Acute painful diabetic neuropathy: an uncommon, remittent type of acute distal small fibre neuropathy
DOI: https://doi.org/10.4414/smw.2015.14131
Christel
Tran, Jacques
Philippe, François
Ochsner, Thierry
Kuntzer, André
Truffert
Summary
INTRODUCTION: Acute painful diabetic neuropathy (APDN) is a distinctive diabetic polyneuropathy and consists of two subtypes: treatment-induced neuropathy (TIN) and diabetic neuropathic cachexia (DNC). The characteristics of APDN are (1.) the small-fibre involvement, (2.) occurrence paradoxically after short-term achievement of good glycaemia control, (3.) intense pain sensation and (4.) eventual recovery. In the face of current recommendations to achieve quickly glycaemic targets, it appears necessary to recognise and understand this neuropathy.
METHODS AND RESULTS: Over 2009 to 2012, we reported four cases of APDN. Four patients (three males and one female) were identified and had a mean age at onset of TIN of 47.7 years (±6.99 years). Mean baseline HbA1c was 14.2% (±1.42) and 7.0% (±3.60) after treatment. Mean estimated time to correct HbA1c was 4.5 months (±3.82 months). Three patients presented with a mean time to symptom resolution of 12.7 months (±1.15 months). One patient had an initial normal electroneuromyogram (ENMG) despite the presence of neuropathic symptoms, and a second abnormal ENMG showing axonal and myelin neuropathy. One patient had a peroneal nerve biopsy showing loss of large myelinated fibres as well as unmyelinated fibres, and signs of microangiopathy.
CONCLUSIONS: According to the current recommendations of promptly achieving glycaemic targets, it appears necessary to recognise and understand this neuropathy. Based on our observations and data from the literature we propose an algorithmic approach for differential diagnosis and therapeutic management of APDN patients.
Abbreviations
ASFSN small fibre sensory neuropathy
APDN acute painful diabetic neuropathy
DSP distal symmetric polyneuropathy
DNC diabetic neuropathic cachexia
ENMG electroneuromyography
GAD glutamic acid decarboxylase
HbA1c glycated haemoglobin
IENFd intraepidermal nerve fibre density
SNRI serotonin-noradrenaline reuptake inhibitor
TIN treatment-induced neuropathy
Introduction
Peripheral neuropathies are well-known complications of diabetes [1]. The two most common types are the chronic, slowly progressive, length-dependent, axonal (or dying back) polyneuropathy involving all nerve fibre types, often referred to as distal symmetric polyneuropathy (DSP), and the more recently recognised small fibre neuropathy [2]. Less common types include subacute onset diabetic lumbosacral radiculoplexus neuropathy [3], truncal neuropathy resembling herpes zoster, and acute painful diabetic neuropathy (APDN). APDN is reported in diabetes type 1 and 2 patients and is associated with the rapid decrease of high glycaemic levels. APDN was initially reported in 1933 by Caravati [4] as “insulin neuritis” in a woman with diabetes who had acute pain in the lower extremities that appeared 4 weeks after insulin initiation. The pain resolved within 3 days of stopping insulin concurrent with severe hyperglycaemia. Since then, similar observations have been reported either in patients treated with exogenous insulin [5–8] or oral antidiabetic agents or even after a change of diet [9, 10], suggesting that insulin itself might not be the primary inducing factor. Therefore “treatment-induced neuropathy” (TIN) appears to be a more appropriate term for this subtype of APDN. A recent investigation of TIN in 16 patients suggested a predominantly nociceptive and autonomic small nerve neuropathy [11].
Four decades after the first TIN description, Ellenberg reported a case series of 6 patients with painful diabetic neuropathy, depression and cachexia arising at the time of clinical diagnosis of diabetes [12]. Since then several cases with a similar history and clinical picture have been reported, which is described as diabetic neuropathic cachexia (DNC) and considered a subtype of APDN [13–15].
We report four cases of APDN and include pathological and electrophysiological descriptions at the time of diagnosis and at follow-up in one case; thus we can better delineate the type of neuropathy and its evolution over time. Our aim is to provide an algorithmic approach and diagnostic criteria for evaluation and differential diagnosis of a patient with APDN.
Methods
Cases of APDN were identified in Lausanne and Geneva University Hospitals Neuromuscular Units over a 5-year period from 2009 to 2013. Charts of these patients were analysed for history, and clinical, laboratory, electrophysiological and pathological findings. The following were recorded: age at onset of APDN, diabetes type, diabetes duration; HbA1c prior to and after treatment initiation, estimated time to correct HbA1c, onset of neuropathic pain, diabetic and pain medications, other symptoms such as cachexia and/or dysautonomic symptoms; electrophysiological findings (electroneuromyography [ENMG] descriptions), intraepidermal nerve fibre density (IENFd) and sural nerve biopsy findings if reported.
Results
Based on the average annual activity of our two neuromuscular units and on the proportion of diabetic patients observed in a sample of patients seen over a 1-week period, we estimated the number of diabetic patients examined in our neuromuscular units from 2009 to 2013 to be approximately 1,500. This corresponds to a rough prevalence of around 10% for diabetes in our neuromuscular units, which is nearly double the admitted extrapolated prevalence of diabetes in our country in 2011 of 4.9% [16]. Four patients (three male and one female) fulfilling criteria for APDN could be detected over this 5-year period. Table 1 shows the patient characteristics. Mean age at onset of APDN symptoms was 47.7 years (±6.99), 50% (n = 2) of patients had type 1 diabetes and 50% (n = 2) type 2 diabetes. Mean baseline HbA1c was 14.2% (±1.42; reference value 4.8%–5.9%) before treatment initiation and 7.0% (±3.60%) after treatment. Mean estimated time to correct HbA1c was 4.5 months (±3.82 months). Time for resolution of symptoms in three patients was 12.7 months (±1.15 months). Neuropathic pain management included specific pharmacological treatment (anticonvulsants, antidepressants and opioids).
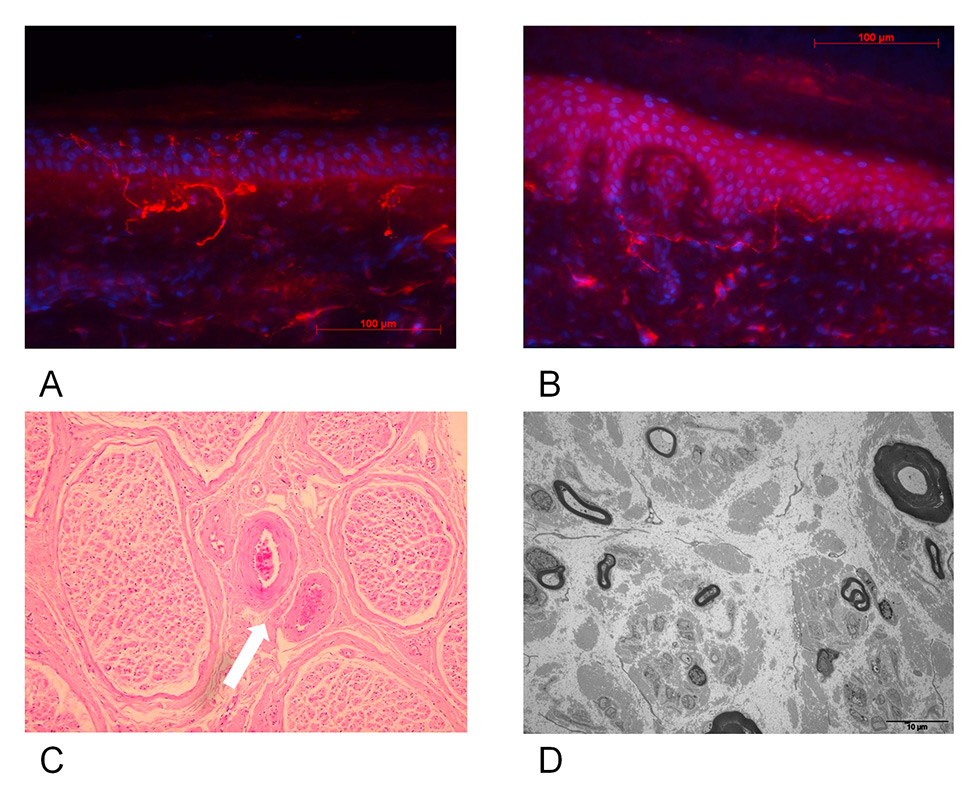
Figure 1
Distal lower limb skin and sural nerve biopsy in normal subject compared with patient 4.
A: Distal lower limb biopsy of a normal subject (FO). Magnification 40x. Average intraepidermal nerve fibre density (IENFDd) = 12.7 s/mm.
B: Distal lower limb skin biopsy of patient 4. Note rarefaction of intraepidernal nerve fibres. Magnification 40x. Average IENFd = 2.8 s/mm.
C: Sural nerve biopsy of patient 4. Optical microspcopy (haematoxylin and eosin staining, orginal magnification 40x). Note microthrombosis of vasa vasorum (white arrow).
D: Sural nerve biopsy of patient 4, electron microscopy. Severe loss of myelinated and unmyelinated axons.
Detailed case reports
Case 1
A 44-year-old African male was diagnosed with type 1 diabetes after 2 weeks of polydipsia, polyuria and generalised weakness. The initial blood glucose value was 24 mmol/l. Subcutaneous insulin therapy was started and symptoms resolved rapidly. Glycated haemoglobin (HbA1c) decreased from 15.1% to 7.2% within 5 months. After 4 to 6 weeks of insulin treatment, he complained of intermittent sharp shooting pain and a severe burning sensation in the soles of both feet, associated with intense discomfort to touch sensation in both legs, which propagated upwards to the thighs. Weight loss of 11 kg in 6 months associated with depression was reported. Investigations to identify a tumour, or infectious, digestive or endocrinological aetiology were all negative except for vitamin D deficiency. Neurological examination revealed absent Achilles tendon reflexes on both sides with no motor or sensory deficits. Orthostatic hypotension was noted. ENMG examination with standard nerve conduction studies was normal. Pregabalin (300 mg/d) and gabapentin (3 x 300 mg/d) were not effective for the lower limb pain. With duloxetine (60 mg/d) and opioids the neuropathic pain gradually improved but never resolved completely. Weight regain was 2 kg. After 5 months of therapy ENMG was performed a second time because of persistent symptoms, which disclosed marked abnormalities suggestive of an axonal and myelinic sensory motor polyneuropathy. Amplitudes of motor and sensory responses recorded on distal parts of the lower limbs and nerve conduction velocities were markedly decreased. There was diffuse hypoexcitability of nerve trunks. With the same medication, mild improvement of the pain was gradually observed, over a 1-year follow-up.
Case 2
A 57-year-old Caucasian male with a 5-year history of poorly controlled type 2 diabetes and alcohol abuse complained of tingling and burning in the feet and erectile dysfunction. Clinical examination showed decreased pallaesthesia of 4/8. HbA1c was 12.0%. He adopted a healthier lifestyle by avoiding alcohol and became compliant with medications. Subsequently, his HbA1c fell from 12% to 8% between April and November 2009 and he described several episodes of postprandial symptomatic hypoglycaemia three to four times a week. In the meantime, the polyneuropathy symptoms worsened with increased pain, symmetric paraesthesia and hyperaesthesia of the lower limbs, and nocturnal leg cramps. He also reported symptoms of gastroparesis including early satiety and postprandial fullness. Symptoms decreased with dietary change. The neuropathy manifestations gradually improved, disappearing almost completely after 14 months without therapy.
Case 3
A previously healthy 49-year-old male was diagnosed with type 2 diabetes after 4 months of polydipsia, polyuria and general weakness. Symptoms disappeared after insulin therapy was started and oral antidiabetic drugs were introduced. HbA1c dropped from 15.1% to 6.1%. Several weeks after metformin was introduced the patient began to experience severe and disabling burning pain and cramps in the feet. He also complained of erectile dysfunction. On neurological examination, vibratory sense was slightly diminished in both feet. Patellar and Achilles reflexes were reduced. Primary sensory modalities were preserved. ENMG showed slowed motor conduction velocities and reduced amplitudes of sural nerve sensory responses, absent peroneal sensory responses and diffuse slowing of motor conduction velocities mostly in the lower limbs, consistent with a predominantly axonal and sensory length-dependent polyneuropathy. Duloxetine quickly improved the symptoms except for persistence of shooting pain in the legs. Two years after diagnosis, the patient was asymptomatic except for rare, mild foot pain. Treatment was then discontinued.
Case 4
A 41-year-old woman initially sought medical advice for weight loss of 39 kg within 12 weeks together with polydipsia, polyuria and marked asthenia. Her blood glucose level reached 25 mmol/l and HbA1c was 14.3%. Serum anti-glutamic acid decarboxylase (GAD) antibodies were very high (2,000 U/l, normal <10 U/l) indicating type 1 diabetes mellitus. With insulin therapy, her symptoms quickly improved, with HbA1c levels returning to 5.2% within 2 weeks. After 1 month of treatment she suddenly developed onset of burning pain associated with deep aching pain over the four extremities and trunk with predominantly lower limb involvement. Pain intensity was reported between 8 and 10 on a visual analogue scale and its severity caused insomnia. There was some improvement in warm temperatures but worsening with contact or touch. On neurological examination, the patient had dry skin on palms and soles, Achilles reflexes were absent and all other tendon reflexes were normally evoked. Blood pressures were normal without orthostatic changes. ENMG showed absent sural nerve action potentials bilaterally, but nerve conduction parameters were otherwise normal in lower and upper extremities. The sural nerve cutaneous silent period was delayed and shortened, but RR interval variability and sympathetic skin responses recorded from palms and soles were normal. Intraepidermal nerve fibre density (IENFd) values from thigh and leg skin biopsies were 11.2 IENF/mm on the thigh (normal 20.4 ± 6.9) and 5.8 IENF/mm on the leg (normal 11.7 ± 4.1) (fig. 1 A and B). A superficial peroneal nerve biopsy was performed and showed loss of large myelinated fibres as well as unmyelinated fibres, and signs of microangiopathy (fig. 1C and D). She was given pregabalin (150 mg/d) together with amitryptiline (75 mg/d) and showed progressive improvement within the next 6 weeks, accompanied by an 8 kg gain in weight. One year after onset of APDN the patient was almost asymptomatic with normal HbA1c.
Discussion
We report four cases of APDN identified in specialised neuromuscular units over a 5-year period. Although the design of our study does not allow accurate epidemiological data to be calculated, because of the absence of systematic screening for APDN and possible selection biases (more diabetic patients may be referred to our units for investigation, so that their proportion in our units may be above the prevalence of diabetes in general population), it can be inferred from this number that the incidence of APDN is probably below 1 in 1,000 diabetic patients, which suggests that this condition may be very rare. However, a recent retrospective study using a systematic approach indicates that APDN incidence may by greatly underestimated, as it was observed in as many as 10.9% of patients examined for diabetic neuropathy [17]. All four patients presented with signs associated with TIN: sudden onset of neuropathic pain and rapid drop of HbA1c values from more than 10.0% to less than 7.0% following insulin or oral antidiabetic treatment, abnormal nerve conduction studies in three patients showing features usually described for DSP [2] and resolution of symptoms within 14 months. Severity of TIN is associated with the magnitude of the change of HbA1c and the estimated time to correct HbA1c. We did a short survey of existing publications on the topic of TIN/APDN. Overall, reports of TIN have been predominantly small observational case studies with variable follow-up [4–6, 8–14, 17–27]. Interestingly cases 1 and 4 developed APDN soon after newly diagnosed and treated diabetes type 1 as reported in few cases [7, 13]. Indeed, duration of diabetes is not a predictive factor of developing APDN in diabetic type 1 patients, in contrast to peripheral diabetic neuropathy, screening for which is recommended only within the first 5 years after diagnosis [28]. The neuropathic pain has an acute onset, appearing within 8 weeks of glycaemic change in contrast to the more insidious onset of the distal sensory-motor polyneuropathy [29].
When looking more specifically at the magnitude of change of HbA1c, all patients reported in those studies presented with baseline HbA1c higher than 10% and a minimum drop of 2%. Gibbons et al. reported the largest cohort of patients with TIN including 16 subjects (male and female with diabetes type 1 and 2). Mean baseline HbA1c was 15.5% for diabetes type 1 and 13% for diabetes type 2 vs 6.4% and 7.5%, respectively, after treatment. More recently, Gibbons et al. published a retrospective review of 104 individuals with TIN referred to a tertiary centre over 5 years. With a decrease in HbA1c of >4 percentage points over 3 months the absolute risk of developing TIN exceeded 80% [17].
Although autonomic dysfunction was not tested for in our patients, it is classically reported in patients with APDN and seems to be correlated with the magnitude of decrease in HbA1c [17]. Autonomic dysfunction is reversible in some patients [10, 11].
At onset, TIN may present as an acute small fibre sensory neuropathy (ASFSN), raising the problem of differential diagnosis with the other causes of ASFSN such as Guillain-Barré variant [30], acute steroid-responsive small fibre sensory neuropathy [31] or non-systemic nerve vasculitis. Several weeks after onset, the neuropathy pattern may change, with the appearance of more classical length-dependent axonal and demyelinating sensory and motor neuropathies. Interestingly, early nerve conduction studies in two of the patients (cases 1 and 4) were normal, as observed in five of the six patients reported by Dabby et al. [10], suggesting predominant involvement of small nerve fibres at the initial stages of TIN. In case 4, specific electrophysiological tests for small nerve fibre assessment revealed abnormalities in the cutaneous silent period but not the autonomic nervous system tests suggesting that, in this patient, small nerve fibre damage involved nociceptive fibres rather than autonomic fibres. Unlike the other three patients, patient 4 had no conspicuous symptoms of dysautonomia. To explore this observation, quantification of intraepidermal nerve fibres (IENF) was performed on skin biopsies and showed a significant decrease in IENF density. The role of IENF in sensory neuropathy is not well understood, but some studies found a relationship between pain intensity and the amount of small fibre loss [32, 33]. Nerve biopsy showed not only that small fibre involvement was not exclusive, but also signs of nerve vasculopathy, indicating that axonal lesions and wallerian degeneration of small nerve fibres may actually take place, also observed by Gibbons et al. in 8 of their 16 patients [11].
Normal nerve conduction, despite severe clinical symptoms followed by resolution, has been attributed to haemodynamic factors rather than changes in nerve fibre structure. Tesfaye et al. described epineural arteriovenous shunting, which results in a “steal effect”, which causes increased epineural blood flow and, consequently, endoneural hypoxia of small fibres [34]. Insulin therapy itself has been reported to be the cause of arteriovenous shunting and nerve ischaemia [35]. However, the same clinical picture may occur without insulin treatment, and endoneural hypoxia is not specific to acute painful diabetic neuropathy, but also demonstrated in painful DSP [36]. Indeed, rapid glycaemic control with consecutive haemodynamic components is likely to be the cause of the hypoxia rather than insulin itself.
Similar to TIN, it is known that intensive treatment of diabetes can transiently worsen pre-existing diabetic neuropathy [24, 37], as observed in case 2. Many clinical studies provide evidence for an imbalance between pro- and anti-inflammatory cytokines in neuropathic pain [38]. Based on these findings, it is hypothesised that hypoxia, which up-regulates proinflammatory cytokines, associated with intensive glycaemic control contributes to the pathogenesis of TIN, in a process similar to that responsible for the early worsening of diabetic retinopathy [11, 34].
DNC shares similarities with TIN: a sensorimotor painful polyneuropathy following recent diabetes onset associated with autonomic dysfunction and a history of eating disorder. In contrast to APDN, changes in glycaemic control are not necessarily associated with DNC. Profound weight loss (>10% of the body weight) is present in all patients. Cases have been reported in patients with type 2 diabetes, predominantly male patients in their sixth decade, and in younger male and female type 1 diabetes patients. Weight loss usually resolves within 1–2 years in parallel with resolution of the painful neuropathic symptoms [12, 14, 19, 22, 26]. The aetiology of weight loss is unclear and has been attributed to exaggerated protein catabolism due to poor diabetes control, pronounced dysautonomia (gastroparesis) and/or malabsorption as a result of exocrine pancreas insufficiency [23].
Neural and muscle biopsies in patients with DNC have shown neurogenic atrophy in muscle involving both small and large fibres and axonal degeneration, but no inflammatory and normal vasa nervosum [25]. Cases 1 and 4 showed features of DNC including severe weight loss (cases 1 and 4), depression [12, 19, 27] and autonomic impairment (case 1).
|
Table 1: Patient characteristics. |
|
Patient ID
|
Age at onset (y)/sex
|
Type of diabetes
|
Diabetes duration (y)
|
Initial HbA1c (%)
|
Treatment
|
HbA1c following treatment initiation (%)
|
Estimated time to correct HbA1c (months)
|
Onset of neuropathic pain (weeks from treatment)
|
Time to symptoms resolution (months)
|
Other symptoms
|
ENMG/peroneal nerve biopsy
|
Pain medications
|
| 1 |
44/M |
Type 1 |
|
15.1 |
Insulin |
7.2 |
5 |
4–6 |
Persistent |
Cachexia (weight loss of 11 kg) |
First ENMG normal
Second ENMG: axonal and myelinic sensory neuropathy |
Pregabalin
Gabapentin
Replaced with duloxetine and opioids |
| 2 |
57/M |
Type 2 |
5 |
12.0 |
Sulfonylurea
Metformin
Insulin
Lifestyle |
8 |
8 |
4–6 |
14 |
Autonomic dysfunction |
– |
No medication |
| 3 |
49/M |
Type 2 |
|
15.1 |
Insulin
Metformin |
6.1 |
3 |
4–6 |
12 |
Erectile dysfuntion |
ENMG: axonal and myelinic neuropathy |
Duloxetine |
| 4 |
41/F |
Type 1 |
|
14.3 |
Insulin |
5.2 |
0.5 |
4 |
12 |
Cachexia (weight loss of 39 kg) |
ENMG:
Normal
IENFd: decreased on the thigh and the leg
Peroneal nerve biopsy: loss of myelinated fibres |
Pregabalin
Amitryptiline |
| ENMG = electroneuromyography; F = female; HbA1c = glycated haemoglobin; ID = identity; IENFd = intraepidermal nerve fibre density; M = male |
|
Table 2:Proposed diagnostic criteria for acute painful diabetic neuropathy (APDN): treatment induced neuropathy and diabetic neuropathy cachexia. |
|
|
Treatment induced neuropathy
|
Diabetic neuropathy cachexia
|
|
History
|
1. Improved control of diabetes (type 1 or 2) following insulin or oral antidiabetic treatment
And:
2. Onset of neuropathic pain (weeks from treatment): <6 months
3. Autonomic symptoms
4. History of eating disorder in diabetes type 1 |
1. Recent diabetes onset (type 1 or 2)
And:
2. Severe unintentional weight loss
3. Autonomic symptoms
4. History of eating disorder in diabetes type 1 |
|
Clinical criteria
|
1. Painful paraesthesia
And:
2. Symptoms suggestive of autonomic polyneuropathy |
1. Painful paraesthesia
And:
2. Symptoms suggestive of autonomic polyneuropathy
And:
3. Weight loss >10% of baseline body weight |
|
Electrodiagnostic criteria
|
1. Consistent with sensorimotor polyneuropathy* |
1. Consistent with sensorimotor polyneuropathy* |
|
Autonomic testing
|
1. Parasympathetic and sympathetic dysfunction |
1. Parasympathetic and sympathetic dysfunction |
|
Laboratory
|
1. Baseline HbA1c >10%
And:
2. HbA1c after treatment <9%
Or:
3. Drop of HbA1c >2% within 3 months
And:
4. Normal values of TSH, ANA, vitamin B12, vitamin B1, protein electrophoresis |
1. Baseline HbA1c ≥6.5%
And:
2. Normal values of TSH, ANA, vitamin B12, vitamin B1, protein electrophoresis |
|
Alternative diagnosis excluded
|
Other polyneuropathies |
Pancreatic carcinoma, coeliac disease, other causes of severe weight loss |
|
*Electroneuromyography (ENMG) can be normalat the initial stage of the disease
ANA = antinuclear antibodies; HbA1c = glycated haemoglobin; TSH = thyroid stimulating hormone |
Hints for diagnosis and care management of APDN
Based on previous literature, we propose diagnostic criteria (table 2) and an algorithmic approach to the evaluation and differential diagnosis of APDN (fig. 2). Careful history-taking and clinical examination are essential to exclude other potentially treatable causes of leg pain, such as peripheral vascular diseases or other neurological disorders, like lumbar stenosis, or generalised neuropathies. The European Federation of Neurological Societies guidelines on neuropathic pain assessment should orientate the initial evaluation [39]. Severe pain combined with symptoms of polyneuropathy accompanying rapid control of hyperglycaemia or a drop in HbA1c and/or glycaemia levels support TIN diagnosis (table 2). In addition to HbA1c and plasma glucose, initial laboratory tests should include vitamin B12 and B1 levels, thyroid stimulating hormone, serum protein electrophoresis and light chain serum levels, antinuclear antibody and erythrocyte sedimentation rate [40]. ENMG should be performed in all patients with symptoms and signs of polyneuropathy, but normal results do not rule out the diagnosis. If standard nerve conduction studies are negative, additional, more specific functional or electrophysiological investigations may be of interest in disclosing small nerve fibre dysfunction, such as quantitative sensory tests [41], cutaneous silent period studies [42], laser evoked potentials or autonomic tests [43]. Currently, the gold standard for positive small nerve fibre neuropathy diagnosis is a skin biopsy with IENFd determination [44]. However, in-vivo confocal microscopy of the cornea might be a valuable alternative as it gives the same information and is noninvasive [45–47]. Moreover, as the pathogenesis of APDN may be similar to that of diabetic retinopathy, it is of great interest that microvascular abnormalities of the diabetic retina were recently described to be strongly correlated with the corneal neuropathy observed in diabetic neuropathies [48]. Therefore, ophthalmological assessment, with classical fundoscopy and videofluoroscopy might provide interesting pathology.
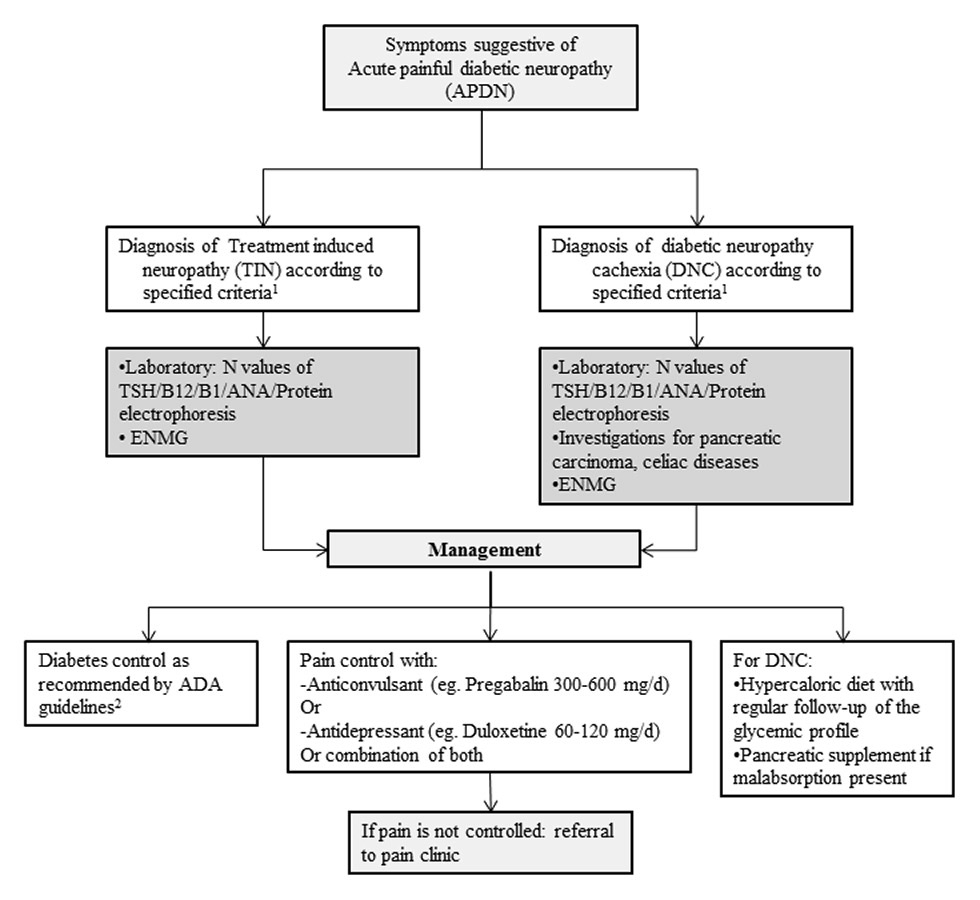
Figure 2
Alogarithm of the approach to acute painful diabetic neuropathy (APDN).
ANA = antinuclear antibody; B1 = vitamin B1; B12 = vitamin B12; ENMG = electroneuromyography; N = normal; TSH = thyroid stimulating hormone
1Reference: Table 2
2ADA = American diabetes association
Patients with suspected DNC should have an extensive work-up to exclude alternative diagnoses of weight loss such as cancer or celiac disease (table 2). Autonomic testing should be considered in patients with symptoms such as orthostatic hypotension or gastrointestinal tract paresis [15].
Treatment options for APDN are similar to those used for chronic painful diabetic neuropathy [49, 50] (fig. 2). They combine good glycaemic control, foot care and pharmacological treatment. Good glycaemic control, in addition to its proven benefit in diabetic polyneuropathy, also helps to relieve pain [51]. A previous study has highlighted the importance of fluctuations in glucose concentrations, which may affect neuropathic pain [52]. However, the benefit of improved glycaemic control on neuropathic pain has been investigated only in small studies. With the exceptions of hyperglycaemic emergencies, such as diabetic ketoacidosis, it is safe to decrease glycaemic control slowly and gradually towards the normal range.
Regular foot examination should be done by physicians and patients should learn to inspect their feet for signs of early infection or ulceration.
Specific pharmacological treatment of neuropathic pain includes anticonvulsants, antidepressants and opioids. The anticonvulsant pregabalin and the antidepressant serotonin-norepinephrine reuptake inhibitor (SNRI) duloxetine have been approved by the American Food and Drug Administration (FDA) for treatment of diabetic peripheral neuropathic pain [53]. Antidepressants are of great help in the management of depression in DNC and, equally, the patient should be referred to a dietician to increase caloric intake without worsening glycaemic control [21].
ASFSN can improve dramatically with steroid therapy, which underscores the possible role of small nerve vessel inflammation in the pathophysiology of APDN [31], as observed in diabetic lumbosacral radiculoplexus neuropathy [3]. On the other hand, the use of steroids in APDN is questionable because glucose control, which is an important therapeutic target in diabetic polyneuropathy [2], may be compromised by this treatment.
Conclusion
The major precipitating factor for TIN is a rapid drop in glycaemia from a previously unusually high level. Previous studies such as The Diabetes Control and Complications Trial (DCTT) [54] have emphasised the benefits of rapidly achieving glycaemic control in reducing microvascular complications of diabetes type 1. However, mortality findings in Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial [55] and subgroup analyses of the Veterans Affairs Diabetes Trial (VADT) [56] suggested that the potential risks of intensive glycaemic control may outweigh the benefits in some patients. Therefore the American Diabetes Association recommended in 2015 more or less stringent glycaemic goals for individual patients [28]. This approach might be beneficial in reducing the risk of TIN. Nevertheless, the recommended 3-month period to correct HbA1c without considering the magnitude of change of HbA1c might increase the risk of TIN in some individuals. Awareness of this risk might in turn lead to future adjustment of guidelines for an optimal rate of glycaemic control that would minimise the risk of triggering TIN.
In order to better understand the underlying pathophysiology of TIN further research should (1.) assess peripheral nerve damage, not only in the course of painful neuropathy but also prior to the symptomatic phase by use of IENF quantification and nerve biopsy and (2.) compare these findings with chronic painful DSP and asymptomatic diabetic neuropathy. A better understanding of the underlying pathophysiology of nerve damage and pain will help to develop more effective treatments.
Acknowledgements:The authors wish to thank Dr Cyrille Sottas, for performing the ENMG examination of case 1, Prof. Jean-Michel Vallat, Dr Laurent Magy and Dr Johannes Alexander Lobrinus for their assistance and helpful comments for cutaneous and nerve biopsies of case 4, Mrs Jaina Patel and Dr Melanie Price for reviewing English language.
References
1 Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care. 2006;29(7):1518–22.
2 Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. 2012;11(6):521–34.
3 Dyck PJ, Windebank AJ. Diabetic and nondiabetic lumbosacral radiculoplexus neuropathies: new insights into pathophysiology and treatment. Muscle Nerve. 2002;25(4):477–91.
4 Caravati C. Insulin neuritis: a case report. Va Med Mon. 1933;59:745–6.
5 Llewelyn JG, Thomas PK, Fonseca V, King RH, Dandona P. Acute painful diabetic neuropathy precipitated by strict glycaemic control. Acta Neuropathol. 1986;72(2):157–63.
6 Vital C, Vital A, Dupon M, Gin H, Rouanet-Larriviere M, Lacut JY. Acute painful diabetic neuropathy: two patients with recent insulin-dependent diabetes mellitus. J Peripher Nerv Syst. 1997;2(2):151–4.
7 Wilson JL, Sokol DK, Smith LH, Snook RJ, Waguespack SG, Kincaid JC. Acute painful neuropathy (insulin neuritis) in a boy following rapid glycemic control for type 1 diabetes mellitus. J Child Neurol. 2003;18(5):365–7.
8 Guldiken S, Guldiken B, Arikan E, Altun Ugur B, Kara M, Tugrul A. Complete relief of pain in acute painful diabetic neuropathy of rapid glycaemic control (insulin neuritis) with venlafaxine HCL. Diabetes Nutr Metab. 2004;17(4):247–9.
9 Takayama S, Takahashi Y, Osawa M, Iwamoto Y. Acute painful neuropathy restricted to the abdomen following rapid glycaemic control in type 2 diabetes. J Int Med Res. 2004;32(5):558–62.
10 Dabby R, Sadeh M, Lampl Y, Gilad R, Watemberg N. Acute painful neuropathy induced by rapid correction of serum glucose levels in diabetic patients. Biomed Pharmacother. 2009;63(10):707–9.
11 Gibbons CH, Freeman R. Treatment-induced diabetic neuropathy: a reversible painful autonomic neuropathy. Ann Neurol. 2010;67(4):534–41.
12 Ellenberg M. Diabetic neuropathic cachexia. Diabetes. 1974;23(5): 418–23.
13 Weintrob N, Josefsberg Z, Galazer A, Vardi P, Karp M. Acute painful neuropathic cachexia in a young type I diabetic woman. A case report. Diabetes Care. 1997;20(3):290–1.
14 Wright DL, Shah JH. Diabetic neuropathic cachexia and hypothyroidism in a woman. Mo Med. 1987;84(3):143–5.
15 Blau RH. Diabetic neuropathic cachexia. Report of a woman with this syndrome and review of the literature. Arch Intern Med. 1983;143(10):2011–2.
16 Huber CA, Schwenkglenks M, Rapold R, Reich O. Epidemiology and costs of diabetes mellitus in Switzerland: an analysis of health care claims data, 2006 and 2011. BMC Endocr Disord. 2014;14:44.
17 Gibbons CH, Freeman R. Treatment-induced neuropathy of diabetes: an acute, iatrogenic complication of diabetes. Brain. 2015;138(Pt 1):43–52.
18 Massey EW. Diabetic neuropathic cachexia and diabetic amyotrophy. Acta Diabetol Lat. 1982;19(1):91–5.
19 Blau RH. Diabetic neuropathic cachexia. Report of a woman with this syndrome and review of the literature. Arch Intern Med. 1983;143(10):2011–2.
20 Al-Hajeri T, El-Gebely S, Abdella N. Profound weight loss in a type 2 diabetic patient with diabetic neuropathic cachexia: a case report. Diabetes Metab. 2009;35(5):422–4.
21 Gade GN, Hofeldt FD, Treece GL. Diabetic neuropathic cachexia. Beneficial response to combination therapy with amitriptyline and fluphenazine. JAMA. 1980;243(11):1160–1.
22 Jackson CE, Barohn RJ. Diabetic neuropathic cachexia: report of a recurrent case. J Neurol Neurosurg Psychiatry. 1998;64(6):785–7.
23 D’Costa DF, Price DE, Burden AC. Diabetic neuropathic cachexia associated with malabsorption. Diabet Med. 1992;9(2):203–5.
24 Song KB, Cho SJ, Minn YK, Kwon KH, Park MK. A case of insulin neuritis that developed in a patient under regular insulin treatment on increasing the insulin dose. Insulin neuritis: is it a misnomer? J Neurol. 2009;256(2):274–5.
25 Archer AG, Watkins PJ, Thomas PK, Sharma AK, Payan J. The natural history of acute painful neuropathy in diabetes mellitus. J Neurol Neurosurg Psychiatry. 1983;46(6):491–9.
26 Godil A, Berriman D, Knapik S, Norman M, Godil F, Firek AF. Diabetic neuropathic cachexia. West J Med. 1996;165(6):382–5.
27 Castellanos F, Mascias J, Zabala JA, Ricart C, Cabello A, Garcia-Merino A. Acute painful diabetic neuropathy following severe weight loss. Muscle Nerve. 1996;19(4):463–7.
28 Standards of medical care in diabetes – 2015: summary of revisions. Diabetes Care. 2015;38 Suppl:S4.
29 Tesfaye S. Recent advances in the management of diabetic distal symmetrical polyneuropathy. J Diabetes Investig. 2011;2(1):33–42.
30 Seneviratne U, Gunasekera S. Acute small fibre sensory neuropathy: another variant of Guillain-Barré syndrome? J Neurol Neurosurg Psychiatry. 2002;72(4):540–2.
31 Dabby R, Gilad R, Sadeh M, Lampl Y, Watemberg N. Acute steroid responsive small-fiber sensory neuropathy: a new entity? J Peripher Nerv Syst. 2006;11(1):47–52.
32 Nolano M, Simone DA, Wendelschafer-Crabb G, Johnson T, Hazen E, Kennedy WR. Topical capsaicin in humans: parallel loss of epidermal nerve fibers and pain sensation. Pain. 1999;81(1–2):135–45.
33 Kennedy WR, Wendelschafer-Crabb G. Utility of skin biopsy in diabetic neuropathy. Semin Neurol. 1996;16(2):163–71.
34 Tesfaye S, Malik R, Harris N, Jakubowski JJ, Mody C, Rennie IG, et al. Arterio-venous shunting and proliferating new vessels in acute painful neuropathy of rapid glycaemic control (insulin neuritis). Diabetologia. 1996;39(3):329–35.
35 Kihara M, Zollman PJ, Smithson IL, Lagerlund TD, Low PA. Hypoxic effect of exogenous insulin on normal and diabetic peripheral nerve. Am J Physiol. 1994;266(6 Pt 1):E980–5.
36 Eaton SE, Harris ND, Ibrahim S, Patel KA, Selmi F, Radatz M, et al. Increased sural nerve epineurial blood flow in human subjects with painful diabetic neuropathy. Diabetologia. 2003;46(7):934–9.
37 Leow MK, Wyckoff J. Under-recognised paradox of neuropathy from rapid glycaemic control. Postgrad Med J. 2005;81(952):103–7.
38 Uçeyler N, Sommer C. Cytokine regulation in animal models of neuropathic pain and in human diseases. Neurosci Lett. 2008;437(3):194–8.
39 Cruccu G, Sommer C, Anand P, Attal N, Baron R, Garcia-Larrea L, et al. EFNS guidelines on neuropathic pain assessment: revised 2009. Eur J Neurol. 2010;17(8):1010–8.
40 England JD, Gronseth GS, Franklin G, Carter GT, Kinsella LJ, Cohen JA, et al. Practice Parameter: evaluation of distal symmetric polyneuropathy: role of laboratory and genetic testing (an evidence-based review). Report of the American Academy of Neurology, American Association of Neuromuscular and Electrodiagnostic Medicine, and American Academy of Physical Medicine and Rehabilitation. Neurology. 2009;72(2):185–92.
41 Ragé M, Van Acker N, Knaapen MW, Timmers M, Streffer J, Hermans MP, et al. Asymptomatic small fiber neuropathy in diabetes mellitus: investigations with intraepidermal nerve fiber density, quantitative sensory testing and laser-evoked potentials. J Neurol. 2011;258(10):1852–64.
42 Onal MR, Ulas UH, Oz O, Bek VS, Yucel M, Taslipinar A, et al. Cutaneous silent period changes in Type 2 diabetes mellitus patients with small fiber neuropathy. Clin Neurophysiol. 2010;121(5):714–8.
43 Koytak PK, Isak B, Borucu D, Uluc K, Tanridag T, Us O. Assessment of symptomatic diabetic patients with normal nerve conduction studies: utility of cutaneous silent periods and autonomic tests. Muscle Nerve. 2011;43(3):317–23.
44 Devigili G, Tugnoli V, Penza P, Camozzi F, Lombardi R, Melli G, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain. 2008;131(Pt 7):1912–25.
45 Zhao C, Lu S, Truffert A, Tajouri N, Zhao K, Mateo Montoya A, et al. Corneal nerves alterations in various types of systemic polyneuropathy, identified by in vivo confocal microscopy. Klin Monbl Augenheilkd. 2008;225(5):413–7.
46 Edwards K, Pritchard N, Vagenas D, Russell A, Malik RA, Efron N. Utility of corneal confocal microscopy for assessing mild diabetic neuropathy: baseline findings of the LANDMark study. Clin Exp Optom. 2012;95(3):348–54.
47 Tavakoli M, Petropoulos IN, Malik RA. Corneal confocal microscopy to assess diabetic neuropathy: an eye on the foot. J Diabetes Sci Technol. 2013;7(5):1179–89.
48 Nitoda E, Kallinikos P, Pallikaris A, Moschandrea J, Amoiridis G, Ganotakis ES, et al. Correlation of diabetic retinopathy and corneal neuropathy using confocal microscopy. Curr Eye Res. 2012;37(10):898–906.
49 Veves A, Backonja M, Malik RA. Painful diabetic neuropathy: epidemiology, natural history, early diagnosis, and treatment options. Pain Med. 2008;9(6):660–74.
50 Hartemann A, Attal N, Bouhassira D, Dumont I, Gin H, Jeanne S, et al. Painful diabetic neuropathy: diagnosis and management. Diabetes Metab. 2011;37(5):377–88.
51 Boulton AJ, Drury J, Clarke B, Ward JD. Continuous subcutaneous insulin infusion in the management of painful diabetic neuropathy. Diabetes Care. 1982;5(4):386–90.
52 Oyibo SO, Prasad YD, Jackson NJ, Jude EB, Boulton AJ. The relationship between blood glucose excursions and painful diabetic peripheral neuropathy: a pilot study. Diabet Med. 2002;19(10):870–3.
53 Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28(4):956–62.
54 The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329(14):977–86.
55 Gerstein HC, Miller ME, Byington RP, Goff DC, Bigger JT, Buse JB, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–59.
56 Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–39.

