PCSK9 inhibitors
DOI: https://doi.org/10.4414/smw.2015.14094
Baris
Gencer, Gilles
Lambert, François
Mach
Summary
The discovery of proprotein convertase subtilisin kexin 9 (PCSK9) has considerably changed the therapeutic options in the field of lipid management. PCSK9 reduces LDL receptor recycling, leading to a decrease of low-density lipoprotein cholesterol (LDL-C) receptors on the surface of hepatocytes and a subsequent increase of circulating LDL-C levels. In observational studies, the loss-of-function mutations of PCSK9 have been associated with a reduction of LDL-C levels and cardiovascular disease (CVD) events. In contrast, humans with high levels of PCSK9 have higher level of plasma LDL-C and significantly enhanced CVD risk during their lifetime, gain-of-function mutations on PCSK9 are, for instance, causatively associated with familial hypercholesterolaemia (FH). Inhibition of PCSK9 is therefore a promising therapeutic option for the lowering of LDL-C levels. The clinical development of human monoclonal antibodies against PCSK9 has progressed, with promising results reported from phase 2 clinical studies in patients with FH or intolerant to statin with LDL-C levels not on target levels. Phase I studies demonstrated safety and efficacy. In phase II, a 60%–70% reduction in LDL-C was observed, especially when subcutaneous injections were performed regularly every two weeks. No significant side effects were observed, with the exception of injection site reactions. Three large phase III programmes with the new anti PCSK9 antibodies are currently underway in patients with acute coronary syndrome (ACS) and LDL-C inadequately controlled by standard treatments. The main objective of these studies is to evaluate the effect of PCSK9 inhibition on the occurrence of CVD events in patients with ACS.
Abbreviations and acronyms of trials:
CVD: cardiovascular disease
FH: familial hypercholesterolaemia
GAUSS: goal achievement after utilizing an anti-PCSK9 antibody in statin intolerant subjects
LAPLACE-TIMI 57 LDL-C assessment with pcsk9 monoclonal antibody inhibition combined with statin therapy-thrombolysis in myocardial infarction 57
LDL-C: low-density lipoprotein cholesterol
MENDEL: monoclonal antibody against PCSK9 to reduce elevated LDL-C in patients currently not receiving drug therapy for easing lipid levels
PCSK9: proprotein convertase sutilisin/kexin type 9
RUTHERFORD: reduction of LDL-C with PCSK9 inhibition in heterozygous familial hypercholesterolemia disorder randomized trial
Introduction
Proprotein convertase subtilisin kexin 9 (PCSK9) has received considerable attention in the last decade as a promising therapeutic target in the management of lipid disorders [1, 2]. PCSK9 inhibition offers a novel therapeutic mechanism for the lowering of low-density lipoprotein cholesterol (LDL-C) levels [3]. The clinical development of human monoclonal antibodies against PCSK9 has yielded promising results reported in several phase II clinical studies [4, 5]. The entry of these agents onto the market for the treatment of familial hypercholesterolaemia (FH) and for patients with statin intolerance is expected within the next two years. Based on available data, this new therapeutic approach would reinforce the possibility of treating patients with FH who have poorly controlled LDL-C levels with current evidence based therapies [6]. In addition, PCSK9 inhibitors may reveal themselves to be an alternative for patients intolerant to statin treatment [7]. The impact of the PCSK9 saga might also be particularly important in patients with established cardiovascular disease (CVD) who need intensive control of LDL-C levels in secondary prevention [8]. The ongoing large clinical phase III trials will provide the definite answer for the potential indication of PCSK9 inhibitors in these high-risk patients. In this review, we discuss the role of PCSK9 in FH, the proof-of-concept mechanisms of PCSK9 inhibition, the results of preliminary clinical trials, the summary of ongoing clinical trials and the future perspectives of these agents.
What is PCSK9?
In adults, PCSK9 is highly expressed in liver hepatocytes, and to a lesser extent in the small intestine and kidneys [9]. PCSK9 is a secreted protein (mostly from the liver) that binds the LDL receptor on the surface of hepatocyte, leading to intracellular degradation of the LDL receptor in the lysosomes of hepatocytes [2, 6] (fig. 1). At the molecular level, PCSK9 blocks the LDL receptor in an extended (or open) conformation. The failure of the receptor to adopt a closed conformation results in a slowed recycling to the plasma membrane and degradation of LDL receptor [2]. Given the tight interaction between PCSK9 and LDL-C, the idea of disrupting the interaction between PCSK9 and the LDL receptor might be a therapeutic approach to limit LDL receptor degradation and consequently decrease circulating LDL-C levels [2, 6]. Measuring circulating plasma PCSK9 levels in humans might be clinically relevant, as previous studies have shown an association between PCSK9 and LDL-C levels. In this respect, several methods have been developed to measure PCSK9 levels in humans.
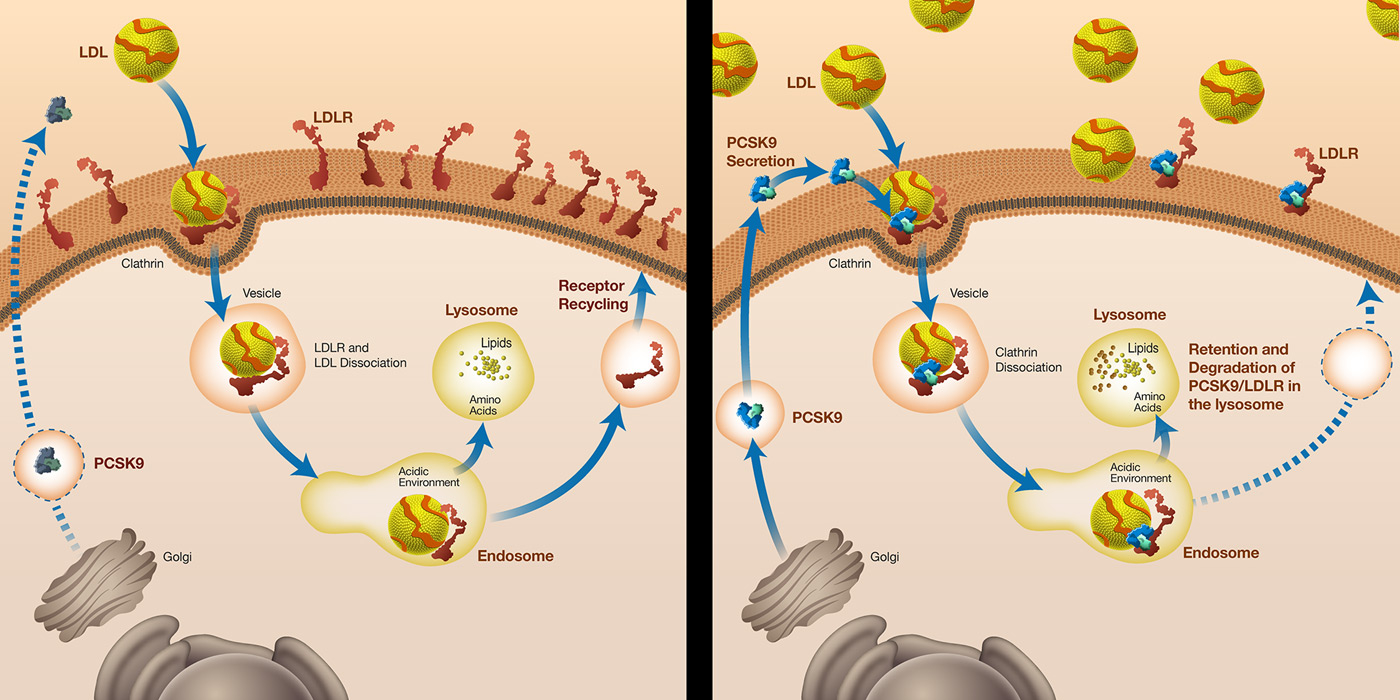
Figure 1
Description of the effect of proprotein convertase subtilisin/kexin type 9 (PCSK9) pathways on the low-density lipoprotein (LDL-C) levels. On the left, loss-of-function mutations or inhibition of PCSK9 result in an increased number of LDL receptors on the cell surface of hepatocytes with lower levels of circulating LDL-C. On the right, gain-of-function of PCSK9 results in a decrease of LCL receptors on the cell surface of hepatocytes, with higher levels of circulating LDL-C.
Reproduced with permission from Marais A.D., et al. [6].
Role of PCSK9 in familial hypercholesterolaemia and risk of cardiovascular disease
The proof-of-concept of the role of PCSK9 in familial hypercholesterolaemia and risk of CVD has been widely established in animal models, as well as in large human cohort studies. It is estimated that PCSK9 mutations represent 1% to 2% of all familial hypercholesterolaemia (FH) cases [10]. Heterozygous FH is an autosomal dominant genetic disorder with an estimated prevalence between 1/200 and 1/500 in the general population [11]. FH can be diagnosed using clinical or genetic criteria [12]. Mutations of the PCSK9 gene are the third cause of FH, after mutations in the LDL receptor or apolipoprotein B (ApoB) genes [10, 13, 14]. It is important to recognise FH cases, as early identification of patients with FH may reduce the risk of morbidity and mortality from premature atherosclerosis with appropriate lipid lowering treatment [15]. In 2003, gain-of-function mutations in the PCSK9 gene were identified in two French families with an autosomal dominant form of FH. Those mutations were associated with a strong effect on the LDL receptor and ApoB metabolism. Other PCSK9 mutations were also reported in FH patients, but the prevalence was very low compared with defects in the LDL receptor and ApoB. The loss-of-function mutations of PCSK9 have been associated with a reduction of circulating low-density LDL-C levels; for instance, mean LDL-C levels were 100 mg/dl in patients carrying the loss-of-function mutation of PCSK9, compared with 130 mg/dl in non-carriers [16]. In the Atherosclerosis Risk in Communities (ARIC) trial, the loss of one functional PCSK9 allele prevented 88% of CVD events. The cardioprotective effect of PCSK9 loss-of-function in terms of atherosclerotic events implies that PCSK9 might be a potential target for the development of new lipid lowering therapies and the improvement of clinical outcomes [14, 16].
Phase I‒II clinical trials of PCSK9 inhibitors
Pharmaceutical companies are testing various approaches in the development of PCSK9 inhibition in clinical or preclinical studies. The most promising approach is the use of monoclonal antibodies directed to PCSK9. A single injection can lower PCSK9 levels by up to 100% for more than a week. Phase I and II clinical trials have been conducted by companies such as Amgen (AMG 145) or Sanofi/Regeneron (SAR236553/REGN727). Phase I studies demonstrated safety and efficacy. In phase II studies, a 60% to 70% LDL-C reduction was confirmed, especially when subcutaneous injections were performed regularly every two weeks. No significant side effects were observed, except a possible local reaction at the injection site.
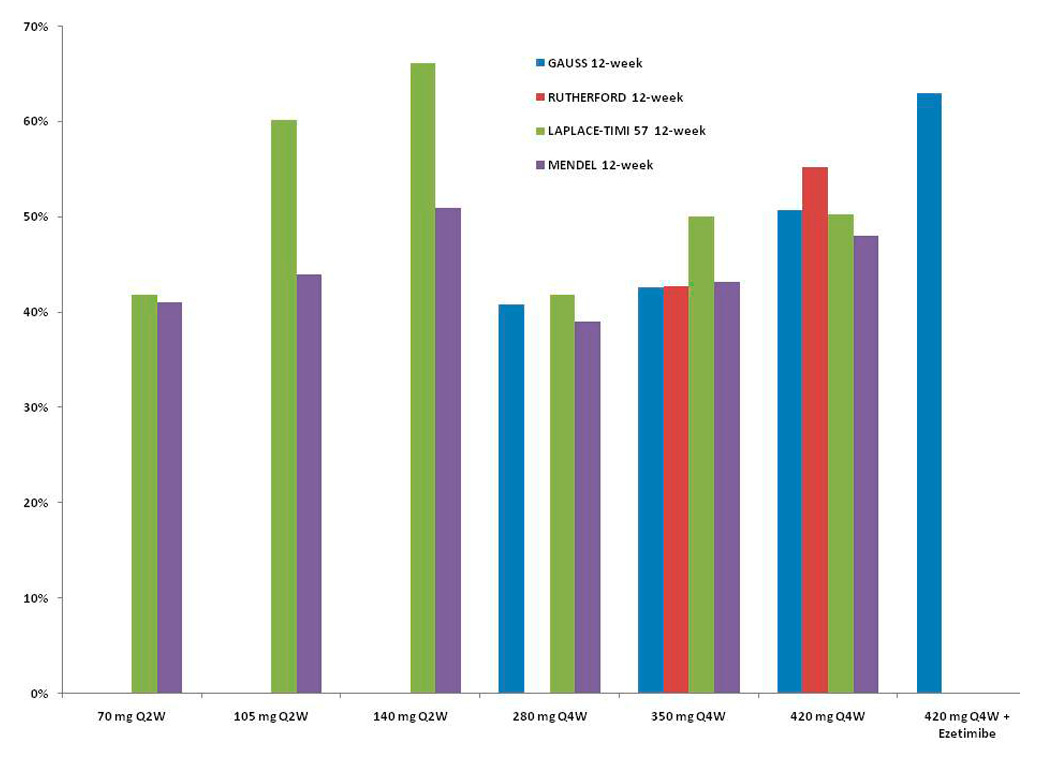
Figure 2
Mean percent decrease from baseline in low-density lipoprotein (LDL) cholesterol levels with the AMG 145 regimen. Results were derived from GAUSS [18], RUTHERFORD [21], LAPLACE-TIMI 57 [19] and MENDEL [20].
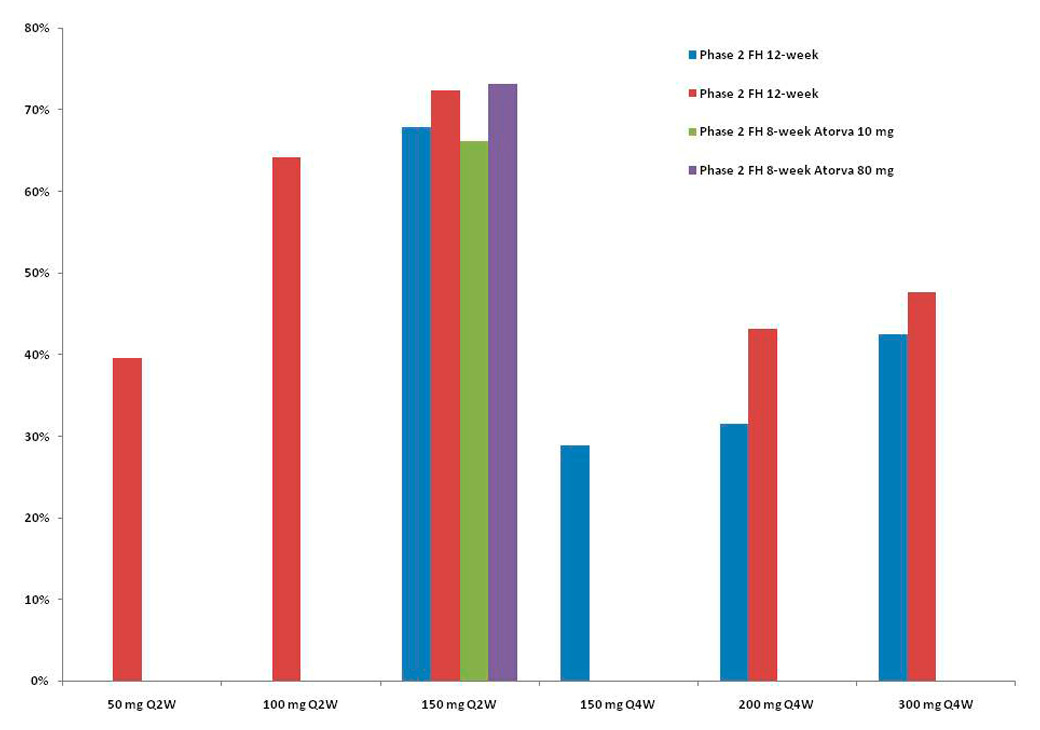
Figure 3
Mean percent decrease from baseline in low-density lipoprotein (LDL) cholesterol levels with the SAR236553/REGN727 regimen. Results were derived from [24–26].
Evolocumab-AMG 145 (fig. 2 and table 1)
In phase I trials, the use of AMG 145 in 113 patients was associated with a reduction in LDL-C of 64% versus placebo, with a similar rate of adverse events in healthy and in hypercholesterolaemic statin-treated subjects, including those with heterozygous familial hypercholesterolaemia [17]. In the GAUSS randomised trial, 160 patients with statin intolerance due to muscle-related side effects were randomised to 5 groups: AMG 145 alone subcutaneously at 280 mg, 350 mg, 420 mg, AMG 145 at 420 mg plus 10 mg ezetimibe once a day, or 10 mg ezetimibe plus placebo. The administration of a monoclonal antibody directed against PCSK9 was significantly associated with a reduction in LDL-C levels ranging from 40% to 65% [18].
In the LAPLACE-TIMI 57 phase II trial, 631 patients with hypercholesterolaemia on statins were tested to different regimen of AMG dosages with varying intervals of administration: AMG 145 70 mg, 105 mg, and 140 mg or matching placebo every two weeks; or AMG 145 280 mg, 350 mg, and 420 mg and matching placebo every four weeks. At week 12, the mean LDL-C concentration reduction was dose-dependent, and ranging from 41.8% to 66.1% every two weeks, and from 41.8% to 50.3% every four weeks [19].
In the MENDEL trial, 406 patients with hypercholesterolaemia and statin intolerance were randomly assigned to AMG 145 70 mg, 105 mg and 140 mg every two weeks; AMG 145 280 mg, 350 mg and 420 mg every four weeks; placebo every two weeks or every four weeks or ezetimibe once a day. AMG 145 significantly reduced LDL-C concentrations in all dose groups with the maximal effect for the regimen of 140 mg every two weeks (–51%). No deaths or serious treatment related adverse events were reported [20].
In the RUTHERFORD trial, 167 patients with heterozygous familial hypercholesterolaemia and uncontrolled LDL-C (≥2.6 mmol/l) with statin were randomised 1:1:1 to AMG 145 350 mg, AMG 145 420 mg or placebo every four weeks. A substantial reduction in LDL-C was observed (43% for 350 mg and 55% for 420 mg) on top of intensive statin use [21].
Recently, the DESCARTES trial, including 901 patients with a range of cardiovascular risks treated with diet alone, atorvastatin 10 mg, 80 mg or 80 mg plus ezetimibe once a day were randomised to 420 mg evolocumab or placebo every four weeks. At 52 weeks, evolocumab significantly reduced LDL-C levels with all previous described regimens (from 48.5% to 61.6%), as well as apolipoprotein B, non-high-density lipoprotein cholesterol and lipoprotein(a) [22]. The most common adverse events were nasopharyngitis, upper respiratory tract infection, influenza and back pain. All active, ongoing and recruiting clinical trials with evolocumab registered at clinicaltrials.gov are presented in table 1.
Alirocumab-REGN727/SAR236553 (fig. 3 and table 2)
Three phase I clinical trials of a monoclonal antibody directed against PCSK9 in healthy volunteers and in subjects with familial or nonfamilial hypercholesterolaemia showed a significant reduction of LDL-C, ranging from 39.2% for 50 mg to 61% for 150 mg [23].
Seventy-seven patients with heterozygous familial hypercholesterolaemia on a stable statin dose with or without ezetimibe therapy were randomised to REGN727 150 mg, 200 mg and 300 mg every four weeks, or 150 mg every two weeks, or placebo every two weeks. The mean LDL-C reduction was significantly higher in the AMG 145 groups, with the highest effect in the group receiving 150 mg every two weeks (67.9%, p-value <0.001) [24].
In another phase II, multicentre, double-blind, placebo-controlled trial involving 92 patients with primary hypercholesterolaemia who had uncontrolled LDL (higher than 100 mg/dl) with 10 mg atorvastatin were randomised to receive eight weeks of treatment with 80 mg atorvastatin daily plus SAR236553 once every two weeks, 10 mg atorvastatin daily plus SAR236553 once every two weeks, or 80 mg atorvastatin daily plus placebo every two weeks. All patients who received SAR236553 attained the goal for LDL-C levels of 100 mg/dl, compared with 52% of those who received 80 mg atorvastatin plus placebo. Taking the more stringent target of 70 mg/dl, 90% of the patients who received SAR236554 reached the target, compared with 17% who received 80 mg atorvastatin plus placebo. No life-threatening events were observed in either trial [25].
Finally, among 183 patients with primary hypercholesterolaemia and LDL-C ≥2.6 mmol/l on a stable dose of atorvastatin 10, 20 or 40 mg for ≥6 weeks, SAR236553 was significantly associated with LDL-C reduction: 40% for 50 mg, 63% for 100 mg and 72% for 150 mg every two weeks, and 43% for 200 mg and 48% for 300 mg [26]. In this trial, the authors reported a significant decrease of non-HDL-C, apolipoprotein B and lipoprotein(a). All active, ongoing and recruiting clinical trials with alirocumab registered at clinicaltrials.gov are presented in table 2.
Bococizumab-PF-4950615/RN-316 (table 3)
Another anti-PCSK9 monoclonal antibody is currently being tested in an all active, ongoing and recruiting clinical trial with bococizumab registered at clinicaltrials.gov and presented in table 3. No data have yet been published with this antibody [27].
|
Table 1:Phase III clinical trials of evolocumab (AMG 145), developed by Amgen. |
|
Study description
|
Clinical trial no.
|
Status
|
| Study of LDL-Cholesterol Reduction Using Evolocumab (AMG 145) in Japanese Patients With Advanced Cardiovascular Risk |
NCT01953328 |
Active, not recruiting |
| Evaluating PCSK9 binding antibody influence on cognitive health in high cardiovascular Risk Subjects (EBBINGHAUS) |
NCT02207634 |
Active, not recruiting |
| Effects on Lipoprotein Metabolism From PCSK9 Inhibition Utilizing a Monoclonal Antibody (FLOREY) |
NCT02189837 |
Recruiting |
| Trial Assessing Long Term Use of PCSK9 Inhibition in Subjects with genetic LDL Disorders (TAUSSIG) |
NCT01624142 |
Recruiting |
| Global assessment of plaque regression with a PCSK9 antibody as measured by intravascular ultrasound (GLAGOV) |
NCT01813422 |
Recruiting |
| Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER) |
NCT01764633 |
Recruiting |
| Goal Achievement After Utilizing an Anti-PCSK9 Antibody in Statin Intolerant Subjects-3 |
NCT01984424 |
Recruiting |
| Open Label Study of Long Term Evaluation Against LDL-C Trial-2 (OSLER-2) |
NCT01854918 |
Recruiting |
| A Randomized, Multi-centre Clinical Study in Subjects With Hypercholesterolemia or Mixed Dyslipidaemia |
NCT01879319 |
Completed |
| Trial Evaluating PCSK9 Antibody in Subjects With LDL Receptor Abnormalities (TESLA) |
NCT01588496 |
Completed |
| A Multi-centre, Randomized Study in Subjects With Primary Hypercholesterolemia or Mixed Dyslipidaemia |
NCT01849497 |
Completed |
| Durable Effect of PCSK9 Antibody Compared with placebo Study (DESCARTES) |
NCT01516879 |
Completed |
| Goal Achievement After Utilizing an Anti-PCSK9 Antibody in Statin Intolerant Subjects -2 (GAUSS-2) |
NCT 01763905 |
Completed |
| Monoclonal Antibody Against PCSK9 to Reduce Elevated LDL-C in Subjects Currently Not Receiving Drug Therapy for Easing Lipid
Levels-2 (MENDEL-2) |
NCT01763827 |
Completed |
| LDL-C Assessment w/ PCSK9 Monoclonal Antibody Inhibition Combined With Statin Therapy-2 (LAPLACE-2) |
NCT01763866 |
Completed |
| Reduction of LDL-C With PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder Study-2 (RUTHERFORD-2) |
NCT01763918 |
Completed |
| Table was updated from the site https://clinicaltrials.gov on 22 September 2014 |
|
Table 2:Phase III clinical trials of alirocumab (REGN727, SAR236553), developed by Regeneron/Sanofi. |
|
Study description
|
Clinical trial no.
|
Status
|
| Study to Evaluate the Efficacy and Safety of an Every Four Weeks Treatment Regimen of Alirocumab in Patients With Primary Hypercholesterolemia (ODYSSEY CHOICE 1) |
NCT01926782 |
Active, not recruiting |
| Phase III Study To Evaluate Alirocumab in Patients With Hypercholesterolemia Not Treated With a Statin (ODYSSEY CHOICE II) |
NCT02023879 |
Active, not recruiting |
| Efficacy and Safety of Alirocumab Versus Ezetimibe on Top of Statin in High Cardiovascular Risk Patients With Hypercholesterolemia (ODYSSEY Combo II) |
NCT01644188 |
Active, not recruiting |
| Efficacy and Safety of Alirocumab Versus Placebo on Top of Lipid-Modifying Therapy in Patients With Heterozygous Familial Hypercholesterolemia (ODYSSEY High FH) |
NCT01617655 |
Active, not recruiting |
| Efficacy and Safety of Alirocumab Versus Placebo on Top of Lipid-Modifying Therapy in Patients With Heterozygous Familial Hypercholesterolemia Not Adequately Controlled With Their Lipid-Modifying Therapy (ODYSSEY FH I) |
NCT01623115 |
Active, not recruiting |
| Study of Alirocumab (REGN727/ SAR236553) in Patients With heFH (Heterozygous Familial Hypercholesterolemia) Who Are Not Adequately Controlled With Their LMT (Lipid-Modifying Therapy) (Odyssey FH II) |
NCT01709500 |
Active, not recruiting |
| Study of Alirocumab (REGN727/ SAR236553) in Patients With Primary Hypercholesterolemia and Moderate, High, or Very High Cardiovascular (CV) Risk, Who Are Intolerant to Statins (Odyssey Alternative) |
NCT01709513 |
Active, not recruiting |
| Long-term Safety and Tolerability of Alirocumab SAR236553 (REGN727) Versus Placebo on Top of Lipid-Modifying Therapy in High Cardiovascular Risk Patients With Hypercholesterolemia (ODYSSEY Long Term) |
NCT01507831 |
Active, not recruiting |
| ODYSSEY Outcomes: Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab |
NCT01663402 |
Recruiting |
| Open Label Study of Long Term Safety Evaluation of Alirocumab (ODYSSEY OLE) |
NCT01954394 |
Recruiting |
| Efficacy and Safety Evaluation of Alirocumab in Patients With Heterozygous Familial Hypercholesterolemia or High Cardiovascular Risk Patients With Hypercholesterolemia on Lipid Modifying Therapy |
NCT 02107898 |
Recruiting |
| Efficacy and Safety of Alirocumab Versus Ezetimibe in Patients With Hypercholesterolemia (ODYSSEY Mono) |
NCT01644474 |
Completed |
| Efficacy and Safety of Alirocumab SAR236553 (REGN727) Versus Placebo on Top of Lipid-Modifying Therapy in Patients With High Cardiovascular Risk and Hypercholesterolemia (ODYSSEY Combo I) |
NCT01664175 |
Completed |
| Study of Alirocumab added-on to Rosuvastatin Versus Other Lipid Modifying Treatments |
NCT01730053 |
Completed |
| Study of the Efficacy and Safety of REGN727 (SAR236553) in Combination With Other Lipid-modifying Treatments |
NCT 01730040 |
Completed |
| Table was updated from the site https://clinicaltrials.gov on 22 September 2014 |
|
Table 3: Phase III clinical trials of bococizumab (PF-4950615, RN-316), developed by Pfizer. |
|
Study
|
Clinical Trials
|
Status
|
| Randomized Clinical Trial of RN-316 (PF-4950615) in Subjects Who Are Intolerant to Statins (SPIRE-SI) |
NCT02135029 |
Recruiting |
| A 52 Week Study To Assess The Use of RN-316 (PF-4950615) in Subjects With Heterozygous Familial Hypercholesterolemia (SPIRE-HF) |
NCT01968980 |
Recruiting |
| Randomized Clinical Trial of RN-316 (PF-4950615) in Subjects With Hyperlipidaemia or Mixed Dyslipidaemia at Risk of Cardiovascular Events (SPIRE-LDL) |
NCT01968967 |
Recruiting |
| Randomized Clinical Trial of RN-316 (PF-4950615) in Subjects With Primary Hyperlipidaemia or Mixed Dyslipidaemia at Risk of Cardiovascular Events (SPIRE-LL) |
NCT02100514 |
Recruiting |
| Randomized Clinical Trial of RN-316 (PF-4950615) in Subjects With Hyperlipidaemia or Mixed Dyslipidaemia at Risk of Cardiovascular Events (SPIRE-HR) |
NCT01968954 |
Recruiting |
| The Evaluation of PF-4950615 (RN-316), in Reducing The Occurrence of Major Cardiovascular Events in High Risk Subjects (SPIRE-1) |
NCT01975376 |
Recruiting |
| The Evaluation of PF-4950615 (RN-316) in Reducing The Occurrence of Major Cardiovascular Events in High Risk Subjects (SPIRE-2) |
NCT01975389 |
Recruiting |
| Table was updated from the site https://clinicaltrials.gov on 22 September 2014 |
Ongoing phase III clinical trials of PCSK9 inhibitors
Three large phase III programmes with the new PCSK9 antibodies are currently ongoing: the PROFICIO programmes with evolocumab (table 1), the ODYSSEY programme with alirocumab (table 2) and the bococizumab programme (SPIRE 1 and 2) (table 3). Two large trials – FOURIER with evolocumab and ODYSSEY-OUTCOMES with alirocumab – are ongoing in patients hospitalised following with ACS inadequately controlled LDL-C on evidence-based treatment. The main objective of these studies is to evaluate the effect of PCSK9 inhibition on the occurrence of cardiovascular events (composite endpoint of coronary heart death, non-fatal myocardial infarction, fatal and non-fatal stroke, unstable angina requiring hospitalisation) in patients with ACS.
Perspectives
Although injections might not be particularly attractive for lifelong treatment, this approach would provide a valuable option for patients who suffer from the side effects of statins or for high risk patients who need to a achieve stringent LDL-C target level, as recommended by the European guidelines. For example, patients with FH started with LDL-C levels ≈3–4 times higher than the general population. Although low PCSK9 levels or their genetic deficiency appear to be safe on the basis of available data, the impact of PCSK9 inhibition in individuals with genetically normal PCSK9 has yet to be clarified [28]. The long-term impact of very low LDL-C levels such as <1 mmol/l is unclear. However, this reflects circulating cholesterol and not the intracellular cholesterol levels involved in physiological functions (e.g. synthesis of hormones and vitamins). Another source of controversy surrounding the PCSK9 inhibitors might be the incremental increased risk of diabetes resulting from the intracellular accumulation of lipids in the pancreas cells. However, at the current time more data are needed from large trials to detect such unexpected adverse effects of PCSK9 inhibitors.
It is well known that statin treatment enhances PCSK9 levels via a contra-regulatory mechanism that contributes to the observed resistance to their own lipid-lowering effect. Statin therapy results in increased plasma PCSK9 levels, but lower overall LDL-C levels, suggesting that lowering PCSK9 might enhance the efficacy of statins. PCSK9 inhibitors might be the key actors complementing statin therapy to improve the control of LDL-C levels [14]. This is of particular importance, as lowering LDL-C is associated with a reduction of CVD events, especially in patients at high CVD risk [29]. The European Society of Cardiology dyslipidaemia guidelines recommend a target of <1.8 mmol/l (70 mg/dl), or a ≥50% LDL-C reduction from baseline [12]. However, previous observational studies have shown that poorly controlled LDL-C levels after ACS are common and statin intensification often suboptimal [30]. The preliminary data of PCSK9 trials suggest a clear benefit of PCSK9 antibodies in patients with LDL-C poorly controlled by statins alone, either because of an inadequate lipid-lowering response to a high-dose statin or because of unacceptable side effects. The latter are a large and growing problem in routine clinical practice.
Conclusion
The discovery of PCSK9 has considerably changed the therapeutic reality in the lipid field. PCSK9 reduces LDL receptor recycling and increases the levels of LDL-C in the blood. Humans with low levels of PCSK9 have reduced levels of circulating LDL-C and a significantly lower risk of developing CVD. Monoclonal antibodies are at present the most advanced PCSK9 inhibitors in terms of pharmacological development and clinical response of LDL-C. Large clinical trials with long term follow-up are currently assessing their impact on clinical outcomes, especially in term of CVD events reduction. Despite their safety, there are some concerns about the potential side effects of lowering LDL-C and PCSK9 to “unphysiological” levels. Nevertheless, since the discovery of statin treatment, it has been a long time since a new therapy could be shown to reduce cholesterol levels so efficiently.
References
1 Vogel RA. PCSK9 inhibition: the next statin? J Am Coll Cardiol. 2012;59(25):2354–5.
2 Lambert G, Sjouke B, Choque B, Kastelein JJ, Hovingh GK. The PCSK9 decade. J Lipid Res. 2012;53(12):2515–24.
3 Urban D, Poss J, Bohm M, Laufs U. Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis. J Am Coll Cardiol. 2013;62(16):1401–8.
4 Dadu RT, Ballantyne CM. Lipid lowering with PCSK9 inhibitors. Nat Rev Cardiol. 2014;11(10):563–75.
5 Farnier M. PCSK9 inhibitors. Curr Opin Lipidol. 2013;24(3):251–8.
6 Marais AD, Kim JB, Wasserman SM, Lambert G. PCSK9 inhibition in LDL cholesterol reduction: Genetics and therapeutic implications of very low plasma lipoprotein levels. Pharmacology & Therapeutics 2014.
7 Farnier M. PCSK9: From discovery to therapeutic applications. Arch Cardiovasc Dis. 2014;107(1):58–66.
8 Sahebkar A, Watts GF. New LDL-cholesterol lowering therapies: pharmacology, clinical trials, and relevance to acute coronary syndromes. Clinical therapeutics. 2013;35(8):1082–98.
9 Cariou B, Le May C, Costet P. Clinical aspects of PCSK9. Atherosclerosis. 2011;216(2):258–65.
10 Cohen J, Pertsemlidis A, Kotowski IK, Graham R, Garcia CK, Hobbs HH. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nature genetics. 2005;37(2):161–5.
11 Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J. 2013;34(45):3478–90a.
12 Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32(14):1769–818.
13 Abifadel M, Varret M, Rabes JP, Allard D, Ouguerram K, Devillers M, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nature genetics. 2003;34(2):154–6.
14 Seidah NG, Awan Z, Chretien M, Mbikay M. PCSK9: a key modulator of cardiovascular health. Circ Res. 2014;114(6):1022–36.
15 Hovingh GK, Davidson MH, Kastelein JJ, O’Connor AM. Diagnosis and treatment of familial hypercholesterolaemia. Eur Heart J. 2013;34(13):962–71.
16 Cohen JC, Boerwinkle E, Mosley TH, Jr., Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354(12):1264–72.
17 Dias CS, Shaywitz AJ, Wasserman SM, Smith BP, Gao B, Stolman DS, et al. Effects of AMG 145 on low-density lipoprotein cholesterol levels: results from 2 randomized, double-blind, placebo-controlled, ascending-dose phase 1 studies in healthy volunteers and hypercholesterolemic subjects on statins. J Am Coll Cardiol. 2012;60(19):1888–98.
18 Sullivan D, Olsson AG, Scott R, Kim JB, Xue A, Gebski V, et al. Effect of a monoclonal antibody to PCSK9 on low-density lipoprotein cholesterol levels in statin-intolerant patients: the GAUSS randomized trial. JAMA. 2012;308(23):2497–506.
19 Giugliano RP, Desai NR, Kohli P, Rogers WJ, Somaratne R, Huang F, et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study. Lancet. 2012;380(9858):2007–17.
20 Koren MJ, Scott R, Kim JB, Knusel B, Liu T, Lei L, et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 as monotherapy in patients with hypercholesterolaemia (MENDEL): a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2012;380(9858):1995–2006.
21 Raal F, Scott R, Somaratne R, Bridges I, Li G, Wasserman SM, et al. Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) randomized trial. Circulation. 2012;126(20):2408–17.
22 Blom DJ, Hala T, Bolognese M, Lillestol MJ, Toth PD, Burgess L, et al. A 52–week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med. 2014;370(19):1809–19.
23 Stein EA, Mellis S, Yancopoulos GD, Stahl N, Logan D, Smith WB, et al. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Engl J Med. 2012;366(12):1108–18.
24 Stein EA, Gipe D, Bergeron J, Gaudet D, Weiss R, Dufour R, et al. Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial. Lancet. 2012;380(9836):29–36.
25 Roth EM, McKenney JM, Hanotin C, Asset G, Stein EA. Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia. N Engl J Med. 2012;367(20):1891–900.
26 McKenney JM, Koren MJ, Kereiakes DJ, Hanotin C, Ferrand AC, Stein EA. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J Am Coll Cardiol. 2012;59(25):2344–53.
27 Reichert JM. Antibodies to watch in 2014. mAbs 2014;6(1):5–14.
28 Lambert G, Petrides F, Chatelais M, Blom DJ, Choque B, Tabet F, et al. Elevated plasma PCSK9 level is equally detrimental for patients with nonfamilial hypercholesterolemia and heterozygous familial hypercholesterolemia, irrespective of low-density lipoprotein receptor defects. J Am Coll Cardiol. 2014;63(22):2365–73.
29 Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–81.
30 Martin SS, Gosch K, Kulkarni KR, Spertus JA, Mathews R, Ho PM, et al. Modifiable factors associated with failure to attain low-density lipoprotein cholesterol goal at 6 months after acute myocardial infarction. Am Heart J. 2013;165(1):26–33 e3.


