Kidney paired donation: a plea for a Swiss National Programme
DOI: https://doi.org/10.4414/smw.2015.14083
Karine
Hadaya, Thomas
Fehr, Barbara
Rüsi, Sylvie
Ferrari-Lacraz, Jean
Villard, Paolo
Ferrari
Summary
Growing incidence of end-stage renal disease, shortage of kidneys from deceased donors and a better outcome for recipients of kidneys from living donor have led many centres worldwide to favour living donor kidney transplantation programmes. Although criteria for living donation have greatly evolved in recent years with acceptance of related and unrelated donors, an immunological incompatibility, either due to ABO incompatibility and/or to positive cross-match, between a living donor and the intended recipient, could impede up to 40% of such procedures. To avoid refusal of willing and healthy living donors, a number of strategies have emerged to overcome immunological incompatibilities. Kidney paired donation is the safest way for such patients to undergo kidney transplantation. Implemented with success in many countries either as national or multiple regional independent programmes, it could include simple exchanges between any number of incompatible pairs, incorporate compatible pairs and non-directed donors (NDDs) to start a chain of compatible transplantations, lead to acceptance of ABO-incompatible matching, and integrate desensitising protocols. Incorporating all variations of kidney paired donation, the Australian programme has been able to facilitate kidney transplantation in 49% of registered incompatible pairs. This review is a plea for implementing a national kidney paired donation programme in Switzerland.
Background
Kidney transplantation is the treatment of choice for patients with end-stage renal disease; it reduces the mortality risk compared to dialysis in all age groups and improves the quality of life, offering benefits in terms of life expectancy [1, 2]. Living donor kidney transplantation is associated with superior long-term recipient and graft survivals compared to deceased kidney donors [3], possibly because of the shorter dialysis waiting-time or avoidance of dialysis [4]. The global shortage of deceased donor organs has led to increased reliance on living kidney donation programmes [5]. In Switzerland, the number of living donors has exceeded that from deceased donors over the last decade. Unfortunately, ABO blood group incompatibility or pre-existing donor specific antibodies (DSA) to human leukocyte antigen (HLA) as a result of prior transplants, pregnancies or blood transfusions, are major barriers to living donor kidney transplantation, excluding up to 54% of otherwise appropriate pairs [6]. Different strategies have emerged to overcome these immunologic incompatibilities. ABO-incompatible kidney transplantation with long-term outcomes equivalent to ABO-compatible kidney transplantation can now be achieved using strategies that include removal of anti-blood group antibody using plasmapheresis or specific immunoabsorption, rituximab [7, 8], and long-term standard immunosuppression [9]. In contrast, HLA-incompatible transplantation in the presence of preformed DSA resulting in positive cell-based cross-match (either by flow cytometry or complement-dependent cytotoxicity CDC) is still associated with high rates of antibody-mediated rejection, early graft loss and reduced long-term graft survival [10]. Despite desensitisation strategies using a combination of plasmapheresis, intravenous immunoglobulin and T and B cell depleting agents [11–13] in order to reverse positive cross-matches [14], up to 50% of surviving grafts show chronic active antibody-mediated rejection and premature graft failure after 5 years [10, 15]. Highly immunised patients with a panel-reactive antibody (PRA) level of 80% or more are particularly disadvantaged translating into a waiting time in excess of 10 years on the transplant waitlist. Allowing sensitised patients against their willing living donor to undergo a safely kidney transplantation is only possible through kidney paired donation (KPD). Also known as paired kidney exchange, crossover transplantation or closed-loop kidney swaps, this procedure can overcome immunological barriers and resort to all potential living donors [16–18]. Variations of KPD incorporate compatible pairs and non-directed donors (NDDs) to start a chain of compatible transplantations. In this overview we argue in favour of the establishment of a national KPD registry in Switzerland and discuss key ingredients that are critical for a successful programme.
A brief history of kidney paired donation
Although the idea of KPD was originally proposed by Rapaport in 1986 [19], it was not until 1991 that the first successful living donor exchange programme was developed in Korea, a country largely dependent on living donation as a result of limited deceased donation [20]. This programme, despite being referred to as the Korean KPD programme, is managed at a single centre in Seoul and does not have the structure of a national programme integrating multiple units involved with living donor kidney transplantation [20, 21]. Single centre programmes have also been reported from several other countries including Romania [22, 23] Turkey [24, 25] and India [26–28]. Since 2000, multiple single centres or regional KPD programmes were started in the United States (US) leading to 2095 transplantations [29–33]. Networked, multi-centre, national KPD registries exist in the Netherlands [16, 34, 35], the United Kingdom (UK) [36], Canada [37] and Australia [38–41]. Switzerland has played an important role in KPD, since the first crossover transplantation in the Western World was performed on May 23rd, 1999, at Basel University Hospital [42]. One of the most successful national KPD programmes, the Australian paired kidney exchange programme [17, 38–41, 43] was established in August 2010 and is managed by a Swiss physician and co-author of this review.
Conventional kidney paired donation
KPD is a strategy that helps patients to find a suitable kidney donor when their only intended living donor is unsuitable for them, for immunological reasons. In a conventional or balanced crossover procedure, two incompatible pairs simply exchange donors (two-way kidney donor exchange), creating two compatible matches (fig. 1 A) [19]. In a more complex procedure, three or more incompatible pairs can be matched with other incompatible pairs such that multiple compatible transplantations can be performed (three-, four-, n-way KPD exchanges) (fig. 1 B). In any n-way exchange, three prerequisites are essential: each patient must have a healthy and willing live donor, each live donor must be compatible with a recipient, and all pairs must have agreed to accept indirect living kidney donation from a stranger who is a willing but incompatible donor to his intended recipient. The probability of finding the optimal number of suitable matches depends on the size of the pool of incompatible pairs and the rules and conditions built into the sophisticated matching software [36, 40, 44–46]. Withdrawal of a donor from the exchange agreement after his original recipient has been transplanted would harm the remaining recipient on two levels: firstly, the patient would not receive the promised kidney transplant and secondly he/she would also lose their willing, though incompatible living donor as the “bargaining chip’ for another alternative KPD. Thus, the only way to ensure that all recipients in a KPD procedure will be transplanted is to perform all live donor surgical procedures simultaneously. Logistics is therefore the cornerstone of such procedures requiring high availabilities of surgeons, anaesthetists and operating rooms.
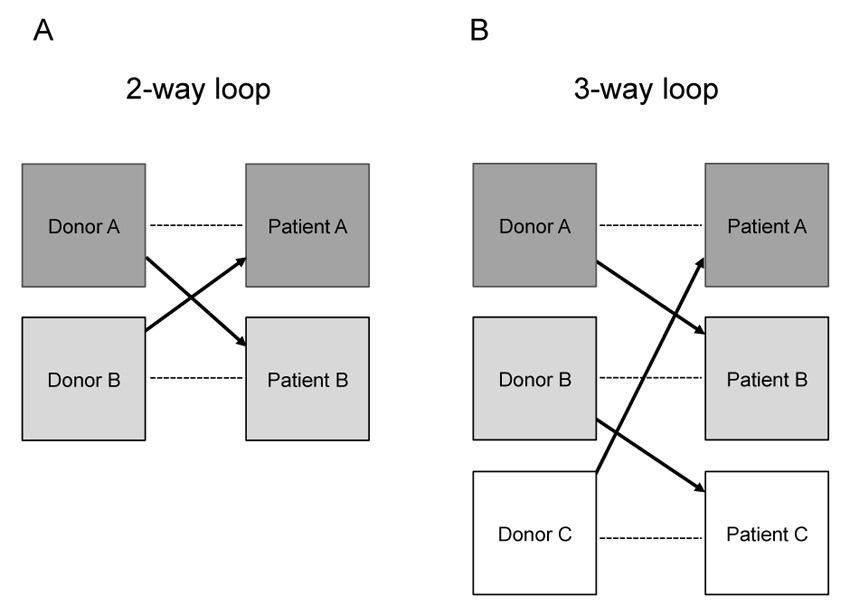
Figure 1
Exchange strategies in kidney paired donation: A conventional two-way loop exchange between two incompatible donor-recipient pairs (A) a three-way loop exchange among three incompatible donor-recipient pairs (B). Multi-way loops can be arranged with three, four, five or more pairs. --- incompatible pair; -> compatible pair.
Unbalanced kidney paired donation
Another variant of KPD mixing compatible and incompatible donor/recipient pairs is labelled altruistically unbalanced paired donation [47–49]. In this instance, a transplant candidate with an ABO/HLA compatible living donor may benefit from receiving a transplant either from a younger donor age [50] or with a better HLA match [49, 51]. The latter case is particularly attractive to otherwise compatible pairs who have a high degree of HLA-mismatch, as is the case between spouses, or who are at high immunologic risk combination, such as husband-to-wife. While improved HLA matching may or may not [52] be associated with better long-term outcomes, selection of a better matched donor is important for those likely to require repeat transplantation. Unbalanced KPD was first proposed as a possible solution to help O recipients in the KDP pool [47–49] to find a match. Indeed, as O donors will rarely enter a KPD pool, with the exception being those who have positive crossmatch with their recipient, scarce O donors are available for O recipients. In unbalanced KPD, one ABO-incompatible pair (e.g. A-donor to O-recipient) and one compatible but not identical pair (e.g. O-donor to A-recipient) could exchange (fig. 2), resulting in 2 ABO identical living donor kidney transplants. However, there may be a delay of a few months in donation/transplant surgeries of HLA/ABO compatible pairs participating in a KPD programme, until they find a better match. Although there are a number of challenges to overcome, this approach is consistent with accepted ethical tenets and it has been shown that inclusion of even a small number of HLA/ABO-compatible pairs in KPD programme can result in a substantial increase of incompatible pair match rates [33, 53].
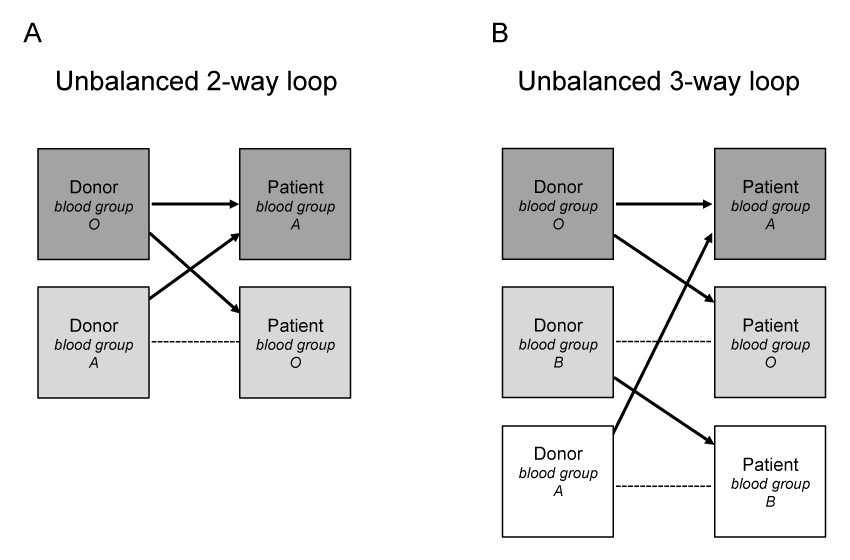
Figure 2
Exchange strategies in kidney paired donation. Unbalanced n-way loop exchanges between one compatible and one incompatible donor-recipient pairs (A) or one compatible and two incompatible donor-recipient pairs (B). --- incompatible pair; -> compatible pair.
|
Table 1:Key ingredients for a successful national kidney paired donation (KPD) programme. |
| Agreement for absolute donor criteria between the transplant centres |
| Full donor evaluation before registration in a match cycle |
| Simultaneous anaesthetic induction time for donor surgeries |
| A National Coordination Centre organising KPD activities between transplant centres and HLA laboratories |
| A computer allocation system using virtual crossmatch and ranking criteria algorithm |
Non-directed donors chains
Incorporation of NDD also known as Good Samaritans or altruistic donors [54, 55] into a KPD registry can initiate a chain of transplants [56, 57]. As an NDD is not associated with any indented recipient, it results in a minimum of two transplants via “domino” chains. Such chains are either closed when the last donor in the chain gives to a patient on the deceased-donor waitlist [58, 59] or opened (so called “never ending”) when the final donor, also called “bridge donor” will wait to initiate a future NDD chain (fig. 3). Allocation of NDD into a KPD registry has been shown to facilitate a much larger number of transplants [54, 55], on average up to 3.5 transplants per NDD chain [18, 56, 57]. For the NDD, donation of their kidney in an NDD chain amplifies their feelings of self-esteem and well-being [55]. For these reasons, given the optimising effect associated with NDD chains, some advocate that NDD should preferentially be allocated to KPD registry, an approach that is customary in the United States [58]. An important ethical issue regarding NDD participation in KPD is the diversion of NDD kidneys from highly sensitised patients on the waiting list [60]. Agreeing on a pathway where an NDD is first allocated within a defined group of highly sensitised unpaired recipients on the deceased donor wait list before being included in the KPD registry, as is the case in the UK [36], could help mitigate the ethical issue. The autonomy of each NDD also needs to be taken into consideration, and donors with very specific time constraints should be given the choice to donate directly to a patient on the waiting list. NDD chains also have the advantage of facilitating transplantations of incompatible pairs who cannot be matched in conventional KPD loops, where donors cannot mutually reciprocate in a closed loop arrangement. Arranging the logistics for multiple transplant surgeries within an NDD chain is also easier, because surgeries can be arranged sequentially. The risk of voluntary donor withdrawal and chain breakdown is modest and is offset by the benefits of leveraging one NDD to enable multiple transplants [56]. Unlike conventional KPD, this modest risk does not irreparably harm the transplant candidate, as he/she will still be able to enter into another match cycle, because his/her co-registered donor has not yet undergone nephrectomy. Limiting the waiting periods for sequential donor surgeries to a maximum of 24 hours can minimise the risk of donor reneging.
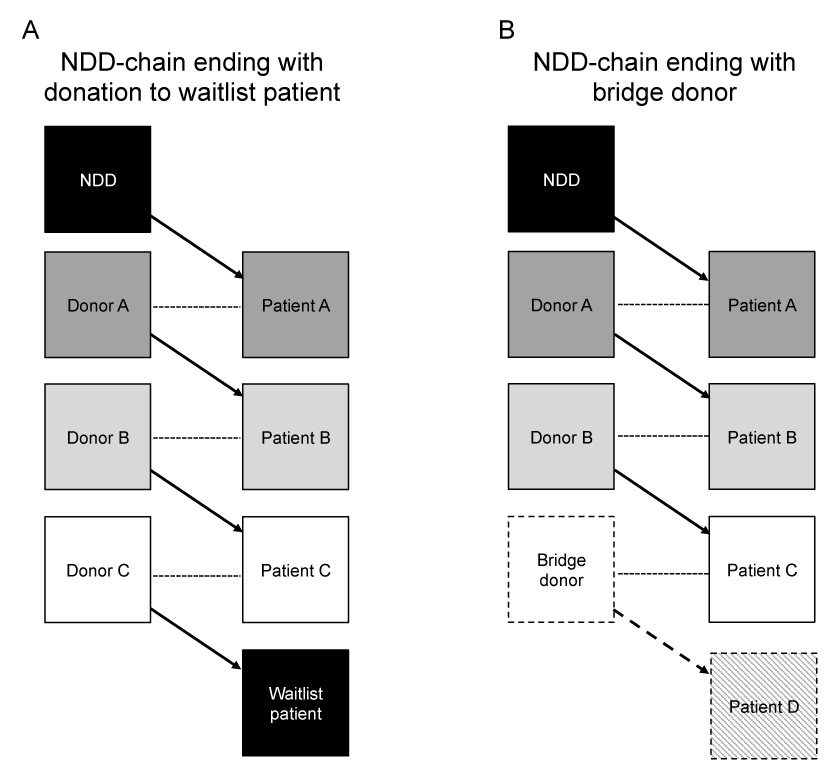
Figure 3
Multi-way exchanges beginning with a non-directed donor (NDD) and including multiple donor-recipient pairs. The closed chain donation model ends with the final donor donating to a patient on a deceased-donor wait list (A). The open chain model ends with a bridge donor that will start a new chain of transplants (B). --- incompatible pair; -> compatible pair; ---> next compatible chain.
Kidney paired donation registries
As the match probability increases with the number of registered incompatible pairs in any given KPD pool [29, 44, 46], countries with a relatively small population like Switzerland will benefit from a national KPD programme, as multiple independent regional registries would not reach a critical mass of registered incompatible pairs. Worldwide there are currently four national KPD programmes in The Netherlands, the UK, Canada and Australia [18], which could be used as models for a Swiss KPD registry. All have an oversight body, which is part of their national government health system or which is managed by a national organisation. Matching cycles occur every 3–4 months in each of these registries, unlike other registries that use revolving, real-time computer matching [32, 61]. All four countries use a matching algorithm whose primary allocation criteria are based on virtual cross-match [18]. In the Netherlands and Canada, donors travel to the recipient’s centre for surgery, whereas kidneys are transported between centres in the UK and Australia. All programmes endeavour to ensure that surgeries take place on the same day, and that anaesthetic induction time is the same [18]. Commonalities and differences of multiple programmes in the US have been reviewed in detail elsewhere [30, 33, 61–64]. The undeniable success of some of the American programmes has been dependent on the inclusion of compatible pairs [33] and NDD, using bridge donors [64], and integrating KPD into desensitisation protocols [63, 65].
Kidney paired donation in Australia
The Australian KPD programme is known as the Australian paired Kidney eXchange (AKX) Programme and was established in 2010 following the initial experience of a regional pilot programme in Western Australia [41]. From October 2010 until August 2014, the Australian KPD programme has facilitated 101 kidney transplants (91 completed, 10 awaiting surgery) among the 207 registered pairs (49% of registered KPD candidates) and 4 waitlist recipients; 89 transplants were achieved using N-way chains and 16 transplants were performed through NDD chains (15%). This relatively high proportion of transplants was achieved despite a pool consisting primarily of highly sensitised, HLA-incompatible pairs (35% of registered patient had cPRA 95–100%), compared to non-sensitised, ABO-incompatible pairs. Overall transplant rates have been excellent, and the proportion of patients with cPRA 50–96% being transplanted (62%) is fairly similar to the proportion of transplanted patients with cPRA 0–50% (73%), although, not surprisingly, the proportion of transplants among extremely highly sensitised candidates with cPRA ≥97% has been low (25%) (fig. 4). NDD chains have been a minor driver in the Australian programme due to the low number of NDDs included. The Australian programme compares favourably with the Dutch programme, in which 242 kidney transplants over a 10-year period have been facilitated among the 655 registered pairs using ≥2–way chains (transplant rate 37%) [18]. It also outperforms the UK programme, in which between April 2007 and 2014, 284 of the 991 registered patients (29%) proceeded to receive a kidney transplant through 2–way and 3–way loops and 36 through NDD chains (3.6%) [18]. The Canadian programme, active since 2009, shares many commonalities with the Australian programme: its KPD pool is mainly composed of highly sensitised, HLA-incompatible pairs and the proportion of KPD transplant among registered transplant candidates is equally excellent (44%) [18]. A major driver of the success in Canadian registry is the inclusion of a large number of NDDs (n = 54 at the end of 2013), facilitating 62% of the registered recipient transplants versus 38% in N-way exchanges [18, 37]. Comparison between the four national programmes [18] would suggest that match and transplant rates from N-way loops does not only rely upon incompatible pair pool size. Indeed, transplant rate from loops is 41% in the Australian programme despite inclusion of only 40 to 50 patients per match cycle, whereas transplant rates from loops were only 25% in the UK programme despite inclusion of 160 to 180 patients in each allocation round. The most plausible explanation for this success is wide acceptance of ABO-incompatible matching in the Australian programme [38]. On the other hand, the power of NDD domino chains in KPD programme is clearly demonstrated by the Canadian programme, where 62% of all KPD transplants were facilitated by NDD chains [18, 37].
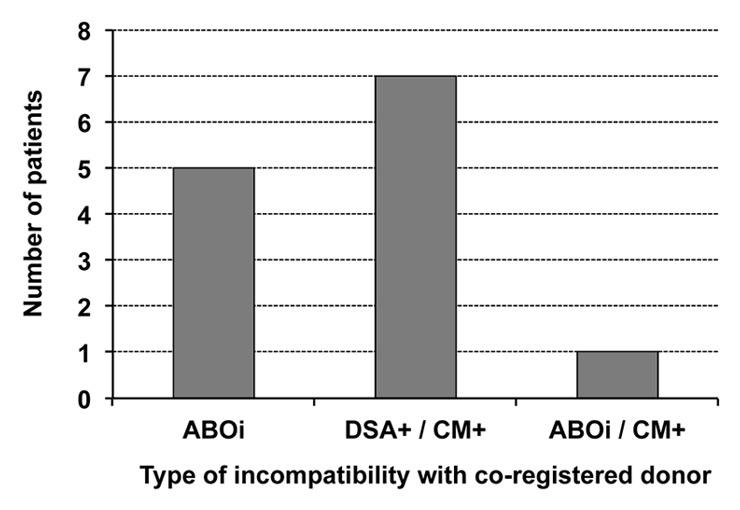
Figure 4
Type of immunological incompatibilities of 13 living donor pairs in Switzerland.
ABOi = ABO incompatible; CM+ = positive crossmatch; DSA+ = donor specific antibodies.
Kidney paired donation in Switzerland
In Switzerland, the first paired kidney exchange procedure took place at Basel University Hospital on the 23rd May 1999 between a Swiss and a German couple. Thereafter, it was not until September 2011 that the next crossover procedure between 2 incompatible couples was carried out at Geneva University Hospital. The first inter-hospital paired kidney exchange in Switzerland was successfully completed through a combined effort of the Zurich and Geneva transplant teams. After the success of the third KPD procedure, representatives of all Swiss kidney transplant units, Swisstransplant and the Council of the Swisstransplant Foundation joined efforts to promote the establishment of a Swiss national KPD programme. It is worth noting that at the time, a national protocol for ABO incompatible kidney transplantation had already been established in Switzerland with success since 2005.
Since the effectiveness of a KPD programme depends largely upon the pool’s size of incompatible living donors’ couples, it was important to gauge the likely referral base of HLA and ABO incompatible pairs. Thus, all 6 Swiss kidney transplant centres were surveyed with regard to their own potential incompatible pairs. The survey showed that in 2012 there were at least 38 patients with incompatibilities to their intended donor, either due to preformed DSA with positive crossmatch and/or ABO incompatibility. While this relatively small number may seem discouraging, it is worth noting that in Australia, a country with a population of 22 million, the input of new pairs per match cycle in the last 12 months has averaged 14 added each time on a pool of 32–38 existing pairs. Thus, the projected enrolment of at least 38 incompatible pairs in Switzerland, a country of 7 million inhabitants, was thought adequate to warrant the establishment of a national KPD programme. Discussions with the Federal Public Health Office to implement such a programme and the need for a national platform in accordance to existing Swiss Transplantation Law are currently underway.
While a formal national KPD registry is yet to be finalised, exchange of information between the transplant centres on incompatible pairs has led to 13 crossover transplantations: two 2–way and three 3–way loop exchanges, between September 2011 and October 2013 (fig. 1). Matching was performed manually using a virtual crossmatch approach that takes into consideration preformed anti-HLA antibodies and donor and recipient blood groups. Immunological suitability of identified matches was confirmed by cell-based crossmatches performed at the Swiss National Reference Laboratory in Geneva. All donations and all transplantations took place on the same day at the same anaesthetic induction time. All donors travelled to the recipient’s centre for their operations. Choice was given to the new donors-recipients pairs to meet: 10 of 13 pairs met before surgery. Reasons for crossover procedures are shown in (fig. 4); KPD allowed in all cases to overcome the immunological barrier (positive crossmatch and/or ABO incompatibility) each recipient had with his intended donor. One year graft and patient survival are 100% and the matched pairs that met are still in touch.
Special considerations
Allocation algorithms in kidney paired donation
The ability of KPD to match incompatible pairs depends upon the pool size [29, 46], the ratio of ABO-incompatible to HLA-incompatible pairs, the level of sensitisation of transplant candidates and the algorithm-specific allocation rules. Using N-way exchanges, the match probability for ABO-incompatible or sensitised non-O recipients is around 60%, but can be as low as 20% for ABO-incompatible O recipients [66]. This observation has been used as an argument to preferentially match scarce O donors in KPD registry to O recipients in the interest of fairness [44, 67]. As many ABO-incompatible pairs may not accept participation in KPD programme to undergo living donor kidney transplantation [7–9], an important source of unsensitised recipients is removed from KPD pool, leading to a high proportion of highly sensitised pairs [38, 68]. When the number of pairs referred for KPD because of HLA incompatibility outnumbers the pairs with ABO incompatibility the match probability decreases [36]. A strategy to minimise this problem would be to offer ABO incompatible pairs the option to be entered first in the KPD registry with the aim of improving HLA matching and to resort to directed ABO-incompatible transplantation if no suitable match is identified within an agreed number of match cycles.
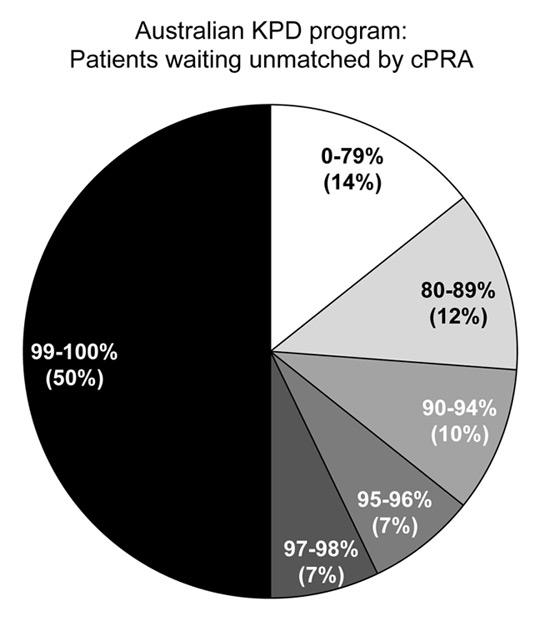
Figure 5
Level of sensitisation of 38 transplant candidates still waiting unmatched in the Australian KPD programme after at least two match cycles. Two thirds of patients waiting are highly sensitised with a cPRA >95%.
The key ingredient of any KPD programme is the matching algorithm selecting pairs within the pool. The ideal algorithm should identify the maximum number of possible transplants, while minimising the probability of unexpected post-match positive cell-based crossmatches and simultaneously promoting high quality exchanges. These difficult goals require sophisticated KPD software that takes into account two critical elements of priority for matching: blood group matching and negative crossmatch [40, 44] (table 1). Virtual crossmatch approach is widely accepted to allocate suitable donors in the pool to registered transplant candidates, although the extent of HLA-antigens included in this virtual crossmatch algorithm varies between registries [18, 32, 36, 37, 40, 44, 56]. The Australian KPD programme uses the computer platform of the National Organ Matching System (NOMS), which is also responsible for deceased donor organ allocation, with a purpose-built KPD allocation module [40]. The NOMS computer programme matches each recipient with any donors using a two–step process: (1.) ABO-compatibility or acceptable donors matching (in case of approved ABO-incompatibility matching) and (2.) HLA virtual crossmatches among these ABO-acceptable donors and recipients. Next, the NOMS programme generates ≥2–way exchanges using six ranking criteria: (1.) prioritising combinations that maximise the number of potential transplants; (2.) selecting matches for recipients with low match probabilities (high cPRA); (3.) maximising the number of ABO identical pairs, giving O-to-O pairs priority; (4.) minimising the number of simultaneous transplants within a chain in a single hospital; (5.) maximising the number of short chains; and (6.) promoting matches for patients with longer waiting times. Despite the relatively small pool of incompatible pairs, the high proportion of sensitised patients and the small number of NDDs, the Australian programme has been able to facilitate kidney transplantation in 49% of registered patients [39, 43].
Highly sensitised recipients: integration of desensitisation and ABOi matching
Despite desensitisation protocols or inclusion in a KPD registry, highly sensitised patients with cPRA ≥97% remain difficult to undergo living kidney transplantation. In the Australian programme, 50% of unmatched pairs still waiting in the registry have a cPRA of 99%–100% (fig. 5). It is obvious that for these patients either strategy alone will not be able to find a suitable crossmatch-negative donor without any detectable DSA. Several strategies to help these highly sensitised patients include reducing constraints in the matching algorithm such as allowing ABO-incompatible matching [38], expanding the KPD pool size through international collaboration [69], including all NDDs and large numbers of compatible pairs [29, 53] and allowing DSA positive KPD transplants in order to let them undergo acceptable, albeit not ideal kidney transplantation. While traditionally most KPD programmes use virtual crossmatch criteria to identify fully compatible matches that will avoid even low-strength DSA [36, 37, 40, 44], some KPD programmes have already successfully explored the option of a hybrid strategy combining KPD with low immunologic risk desensitisation [11, 63, 65, 70–72]. With this strategy, although KPD recipients would have a positive virtual crossmatch based on the DSA identified by SAB testing, they can be safely transplanted in most instances in the presence of a negative CDC crossmatch and in many cases even a negative flow cytometric crossmatch [72]. This hybrid strategy used quite extensively at Johns Hopkins Hospital in Baltimore [63, 65] and in a few cases in Australia, was proving effective in transplanting patients who are both hard to match and difficult to desensitise.
Legal framework
Successful kidney transplantation was first reported when a patient with kidney failure received a kidney from his identical homozygous twin in 1954. Living donor kidney transplantation remained restricted to haploidentical siblings and first degree related donors until introduction of potent immunosuppressive drugs in the mid 1980s. Since then, transplantation laws and relationships between live donors and recipients have evolved in line with developments in biology and pharmacotherapy. In directed living donor kidney transplantation, a genetically or emotionally related donor knows the recipient beforehand. When considering a KPD programme, which is an expansion of conventional living kidney donation, it is important to be aware of possible legislative barriers and commitment of politicians in changing their national organ transplant act. The fact that the donor and the recipient of the matched pair are strangers to one another could hurdle KPD programme establishment. In theory, a KPD could be considered an arrangement akin to a bilateral contract, where a donor would agree to donate a kidney to a stranger, if his/her co-registered recipient would receive a kidney in return; this is generally known as ‘valuable consideration’. In the US, the National Organ Transplant Act (NOTA) of 1984 prohibited “any person to knowingly acquire, receive, or otherwise transfer any human organ for valuable consideration for use in human transplantation.” Although the concept of “valuable consideration” applies to monetary value, an exchange of organs was also considered valuable, as ‘one pair is paying with a kidney in order to receive a kidney”. In 2007, a bill was passed both in the House and in the Senate that amends NOTA to clarify that “kidney exchange shall not be considered to involve the transfer of a human organ for valuable consideration”. In Australia, there is no Federal Legislation on Organ and Tissue Transplants and each state has their own Transplant Act. All states Transplant Act have a prohibition against trading in human tissues, the majority include a clause mandating that “…a person must not enter into, or offer to enter into, a contract or arrangement under which any person agrees, for valuable consideration, whether given or to be given to any such person or to any other person…”, which basically translates as a prohibition on living donor kidney exchange. However, all Transplant Acts have a special provision for the Minister for Health to grant exemption to allow KPD to occur. To date, a ministerial exemption is required for each pair participating in the programme in all states except Victoria and Queensland. Like most human tissue and transplant acts in developed countries, the Swiss Transplant Law on human organs, tissues and cells, regulates the prohibition on organ trading and similar valuable consideration that could be perceived as trading of organs or tissues for human transplantation (http://www.admin.ch/opc/de/federal-gazette/2004/5453.pdf). In chapter 2, section 1, paragraph 6(d), it is outlined that crossover live donor transplantation is not prohibited as organ trading, because no financial or other gain is being associated. No detail is given regarding how a KPD programme should be implemented and whether NDDs could be integrated to allow initiation of chains.
Conclusions and perspectives
In many countries, KPD has become the fastest growing source of live donor kidney transplantations. By including all healthy, willing but immunologically incompatible living donors, but also non-directed anonymous donors and compatible pairs it is possible to achieve a sufficiently large pool of donors and recipients in order to find the best match for the highest number of recipients, even in a relatively small country like Switzerland. Facilitating immunologically compatible kidney transplants should remain the primary aim of a KPD programme, but for those extremely highly sensitised patients, KPD can enable living donor kidney transplantations with low immunological risk and longer graft survivals. KPD is also beneficial to unpaired patients, as removing all patients with a potential living donor from the deceased donor waitlist will lower their waiting time. Finally, increased access to kidney transplantation will help reduce the demand for illegal commercial transplantation.
In our opinion, it is time to establish a funded Swiss national KPD programme; this view is supported by all 6 transplantations centres, who have agreed to establish a registry, have concurred on a computer software and allocation algorithm and have settled for a central and dedicated coordination through Swisstransplant. Crossover living donor transplantation is not considered as organ trading and thus is not prohibited by the Swiss transplant law. The legal framework is on hold and governmental support is required.
References
1 Oniscu GC, Brown H, Forsythe JL. Impact of cadaveric renal transplantation on survival in patients listed for transplantation. J Am Soc Nephrol. 2005;16:1859–65.
2 Watson CJ, Dark JH. Organ transplantation: historical perspective and current practice. Br J Anaesth. 2012;108(Suppl 1):i29–42.
3 Terasaki PI, Cecka JM, Gjertson DW, et al. High survival rates of kidney transplants from spousal and living unrelated donors. N Engl J Med. 1995;333:333–6.
4 Meier-Kriesche HU, Kaplan B. Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes: a paired donor kidney analysis. Transplantation. 2002;74:1377–81.
5 Harter TD. Overcoming the organ shortage: failing means and radical reform. HEC Forum. 2008;20:155–82.
6 Karpinski M, Knoll G, Cohn A, et al. The impact of accepting living kidney donors with mild hypertension or proteinuria on transplantation rates. Am J Kidney Dis. 2006;47:317–23.
7 Tanabe K. Japanese experience of ABO-incompatible living kidney transplantation. Transplantation. 2007;84(12 Suppl):S4–7.
8 Tyden G, Kumlien G, Genberg H, et al. ABO incompatible kidney transplantations without splenectomy, using antigen-specific immunoadsorption and rituximab. Am J Transplant. 2005;5:145–8.
9 Flint SM, Walker RG, Hogan C, et al. Successful ABO-incompatible kidney transplantation with antibody removal and standard immunosuppression. Am J Transplant. 2011;11:1016–24.
10 Bentall A, Cornell LD, Gloor JM, et al. Five-year outcomes in living donor kidney transplants with a positive crossmatch. Am J Transplant. 2013;13:76–85.
11 Vo AA, Peng A, Toyoda M, et al. Use of intravenous immune globulin and rituximab for desensitization of highly HLA-sensitized patients awaiting kidney transplantation. Transplantation. 2010;89:1095–102.
12 Gloor JM, Winters JL, Cornell LD, et al. Baseline donor-specific antibody levels and outcomes in positive crossmatch kidney transplantation. Am J Transplant. 2010;10:582–9.
13 Montgomery RA, Lonze BE, King KE, et al. Desensitization in HLA-incompatible kidney recipients and survival. N Engl J Med. 2011;365:318–26.
14 Bostock IC, Alberu J, Arvizu A, et al. Probability of deceased donor kidney transplantation based on % PRA. Transpl Immunol. 2013;28:154–8.
15 Neumayer HH, Budde K, Liefeldt L. Human Leukocyte Antigen-Incompatible Kidney Transplantation After “Desensitization”-Hope and Reality. Transplantation. 2014:(ePub).
16 de Klerk M, Keizer KM, Claas FH, et al. The Dutch national living donor kidney exchange program. Am J Transplant. 2005;5:2302–5.
17 Ferrari P, De Klerk M. Paired kidney donations to expand the living donor pool. J Nephrol. 2009;22:699–707.
18 Ferrari P, Weimar W, Johnson RJ, et al. Kidney Paired Donation: Principles, Protocols and Programs. Nephrol Dial Transplantation. 2014:(in press).
19 Rapaport FT. The case for a living emotionally related international kidney donor exchange registry. Transplant Proc. 1986;18(3 Suppl 2):5–9.
20 Kwak JY, Kwon OJ, Lee KS, et al. Exchange-donor program in renal transplantation: a single-center experience. Transplant Proc. 1999;31:344–5.
21 Park K, Lee JH, Huh KH, et al. Exchange living-donor kidney transplantation: diminution of donor organ shortage. Transplant Proc. 2004;36:2949–51.
22 Lucan M. Five years of single-center experience with paired kidney exchange transplantation. Transplant Proc. 2007;39:1371–5.
23 Lucan M, Rotariu P, Neculoiu D, et al. Kidney exchange program: a viable alternative in countries with low rate of cadaver harvesting. Transplant Proc. 2003;35:933–4.
24 Gurkan A, Kacar SH, Varilsuha C, et al. Exchange kidney transplantation: a good solution in living kidney transplantation. Transplant Proc. 2004;36:2952–3.
25 Kacar SH, Eroglu A, Tilif S, et al. A novel experience in living donor renal transplantation: voluntary exchange kidney transplantation. Transplant Proc. 2013;45:2106–10.
26 Gumber MR, Kute VB, Goplani KR, et al. Transplantation with kidney paired donation to increase the donor pool: a single-center experience. Transplant Proc. 2011;43:1412–4.
27 Kute VB, Gumber MR, Patel HV, et al. Outcome of kidney paired donation transplantation to increase donor pool and to prevent commercial transplantation: a single-center experience from a developing country. Int Urol Nephrol. 2013;45:1171–8.
28 Kute VB, Vanikar AV, Gumber MR, et al. Successful three-way kidney paired donation with compatible pairs to increase donor pool. Ren Fail. 2014;36:447–50.
29 Segev DL, Gentry SE, Warren DS, et al. Kidney paired donation and optimizing the use of live donor organs. JAMA. 2005;293:1883–90.
30 Hanto RL, Reitsma W, Delmonico FL. The development of a successful multiregional kidney paired donation program. Transplantation. 2008;86:1744–8.
31 Rees MA, Bargnesi D, Samy K, et al. Altruistic donation through the Alliance for Paired Donation. Clin Transpl. 2009:235–46.
32 Veale J, Hil G. The National Kidney Registry: 175 transplants in one year. Clin Transpl. 2011:255–78.
33 Bingaman AW, Wright FH, Jr., Kapturczak M, et al. Single-Center Kidney Paired Donation: The Methodist San Antonio Experience. Am J Transplant. 2012;12:2125–32.
34 de Klerk M, Witvliet MD, Haase-Kromwijk BJ, et al. Hurdles, barriers, and successes of a national living donor kidney exchange program. Transplantation. 2008;86:1749–53.
35 de Klerk M, van der Deijl WM, Witvliet MD, et al. The optimal chain length for kidney paired exchanges: an analysis of the Dutch program. Transpl Int. 2010;23:1120–25.
36 Johnson RJ, Allen JE, Fuggle SV, et al. Early experience of paired living kidney donation in the United kingdom. Transplantation. 2008;86:1672–7.
37 Malik S, Cole E. Foundations and principles of the Canadian Living Donor Paired Exchange Program. Can J Kidney Health Dis. 2014; 1:ePub.
38 Ferrari P, Hughes PD, Cohney SJ, et al. ABO-incompatible matching significantly enhances transplant rates in kidney paired donation. Transplantation. 2013;96:821–6.
39 Ferrari P, Fidler S, Holdsworth R, et al. High Transplant Rates of Highly-Sensitised Recipients with Virtual Crossmatching in Kidney Paired Donation. Transplantation. 2012;94:744–9.
40 Ferrari P, Fidler S, Wright J, et al. Virtual Crossmatch Approach to Maximize Matching in Paired Kidney Donation. Am J Transplant. 2011;11:272–78.
41 Ferrari P, Woodroffe C, Christiansen FT. Paired kidney donations to expand the living donor pool: the Western Australian experience. Med J Aust. 2009;190:700–3.
42 Thiel G, Vogelbach P, Gurke L, et al. Crossover renal transplantation: hurdles to be cleared! Transplant Proc. 2001;33:811–6.
43 Ferrari P, Fidler S, Woodroffe C, et al. Comparison of time on the deceased donor kidney waitlist versus time on the kidney paired donation registry in the Australian program. Transplant Int. 2012;25:1026–1031.
44 Keizer KM, de Klerk M, Haase-Kromwijk BJ, et al. The Dutch algorithm for allocation in living donor kidney exchange. Transplant Proc. 2005;37:589–91.
45 Montgomery RA, Zachary AA, Ratner LE, et al. Clinical results from transplanting incompatible live kidney donor/recipient pairs using kidney paired donation. JAMA. 2005;294:1655–63.
46 Saidman SL, Roth AE, Sonmez T, et al. Increasing the opportunity of live kidney donation by matching for two- and three-way exchanges. Transplantation. 2006;81:773–82.
47 Ross LF, Woodle ES. Ethical issues in increasing living kidney donations by expanding kidney paired exchange programs. Transplantation. 2000;69:1539–43.
48 Stegall MD, Dean PG, Gloor JM. ABO-incompatible kidney transplantation. Transplantation. 2004;78:635–40.
49 Kranenburg LW, Zuidema W, Weimar W, et al. One donor, two transplants: willingness to participate in altruistically unbalanced exchange donation. Transpl Int. 2006;19:995–9.
50 Rizzari MD, Suszynski TM, Gillingham KJ, et al. Consideration of donor age and human leukocyte antigen matching in the setting of multiple potential living kidney donors. Transplantation. 2011;92:70–5.
51 Wiebe C, Pochinco D, Blydt-Hansen TD, et al. Class II HLA epitope matching – A strategy to minimize de novo donor-specific antibody development and improve outcomes. Am J Transplant. 2013;13(12):3114–22.
52 Fuggle SV, Allen JE, Johnson RJ, et al. Factors affecting graft and patient survival after live donor kidney transplantation in the UK. Transplantation. 2010;89:694–701.
53 Gentry SE, Segev DL, Simmerling M, et al. Expanding kidney paired donation through participation by compatible pairs. Am J Transplant. 2007;7:2361–70.
54 Matas AJ, Garvey CA, Jacobs CL, et al. Nondirected donation of kidneys from living donors. N Engl J Med. 2000;343:433–6.
55 Bramstedt KA, Dave S. The silence of Good Samaritan kidney donation in Australia: a survey of hospital websites. Clin Transplant. 2013;27:244–8.
56 Rees MA, Kopke JE, Pelletier RP, et al. A nonsimultaneous, extended, altruistic-donor chain. N Engl J Med. 2009;360:1096–101.
57 Roodnat JI, Zuidema W, van de Wetering J, et al. Altruistic donor triggered domino-paired kidney donation for unsuccessful couples from the kidney-exchange program. Am J Transplant. 2010;10:821–7.
58 Montgomery RA, Gentry SE, Marks WH, et al. Domino paired kidney donation: a strategy to make best use of live non-directed donation. Lancet. 2006;368:419–21.
59 Roth AE, Sonmez T, Unver MU, et al. Utilizing list exchange and nondirected donation through “chain” paired kidney donations. Am J Transplant. 2006;6:2694–705.
60 Woodle ES, Daller JA, Aeder M, et al. Ethical considerations for participation of nondirected living donors in kidney exchange programs. Am J Transplant. 2010;10:1460–7.
61 Mierzejewska B, Durlik M, Lisik W, et al. Current approaches in national kidney paired donation programs. Ann Transplant. 2013;18:112–24.
62 Wallis CB, Samy KP, Roth AE, et al. Kidney paired donation. Nephrol Dial Transplant. 2011;26:2091–9.
63 Montgomery RA, Lonze BE, Jackson AM. Using donor exchange paradigms with desensitization to enhance transplant rates among highly sensitized patients. Curr Opin Organ Transplant. 2011;16:439–43.
64 Leeser DB, Aull MJ, Afaneh C, et al. Living donor kidney paired donation transplantation: experience as a founding member center of the National Kidney Registry. Clin Transplant. 2012;26:E213–22.
65 Sharif A, Zachary AA, Hiller J, et al. Rescue kidney paired donation as emergency salvage for failed desensitization. Transplantation. 2012;93:27–9.
66 Roodnat JI, van de Wetering J, Claas FH, et al. Persistently low transplantation rate of ABO blood type O and highly sensitised patients despite alternative transplantation programs. Transpl Int. 2012;25:987–93.
67 de Klerk M, Witvliet MD, Haase-Kromwijk BJ, et al. A highly efficient living donor kidney exchange program for both blood type and crossmatch incompatible donor-recipient combinations. Transplantation. 2006;82:1616–20.
68 Baxter-Lowe LA, Cecka M, Kamoun M, et al. Center-Defined Unacceptable HLA Antigens Facilitate Transplants for Sensitized Patients in a Multi-Center Kidney Exchange Program. Am J Transplant. 2014;14:1592–8.
69 Garonzik-Wang JM, Sullivan B, Hiller JM, et al. International kidney paired donation. Transplantation. 2013;96:55–6.
70 Montgomery RA. Renal transplantation across HLA and ABO antibody barriers: integrating paired donation into desensitization protocols. Am J Transplant. 2010;10:449–57.
71 Yabu JM, Pando MJ, Busque S, et al. Desensitization combined with paired exchange leads to successful transplantation in highly sensitized kidney transplant recipients: strategy and report of five cases. Transplant Proc. 2013;45:82–7.
72 Blumberg JM, Gritsch HA, Reed EF, et al. Kidney paired donation in the presence of donor-specific antibodies. Kidney Int. 2013;84:1009–16.




