Hyperthermia and reirradiation for locoregional recurrences in preirradiated breast cancers: a single institutional experience
DOI: https://doi.org/10.4414/smw.2015.14133
Niloy Ranjan
Datta, Emsad
Puric, Juerg
Heüberger, Dietmar
Marder, Nicoletta
Lomax, Olaf
Timm, Priska
Memminger, Stephan
Bodis
Summary
QUESTIONS UNDER STUDY: The aim of this retrospective analysis was to evaluate the safety and efficacy of local hyperthermia (HT) and reirradiation (ReRT) in the management of preirradiated locoregional recurrent breast cancers at Kantonsspital Aarau, Switzerland.
METHODS: Twenty-four previously irradiated patients who had developed locoregional recurrences in the chest wall or breast, with or without regional lymph node involvement, were reirradiated to a mean dose of 36.8 Gy (range 20–50 Gy) delivered at a mean dose per fraction of 2.33 Gy (range 1.8–4.0 Gy). All patients received local HT at 41 to 43 °C, once or twice a week prior to radiotherapy. Online thermometry was carried out during the hyperthermia sessions.
RESULTS: An overall objective response rate of 91.7% (22/24) with a complete response in 66.7% (16/24) of patients and partial response in 25% (6/24) of patients was observed. Post-thermoradiotherapy follow-up ranged from 1 to 38 months (median 10 months). The 3-year actuarial local control rate was 59.7%. More patients who attained complete response had sustained locoregional control until their death or last follow-up when compared with those who were partial or non-responders (median local disease-free survival for complete responders not reached; for partial and non-responders 4 months; p <0.001). Post-retreatment median overall survival for all 24 patients was 10 months. Grade III/IV acute toxicity was seen in only one patient and no patient had any significant late morbidity.
CONCLUSIONS: ReRT and HT is an effective and a safe modality to treat locoregional recurrences in previously irradiated breast cancers. The approach can lead to sustainable long-term palliation with minimal morbidity.
Presented at the Annual Conferences of the European Society of Therapeutic Radiology and Oncology (ESTRO), 4–8 April 2014, Vienna, Austria, and the European Society for Hyperthermic Oncology (ESHO), 11–14 June, 2014, Torino, Italy.
Introduction
Around one-third of breast cancer patients develop locoregional recurrences during their follow-up and more than 80% of the recurrences are known to occur within the first 5-years of their primary treatment [1, 2]. Of these recurrences, 85% are located in the original tumour bed with 15% at other sites in the breast or even a de novo tumour [3]. Various therapeutic approaches have been advocated to treat these locoregional recurrences. These include surgery, radiation therapy (RT) and chemotherapy either alone or in combination [1, 4–8].
Mastectomy, considered a gold standard for treating recurrences after breast conservation, may result in a second local recurrence in 2% to 31% of patients [9]. For those who had undergone mastectomy as part of their initial treatment, repeat surgery for recurrences provides local control in only one-third of the patients [10]. In preirradiated patients, reirradiation (ReRT) increased the risk of acute and late toxicity owing to an increase in cumulative radiation dose. Wahl et al., [8] in a multi-institutional review of ReRT in recurrent breast cancer, reported a complete response rate of 39% even with a median cumulative dose of 106 Gy (range 74.4–137.5 Gy). A Cochrane review did not find conclusive evidence for use of chemotherapy in the management of these locoregional recurrences [6]. Thus, management of locoregional recurrences in breast cancers after primary treatment is indeed a therapeutic challenge.
Hyperthermia (HT), at 41 to 43 °C, is a known radiosensitiser with minimal morbidity and enhances the efficacy of radiation through its unique thermoradiobiological properties [11, 12]. It complements the radiation-induced tumouricidal effects through hypoxic cell sensitisation and reduces repair of radiation-induced DNA damage [12, 13]. This provides the basis for using HT with moderate doses of radiation to treat chest wall recurrences, which has been reported to have reasonable success and minimal morbidity [14–27].
In the literature, the approaches to treatment of these recurrent breast cancers with hyperthermia have been variable [14–27]. Those that were operable were operated upon and then treated with ReRT and HT. Patients with no previous RT have been treated with higher radiation doses. However, those lesions considered inoperable and which had also been exposed to previous RT pose a greater theraputic challenge. Herein, we share our experience of treating inoperable locoregional recurrences with local HT and ReRT in preirradiated breast cancers.
Patients and methods
Patient population
Between 2006 and 2013, a total of 46 breast cancer patients with locoregional recurrences were referred to our institute for further management. Of these, 24 had been previously irradiated and were now considered for local HT and ReRT. The initial diagnosis and treatment of these patients were carried out between 1986 and 2011. The median interval to recurrence after initial treatment was 6 years (range 2–22 years) (table 1). All patients had received RT, with a median dose of 54 Gy (range 30‒70 Gy). They received multimodality treatment including surgery, chemotherapy and hormonal intervention according to their initial disease stage and the treatment policies of the referring hospitals. The primary indication for HT and ReRT was inoperable locoregional recurrent disease following previous irradiation.
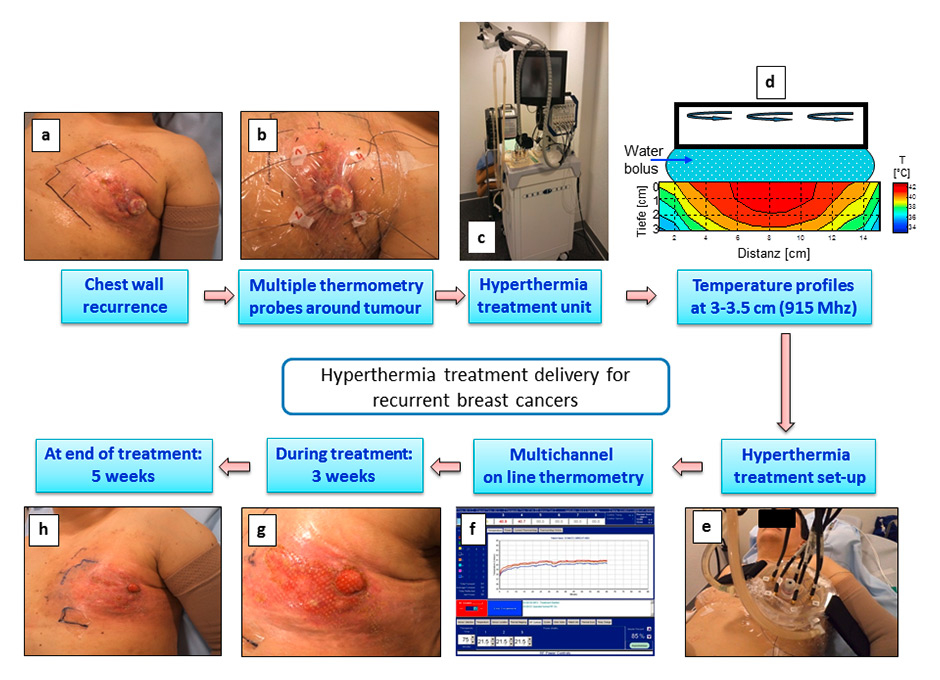
Figure 1
Steps involved in the hyperthermia treatment delivery for a patient with locoregional recurrent breast cancers. (a) Patient with the chest wall recurrence following previous radiotherapy. (b) Placement of multiple thermometry sensors all around the tumour in the area to be treated with hyperthermia. (c) Superficial hyperthermia treatment unit (BSD 500, 915 MHz). (d) Temperature profiles in a phantom showing the heating patterns with 915 MHz radiofrequency applicators. A water bolus has been sandwiched between the applicators and the phantoms to simulate the treatment conditions. A temperature ranging from 40–43 °C could be achieved to a depth of 3 cm. (e) and (f) Actual treatment setup with online recording of the temperature profiles. (g) and (h) Tumour regression observed at 3 weeks and at completion of treatment with minimal skin morbidity. The patient went on to achieve a complete response at 6 weeks of completion of reirradiation and hyperthermia.
Two-thirds of the patients (n = 16) had isolated chest wall recurrence and four had isolated recurrences in the regional nodes. Two patients had recurrences in the chest wall and regional nodes. In addition, two patients had recurrence after breast conservation treatment: one only in the ipsilateral breast, the other in both the breast and regional lymph nodes. The recurrences varied from isolated or multiple ulcerative or nodular lesions to diffuse lesions in the chest wall or breast, with or without regional lymphadenopathy. No surgical intervention was carried out for the recurrences, either because they were deemed inoperable or because surgery was perceived to add no appreciable benefit . None of the patients received any chemotherapy during the ReRT and HT treatments.
Fourteen of these 24 patients (58.3%) also had distant metastasis at the time of referral for treatment of locoregional recurrent disease. The distant metastases occurred mainly as isolated or multiple lesions in bones, lungs, liver and/or brain for which the patients were receiving salvage chemotherapy / hormonal manipulation. Salvage chemotherapy was withheld during the time of ReRT and HT, but patients were allowed to continue hormonal therapies.
Reirradiation
The ReRT dose and fractionation were individualised on the basis of the site of recurrence, previous RT doses received at the recurrence site, time of previous RT, present disease status and the expected survival. Thus, the ReRT dose ranged from 20 to 50 Gy (median 39 Gy) delivered at a dose per fraction of 1.8 to 4 Gy (median 2.07 Gy) in 5 to 25 fractions (median 15.5 fractions) (table 2). Radiotherapy treatment planning was carried out for all patients and, depending on the tumour site and size, patients were treated either with a 6 MV photon beam or with electrons with energy adapted to the tumour thickness and the desired treatment depth. The summated median RT dose of the primary RT and ReRT was 94.5 Gy (range 55‒114 Gy).
Local hyperthermia
Local hyperthermia was administered with the superficial hyperthermia unit, BSD-500 (BSD Medical Corporation, USA), capable of generating microwaves at 915 MHz. The system has three surface applicators with 3, 8 or 24 spiral antennas that cover a diameter of 9 cm, 12 cm or an area of 24 x 20 cm, respectively. Depending on the size and extent of the recurrent lesions, the applicator that would well encompass all the recurrent lesions was selected and placed directly over the treatment volume. At 915 MHz, the desired temperature of 41 to 43 ˚C could be attained within the intervening tissues up to a maximum depth of 3 cm. Thus, all patients considered for HT had lesions not extending beyond a depth of 3 cm from the skin surface. This was ensured from the computed tomography (CT) / positron emission tomography-CT (PET-CT) or magnetic resonance imaging (MRI) scans undertaken before the patients were accepted for the HT treatment.
All patients were quite comfortable during the treatment and did not require any analgesia. The HT applicators provide an inbuilt water bolus with recirculating water for surface cooling. This ensures contact between the applicator and the patient and maintains a controlled temperature of the skin surface. Well-controlled coupling of the microwave field from applicator into tissue is necessary for uniform heating, and the skin cooling helps prevent overheating of the skin surface, which may cause thermal blisters or, in rare cases, deeper burns in subcutaneous tissue.
For online temperature monitoring, four temperature sensors were placed in and around the lesions on the overlying skin and once a temperature of 41 to 43 °C was attained, it was maintained for 60 minutes during the heating session. Patients were treated with HT once a week (7 patients) or twice a week (17 patients), 30 minutes before RT. Thus, for patients on 5 RT fractions a week, HT was administered once or twice a week with RT while on the remaining days, the patients were treated with RT alone. The number of HT sessions ranged from 2 to 11 (median 7 sessions) (table 2). The steps of HT treatment delivery are depicted in figure 1.
Evaluation of local response and associated morbidity
All patients were closely monitored during treatment for any therapy-related acute or late morbidity as in the Common Terminology Criteria for Adverse Events (CTCAE) [28, 29]. Skin and subcutaneous tissue disorders were the primary toxicities evaluated according to the CTCAE guidelines. These included – bullous dermatitis, dryness of skin, erythroderma, skin pigmentation, skin induration, skin ulceration and telangiectasia. Late toxicity was scored only in patients who had a follow-up of more than 90 days from the date of completion of ReRT and HT.
Tumour responses were evaluated at 4 weeks after completion of treatment according to the Response Evaluation Criteria in Solid Tumours (RECIST) [30, 31]. In accordance with the RECIST guidelines, measurable lesions were measured either clinically (on each visit) or on periodic imaging (CT/MRI/PET), and nonmeasurable lesions (diffuse or nodular skin infiltration with each lesion less than 10 mm) were evaluated clinically. A complete response was defined as disappearance of all target lesions at 4 weeks from completion of ReRT and HT.
Statistical considerations
All statistical calculations were performed using IBM SPSS software package, version 21.0. For survival analysis, the status of all patients at last follow-up was considered. Actuarial local control represented the status of the local recurrence at the time of last follow-up or death. All patients with a complete locoregional response at their last follow-up or death were considered as “censored”, whereas those with partial or no response were considered as “events.” For computation of overall survival, all patients surviving were considered as “censored,” and those dead from any cause or lost to follow-up were considered as “events.” Kaplan-Meier estimates were used to test the differences in survival outcomes between complete responders and non-complete responders [32]. A logistic regression was carried out to identify any predictive variable for complete response at the end of ReRT and HT. Various patient and treatment related factors (age, tumour grade, hormone receptor [ERPR] status, HER2 status, prior treatment offered, ReRT dose [in terms of biological equivalent dose with tumour α/β = 10], number of HT sessions per week, total number of HT sessions received, time gap between the previous RT and the present ReRT) were entered into the logistic regression using forward (conditional) method. The multivariate analysis for actuarial local control was performed using Cox’s proportional hazard model [33].
|
Table 1:Patient characteristics at the time of referral for reirradiation and hyperthermia. |
|
Parameter
|
Value
|
| Age |
38–88 years (66.0 ± 15.1)* |
| Interval between 1st treatment and retreatment |
2–22 years (7.6 ± 5.7)* |
| Site of recurrence |
| |
Chest wall |
16 (66.6%) |
| |
Regional lymph nodes |
4 (16.7%) |
| |
Chest wall and regional lymph nodes |
2 (8.3%) |
| |
Breast |
1 (4.2%) |
| |
Breast and regional lymph nodes |
1 (4.2%) |
| Initial group stage#
|
| |
Stage I |
6 (25%) |
| |
Stage II |
3 (12.5%) |
| |
Stage III |
13 (54.2%) |
| |
Stage IV |
2 (8.3%) |
| Previous treatment received (alone or in combination) |
| |
Radiotherapy |
24 (100%) |
| |
Surgery |
23 (95.8%) |
| |
Chemotherapy |
20 (83.3%) |
| |
Hormonal therapy |
15 (62.5%) |
| Previous radiotherapy dose |
30–70 Gy (53.7 ± 10.5)* |
| * Mean ± standard deviation
# Stage of the disease at the time of initial presentation |
|
Table 2: Retreatment offered with reirradiation and local hyperthermia. |
|
Treatment offered
|
Values
|
| Reirradiation |
| |
ReRT dose (Gy) |
20–50 (36.8 ± 8.5)* |
| |
No. of fractions |
5–25 (17.2 ± 5.7)* |
| |
Dose per fractions (Gy/fr.) |
1.8–4.0 (2.3 ± 0.5)* |
| Hyperthermia |
| |
Total no. of sessions |
2–11 (7.3 ± 2.3)* |
| |
Average temperature (°C) |
41–43.5 (40.6 ± 0.9)* |
| Total radiotherapy dose (Previous RT + ReRT) (Gy) |
55–114 (90.6 ± 14.1)* |
| ReRT = reirradiation; RT = radiotherapy
* Mean ± standard deviation |
Results
Post-HT and -ReRT locoregional response and survival
After local thermoradiotherapy, 22 of the 24 patients (91.7%) demonstrated a clinical response to ReRT and HT. Sixteen patients (66.7%) had a complete regression and 6 (25%) had partial regression (fig. 2). During the median follow-up period of 10 months (range 1‒36 months), all patients with a complete response continued to maintain their complete remission until their last follow-up or death. One patient who had a partial response at 4 weeks from treatment completion went on to have a complete response by 8 weeks. Thus, 17 of the 24 patients who at the time of their last follow up had complete locoregional control were considered as “censored” for the computation of their actuarial local control by means of Kaplan-Meier estimates. The actuarial local control rates at 2 and 3 years was estimated to be 76.8% and 59.7%, respectively. There was a significant difference between the complete responders and others (p <0.001, fig. 3). Median overall survival for all patients was 12 months, 23 months for complete responders and 4 months for incomplete responders (p = not significant). Seven patients were alive and 13 had died from disease progression. The follow-up status of four patients could not be obtained and hence these patients were considered as lost to follow-up. They were considered as “event” for computation of overall survival, as because of their advanced disease status, it may be more realistic to consider them as events.
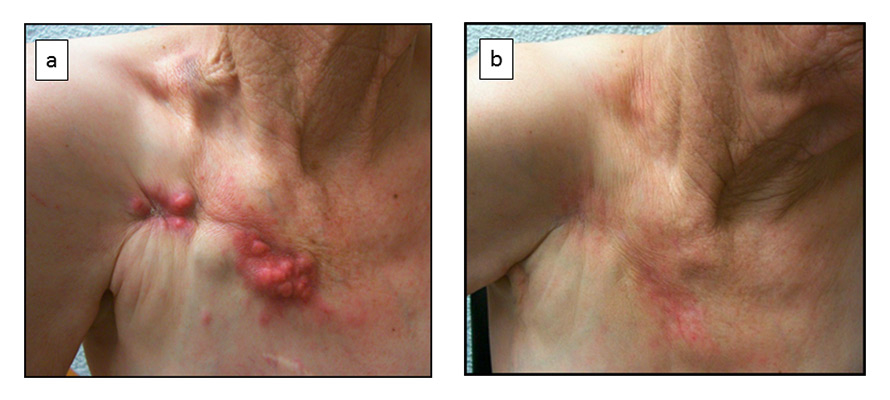
Figure 2
(a) Patient with isolated chest wall recurrence. (b) Complete locoregional response was achieved and sustained after reirradiation and hyperthermia at 2 years after the treatment.
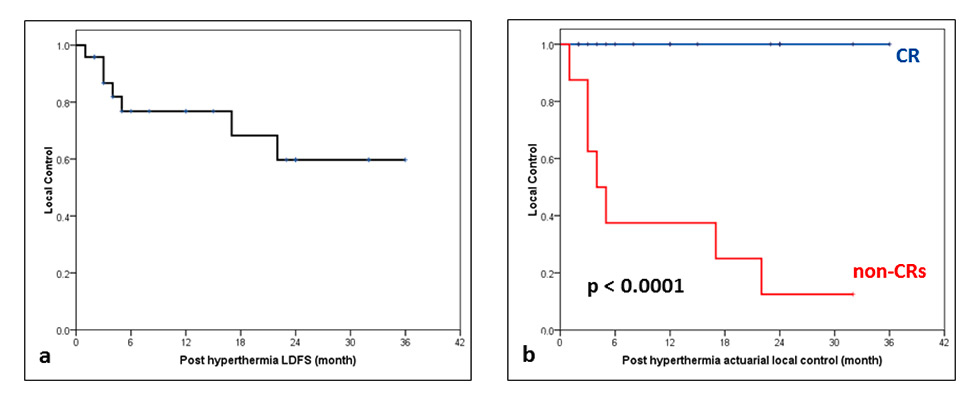
Figure 3
(a) Actuarial local control for all patients of recurrent breast cancers following reirradiation and hyperthermia. (b) Actuarial local control in complete responders (CRs) vs non-CRs (p <0.001).
LDFS = local disease-free survival
No significant predictive factor could be detected in multivariate logistic regression for complete responders. In Cox’s proportional hazard model, the only variable that was included in the regression model was response at end of ReRT and HT (exp (β) = 0.004; 95% confidence interval [CI] 0.000‒7.227, p = 0.150). The same factor was also significant in univariate analysis as evident from the Kaplan-Meier survival estimates (p <0.001).
Acute and late morbidity
All patients tolerated the treatment, and were able to complete the assigned HT treatment sessions and ReRT without any appreciable problems. They had good compliance with ReRT and HT. Acute skin grade I and II toxicity was experienced by 23 of the 24 patients, but only 1 (4.1%) patient had a grade III acute skin morbidity. The lesion healed within 4 weeks with conservative treatment. Late toxicity at more than 90 days after completion of ReRT and HT was evaluable in 22 patients and none had any significant late morbidity attributable to local HT and ReRT.
Discussion
Locoregional recurrences in previously irradiated breast cancers pose a delicate problem of delivering adequate radiation doses for tumour control and at the same time minimising the acute and late toxicity that could result from the cumulative dose of primary RT and ReRT. Patients with locoregional recurrences are known to survive after control of their local disease and hence development of such lesions may not always indicate a fatal outcome and signal institution of purely palliative measures. Willner et al. [34] in a study of 145 patients with locoregional recurrence have reported that 36% of the patients were alive and free of distant disease at 10 years. Hence, it may be desirable to treat these local recurrences effectively to achieve a long-term local disease control and to expect the patient to have a reasonable quality of life. Treatment approaches thus need to be tailor-made not only to be effective, but also to have the least acute and late morbidities. Increasing the ReRT doses or using a higher dose per fraction could increase the risks of acute and long-term late toxicities and thereby be counterproductive.
Hyperthermia with ReRT is one of the modalities that have been tried to treat these lesions. A common approach has been the use of moderate doses of ReRT and HT and this has given reasonable complete response ratess varying from 33.3% to 67% with a range of ReRT dose schedules and HT treatment parameters [8, 14–17, 19–23, 25–27]. However, the acute and late toxicities are a matter of concern with ReRT, especially for previously irradiated patients. The choice of the optimal thermal and ReRT treatment parameters thus requires special consideration as a higher temperature and a higher ReRT dose could result in increased morbidity.
Locoregional recurrence in the breast has also been treated with RT alone [1]. However, addition of HT with ReRT improves the outcomes as a result of the thermoradiobiological advantages of thermoradiotherapy as evident from randomised trials comparing ReRT and HT with RT alone. The International Collaborative Hyperthermia Group reported a complete response rate of 57% with thermoradiotherapy versus 31% with ReRT alone (odds ratio 4.7; CI 2.4–9.5) in previously irradiated patients [14]. In a study from Duke’s University, the complete response was reported to be 66.1% with ReRT and HT versus 42.3% with ReRT alone (p = 0.02) [18]. In contrast, the randomised trial by the Radiation Therapy Oncology Group (RTOG), protocol 8104, reported a nonsignificant difference in complete response of 33.3% with ReRT and HT versus 30.7% with ReRT alone [26]. This has been ascribed to the suboptimal heating quality and thermometry during HT sessions [35, 36].
One of the other approaches that have also been successfully advocated is surgical excision of the recurrences followed by ReRT and HT. This has been shown to produce sustainable local control [37, 38]. However, this is possible only in patients with operable isolated and gross recurrent lesions where a complete excision, preferably with negative margins, could be effectively controlled by moderate doses of ReRT with HT.
Chemotherapy has also been tried to treat recurrent breast cancer lesions. The Cochrane review failed to document conclusive evidence of benefit with chemotherapy alone [6]. A pilot study of weekly paclitaxel and HT in seven patients reported a complete response in four with no significant toxicity [39]. In a phase I/II trial, liposomal doxorubicin with ReRT and HT produced a complete response in 3 out of the 15 patients [40]. A triple modality approach with chemotherapy (epirubicin and ifosphamide), HT and ReRT has also been reported by Feyerabend et al. [41]. They reported a complete response rate of 44%, especially in noninflammatory recurrent breast cancers. Borenstein et al. [42] observed a complete response of 53% with thermochemoradiotherapy using cisplatin with or without bleomycin or etoposide in recurrent breast cancers. However, they observed a higher toxicity with this approach and concluded that in preirradiated patients, the triple combination appeared to be more toxic than combined ReRT and HT.
It is desirable to tailor the radiation dose and fractionation schemes, and take into account the previous RT doses to the site of reirradiation, the time interval between the primary and the retreatment, present disease status, expected survival and also other comorbid conditions to achieve an optimal outcome. As evident from our data, a range of ReRT dose schedules have been used to adapt to individual patient profiles. A satisfactory sustainable local control was achieved in two-thirds of our patients with minimal acute morbidity (Grade III acute toxicity: 1/24). Moreover, the complete response to ReRT and HT was maintained until the time of death or last follow-up. Similar observations have also been reported by Wahl et al. [8]. None of our patients had any long-term toxicity during the median follow up of 10 months (range 1–38 months). Moreover, in patients with partial responses, the local disease was reasonably controlled, which provided a reasonable palliation (fig. 3). Since 58.3% of the patients (14/24) already had metastatic disease at the time of recurrence, local treatment with ReRT and HT should not be expected to improve the overall survival, but at least to provide effective palliation without any superimposed morbidity. Addition of HT to ReRT has been, therefore, an effective therapeutic modality to treat postirradiated locoregional recurrent breast cancers. Even with a moderate dose of radiation, thermoradiotherapy produces a sustained complete regression in nearly two-thirds of the patients without any significant additional morbidity. This further demonstrates that HT is one of the most potent radiosensitizers [12].
Thus, a delicate balance of the various retreatment parameters – namely ReRT dose schedules and HT treatment variables ‒ needs to be tailored to the patient’s disease status to achieve the optimal outcome for these patients. ReRT with local HT can certainly be a viable and effective treatment strategy for treating locoregional recurrent breast cancers. A multicentre well designed clinical trial with adequate sample size to address these issues is certainly warranted.
To date, only a few centres have hyperthermia treatment facilities. This could rapidly change as encouraging results of HT in various tumour sites are being reported [43]. Moreover, with the recent developments in hardware and software, HT could fast emerge as an effective and a safer addendum to the existing treatment modalities, not only for recurrent breast tumours but also for tumours in other sites.
References
1 Siglin J, Champ CE, Vakhnenko Y, Anne PR, Simone NL. Radiation therapy for locally recurrent breast cancer. Int J Breast Cancer. 2012;2012:571946.doi: 10.1155/2012/571946.
2 Cheng L, Swartz MD, Zhao H, Kapadia AS, Lai D, Rowan PJ, et al. Hazard of recurrence among women after primary breast cancer treatment – a 10–year follow-up using data from SEER-Medicare. Cancer Epidemiol Biomarkers Prevent. 2012;21(5): 800–9.
3 Philippson C, Simon S, Vandekerkhove C, Hertens D, Veys I, Noterman D, et al. Early invasive cancer and partial intraoperative electron radiation therapy of the breast: experience of the Jules Bordet Institute, Int J Breast Cancer. 2014;2014:627352.doi: 10.1155/2014/627352.
4 Bethke KP. Breast conservation: predictors and treatment of local recurrence. Semin Surg Oncol. 1996;12(5):332–8.
5 Plasswilm L, Seegenschmiedt MH, Ganssauge F, Sauer R. Simultaneous radiochemotherapy for recurrent and metastatic breast carcinoma: evaluation of two treatment concepts. Am J Clin Oncol. 1996;19(4):403–7.
6 Rauschecker H, Clarke M, Gatzemeier W, Recht A. Systemic therapy for treating locoregional recurrence in women with breast cancer. Cochrane Database Syst Rev. 2001;(4):CD002195.
7 Schwaibold F, Fowble BL, Solin LJ, Schultz DJ, Goodman RL. The results of radiation therapy for isolated local regional recurrence after mastectomy. Int J Radiat Oncol Biol Phys. 1991;21(2):299–310.
8 Wahl AO, Rademaker A, Kiel KD, Jones EL, Marks LB, Croog V, et al. Multi-institutional review of repeat irradiation of chest wall and breast for recurrent breast cancer, Int J Radiat Oncol Biol Phys. 2008;70(2):477–84.
9 Kuerer HM, Arthur DW, Haffty BG. Repeat breast-conserving surgery for in-breast local breast carcinoma recurrence: the potential role of partial breast irradiation, Cancer. 2004;100(11):2269–80.
10 Dahlstrøm KK, Andersson AP, Andersen M, Krag C. Wide local excision of recurrent breast cancer in the thoracic wall. Cancer. 1993;72(3):774–7.
11 Overgaard J. The current and potential role of hyperthermia in radiotherapy, Int J Radiat Oncol Biol Phys. 1989;16(3):535–49.
12 Overgaard J. The heat is (still) on – the past and future of hyperthermic radiation oncology. Radiother Oncol. 2013;109(2):185–7.
13 Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, et al. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002;3(8):487–97.
14 Vernon CC, Hand JW, Field SB, Machin D, Whaley JB, van der Zee J, et al. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: results from five randomized controlled trials. International Collaborative Hyperthermia Group. Int J Radiat Oncol Biol Phys. 1996;35(4):731–44.
15 Lee HK, Antell AG, Perez CA, Straube WL, Ramachandran G, Myerson RJ, et al. Superficial hyperthermia and irradiation for recurrent breast carcinoma of the chest wall: prognostic factors in 196 tumors, Int J Radiat Oncol Biol Phys. 1998;40(2):365–75.
16 Hehr T, Lamprecht U, Glocker S, Classen J, Paulsen F, Budach W, et al. Thermoradiotherapy for locally recurrent breast cancer with skin involvement, Int J Hyperthermia. 2001;17(4):291–301.
17 Ben-Yosef R, Vigler N, Inbar M, Vexler A. Hyperthermia combined with radiation therapy in the treatment of local recurrent breast cancer. Isr Med Assoc. 2004:6(7):392–5.
18 Jones EL, Oleson JR, Prosnitz LR, Samulski TV, Vujaskovic Z, Yu D, et al. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol. 2005;23(13):3079–85.
19 Gabriele P, Ferrara T, Baiotto B, Garibaldi E, Marini PG, Penduzzu G, et al. Radio hyperthermia for re-treatment of superficial tumours. Int J Hyperthermia. 2009;25(3):189–98.
20 Perez CA, Kuske RR, Emami B, Fineberg B. Irradiation alone or combined with hyperthermia in the treatment of recurrent carcinoma of the breast in the chest wall: a nonrandomized comparison. Int J Hyperthermia. 1986;2(2):179–87.
21 van der Zee J, Treurniet-Donker AD, The SK, Helle PA, Seldenrath JJ, Meerwaldt JH, et al. Low dose reirradiation in combination with hyperthermia: a palliative treatment for patients with breast cancer recurring in previously irradiated areas. Int J Radiat Oncol Biol Phys. 1988;15(6):1407–13.
22 Lindholm CE, Kjellen E, Nilsson P, Hertzman S. Microwave-induced hyperthermia and radiotherapy in human superficial tumours: clinical results with a comparative study of combined treatment versus radiotherapy alone. Int J Hyperthermia. 1987;3(5):393–411.
23 Dragovic J, Seydel HG, Sandhu T, Kolosvary A, Blough J. Local superficial hyperthermia in combination with low-dose radiation therapy for palliation of locally recurrent breast carcinoma. J Clin Oncol. 1989;7(1):30–5.
24 Masunaga S, Hiraoka M, Takahashi M, Jo S, Akuta K, Nishimura Y, et al. Clinical results of thermoradiotherapy for locally advanced and/or recurrent breast cancer- comparison of results with radiotherapy alone. Int J Hyperthermia. 1990;6(3):487–97.
25 Kapp DS, Barnett TA, Cox RS, Lee ER, Lohrbach A, Fessenden P. Hyperthermia and radiation therapy of local-regional recurrent breast cancer: prognostic factors for response and local control of diffuse or nodular tumors. Int J Radiat Oncol Biol Phys. 1991;20(5):1147–64.
26 Perez CA, Pajak T, Emami B, Hornback NB, Tupchong L, Rubin P. Randomized phase III study comparing irradiation and hyperthermia with irradiation alone in superficial measurable tumors: final report by the Radiation Therapy Oncology Group. Am J Clin Oncol. 1991;14(2):133–41.
27 Phromratanapongse P, Steeves RA, Severson SB, Paliwal BR. Hyperthermia and irradiation for locally recurrent previously irradiated breast cancer. Strahlenther Onkolol. 1991;167(2):93–7.
28 Cancer Therapy Evaluation Programme, Common Terminology Criteria for Adverse Events, version 3.0, DTCD, NCI, NIH, DHHS, March 31, 2003, published date, August 9,2006. Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf (Accessed on 17 July, 2014)
29 US Department of Health and Human Services, National Institutes of Health, National Cancer Institute, Cancer Therapy Evaluation Programme, “Common Terminology Criteria for Adverse Events”, version 4.1, published date, June 14, 2010. Available from http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010–06–14_QuickReference_5x7.pdf (Accessed on 17 July, 2014)
30 Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16.
31 Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
32 Kaplan EL, Meier P. Nonparametric estimations from incomplete observations. J Am Stat Assoc. 1958;53(282):457–81.
33 Cox DR. Regression models and life-tables. J R Stat Soc Series B Stat Methodol. 1972;34(2):187–220.
34 Willner J, Kiricuta IC, Kölbl O. Locoregional recurrence of breast cancer following mastectomy: always a fatal event? Results of univariate and multivariate analysis. Int J Radiat Oncol Biol Phys. 1997;37(4):853–63.
35 Perez CA, Gillespie B, Pajak T, Hornback NB, Emami B, Rubin R. Quality assurance problems in clinical hyperthermia and their impact on therapeutic outcome: a report by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 1989;16(3):551–8.
36 Zagar TM, Oleson JR, Vujaskovic Z, Dewhirst MW, Craciunescu OI, Blackwell KL, et al. Hyperthermia combined with radiation therapy for superficial breast cancer and chest wall recurrence: a review of the randomised data. Int J Hyperthermia. 2010;26(7):612–7.
37 Linthorst M, van Geel AN, Baaijens M, Ameziane A, Ghidey W, van Rhoon GC, et al. Re-irradiation and hyperthermia after surgery for recurrent breast cancer. Radiother Oncol. 2013;109(2):188–193.
38 Müller AC, Eckert F, Heinrich V, Bamberg M, Brucker S, Hehr T. Re-surgery and chest wall re-irradiation for recurrent breast cancer: a second curative approach. BMC Cancer. 2011;11:197. doi: 10.1186/1471–2407–11–197.
39 Zoul Z, Filip S, Melichar B, Dvorák J, Odrázka K, Petera J. Weekly paclitaxel combined with local hyperthermia in the therapy of breast cancer locally recurrent after mastectomy – a pilot experience. Onkologie. 2004;27(4):385–8.
40 Kouloulias VE, Dardoufas CE, Kouvaris JR, Gennatas CS, Polyzos AK, Gogas HJ, et al. Liposomal doxorubicin in conjunction with reirradiation and local hyperthermia treatment in recurrent breast cancer: a phase I/ II trial. Clin Cancer Res. 2002;8(2):374–82.
41 Feyerabend T, Weidemann GJ, Jäger B, Vesely H, Mahlmann B, Richter E. Local hyperthermia, radiation, and chemotherapy in recurrent breast cancer is feasible and effective except for inflammatory disease. Int J Radiat Oncol Biol Phys. 2001;49(5):1317–25.
42 Borenstein BA, Zouranjian PS, Hansen JL, Fraser SM, Gelwan LA, Teicher BA, et al. Local hyperthermia, radiation therapy, and chemotherapy in patients with local-regional recurrence of breast carcinoma. Int J Radiat Oncol Biol Phys. 1993;25(1):79–85.
43 Hurwitz M, Stauffer P. Hyperthermia, radiation and chemotherapy: the role of heat in multidisciplinary cancer care. Semin Oncol. 2014;41(6):714–29.


