Treatment intensification with insulin glargine in patients with inadequately controlled type 2 diabetes improves glycaemic control with a high treatment satisfaction and no weight gain
DOI: https://doi.org/10.4414/smw.2015.14114
Daniela
Riebenfeld, David
Spirk, Alexandra
Mathis, Lukas
Villiger, Philipp
Gerber, Urs Erwin
Gasser, Roger
Lehmann
Summary
PRINCIPLES: We aimed to evaluate the efficacy of, and treatment satisfaction with, insulin glargine administered with SoloSTAR® or ClikSTAR® pens in patients with type 2 diabetes mellitus managed by primary care physicians in Switzerland.
METHODS: A total of 327 patients with inadequately controlled type 2 diabetes were enrolled by 72 physicians in this prospective observational study, which aimed to evaluate the efficacy of a 6-month course of insulin glargine therapy measured as development of glycaemic control (glycosylated haemoglobin [HbA1c] and fasting plasma glucose [FPG]) and weight change. We also assessed preference for reusable or disposable pens, and treatment satisfaction.
RESULTS: After 6 months, the mean daily dose of insulin glargine was 27.7 ± 14.3 U, and dose titration was completed in 228 (72.4%) patients. Mean HbA1c decreased from 8.9% ± 1.6% (n = 327) to 7.3% ± 1.0% (n = 315) (p <0.0001), and 138 (43.8%) patients achieved an HbA1c ≤7.0%. Mean FPG decreased from 10.9 ± 4.5 to 7.3 ± 1.8 mmol/l (p <0.0001). Mean body weight did not change (85.4 ± 17.2 kg vs 85.0 ± 16.5 kg; p = 0.11). Patients’ preference was in favour of the disposable SoloStar® pen (80%), as compared with the reusable ClickStar® pen (20%). Overall, 92.6% of physicians and 96.3% of patients were satisfied or very satisfied with the insulin glargine therapy.
CONCLUSIONS: In patients with type 2 diabetes insulin glargine administered by SoloSTAR® or ClikSTAR® pens, education on insulin injection and on self-management of diabetes was associated with clinically meaningful improvements in HbA1c and FPG without a mean collective weight gain. The vast majority of both patients and primary care physicians were satisfied with the treatment intensification.
Introduction
Diabetes mellitus represents a fast growing worldwide epidemic: In 2010, an estimated 285 million people were affected by type 2 diabetes. The projected numbers for 2030 reach 439 million people, owing to a marked increase of prevalence in young adults and adolescents and, in certain regions such as Africa, the Middle East and Asia [1]. In Switzerland, the prevalence of type 2 diabetes is estimated at 5.7–7% of the overall population, corresponding to approximately 500,000 people [2]. In patients with type 2 diabetes, hyperglycaemia enhances the risk of vascular disease, acute myocardial infarction, stroke, lower limb amputation and microvascular complications [2–6]. Real-life data from a survey in 157,000 American patients with type 2 diabetes, however, indicate that over two-thirds of the patients have HbA1c concentrations >6.5%, which may cause considerable long-term complications and healthcare costs [7].
Once life-style intervention and one or more oral antidiabetic drugs (OADs) become ineffective to lower HbA1c to target levels, the addition of basal insulin, particularly in patients with high fasting blood glucose (FBG) levels, is highly recommended [8]. Good glycaemic control over time is required to reduce the risk of diabetes-associated complications [8, 10]. Type 2 diabetes guidelines and algorithms that highlight the importance of basal insulin for the management of the disease have been published in recent years jointly by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Based on the above mentioned guidelines, the SSED (Swiss Society of Endocrinology and Diabetes) has published its own recommendations [12, 13]. Whereas the addition of basal insulin to existing OAD treatment was recommended in patients with HbA1c levels above 7% in the former SSED guidelines from 2009 [12], individual target levels between 6% and 8% are recommended in the new SSED guidelines published in 2013 [13].
Clinical trials demonstrated the benefit of insulin glargine, a basal-insulin analogue [14], to achieve HbA1c levels of <7% in patients with uncontrolled type 2 diabetes mellitus [8, 15–27]. Optimisation and long-term maintenance of glycaemic control with insulin glargine was confirmed in large cohorts of patients with type 2 diabetes [10, 22, 23], and advantages of insulin glargine compared with other insulins and antidiabetic medication were described in several studies [8, 15, 17, 19, 20, 24, 25].
Patients requiring insulin are challenged by complex interventions for their disease. They have to manage multiple blood glucose measurements and to balance carbohydrate intake and daily insulin injections [8]. Failure to initiate or noncompliance with insulin treatment regimens represents a common and critical issue for reaching therapeutic goals. Insulin use by patients is often limited by factors such as fear of injection and hypoglycaemia, but could also be also related to the injection device itself. Therefore, easy-to-use devices may positively influence physicians’ insulin prescribing habits and patient compliance [8]. The convenient and simple handling of SoloSTAR® and ClikSTAR® represents a big step forward towards easy self-management of diabetes and lead to increased treatment adherence and higher patient satisfaction [8, 26]. Thus, administration of insulin glargine with SoloSTAR® and ClikSTAR® pens contributes to the optimisation of diabetes treatment [27].
In addition, education on self-management of diabetes reflects a further important aspect of the current patient-centred approach. The later focuses on the patient’s needs and abilities with the aim to improve their knowledge and skills, thus enabling better self-management of treatment regimens and life-style interventions [8, 28, 29].
The aim of the present study was to evaluate the efficacy of a 6-month course of insulin glargine therapy measured as development of the glycaemic control (HbA1c and FPG) and weight change. We also assessed, whether patients will choose a reusable or a disposable pen, and the treatment satisfaction with SoloSTAR® (disposable pre-filled insulin pen) and ClikSTAR® (reusable insulin pen for 3 ml insulin cartridges) in patients with type 2 diabetes mellitus managed by primary care physicians in various regions of Switzerland.
Patients and methods
Patients
Seventy-two physicians in primary medical care across Switzerland participated in the present prospective observational study conducted between November 2009 and September 2011. All patients with poorly controlled type 2 diabetes mellitus despite prior treatment with OAD(s) or insulin were eligible to participate. Inclusion criteria were age >18 years, HbA1c >7%, and the patient’s informed consent. There were no exclusion criteria. The reasons for nonparticipation in the study were not systematically captured. In accordance with local regulations, informed consent was provided by the participating patients. Treatment with insulin glargine (Lantus®, Sanofi-Aventis (Schweiz) AG, Vernier, Switzerland) and pens for subcutaneous administration (SoloSTAR® and ClikSTAR®, Sanofi-Aventis (Schweiz) AG, Vernier, Switzerland) was prescribed as a part of routine medical care and used according to the product information [30]. Participating physicians were encouraged to provide education on the appropriate administration of insulin glargine, the use of pens and concomitant life-style interventions to enable patients to self-manage their disease and to assist patients to choose which pen to use (the disposable (SoloSTAR) or the reuseable (ClikSTAR) pen). Information on self-management of diabetes was at the discretion of the treating physician. The first patient was included in the observation study on 1 November 2009 and the last patient was included on 31 March 2011. With the exception of the first visit and a visit at after 6 months of insulin glargine treatment, there was no systematic monitoring of treatment with insulin glargine other than routine clinical visits as ordered by the participating physicians. The study was approved by an Independent Ethics Committee according to local regulations.
Outcome measures
Patient demographics, vital signs (systolic and diastolic blood pressure) and lipids (triglycerides, high and low density lipoprotein cholesterol), medical history and complications of diabetes, concomitant diseases, previous treatment of diabetes, and recommended dose titration of insulin glargine were recorded at the baseline (BL) visit. Initial dose, titration scheme and actual dose of insulin glargine were recorded at the follow-up (FU) routine medical care visit after 6 months. The effectiveness parameters (HbA1c and FPG) were recorded at the BL and FU visits. There was no reference laboratory used for the biochemical analysis, but rather the laboratory in the doctor’s office or an external laboratory used by the doctor’s office. Safety events were reported directly to the Swissmedic Pharmacovigilance centres according to national regulatory requirements. Satisfaction was evaluated using the four-point Likert scale (very dissatisfied, dissatisfied, satisfied, very satisfied) by interview of the patients and by questionnaire for the physicians. The results were entered into the case report form (CRF) directly by participating physicians.
Statistical analysis
All patients with data records at BL and FU visits were included in the intention-to-treat (ITT) analysis (n = 315), and all patients compliant with the eligibility criteria and appropriate visit interval were considered for the per-protocol (PP) analysis (n = 300). Baseline characteristics, antidiabetic treatment and safety were assessed in the ITT population. Efficacy (HbA1c and FPG) and treatment satisfaction were analysed using the PP population. Continuous variables with a normal distribution were described as means with standard deviations (SDs), and group comparisons were performed with the independent or paired t-test; continuous variables with a skewed distribution were presented as median values. Changes in HbA1c between different titration groups was analysed by using multiple linear regression, controlled for baseline HbA1c with and Bonferroni-correction of the significance level if more than two groups were compared. Discrete variables were presented as frequencies and percentages, and group comparisons were performed using the chi-square test. All reported p-values are two-sided. Patient data were entered into the CRF directly by the treating physicians. Data management and analysis was performed by Ulrich Kreuter Statistik GmbH, Schwarzenburg. Data were analysed using SAS 9.1, SAS Institute Inc., SAS Campus Drive, Cary, North Carolina 27513, USA.
|
Table 1: Demographics, blood pressure, lipids, medical history (mean ± SD) and diabetic complications (No. and %) at the baseline visit (intention to treat population, n = 315). |
|
Demographics
|
No.
|
Mean
|
Standard deviation
|
| Gender: Female |
147 (46.7%) |
|
|
| Male |
168 (53.3%) |
|
|
| Age (years) |
315 |
62.2 |
11.4 |
| Weight (kg) |
315 |
85.4 |
17.2 |
| Height (cm) |
315 |
161.1 |
16.3 |
| Body mass index (kg/m2) |
315 |
29.7 |
5.4 |
| Smoking (% of study population) |
314 |
85 (27.1) |
|
|
Blood pressure
|
|
|
|
| Systolic blood pressure (mm Hg) |
309 |
139.4 |
14.2 |
| Diastolic blood pressure (mm Hg) |
309 |
83.1 |
9.4 |
|
Lipids
|
|
|
|
| Triglycerides (µmol/l) |
215 |
2.52 |
1.36 |
| High density lipoproteins (µmol/l) |
210 |
1.18 |
0.62 |
| Low density lipoproteins (µmol/l) |
199 |
3.23 |
1.13 |
|
Medical history
|
|
|
|
| Diabetes duration (years) |
|
7.26 |
6.28 |
|
Diabetic complications (micro- and macro-vascular)
|
|
No.
|
%
|
| Retinopathy |
315 |
38 |
12.1 |
| Nephropathy |
315 |
67 |
21.3 |
| Known microalbuminuria |
315 |
106 |
33.7 |
| Neuropathy |
315 |
66 |
21.0 |
| Coronary heart disease |
315 |
82 |
26.0 |
| --- Myocardial infarction |
82 |
29 |
35.4 |
| Stroke |
315 |
5 |
1.6 |
| Peripheral arterial occlusive disease |
315 |
22 |
7.0 |
|
Table 2: Use of antidiabetic drugs prior to enrolment and reasons to change the antidiabetic treatment indicated at baseline visit. Figures represent the number and percent of patients in the intention to treat population (n = 315). |
|
(a) Previous treatments
|
|
Oral antidiabetics
|
282 |
89.5% |
Insulin
|
47 |
14.9% |
| Metformin |
259 |
82.2% |
Short-acting |
11 |
3.5% |
| Sulfonylurea |
126 |
40.0% |
Intermediate-acting |
19 |
6.3% |
| Glitazones |
100 |
31.7% |
Long-acting |
30 |
9.5% |
| Glinides |
27 |
8.6% |
|
|
|
| GLP-1 agonists |
3 |
1.0% |
|
|
|
| DPP-4 Inhibitors |
53 |
16.8% |
|
|
|
|
(b) Reasons to change treatment
|
|
Oral antidiabetics
|
282 |
89.5% |
Insulin
|
47 |
14.9% |
| Not on target |
230 |
81.6% |
Not on target |
35 |
74.5% |
| Hypoglycaemia |
5 |
1.8% |
Hypoglycaemia |
9 |
19.1% |
| Skin irritation |
9 |
3.2% |
Skin irritation |
2 |
4.3% |
| Other adverse events |
21 |
7.4% |
Other adverse events |
1 |
2.1% |
| Dissatisfaction |
30 |
10.6% |
Dissatisfaction |
1 |
2.1% |
|
|
|
Pen |
1 |
2.1% |
| DPP-4 = dipeptidyl peptidase-4; GLP-1 = glucagon-like peptide-1 |
Results
Patient disposition
Out of 327 patients included, 12 (3.7%) patients did not return to the FU visit and were considered as lost to follow-up. In 9 (2.8%) out of 315 patients (ITT) early termination due to intermittent disease, patient’s decision, noncompliance or short visit interval (<8 weeks) was recorded. Furthermore, 6 (1.9%) patients with HbA1c <7.0% at the BL visit (representing a protocol violation) were excluded from the PP population (300 patients). The mean number of patients enrolled per centre was 4.4 (median 4). With regard to the distribution across regions, all major areas and language regions of Switzerland were represented in this study: 244 (77.5%) patients were included from the German-speaking, 39 (12.4%) from the French-speaking, and 32 (10.1%) from the Italian-speaking parts of Switzerland.
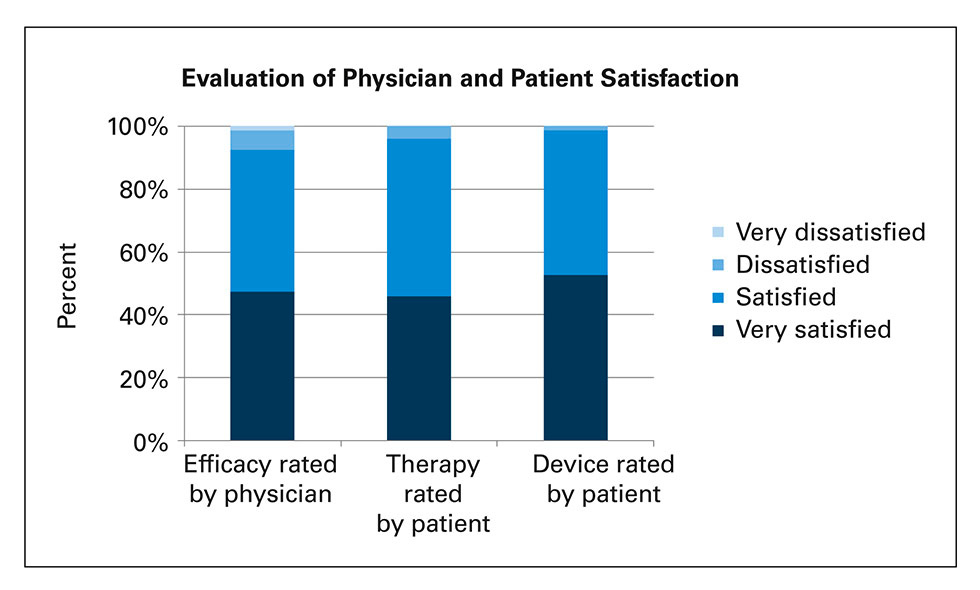
Figure 1
Assessment of efficacy (blood glucose control) by physician, evaluation of satisfaction with therapy and device by patients: Proportions of physicians and patients who were “very satisfied”, “satisfied”, “dissatisfied” or “very dissatisfied” (per protocol population, n = 300).
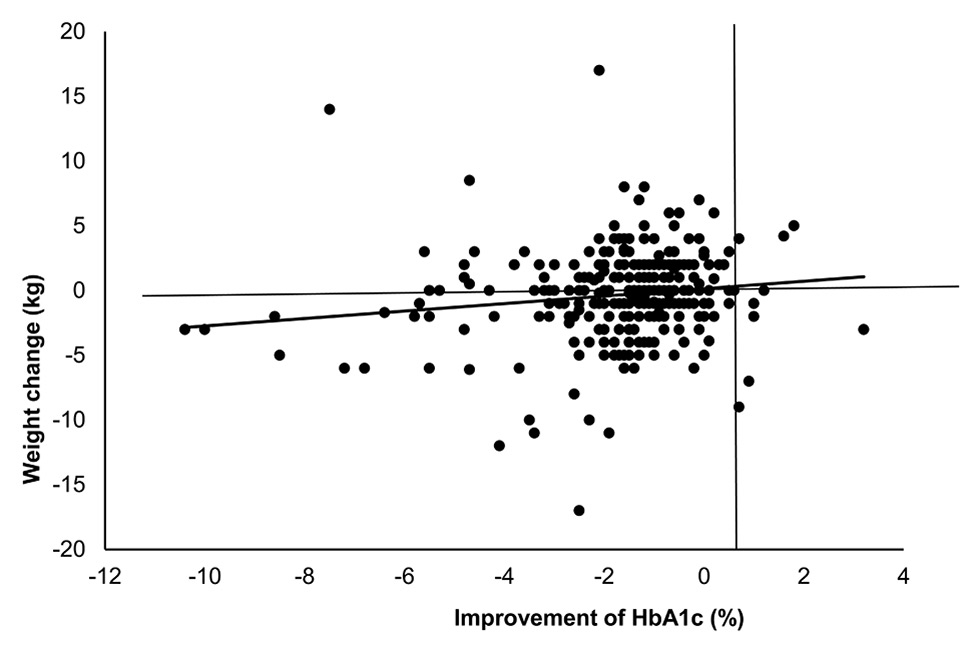
Figure 2
Weight change in relation to improvement of HbA1c. Correlation of weight change and change in HbA1c, p = 0.01.
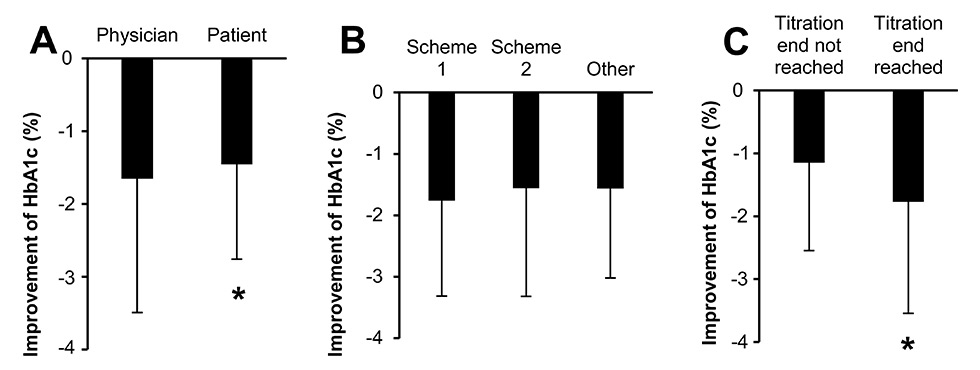
Figure 3
Improvement of HbA1c with the different titration schemes.
A) Physician = titration managed by physician (n = 221), patient = titration managed by patient (n = 91); p = 0.049, adjusted for baseline HbA1c.
B) Improvement of HbA1c in the different titration groups. 1 = change of 2 U every 3 days (n = 58), 2 = weekly adjustment (n = 230), 2 = other titration scheme (n = 27), p = not significant, adjusted for baseline HbA1c.
C) Titration end not reached (n = 87), Titration end reached (n = 228); p <0.001, adjusted for baseline HbA1c.
Demographics and disease characteristics
Baseline characteristics are presented in table 1. The mean age (±SD) was 62.2 ± 11.4 years (range 17‒92 years) and the mean body mass index (BMI) was 29.7 ± 5.4 (range 17‒56). The mean systolic and diastolic blood pressures were 139 ± 14 and 83 ± 9 mm Hg, respectively, the mean concentrations of triglycerides (TG), high density lipoprotein (HDL) and low density lipoprotein (LDL) cholesterol were 2.52 ± 1.36 µmol/l, 1.18 ± 0.62 µmol/l and 3.23 ± 1.13 µmol/l, respectively. The average time from the diagnosis of diabetes was 7.3 ± 6.3 years and the following diabetic complications were reported at baseline: microalbuminuria (33.7%), coronary heart disease (26.0%) including myocardial infarction in 9.2%), peripheral arterial occlusive disease (7.0%), stroke (1.6%), neuropathy (21.3%), nephropathy (21.0%) and retinopathy (12.1%). In total, 27.0% of patients were smokers.
Treatments
At the BL visit, 282 (89.9%) patients were treated with one or more OADs and 47 (14.9%) patients with insulin. The proportions of patients using metformin, sulfonylureas, glitazones, DPP-4–inhibitors, glinides, GLP-1–agonists and any insulin are shown in table 2a. Sulfonylureas were prescribed initially to 126 (40.0%) patients and at 6 months to 87 patients (27.6%), of whom 5 had a dose reduction, and 3 were additionally started on sulfonylureas. The reasons to change OAD or insulin treatment are summarised in table 2b.
Titration of insulin glargine and therapeutic education of patients
The initial dose of insulin glargine was either 10 U (61.9%) or 0.2 U/kg (26.7%) according to protocol, and in 11.4% of the patients the initial insulin dose was higher (mean ± SD 16.5 ± 8.6 U), mostly in patients that were previously on insulin treatment. The titration of glargine was managed by physicians (71.1%) or by patients (28.9%), either with weekly adjustment (73.0%) or a change of 2 U every 3 days (18.4%), and was completed at the FU visit after 6 months in 72.4% of the patients. These patients had a lower HbA1c level as compared with the patients not finishing titration (fig. 3C). The insulin dose was adjusted according to two established algorithms, which could be chosen by the physician: Once weekly, based on the FPG values during the two previous days (table 3) or, more simply, an increase of 2 U every three days, if the FBG was not on target. The titration scheme was not associated with a different HbA1c (fig. 3B). However, titration by the physician resulted in a slightly higher HbA1c improvement as compared with titration by the patient (fig. 3A). The mean daily dose of insulin glargine was 27.7 ± 14.3 U and the mean duration of treatment was 178 ± 56 days. ClickSTAR® and SoloSTAR® pens were used in 20% and 80% of patients, respectively. The proportion of patients treated with OADs remained stable during the study period (89.9% at BL vs 88.9% at the FU visit).
At the BL visit, education on self-management of diabetes was carried out in 303 (96.2%) patients, mainly by physicians (95.4%), diabetes experts (37.3%), diet experts (28.1%) and psychotherapists (1.0%). A similar pattern of educational support was recorded at the FU visit.
Efficacy
In the ITT population, the mean HbA1c decreased from 8.9% ± 1.6% at the BL visit to 7.3% ± 1.0% at the FU visit, resulting in a significant difference of –1.6% ± 1.7% (p <0.0001). The mean FPG decreased from 10.8 ± 4.4 mmol/l at the BL visit to 7.3 ± 1.8 mmol/l at the FU visit with a significant change of –3.6 ± 4.5 mmol/l (p <0.0001). The proportion of patients with an HbA1c level ≤7.0% increased from 4.8% to 43.8% (p <0.0001), and the proportion of patients with FPG ≤7.0 mmol/l increased from 9.6% to 50.3% (p <0.0001). The mean body weight remained stable during the study period (85.4 ± 17.2 kg at the BL vs 85.0 ± 16.5 kg at the FU visit; p = 0.11).
In the PP population, the mean HbA1c decreased from 8.9% ± 1.6% at the BL visit to 7.3% ± 1.0% at the FU visit resulting in a significant difference of –1.6% ± 1.7% (p <0.0001) (table 4). At the BL visit, the mean HbA1ccorrelated significantly (p = 0.022) with age group, being the highest (9.5% ± 1.8%) in patients ≤49 years and the lowest (8.6% ± 1.4%) in patients ≥ 70 years. The differences from the BL visit to the FU visit showed a trend (p = 0.19) to greatest prominence in the youngest compared with the oldest age, resulting in a similar mean HbA1c at the FU visit (table 4c). The mean FPG decreased from 10.9 ± 4.5 mmol/l at the BL visit to 7.3 ± 1.8 mmol/l at the FU visit with a significant change of –3.6 ± 4.6 mmol/l (p <0.0001). The proportion of patients with an HbA1c level ≤7.0% increased from 3.0% to 45.3% (p <0.0001), and the proportion of patients with FPG ≤7.0 mmol/l increased from 9.2% to 50.7% (p <0.0001). The mean body weight remained stable during the study period (85.4 ± 17.2 kg at BL vs 85.0 ± 16.5 kg at the FU visit; p = 0.11) (fig. 2).
Tolerability
At the beginning of this prospective observational study, participating physicians were informed about their obligation to report spontaneous serious safety events directly to the Swissmedic Pharmacovigilance centres using the specific Swissmedic form. Hypoglycaemia was reported in one (0.3%) patient in the comment section of the CRF. Otherwise, no adverse drug reactions have been documented.
Outcomes assessments and patient satisfaction
Overall, 92.7% of treating physicians and 96.3% of patients were satisfied or very satisfied with the insulin glargine therapy (fig. 1); 45.3% physicians and 50.3% patients were satisfied whilst 47.3% physicians and 46.0% patients were very satisfied with the insulin glargine therapy. Similarly, 99.0% of patients were satisfied or very satisfied with the use of SoloSTAR® or ClikSTAR®pens; 46.3% patients were satisfied and 52.7% were very satisfied with the SoloSTAR® or ClikSTAR®pens. The patients’ preference was in favour for the disposable SoloSTAR® pen (80%), as compared with the reusable ClickSTAR® pen (20%).
|
Table 3:Weekly insulin titration schedule based on self-measured fasting blood glucose (FBG) [15]. |
|
Average FBG during the past 2 days
|
Change in insulin dose (U/day)
|
| ≥10 mmol/l |
8 |
| 7.8–9.9 mmol/l |
6 |
| 6.7–7.7 mmol/l |
4 |
| 5.6–6.6 mmol/l |
2 |
| 4.5–5.5 mmol/l |
|
| <4.5 mmol/l |
–2 |
|
Table 4: (a) Mean (standard deviation; SD) HbA1c and fasting blood glucose (FPG) at baseline (BL) and follow-up (FU) visits. The paired differences in mean (SD) HbA1c and FPG from BL visit to FU visit after 6 months. The reported p-values were calculated using the paired t-test. (b) Number and frequency of patients allocated to defined groups of HbA1c and FPG levels at BL and FU visits. (c) Mean (SD) HbA1c by age group at BL and FU visits and mean change from BL visit to FU visit after 6 months. Figures represent the number and percent of patients of the per protocol population (n = 300). |
|
(a) Mean values
|
Visit
|
No.
|
Mean
|
SD
|
95% confidence interval
|
p-value
|
| HbA1c (%) |
BL |
300 |
8.9 |
1.6 |
8.7–9.1 |
|
| FU |
300 |
7.3 |
1.0 |
7.1–7.4 |
|
| Difference |
FU ‒ BL |
300 |
-1.6 |
1.7 |
1.8–1.5 |
<0.0001 |
| FPG (mmol/l) |
BL |
295* |
10.9 |
4.5 |
10.4–11.4 |
|
| FU |
294* |
7.3 |
1.8 |
7.0–7.5 |
|
| Difference |
FU – BL |
293* |
–3.7 |
4.6 |
4.2–3.1 |
<0.0001 |
|
(b) Distribution
|
Visit
|
No.
|
≤6.5%
|
≤7.0%
|
≤8.0%
|
>8.0%
|
| HbA1c (%) |
BL |
300 |
0 (0.0%) |
9 (3.0%) |
97 (32.3%) |
194 (64.7%) |
| FU |
300 |
63 (21.0%) |
73 (24.3%) |
122 (40.7%) |
42 (14.0%) |
|
|
≤5.5
|
≤7.0
|
≤8.0
|
>8.0
|
| FPG (mmol/l) |
BL |
295* |
4 (1.4%) |
23 (7.8%) |
38 (12.9%) |
230 (78.0%) |
| FU |
294* |
37 (12.6%) |
112 (38.1%) |
78 (26.5%) |
67 (22.8%) |
|
HbA1c by age group
|
Visit
|
Age group
|
No.
|
Mean
|
SD
|
p-value
|
| HbA1c (%) |
BL |
≤49 |
35 |
9.5 |
1.8 |
|
|
50–59 |
77 |
9.1 |
1.9 |
|
|
60–69 |
108 |
8.8 |
1.4 |
|
|
≥70 |
80 |
8.6 |
1.4 |
0.022 |
| HbA1c (%) |
FU |
≤49 |
35 |
7.5 |
0.9 |
|
|
50–59 |
77 |
7.2 |
0.9 |
|
|
60–69 |
108 |
7.2 |
0.8 |
|
|
≥70 |
80 |
7.2 |
1.1 |
0.49 |
| HbA1c (%) |
FU – BL |
≤49 |
35 |
–2.1 |
1.9 |
|
|
50–59 |
77 |
–1.8 |
2.1 |
|
|
60–69 |
108 |
–1.6 |
1.3 |
|
|
≥70 |
80 |
–1.4 |
1.6 |
0.19 |
| * Lower numbers are due to missing values. |
Discussion
In this prospective, observational study conducted in a large cohort of patients with previously uncontrolled type 2 diabetes mellitus in a primary care setting, a 6-month course of insulin glargine administered with SoloSTAR® or ClikSTAR® pens, and education on insulin injection with these devices and on self-management of diabetes was associated with a clinically relevant improvement in glycaemic control (fasting plasma glucose and HbA1c) without an increase in mean body weight of the entire cohort. The vast majority of patients and physicians were satisfied with the treatment.
Comparison of randomised studies with real world results of this observational study
Our study presents “real world” results of a patient-centred approach encompassing treatment with insulin glargine using ClickSTAR® and SoloSTAR® pens and education by physicians to enable patients to self-manage the disease. Initiation of insulin glargine treatment and dose titration was managed in 73% of patients according the scheme of Riddle et al. [15] and in 18% of patients following the scheme of the ADA/EASD [11]. Interestingly, self-management of dose titration was reported more frequently in the present study than in a previous study [22] conducted between 2005 and 2007 in Switzerland (29% vs 17%). This observation mirrors a trend in the medical community to increasingly promote therapeutic education and self-empowerment of patients. However, our results demonstrate that titration by the physician resulted in a slightly higher improvement of HbA1c, in contrast to the choice of titration scheme, which did not influence the outcome. The titration was completed after 6 months in 72% of patients with a mean insulin glargine dose of 27.7 ± 14.3 U, corresponding to a mean of 0.32 ± 0.17 U/kg. This resembles the lower range of insulin replacement in patients with initial treatment intensification. The patients who completed the titration scheme had a significantly lower HbA1c level.
In agreement with results from large clinical trials [15, 17, 20, 21], treatment with insulin glargine during a period of 24 weeks resulted in a clinically relevant decrease in HbA1c and FPG. Surprisingly, despite a marked improvement in glycaemic control, body weight remained stable during the treatment period in our study, which supports the argument that weight gain is not always a complication of insulin treatment, particularly when using not very high insulin doses.
The SSED guidelines of 2009 [12], which applied when the study started, recommended an HbA1c target level of ≤7.0%, and the current SSED guidelines [13] recommend an individual target level of 6% to 8%, whereas for most of the patients a value of ≤7.0% is still desirable nowadays. In our study, 45% of patients achieved an HbA1c level of ≤7.0%. The mean HbA1c level after 6 months treatment with insulin glargine was 7.3% ± 1.0% and was similar across all age groups. A similar response rate was observed in previous studies [20, 21], whereas response rates between 60% to 77% were reported elsewhere [15, 22].
Recent studies (ACCORD, ADVANCE und VADT) [32–34] resulted in the new joint recommendations from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) [8] with individual aims for HbA1c of higher than <7% in patients with pre-existing cardiovascular complications, long duration of diabetes and a history of hypoglycaemia. During our study these recommendations [8] were not yet available. The ideal HbA1c range now recommended is between 6%–7.5% with the lowest complications at an HbA1c of 6.5% for microvascular and 7% for macrovascular complications. Only for a short diabetes duration without cardiovascular complications and without frequent hypoglycaemia is an HbA1c of <7% still recommended.
Aggressive treatment with an HbA1ctarget of ≤6.5% was shown to have an increased risk of mortality, probably because of hypoglycaemia. In our study, the median duration of diabetes was 6.2 years (and a large proportion of patients already had cardiovascular disease at the time of enrolment, suggesting a study population at high risk for cardiovascular events). We did not analyse individual patient’s HbA1c targets, but obviously, many physicians chose a less stringent target in patients with a longer duration of the disease even before the new recommendations were published. Otherwise, the proportion of patients in whom the dose titration was deemed to be completed would have been higher than 72%.
Limitations of the study
The absence of any further safety information represents the major limitation of this study, in particular the lack on hypoglycaemic events. Since this is a prospective observational study, serious adverse events such as hypoglycaemia have to be reported directly to Swissmedic Pharmacovigilance centres and can, therefore, not be analysed. One single report of hypoglycaemia, considered to be non serious was recorded by a physician as a comment in the CRF. The fact that no more comments on serious events were reported in the CRF, could be for various reasons: (a) an indication that the primary care physicians adopted a less aggressive and therefore safe titration method, or (b) patients chose a lower insulin dose than prescribed by the physician or suggested by the algorithm scheme, as documented by the low mean insulin dose at the end of follow-up of 28 units, which corresponds to only nine titration steps by two units, whereas 26 or 60 steps would have been possible according to the two different titration algorithms suggested. There was no systematic monitoring of insulin glargine treatment during the 6-month observation other than routine clinical visits at baseline and at the end of the study period. The fact that no reference laboratory was involved and the participating physicians used their own arrangements for measuring blood sugar parameters represents a limitation of the study because of potential differences in measurement methods and bias, but it will most likely not affect the calculated differences. Since the HbA1c at baseline was 8.9% in the present study, we considered it unethical not to give insulin in this group of patients with a long diabetes duration. Therefore, the study lacks a direct comparison group. Although we have included more than 300 patients treated by 72 different physicans from all three major language regions of Switzerland, further studies in randomised populations would be needed before the results can be applied to the wider type 2 diabetes population in Switzerland.
Treatment satisfaction
As in a previous study [22], the vast majority of treating physicians and patients were satisfied or very satisfied with the insulin glargine therapy. It is interesting that 80% of patients chose a disposable pen for initial insulin treatment. In addition, 99% of patients were satisfied or very satisfied with use of the SoloSTAR® or ClikSTAR®pens in the present study. The preference for disposable pens and the satisfaction underlines the simplicity of education in the handling of these devices and their acceptance by the patients.
Whereas randomised comparative trials reflect a methodical approach with defined patient populations, systematic data collection and head-to-head comparisons of treatments, “real life” outcomes generated in observational studies provide insights into day-to-day medical practice in unselected patient populations.
In conclusion,treatment with insulin glargine administered with SoloSTAR® or ClikSTAR® pens and education on insulin injection with these devices and on self-management of diabetes was associated with clinically meaningful improvements in HbA1c and fasting plasma glucose without a mean collective weight gain during a 6-month study period in patients with type 2 diabetes mellitus, who has previously failed to reach local targets under OAD treatment. In primary care practice, this treatment offers good glycaemic control at a stable body weight, thus contributing to a high satisfaction of both patients and treating physicians.
Acknowledgement:We thank Ulrich Kreuter Statistik GmbH, Schwarzenburg for the statistical analysis.
Authors’ contribution: DR and DS contributed equally.
References
1 Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus, present and future perspectives. Nat Rev Endocrinol. 2011;8(4):228–36.
2 Kaiser A, Vollenweider P, Waeber G, Marques-Vidal P. Prevalence, awarness and treatment of type 2 diabetes mellitus in Switzerland: the CoLaus study. Diabet Med. 202;29:190–7.
3 Stratton IM, Adler AI, Neil HAW, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macro- vascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–12.
4 Saydah S, Tao M, Imperatore G, Gregg E. GHb level and subsequent mortality among adults in the U.S. Diabetes Care. 2009;32:1440–6.
5 Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, et al. Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–22.
6 Gerstein HC, Islam S, Anand S, Almahmeed W, Damasceno A, Dans A, et al. Dysglycaemia and the risk of acute myocardial infarction in multiple ethnic groups: an analysis of 15,780 patients from the INTERHEART study. Diabetologia. 2010;53:2509–17.
7 Blonde L. State of diabetes health. 2006 Available from http://www.stateofdiabetes.com/state_compare.htm .
8 Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al.; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35(6):1364–79 / Diabetologia. 2012;55(6):1577–96.
9 Aschner P, Chan J, Owens DR, Picard S, Wang E, Dain M-P, et al., on behalf of the EASIE investigators. Insulin glargine versus sitagliptin in insulin-naive patients with type 2 diabetes mellitus uncontrolled on metformin (EASIE): a multicentre, randomised open-label trial. Lancet. 2012;379:2262–6229.
10 Riddle C (on behalf of the ORIGIN trial investigators). Characteristics Associated With Maintenance of Mean A1C <6.5% in People with Dysglycemia in the ORIGIN Trial. Diabetes Care. 2013;36(10):2915–22.
11 Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, et al. Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. American Diabetes Association, European Association for the Study of Diabetes. Diabetologia. 2009;52(1):17–30.
12 Philippe J, Brändle M, Carrel J, Diem P, Keller U, Kuntschen F, et al. Massnahmen zur Blutzuckerkontrolle bei Patienten mit Typ-2–Diabetes-Mellitus: Consensus Statement der Schweizerischen Gesellschaft für Endokrinologie und Diabetes (SGED): Schweiz Med Forum. 2009;9(3):50–5. German.
13 Lehmann R, Henzen Ch, Christ E. Neue Richtlinien der SGED/SSED zur Therapie des Typ 2 Diabetes mellitus. info@herz+gefäss 2013;4:20–4. German.
14 Wang F, Vergara CM. Insulin glargine: a systematic review of a long-acting insulin analogue. Clin Ther. 2003;25:1541–77.
15 Riddle MC, Rosenstock J, Gerich J. The Treat-to-Target Trial: Randomised addition of Glargine or human NHP insulin to oral therapy of type 2 diabetic patients Diabetes Care. 2003;26(11):3080–6.
16 Yki-Jarvinen H, Yki-Järvinen H, Juurinen Ll, Alvarsson M, Bystedt T, Davies M, et al. Initiate Insulin by Aggressive Titration and Education (Initiate a Randomized Study to Compare Initiation of Insulin Combination Therapy in Type 2 Diabetic Patients Individually And in Groups. Diabetes Care. 2007;30:1364–70.
17 Bretzel RG, Nuber U, Landgraf W, Owens DR, Bradley C, Linn Th. Once-daily basal insulin glargine versus thrice-daily prandial insulin lispro in people with type 2 diabetes on oral hypoglycaemic agents (APOLLO): an open randomised controlled trial. Lancet. 2008;371:1073–84.
18 Fritsche A. Langwirkende Analoginsuline: Ergebnisse mit Insulin glargine: Dtsch Med Wochenschr. 2008;133:S101–S105. German.
19 Davies M, Lavalle-Gonzalez F, Storms F, Gomis R. (on behalf of the AT.LANTUS Study Group). Initiation of insulin glargine therapy in type 2 diabetes subjects suboptimally controlled on oral anti-diabetic agents: results from the AT.LANTUS trial. Diabetes, Obesity and Metabolism. 2008;10:387–99.
20 Rosenstock M, Davies P, Home D, Larsen J, Koenen C, Schernthaner G. A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naive people with type 2 Diabetes. Diabetologia. 2008;51:408–16.
21 Fonseca V, J. Gill J, Zhou R, Leahy J. An analysis of early insulin glargine added to metformin with or without sulfonylurea: impact on glycaemic control and hypoglycaemia. Diabetes Obes Metab. 2011;13:814–22.
22 Spirk D, Lareida J, Scheidegger K, Diem P. Optimierung der glykämischen Kontrolle und Dosistitration mit Insulin Glargine (Lantus®) durch frei praktizierende Ärzte in der Schweiz: Resultate des OPTI-LAN Praxiserfahrungsberichtes. Praxis. 2009;98:315–20. German.
23 Gerber P, Spirk D, Braendle M, Thoenes M, Lehmann R, Keller U. Regional differences of glycaemic control in patients with type 2 diabetes mellitus in Switzerland. Swiss Med Wkly. 2011, doi:10.4414/smw.2011.13218
24 Home PD, Fritsche A, Schinzel S, Massi-Benedetti M. Meta-analysis of individual patient data to assess the risk of hypoglycaemia in people with type 2 diabetes using NPH insulin or insulin glargine. Diabetes Obes Metab. 2010;12:772–9.
25 Polonsky W, Traylor L, Wei W, Shi R, Ameer B, Vlajnic A, et al. More satisfied, but why? A pooled patient-level analysis of treatment satisfaction following the initiation of insulin glargine versus comparators in insulin-naïve patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2013; doi: 10.1111/dom.12214.
26 Hancu N, Czupryniak L, Genestin E, Sourij H. A Pan-European and Canadian prospective survey to evaluate patient satisfaction with the SoloSTAR insulin injection device in type 1 and type 2 diabetes. J Diabetes Sci Technol. 2011;5:1224–34. German.
27 Böhler S, Landgraf W, Schreiber SA. Bewertung des neuen Insulin- Fertigpens und Injektionsgewohnheiten von Diabetikern in der täglichen Praxis. MMW-Fortschritte der Medizin Originalen 2009;IV:179–87. German.
28 Lagger G, Zoltan P, Golay A. Efficacy of therapeutic patient education in chronic disease and obesity. Patient Educ Couns. 2010;79:283–6.
29 Golay A, Lagger G, Chambouleyron, Carrard I, Lasserre-Moutet A. Therapeutic education of diabetic patients. Diabetes Metab Res Rev. 2008;24:192–6.
30 http://www.swissmedicinfo.ch . Fachinformation für Lantus®ClikSTAR und Lantus® SoloSTAR®. Sanofi-Aventis (Schweiz) AG. German.
31 American Diabetes Association. Standards of medical care in diabetes – 2012. Diabetes Care. 2012;35(Suppl 1):S11–63.
32 Effects of Intensive Glucose Lowering in Type 2 Diabetes The Action to Control Cardiovascular Risk in Diabetes Study Group. N Engl J Med. 2008;358:2545–59.
33 Intensive Blood Glucose Control and Vascular Outcomes in Patients with Type 2 Diabetes. The ADVANCE Collaborative Group. N Engl J Med. 2008;358:2560–72.
34 Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al.; VADT Investigators. N Engl J Med. 2009;360(2):129–39.


