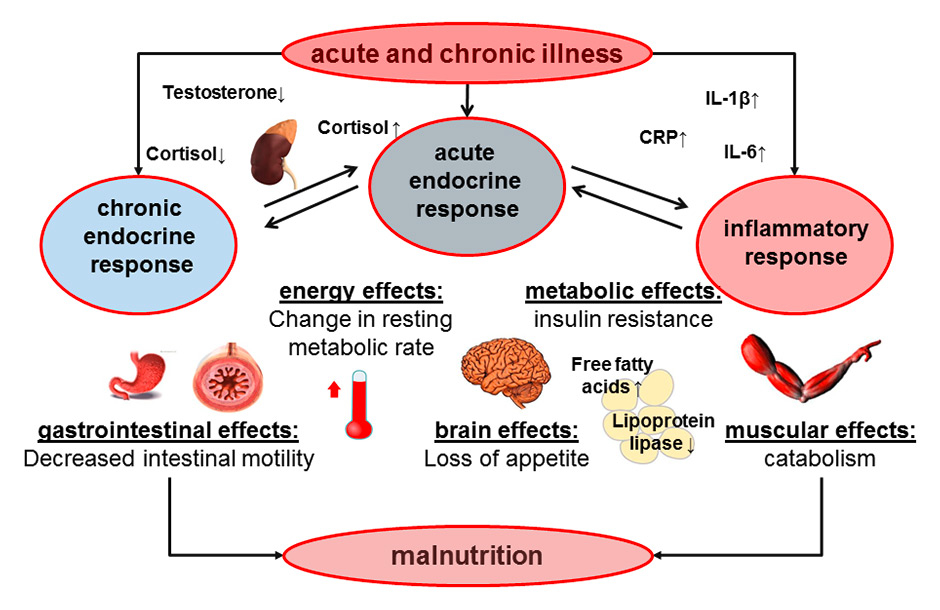
Figure 1
Summary of physiopathological mechanisms occurring during acute and chronic illnesses including endocrine and inflammatory pathways leading to malnutrition.
CRP = C-reactive protein; IL = interleukin
DOI: https://doi.org/10.4414/smw.2015.14132
Acute and chronic illness is associated with loss of appetite and body weight, which increases the risk for malnutrition, particularly in the elderly and frail medical patient population [1]. This relationship between acute disease and eating behaviour / nutritional status may well be bidirectional, not only with illness affecting nutritional status, but also dietary factors influencing the course of illness (reviewed in [2]). For example, cytokines, such as interleukin (IL)-6 and tumour-necrosis-factor (TNF)-alpha, affect the brain circuitries that control food intake, delayed gastric emptying and skeletal muscle catabolism (fig. 1) [3, 4]. From an evolutionary perspective based on the principals of Charles Darwin, it is tempting to hypothesise that loss of appetite during acute illness may well bring a natural selection advantage and increases a patient’s ability to survive. Whether loss of appetite associated with acute illness is indeed a protective physiological response or a therapeutic target needing early corrective nutritional therapy is still debated. Particularly, this is also important when asking the question “who ultimately benefits from nutritional interventions?” ‒ malnourished patients, patients at risk of malnutrition or both? This controversy can only be resolved with a large randomised-controlled trial comparing early nutritional therapy with “appetite-guided” nutrition in this patient population.

Figure 1
Summary of physiopathological mechanisms occurring during acute and chronic illnesses including endocrine and inflammatory pathways leading to malnutrition.
CRP = C-reactive protein; IL = interleukin
Malnutrition is common in elderly, chronic and/or polymorbid inpatients and associated with detrimental metabolic consequences, such as catabolism and muscle wasting [1]. Malnutrition per se is associated with higher mortality and morbidity, increased risk for infection and an increased hospital length-of-stay (LOS) [5, 6]. Therefore, prevention of malnutrition is an important goal in patient care and probably much more effective than treating this condition in sick patients. In medical inpatients at risk for malnutrition, our current clinical approach is still to provide nutritional therapy as an alleged strategy to combat malnutrition and associated adverse outcomes. However, unlike the critical care setting, where various large trials have recently been published [7–9], as well as in surgical patients and patients living in long-term facilities [10], there is an important lack of high quality data from large randomised controlled trials (RCTs) in unselected acutely ill medical inpatients to support the early use of nutritional therapy and to shed light on which patient population ultimately benefits from nutritional interventions, i.e. only malnourished patients, patients at risk for malnutrition or both? Also the optimal type, caloric amount and timing of nutritional therapy remain largely undefined and no current guideline exists today to give physicians guidance on the best approach to the polymorbid medical inpatient.
A 2009 meta-analysis focusing on the effects of nutritional therapy in the elderly patient population and including 10,187 randomised participants in 62 trials of mostly poor study quality, found that supplementation may reduce mortality in older people who are undernourished and may lead to lower risk for complications [10]. However, no evidence of improvement in functional benefit or reduction in length of hospital stay was found with supplements [10]. Most trials, however, included outpatients, long-term facility or surgical patients. Similarly, another previous meta-analysis confirmed the important lack of high-quality evidence to endorse or refute nutritional support and give firm recommendations for or against nutritional therapy [11]. Again, this meta-analysis did not specifically focus on the effect of early nutritional therapy in the complex, polymorbid medical inpatient population.
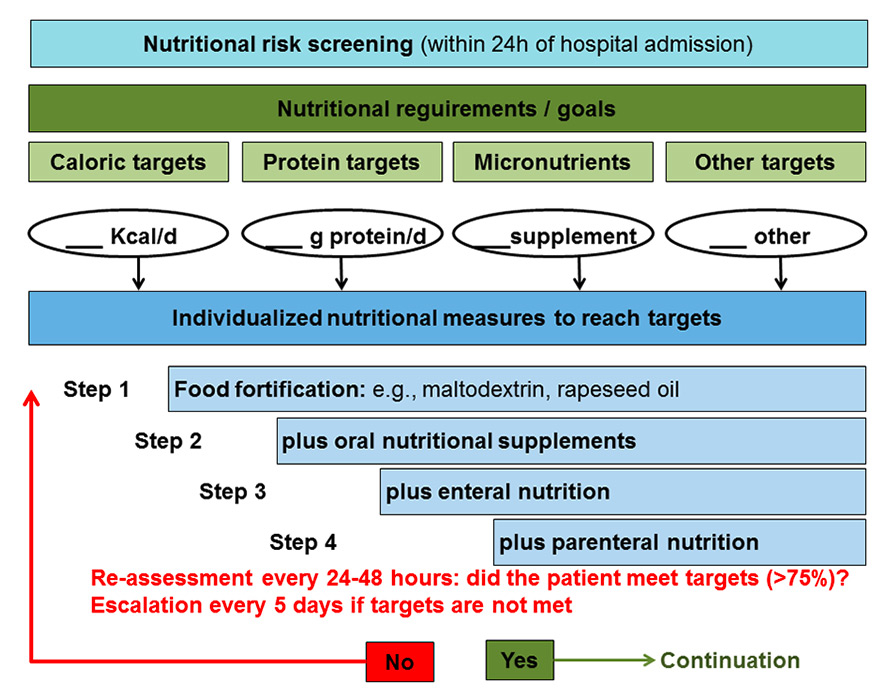
Figure 2
Nutritional strategy in medical inpatients at risk for malnutrition.
Nonetheless, for medical inpatient population, various previous trials have investigated the effects of nutritional strategies on selected patient outcomes, mainly changes in body weight and nutrition-specific quality of life. Table 1 shows a summary of most important nutritional intervention trials based on a recent Cochrane literature search [12]. However, these trials were highly heterogeneous in design, patient populations and type of interventions, lacked power to demonstrate safety and, in aggregate, produced inconclusive results.
| Table 1: Important interventional studies evaluating nutritional therapy in medical inpatients. | ||||||||
| First author, Year | Patient type | No. | Study intervention | Control treatment | Outcomes evaluated | Main effect of study Intervention | Limitations | Ref |
| Starke J, 2011 | Malnourished general medical inpatients | 132 | Individual nutritional support (oral) | Standard nutritional care1 | Caloric intake, weight, vitamin levels, QoL, complications, readmission, mortality (6m) | Higher caloric/protein intake, less weight loss, increase in QoL, fewer complications | Small sample, time-consuming intervention | [32] |
| Hickson M, 2004 | Malnourished medical patients, >65 y | 592 | Nutritional counselling (by healthcare assistants) | Standard nutritional care2 | Weight/BMI, Barthel’s index, infection, LOS, in-hospital mortality | Less antibiotic use, otherwise no effect | Short training for healthcare assistants (15 h) | [33] |
| Norman K, 2008 | Malnourished inpatients, with GI disease | 101 | Dietary counselling with ONS over 3–month period | Dietary counselling without ONS | BMI, muscle strength, readmission, QoL | Improved hand grip strength and PF, fewer readmissions, better QoL | High dropout rate, two intervention groups | [34] |
| Rüfenacht U, 2010 | Malnourished general medical inpatients | 36 | Intensive nutritional counselling with ONS | ONS only | Anthropometrics, energy and protein intake, QoL questionnaire | Higher caloric/protein intake, QoL improvement after hospitalisation | Small sample, two intervention groups | [35] |
| Gariballa S, 2006 | Malnourished inpatients >65 y | 445 | 400 ml ONS/d (995 kcal) | 400 ml placebo/d (60 kcal) | Barthel, readmissions, LOS, mortality | Fewer readmissions, otherwise no effect | Low adherence | [36] |
| Johansen N, 2004 | Malnourished inpatients | 212 | Individualised nutritional therapy | Standard nutritional care2 | Caloric intake, LOS, complications, mortality, QoL | Higher caloric/protein intake associated with shorter LOS | Small sample | [37] |
| BMI = body mass index; d = day(s); GI = gastrointestinal; h = hours(s); LOS = length of stay; m = month(s); ONS = oral nutrition supplement; PF = peak flow; QoL = quality of life; 1 Including the prescription of oral nutritional supplements and nutritional therapy prescribed by the physician according to the routine ward management; 2 Not further specified | ||||||||
Despite the absence of high-quality RCT data, the current clinical approach in unselected medical inpatients is to provide nutritional therapy to reach nutritional requirements including caloric and protein targets, as well as micronutritional requirements (fig. 2). These considerations are mainly based on observational and preclinical studies and the non-ill physiological situation following the basic principles that a deficit in energy or protein metabolism in conjunction with disease-induced inflammation will lead to cachexia and catabolism. Whether during acute illness the same amounts of energy and proteins are needed and beneficial as in healthy patients thereby remains unclear.
In fact, some recent data from critical care suggested even harmful effects of aggressive early (over-) feeding and glutamine supplementation [7, 8, 13]. As a possible explanation for these findings, it has been emphasised that during the acute phase of illness, the body mobilises substrates from muscle and fat tissue to match the increased resting energy expenditure [14]. Exogenous calories then no longer inhibit gluconeogenesis. Excessive nutrition during the acute phase of illness can thus induce occult overfeeding. Still, recent research from Switzerland reported benefit from individually optimised energy supplementation in well-selected patients [9]. The contradictory findings from these trials may be partly explained by the differences in patient populations studied and trial design. An important consideration is, therefore, the fact that every ill patient is different, and nutritional strategies and goals need to be personalised and tailored to the individual patient’s requirements [14].
Importantly, data from critical care cannot unconditionally be transferred to medical inpatients with a lower degree of disease severity. Still, the above-mentioned conflicting observations re-emphasise that nutritional therapy is a medical intervention with associated risks and costs, and call into question todays nutritional approach in medical inpatients. The current lack of strong guideline recommendations [15–28] for type, caloric amount and timing of nutritional therapy in medical inpatients is mainly explained by the paucity of high-level evidence showing such therapy’s efficacy and cost benefits, and the absence of knowledge regarding which patient populations do or do not benefit.
As a result of the conflicting results and gaps in the literature, a pragmatic randomised trial is currently enrolling patients in several hospitals in Switzerland (EFFORT: Effect of Early Nutritional Therapy on Frailty, Functional Outcomes and Recovery of Undernourished Medical Inpatients Trial). The trial aims to assess the effects of early nutritional therapy in regard to effectiveness, safety and costs when applied to the heterogenous, polymorbid medical inpatient population. EFFORT will not only answer the question about overall benefit or harm, but by using a physiopathological mechanistic approach, it also will explore and provide conclusive answers about whether, why, how, and in which patient populations nutritional therapy does and does not work.
Most current nutritional research has focused on selected medical diseases (i.e. pancreatitis). As a consequence, these “clean” results may not be generalisable to “real-life” unselected medical inpatients with multiple comorbidities and illnesses. Comparative effectiveness research aims at improving quality, effectiveness and efficiency of health care and at helping patients and healthcare professionals make informed decisions [29]. To achieve these goals, research must address the patient population that actually consumes the most health care, specifically polymorbid, frail, elderly patients with complex combinations of medical diagnoses. Although this patient population accounts for the majority of costs, it is also the least studied population [30]. To correct this disparity, it has become evident that clinical trials should include large, representative populations, to enable examination of treatment effects within key subpopulations, and to allow robust head-to-head comparison of interventions [29]. Owing to its pragmatic design, EFFORT will close this important gap and because of its large sample size (i.e., 2,000–3,000 patients target sample size) important subgroup analyses will be performed to understand which patients do or do not benefit from therapy. This is an important first step for a more “personalized nutritional care”.
Malnutrition is a major issue in hospital care. Whether, how and why early nutritional therapy affects outcomes of unselected, elderly, frail medical inpatients remains largely unclear today. In the absence of strong evidence for or against nutritional therapy in the medical inpatient population, the following considerations may help to guide physicians’ approach to malnutrition while awaiting more definite trial results (fig. 2): (1.) screening for malnutrition using a validated tool (such as NRS 2002) is recommended [31]; (2.) In patients at risk, a careful assessment of the nutritional situation is recommended to estimate nutritional targets (caloric, protein, micronutrient) and to find modifiable factors (e.g., poor dental status, different food preferences); (3.) If nutritional targets cannot be reached, a team approach including dieticians, nurses and physicians is warranted to optimise nutritional intake in patients; (4.) nutritional therapy should be as physiological as possible starting with between-meal snacking, food enrichment and oral supplements. Enteral and parenteral nutrition should only be used as ultimo ratio when other measures fail.
Hopefully EFFORT will provide more definite answers and will tell us whether such an approach holds its promise and reverses the adverse effects of malnutrition in this frail patient population.
1 Kubrak C, Jensen L. Malnutrition in acute care patients: a narrative review. Int J Nurs Stud. 2007;44(6):1036–54.
2 Schutz P, Bally M, Stanga Z, Keller U. Loss of appetite in acutely ill medical inpatients: physiological response or therapeutic target? Swiss Med Wkly. 2014;144:w13957.
3 Kuhlmann MK, Levin NW. Potential interplay between nutrition and inflammation in dialysis patients. Contrib Nephrol. 2008;161:76–82.
4 Oner-Iyidogan Y, Gurdol F, Kocak H, Oner P, Cetinalp-Demircan P, Caliskan Y, et al. Appetite-regulating hormones in chronic kidney disease patients. J Ren Nutr. 2011;21(4):316–21.
5 Casaer MP, Hermans G, Wilmer A, Van den Berghe G. Impact of early parenteral nutrition completing enteral nutrition in adult critically ill patients (EPaNIC trial): a study protocol and statistical analysis plan for a randomized controlled trial. Trials. 2011;12:21.
6 Villet S, Chiolero RL, Bollmann MD, Revelly JP, Cayeux RNM, Delarue J, et al. Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clin Nutr. 2005;24(4):502–9.
7 Casaer MP, Mesotten D, Hermans G, Wouters PJ, Schetz M, Meyfroidt G, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011;365(6):506–17.
8 Heyland D, Muscedere J, Wischmeyer PE, Cook D, Jones G, Albert M, et al. A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med. 2013;368(16):1489–97.
9 Heidegger CP, Berger MM, Graf S, Zingg W, Darmon P, Costanza MC, et al. Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: a randomised controlled clinical trial. Lancet. 2013;381(9864):385–93.
10 Milne AC, Potter J, Vivanti A, Avenell A. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Syst Rev. 2009(2):CD003288.
11 Koretz RL, Avenell A, Lipman TO, Braunschweig CL, Milne AC. Does enteral nutrition affect clinical outcome? A systematic review of the randomized trials. Am J Gastroenterol. 2007;102(2):412–29; quiz 468.
12 Schuetz P, Blaser Yildirim PZ, Gloy VL, Briel M, Bally MR. Early nutritional therapy for malnourished or nutritionally at-risk adult medical inpatients. Cochrane Metabolic and Endocrine Disorders Group. 2014.
13 Schetz M, Casaer MP, Van den Berghe G. Does artificial nutrition improve outcome of critical illness? Crit Care. 2013;17(1):302.
14 Vincent JL, Preiser JC. When should we add parenteral to enteral nutrition? Lancet. 2013;381(9864):354–5.
15 Volkert D, Berner YN, Berry E, Cederholm T, Coti Bertrand P, Milne A, et al. ESPEN Guidelines on Enteral Nutrition: Geriatrics. Clin Nutr. 2006;25(2):330–60.
16 Vanek VW, Matarese LE, Robinson M, Sacks GS, Young LS, Kochevar M. A.S.P.E.N. position paper: parenteral nutrition glutamine supplementation. Nutr Clin Pract. 2011;26(4):479–94.
17 Vanek VW, Borum P, Buchman A, Fessler TA, Howard L, Jeejeebhoy K, et al. A.S.P.E.N. position paper: recommendations for changes in commercially available parenteral multivitamin and multi-trace element products. Nutr Clin Pract. 2012;27(4):440–91.
18 Sobotka L, Schneider SM, Berner YN, Cederholm T, Krznaric Z, Shenkin A, et al. ESPEN Guidelines on Parenteral Nutrition: geriatrics. Clin Nutr. 2009;28(4):461–6.
19 Plauth M, Cabre E, Riggio O, Assis-Camilo M, Pirlich M, Kondrup J, et al. ESPEN Guidelines on Enteral Nutrition: Liver disease. Clin Nutr. 2006;25(2):285–94.
20 Mueller C, Compher C, Ellen DM. A.S.P.E.N. clinical guidelines: Nutrition screening, assessment, and intervention in adults. JPEN J Parenter Enteral Nutr. 2011;35(1):16–24.
21 McMahon MM, Nystrom E, Braunschweig C, Miles J, Compher C. A.S.P.E.N. clinical guidelines: nutrition support of adult patients with hyperglycemia. JPEN J Parenter Enteral Nutr. 2013;37(1):23–36.
22 Choban P, Dickerson R, Malone A, Worthington P, Compher C. A.S.P.E.N. Clinical Guidelines: Nutrition Support of Hospitalized Adult Patients With Obesity. JPEN J Parenter Enteral Nutr. 2013.
23 Cano NJ, Aparicio M, Brunori G, Carrero JJ, Cianciaruso B, Fiaccadori E, et al. ESPEN Guidelines on Parenteral Nutrition: adult renal failure. Clin Nutr. 2009;28(4):401–14.
24 Brown RO, Compher C. A.S.P.E.N. clinical guidelines: nutrition support in adult acute and chronic renal failure. JPEN J Parenter Enteral Nutr. 2010;34(4):366–77.
25 August DA, Huhmann MB. A.S.P.E.N. clinical guidelines: nutrition support therapy during adult anticancer treatment and in hematopoietic cell transplantation. JPEN J Parenter Enteral Nutr. 2009;33(5):472–500.
26 Arends J, Bodoky G, Bozzetti F, Fearon K, Muscaritoli M, Selga G, et al. ESPEN Guidelines on Enteral Nutrition: Non-surgical oncology. Clin Nutr. 2006;25(2):245–59.
27 Volkert D, Berner YN, Berry E, Cederholm T, Coti Bertrand P, Milne A, et al. ESPEN Guidelines on Enteral Nutrition: Geriatrics. Clin Nutr. 2006;25(2):330–60.
28 Maisonneuve N, Genton L, Karsegard VL, Kyle UG, Dupertuis YM, Pichard C. Role of impedance measurement in nutritional screening. Rev Med Suisse Romande. 2004;124(10):611–5.
29 Tinetti ME, Studenski SA. Comparative effectiveness research and patients with multiple chronic conditions. N Engl J Med. 2011;364(26):2478–81.
30 Van Spall HG, Toren A, Kiss A, Fowler RA. Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review. JAMA. 2007;297(11):1233–40.
31 Kondrup J, Rasmussen HH, Hamberg O, Stanga Z. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22(3):321–36.
32 Starke J, Schneider H, Alteheld B, Stehle P, Meier R. Short-term individual nutritional care as part of routine clinical setting improves outcome and quality of life in malnourished medical patients. Clin Nutr. 2011;30(2):194–201.
33 Hickson M, Bulpitt C, Nunes M, Peters R, Cooke J, Nicholl C, et al. Does additional feeding support provided by health care assistants improve nutritional status and outcome in acutely ill older in-patients? – a randomised control trial. Clin Nutr. 2004;23(1):69–77.
34 Norman K, Kirchner H, Freudenreich M, Ockenga J, Lochs H, Pirlich M. Three month intervention with protein and energy rich supplements improve muscle function and quality of life in malnourished patients with non-neoplastic gastrointestinal disease – a randomized controlled trial. Clin Nutr. 2008;27(1):48–56.
35 Rufenacht U, Ruhlin M, Wegmann M, Imoberdorf R, Ballmer PE. Nutritional counseling improves quality of life and nutrient intake in hospitalized undernourished patients. Nutrition. 2010;26(1):53–60.
36 Gariballa S, Forster S, Walters S, Powers H. A randomized, double-blind, placebo-controlled trial of nutritional supplementation during acute illness. Am J Med. 2006;119(8):693–9.
37 Johansen N, Kondrup J, Plum LM, Bak L, Norregaard P, Bunch E, et al. Effect of nutritional support on clinical outcome in patients at nutritional risk. Clin Nutr. 2004;23(4):539–50.
Disclosures: PS is supported in part by the Swiss National Science Foundation (SNSF Professorship, PP00P3_150531 / 1), the Swiss Academy for Medical Sciences (Schweizerische Akademie der Medizinischen Wissenschaften [SAMW]), and the Research Council of the Kantonsspital Aarau (1410.000.044). No other potential conflict of interest relevant to this article was reported.