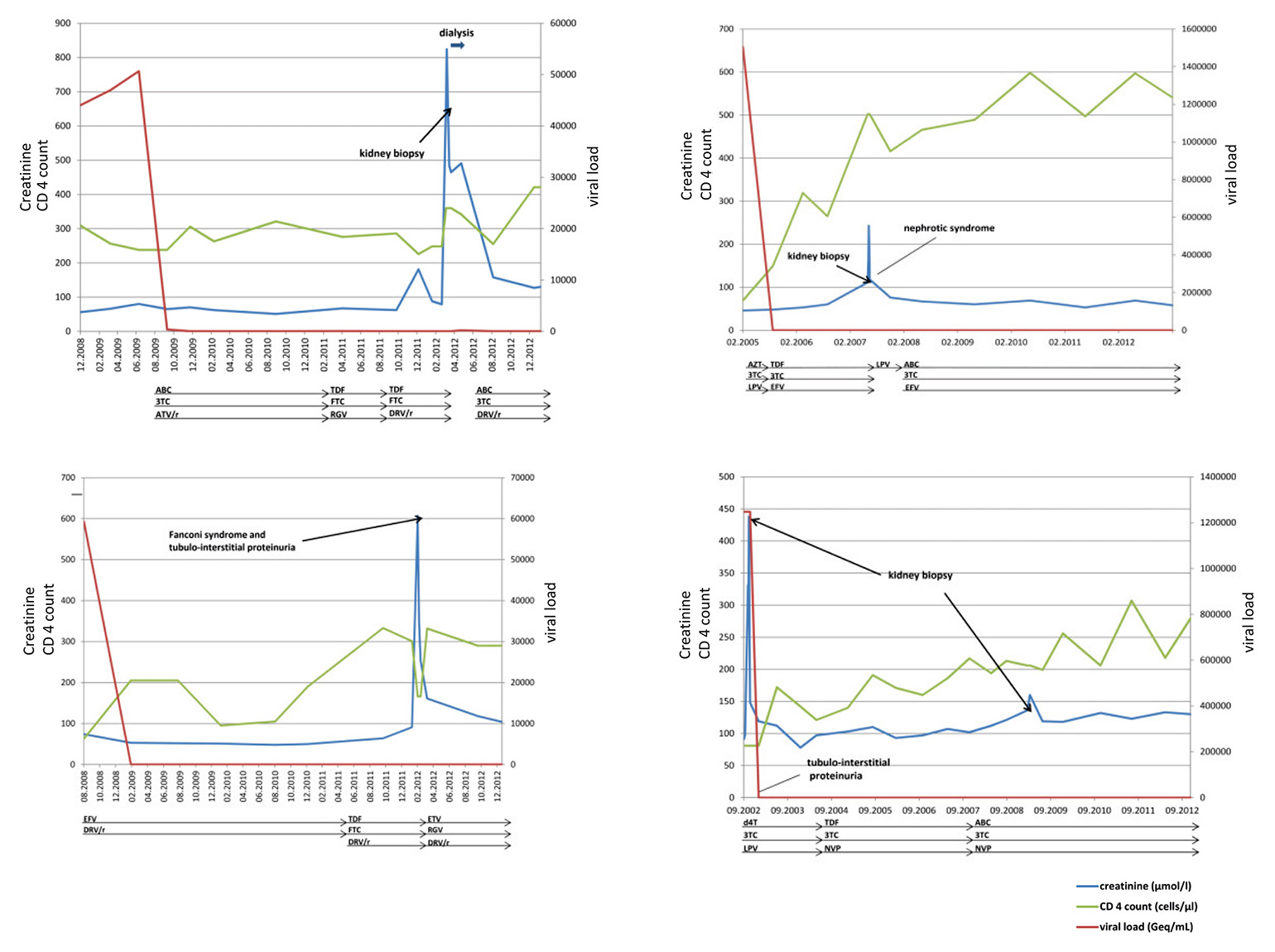
Figure 1
The individual courses of patients 2, 4, 9 and 10. Dialysis, the presence of tubulointerstitial proteinuria and kidney biopsies are indicated by arrows.
3TC = lamivudine; ABC = abacavir; EVF = efavirenz; ATV/ = atazanavir/ritonavir; d4T = stavudin; DRV/r = darunavir/ritonavir; FTC = emtricitabine; LPV/r = lopinavir/ritonavir; NVP = nevirapine; RGV = raltegravir; TDF = tenofovir disoproxil fumarate;
The median eGFR was 87 ml/min (IQR 66‒100) at baseline before development of AKI, 13.0 ml/min (IQR 6‒17) during AKI, and 47 ml/min (IQR 31‒62) 6 months after AKI (calculated for all patients except for patients 11 and 13 who were on chronic dialysis). The median baseline eGFR in the group with a probable association with TDF was 99 ml/min (IQR 87‒100) before AKI, 8 ml/min (IQR 5‒17) during AKI and 42 ml/min (IQR 26–47) 6 months after cessation of TDF therapy. Fanconi’s syndrome was diagnosed in two patients (patients 4 and 5), both with a probable association with TDF therapy, and with the typical findings of urine wasting of solutes, i.e. phosphate, bicarbonate, glucose, low molecular weight proteins and amino acids [9], reflecting the breakdown of solute transport in proximal tubule. Biopsy results of the two patients are described in figure 1.
Among 10 patients exposed to TDF, we identified 6 (patients 1‒6) with a probable association between TDF-based therapy and the onset of AKI. The median TDF exposure time before onset of AKI was 41 months (IQR 13‒82). Patient number 1 developed AKI after 5 months, the remaining five (patients 2‒6) after more than 1 year of drug exposure. Two out of these six patients were in need of temporary dialysis; however, no patient required chronic dialysis because of significantly improved kidney function after discontinuation of TDF. In three additional patients (patients 7‒9) exposed to TDF, AKI was considered to have resulted from other factors such as AA amyloidosis, calcineurin inhibitor-induced nephropathy and minimal change glomerulopathy according to results of kidney biopsy. An association with TDF was uncertain for patient number 12, in whom the cause of kidney disease remained unclear.
In total, 10 of the 13 patients had complete or partial resolution of impairment of kidney function including two out of five patients who discontinued dialysis.
Clinical course of the individual patients
The individual course of patients 2, 4, 9 and 10 is depicted in figure 1. The individual course of all 13 patients is described in the text below.
Patients 1 to 6 showed a probable association of TDF-based cART with the onset of AKI. None of the patients had a known pre-existing kidney disease. Baseline eGFR was >90 ml/min in four out of the six patients and two had mild impairment of kidney function (patients 2 and 3). In all patients kidney function was severely impaired at the time of AKI with an eGFR below 30 ml/min and a significant improvement 6 months after discontinuation of TDF; however, none had a complete normalisation of eGFR 6 months after TDF was stopped. In four out of the six patients, proteinuria was measured and showed a tubulointerstitial pattern of alpha-1 microglobulin and retinol-binding protein measurements. Results of kidney biopsy from four patients showed acute to subacute tubular damage consistent with TDF-induced AKI [17]. In the two remaining patients, biopsy was omitted owing to either the typical clinical presentation with Fanconi syndrome or anticoagulation.
Patients 7, 8 and 9 did not qualify for an association of TDF with the onset of AKI. TDF-exposure might have led to additional impairment of kidney function in patient number 7 with underlying AA amyloidosis due to recurrent cellulitis, as indicated by tubular atrophy and fibrosis in the kidney biopsy. Patient number 8 had underlying calcineurininhibitor-induced nephropathy as a result of the therapy of severe psoriasis. Kidney function deteriorated shortly after TDF exposure, was associated with complete tubulointerstitial proteinuria, and diminished once more after unilateral nephrectomy due to multifocal renal cell cancer. In patient number 9 minimal change glomerulopathy was the underlying kidney pathology. This patient was exposed to TDF at the time of AKI. Histological findings of additional damage to the tubulointerstitial space were absent.
Miliary tuberculosis was the cause of AKI in patient number 10. It completely resolved with antimycobacterial therapy. Seven years later, kidney function declined again owing to hypertensive nephropathy. In patient 11 peritoneal dialysis because had to be started because of HIV-associated focal segmental glomerulosclerosis. Eventually, the patient received a cadaveric kidney transplant. TDF remained part of his antiviral therapy owing to a multiresistant HIV-strain. Patient number 12 demonstrates AKI with underlying chronic kidney failure due to interstitial nephritis of unknown origin. The association with TDF exposure is uncertain. There is a correlation between the drug exposure and worsening of kidney function, and biopsy results were compatible with interstitial nephritis. Biopsy results of patient 13 showed chronic interstitial nephritis and tubular atrophy. The cause of the chronic kidney failure is not understood. This patient now is on chronic dialysis.
|
Table 1: Characteristics of the individual study patients. |
|
Patient
|
Age/
gender
|
CD4
(cells/μl)
|
HCV co-
infection
|
Pre-existing kidney disease
|
Renal risk factors
|
cART-regimen before AKI
|
Association with TDF
|
TDF-exposure before AKI (months)
|
CrCl before AKI/
lowest CrCl/
CrCl 6 months after AKI (ml/min)
|
Biopsy results
|
Dialysis
|
Aetiology
(c)
|
CVD treatment
|
| 1 |
61/m |
491 |
No |
No |
Diabetes mellitus, arterial hypertension |
TDF, FTC, ATV/r |
+ |
5 |
100/4/84 |
Acute tubular necrosis, interstitial nephritis |
Yes |
TDF |
Yes |
| 2 |
72/f |
360 |
No |
No |
Arterial hypertension, arteriosclerosis |
TDF, FTC, DRV/r |
+ |
13 |
87/5/26 |
Acute tubular necrosis, severe arteriolosclerosis |
Yes |
TDF |
Yes |
| 3 |
81/m |
390 |
No |
No |
Arteriosclerosis |
TDF, FTC, LPV/r |
+ |
111 |
61/5/16 |
Subacute tubular necrosis, severe arteriolosclerosis, glomerulosclerosis |
No |
TDF |
Yes |
| 4 |
46/f |
166 |
No |
No |
|
TDF, FTC, DRV/r |
+ |
33 |
123/10/47 |
No biopsy |
No |
TDF |
No |
| 5 |
49/m |
554 |
Yes |
No |
|
TDF, LPV/r |
+ |
49 |
100/17/38 |
Tubular necrosis, interstitial fibrosis, glomerulosclerosis |
No |
TDF |
No |
| 6 |
44/m |
197 |
Yes |
No |
Arterial hypertension |
TDF, FTC, ATV/r |
+ |
82 |
99/18/46 |
No biopsy |
No |
TDF |
No |
| 7 |
49/f |
842 |
Yes |
No |
Arteriosclerosis |
RGV, DRV/r |
– |
2 |
46/6/b |
AA amyloidosis mainly affecting glomeruli, severe interstitial fibrosis and tubular atrophy |
Yes |
AA amyloidosis |
Yes |
| 8 |
42/m |
285 |
No |
Yes |
Arterial hypertensions |
(a) |
– |
1 |
66/17/57 |
Severe glomerular damage, interstitial fibrosis, tubular atrophy |
No |
Calcineurin inhibitor-induced nephropathy |
Yes |
| 9 |
32/f |
502 |
No |
No |
– |
TDF, 3TC, EFV |
– |
24 |
96/23/62 |
Minimal glomerular alteration, no sign of tubular damage |
No |
Minimal change glomerulopathiy |
Yes |
| 10 |
58/m |
81 |
No |
No |
Arterial hypertension, hyperlipidaemia |
(a) |
– |
|
68 /14/86 |
2002: interstitial, granulomatous nephritis, 2009: arteriolosclerosis, interstitial fibrosis, tubular atrophy |
No |
2002: miliary Tbc, 2009: hypertensive nephropathy |
No |
| 11 |
40/m |
366 |
No |
No |
Diabetes mellitus type 2 |
3TC, ddI, EFV, T20 |
– |
|
113/13/b |
Focal segmental glomerular sclerosis |
Yes |
HIV-associated focal segmental sclerosis |
Yes |
| 12 |
45/m |
400 |
No |
No |
|
TDF, 3TC, ATV/r |
– |
11 |
55/39/53 |
Acute interstitial nephritis |
No |
Unclear |
No |
| 13 |
42/m |
59 |
No |
Yes |
Arteriosclerosis |
ETV, RGV, LPV/r |
– |
|
17/7/b |
Chronic interstitial nephritis, tubular and glomerular atrophy, glomerular arteriolosclerosis |
Yes |
Unclear |
Yes |
| AKI = acute kidney injury; ATV/r = atazanavir/ritonavir; Cr Cl = creatinine clearance; ddI = didanosine; DRV/r = darunavir/ritonavir; EVF = efavirenz; FTC = emtricitabine; HCV = hepatitis C virus; LPV/r = lopinavir/ritonavir; RGV = raltegravir; T20 = enfuvirtide; Tbc = tuberculosis; TDF = tenofovir disoproxil fumarate
– no, + probable
a: patients 8 and 10 were not receiving combination antiretroviral therapy at time of sAKI
b: eGFR not calculated for patients on chronic dialysis 6 months after sAKI
c: as assessed by staff nephrologist |
Discussion
In our study, involving 1,153 HIV-infected individuals receiving cART, 13 patients developed AKI KDIGO stage 2 to 3 under treatment. About one-half of the cases were associated with a TDF-containing regimen. Of note, two-thirds of patients with AKI had cardiovascular risk factors, i.e. diabetes mellitus, arterial hypertension or arteriosclerosis. Interestingly, patients usually developed renal disease after more than 1 year of TDF exposure, making prediction of AKI very difficult. Kidney function significantly improved in all patients in whom TDF played a major role 6 months after drug discontinuation, indicating at least a partial reversibility.
TDF has a relatively good renal safety profile and serious adverse events have been reported in fewer than 1% of treated patients and creatinine elevations above AKI KDIGO stage 2 (data of expanded acces programme) in 2% of patients [18]. The D:A:D observational study recently demonstrated that ritonavir-boosted protease inhibitors, i.e. atazanavir and lopinavir, are also independently associated with impaired renal function in patients receiving cART [8].
Despite regular monitoring of SHCS patients, treating physicians were obviously unable to predict and thereby prevent AKI. The cause for this lack of assessment is possibly given by the very wide time range of onset of AKI after TDF exposure of up to 9 years. However, it is possible that other patients have been discontinued from TDF when AKI was imminent. Measurement of tubulointerstitial proteinuria in the form of either retinol binding protein, beta-2 microglobulin or alpha-1 microglobulin [15, 16] might be a useful screening tool for TDF-induced kidney tubulopathy, but its ability to predict AKI remains to be validated in larger studies.
In our study we could show that the involvement of a team of infectious disease physicians, nephrologists and pathologist could adequately diagnose and treat AKI in the majority of cases. This interdisciplinary effort might be necessary for a successful management of AKI.
Our study has several strengths. We investigated the incidence and risk factors of severe AKI in the setting of a prospective cohort study with a systematic long-term follow-up. Importantly the cause of AKI was assessed by a nephrologist and kidney biopsy was performed in most patients, leading to an accurate evaluation of the underlying kidney disease. Misclassification of the effect of TDF was therefore minimised. We acknowledge some limitations. Data on proteinuria prior to AKI were not available in all patients and dip-stick urine tests performed every 6 months within the SHCS detect albuminuria only. The risk of AKI might have been underestimated, as 50 out of 936 (5.3%) patients using TDF had switched their cART regimen because of mild impaired renal function before severe AKI may have developed. Also, we cannot rule out that protease inhibitors contributed to the occurrence of AKI, however, 10 out of 13 patients continued cART with a protease inhibitor treatment which makes an essential contribution unlikely. Finally, the level of causative association is very difficult to assess even with a detailed case history.
In conclusion, the incidence of AKI was associated with TDF-containing cART in our study and may occur without pre-existing renal disease even after a longer drug exposure. Impairment of kidney function is at least partially reversible after discontinuation of TDF in most patients. Close renal monitoring and individual risk assessment are warranted in HIV-infected individuals treated with TDF, in particular with cardiovascular risk factors.
References
1 Fine DM, Atta MG. Kidney disease in the HIV-infected patient. AIDS patient care and STDs. 2007;21:813–24.
2 Kalim S, Szczech LA, Wyatt CM. Acute kidney injury in HIV-infected patients. Semin Nephrol. 2008;28:556–62.
3 Valeri A, Neusy AJ. Acute and chronic renal disease in hospitalized AIDS patients. Clin Nephrol. 1991;35:110–8.
4 Rao TK, Friedman EA. Outcome of severe acute renal failure in patients with acquired immunodeficiency syndrome. Am J Kidney Dis. 1995;25:390–8.
5 Franceschini N, Napravnik S, Eron JJ, Jr., Szczech LA, Finn WF. Incidence and etiology of acute renal failure among ambulatory HIV-infected patients. Kidney Int. 2005;67:1526–31.
6 Estrella MM, Fine DM, Atta MG. Recent developments in HIV-related kidney disease. HIV Ther. 2010;4:589–603.
7 Rodriguez-Novoa S, Alvarez E, Labarga P, Soriano V. Renal toxicity associated with tenofovir use. Expert Opin Drug Saf. 2010;9:545–59.
8 Ryom L, Mocroft A, Kirk O, Worm SW, Kamara DA, Reiss P, et al. Association between antiretroviral exposure and renal impairment among HIV-positive persons with normal baseline renal function: the D:A:D study. J Infect Dis. 2013;207:1359–69.
9 Hall AM. Update on tenofovir toxicity in the kidney. Pediatr Nephrol. 2012;28:1011–23.
10 Khwaja A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Clin Pract. 2012;120:179–84.
11 Swiss HIVCS, Schoeni-Affolter F, Ledergerber B, Rickenbach M, Rudin C, Gunthard HF, et al. Cohort profile: the Swiss HIV Cohort study. Int J Epidemiol. 2010;39:1179–89.
12 Ferguson MA, Waikar SS. Established and emerging markers of kidney function. Clin Chem. 2012;58:680–9.
13 Arora P, Vasa P, Brenner D, Iglar K, McFarlane P, Morrison H, et al. Prevalence estimates of chronic kidney disease in Canada: results of a nationally representative survey. CMAJ. 2013;185:E417–23.
14 Gaspari F, Ferrari S, Stucchi N, Centemeri E, Carrara F, Pellegrino M, et al. Performance of different prediction equations for estimating renal function in kidney transplantation. Am J Transplant. 2004;4:1826–35.
15 Hall AM, Hendry BM, Nitsch D, Connolly JO. Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am J Kidney Dis. 2011;57:773–80.
16 Nishijima T, Shimbo T, Komatsu H, Takano M, Tanuma J, Tsukada K, et al. Urinary beta-2 microglobulin and alpha-1 microglobulin are useful screening markers for tenofovir-induced kidney tubulopathy in patients with HIV-1 infection: a diagnostic accuracy study. J Infect Chemother. 2013;19:850–7.
17 Herlitz LC, Mohan S, Stokes MB, Radhakrishnan J, D’Agati VD, Markowitz GS. Tenofovir nephrotoxicity: acute tubular necrosis with distinctive clinical, pathological, and mitochondrial abnormalities. Kidney Int. 2010;78:1171–7.
18 Nelson MR, Katlama C, Montaner JS, Cooper DA, Gazzard B, Clotet B, et al. The safety of tenofovir disoproxil fumarate for the treatment of HIV infection in adults: the first 4 years. AIDS. 2007;21:1273–81.
