Troubleshooting in extracorporeal life support
DOI: https://doi.org/10.4414/smw.2015.14117
Summary
Since the first clinical use of extracorporeal circulation in the last century by John Gibbon and the first successful mechanical support of the left ventricular function by Forest Dodrill, the progress of techniques and technologies has helped to develop minimised systems for extracorporeal circulatory and respiratory support. However, the fact is that, despite the advanced technologies used for extracorporeal support, successful application in order to be benefit a critically ill population requires highly trained and skilled teams. Application of these highly sophisticated techniques in life-saving situations inside and/or outside the operating room is a procedure with certain pitfalls and dangers.
The aim of this review is to provide a short overview of the technical aspects of extracorporeal circulation, with a look at the recent literature and clinical experiences focusing on technical as well surgical considerations regarding the urgent and/or emergent usage of a central as well as peripheral extracorporeal system.
Introduction
Since the first clinical use of extracorporeal circulation in the last century [1] by John Gibbon and the first successful mechanical support of the left ventricular function by Forest Dodrill [2], the progress of techniques and technologies has helped to develop minimised systems for extracorporeal circulatory and respiratory support.
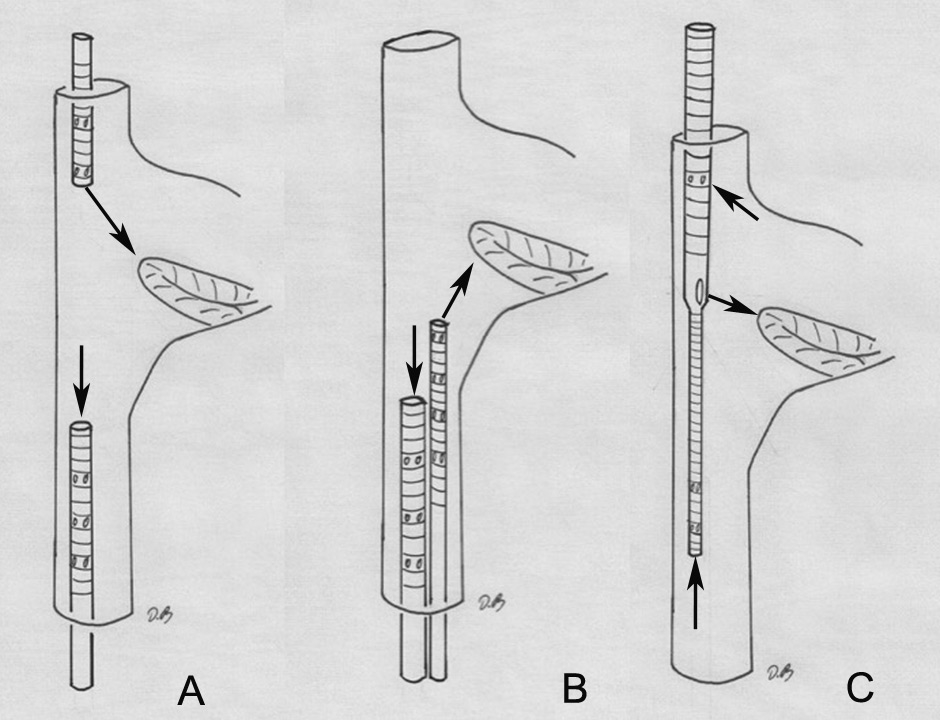
Figure 1
Configuration of the venovenous extracorpareal life support cannulation. (A) Femoroatrial cannulation, where the cannula positioned in the femoral vein serves as a drainage device and is placed with its tip 5 to 10 cm inferior to the angle between the inferior vena cava and right atrium. The oxygenated blood is returned via a cannula positioned in the internal jugular vein. (B) Femorofemoral cannulation. The drainage cannula is positioned with its tip about 5 to 10 cm below the junction between the inferior vena cava and right atrium. The oxygenated blood is delivered by the other cannula that has its tip positioned in the right atrium. (C) Single venovenous cannulation, here the drainage segments are positioned in the superior and inferior vena cava. The oxygenated blood is delivered in the right atrium just in front of the tricuspid valve.
Extracorporeal life support (ECLS) is a therapeutic option used in patients with cardiac, respiratory or cardiorespiratory failure that is refractory to conservative therapy modalities [3, 4]. This therapy option is meant to be a bridge to organ or organ system recovery, to definitive decisions in life-threatening situations, to long-term support device implantation, and to heart, lung-heart and lung transplantation.
Nowadays, two ECLS modalities are well established, venovenous (VV) and venoarterial (VA) ECLS. ECLS is basically a modification of cardiopulmonary bypass where the most essential part of the circuit is an oxygenator with or without a heat exchanger. The venous blood is removed from the central venous system either by cannulation of a peripheral great vein (femoral vein, external jugular and subclavian vein) or by inserting the venous cannula into the right atrium. After the venous blood is oxygenated and carbon dioxide is removed the blood is returned to the body via a peripheral path through cannulation of the femoral artery, or by a central path where the arterial cannula may be inserted directly into the ascending aorta or into the subclavian artery.
It should be noted that the optional modalities of VA ECLS are extracorporeal membrane oxygenator (ECMO) and left ventricular assist devices (VADs), where the VAD stands for haemodynamic support by a centrifugal pump, without inclusion of an oxygenator in the circuit. Although the recent re-interest in ECLS techniques, especially by successful application of VV ECLS in the treatment of an H1N1 influenza pandemic [5], there is a net lack of evidence regarding its routine application. There are a number of factors that limit more frequent usage of ECLS circuits, including correct patient selection, appropriate strategies, appropriate technical aspects of ECLS maintenance and initialisation. All of these factors put a limit on conducting well-controlled clinical trials. The aim of this review is to analyse the pitfalls of recently available ECLS modalities, focusing on technical aspects and the most frequent complications.
Extracorporeal support for respiratory failure
VV ECLS is used for severe acute respiratory distress syndrome (ARDS) refractory to conservative therapy modalities. The advantage of the VV ECLS in ARDS over VA ECLS is that it may be employed via peripheral cannulation, and does not result in upper body hypoxaemia. Upper body hypoxaemia is a well-known phenomenon that may occur in patients with respiratory failure and preserved heart function who are supported with peripheral VA ECLS; this phenomenon is described below.
VV ECLS does not provide any haemodynamic support; however, with successful oxygenation and/or carbon dioxide extraction, the ventilation setting may be reduced down to rest levels, i.e., a peak inspiratory pressure of 20 cm H2O, end expiratory pressure 10 cm H2O, rate 10 breaths per minute and FiO2 30%. This may augment the heart function in two ways. First, with better blood oxygenation, a sufficient amount of oxygen is delivered to the heart, and second, the reduced intrathoracic pressure contributes to better heart function. In the CESAR study [6] in 2009 the treatment of H1N1influenza with VV ECMO contributed to excellent results, with reported survival of 63% versus 47% for those not treated with VV ECMO. Notably, 28 cases out of 57 allocated to the VV ECMO were at the time of device induction in multiorgan failure and sepsis, having low heart output. Less than 10% of the cases needed VA ECMO support because of haemodynamic instability [6].
To date, two modalities of VV ECLS for lung support have been established in daily clinical practice: (1.) double VV ECLS cannulation and (2.) single VV ECLS cannulation techniques. Both support modalities are introduced and analysed below.
Double venovenous cannulation for pulmonary ECLS
Two venous cannulas are used, such that deoxygenated blood is drained from a large central vein, in the majority of cases the inferior vena cava (IVC), and the oxygenated blood is delivered to the right atrium (RA). According to the cannula positioning two further modalities can be differentiated: femoroatrial and femorofemoral cannulation.
Before going into the details of support for the two VV ECSL modalities, it has to be mentioned that independently from support modality, the flow in the circuit depends on the inserted drainage cannula size. This is limited by target vessel size, and for adults the size of commercially available venous femoral cannulas (Bio-Medicus Multi Stage Femoral, Medtronic Inc. USA) ranges between 27F to 29F, with which a maximal circuit flow of around 5 l/min may be achieved. The standard Bio-Medicus wire-reinforced multiorifice cannula is usually positioned with its tip in the right atrium or just underneath it, and consequently all the blood drained inferior to this level has to travel from the inferior body along the IVC to its junction with the RA. Only here, where the multiporus part of the cannula is positioned, may the blood enter the device [7]. Unfortunately, in this way only 70% to 80% of the theoretical target pump-flow can be achieved [8]. In contrast, a self-expanding device (SmartCannula, Lausanne, Switzerland), that has a multiporous grid-like structure and adapts its diameter to the natural vascular diameter in a self-expanding way, drains the IVC side branches in a direct way. As such, the flow achieved may be 40% higher than with a wire-reinforced device [9, 10].
In current routine clinical practice, however, self-expanding cannulas have not gained the place that might be expected, and at a majority of centres wire-reinforced multiporous devices are used. There usage is thus described below.
Femorofemoral cannulation for ECLS:Here two cannulas are inserted into the contralateral femoral veins. The drainage cannula is positioned with its tip about 5 to 10 cm below the junction between the IVC and RA. The oxygenated blood is delivered by the other cannula, which has its tip positioned in the right atrium (figs 1 and 2).
Femoroatrial cannulation for ECLS:Here also, two cannulas are inserted into the central venous target vessel, one in the femoral vein and a second in the internal jugular vein. The cannula positioned in the femoral vein serves as a drainage device and is placed with its tip 5 to 10 cm inferior at the angle between the IVC and RA. The oxygenated blood is returned via a cannula positioned in the internal jugular vein (IJ), and the tip of this device is placed in the right atrium (figs 1 and 2).
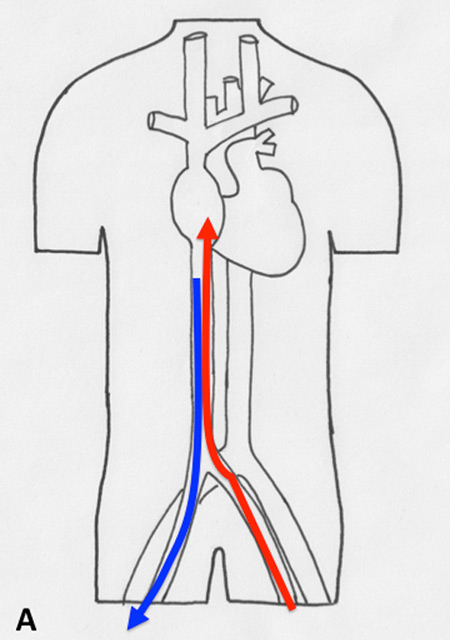
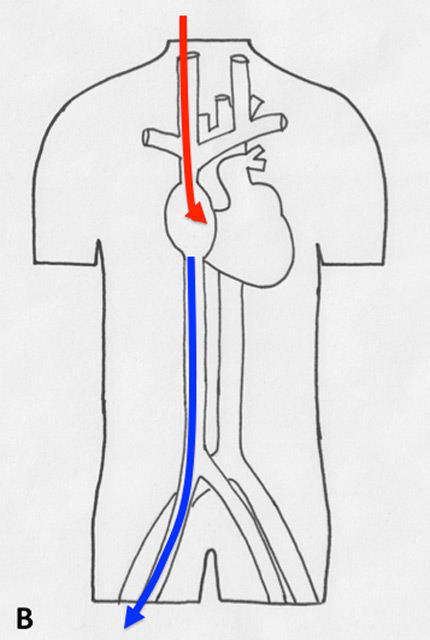
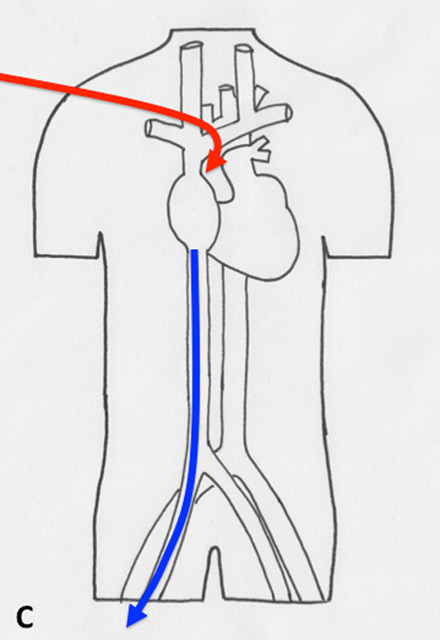
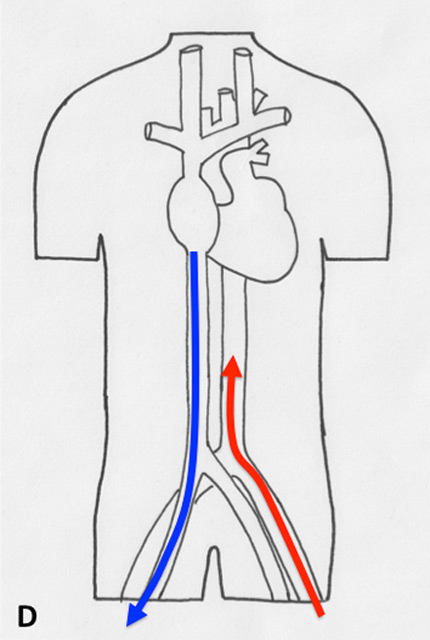
Figure 2
Different types of cannulation for venovenous and venoarterial cannulation. (A) Femorofemoral venovenous extracorporeal life support (VV ECLS) cannulation modality, with drainage cannula just underneath of the junction between the inferior vena cava and right atrium. The inflow cannula is positioned in the right atrium. (B) Venoatrial VV ECLS cannulation modality, where the drainage cannula is positioned just underneath the right atrium. The inflow cannula is placed via the internal jugular vein with its tip just in front of the tricuspid valve. (C) Standard central cannulation for ECLS, here the drainage device is depicted positioned in the femoral vein. Of note is that from the aspect of support this does not show any difference compared with the device being in right atrium. (D) Femorofemoral AV ECLS cannulation modality, with drainage device in the inferior vena cava and the inflow cannula in contralateral femoral artery.
In both cannulation modalities it is more than obvious that the correct tip position has to be verified. This is in order to avoid central vessel and/or heart structure damage and to minimise the recirculation phenomenon. In VV ECLS, ideally all the blood from the inflow cannula should pass through the tricuspid valve into the pulmonary circulation. From here on the oxygenated blood is distributed into the systemic blood stream through ejection by the left ventricle. However, the blood from the inflow cannula positioned in the right atrium does not entirely enter the pulmonary circulation. Part of it enters the drainage cannula that is placed just inferior to the angle between the IVC and RA, and thus does not contribute to systemic oxygenation. This is called the “recirculation” phenomenon. Because of the recirculation phenomenon, in a case when the lung does not contribute to oxygenation it is not always possible to achieve the normal arterial oxygen saturation (SaO2). The commonly accepted target for SaO2 is 85% to 92%. Theoretically, if the recirculation is held at minimal amount, then with 70% of normal cardiac output this target of SaO2 may be achieved. It is easy to understand that patient conditions such as intravascular volume load, patient position, pump flow and the position of the cannulas are the most important factors that influence the grade of recirculation. The recirculation phenomenon may be assessed at the patient’s bedside by comparing the oxygen saturation at the venous side of the oxygenator with SaO2. A high preoxygenantor oxygen saturation SaO2 (>75%) and low SaO2 (<85%) is indicating a significant amount of oxygenated blood escaping the pulmonary circulation. In contrast, if the preoxygenator oxygen saturation is low (<60%) and the SaO2 is also low (<85%), this would mean that either the patient’s cardiac output is high or the pump flow on ECLS is too low. It is important that if recirculation is suspected, the positions of the cannulas be verified. A simple chest anterioposterior image provides basic information about cannula placement; however, thransoesophagial echo (TEE) is a more accurate method. Clearly the reperfusion phenomenon may be reduced by correct positioning of the cannulas, and this may be done under TEE or fluoroscopic control. It seems that TEE echocardiography is not entirely accurate, as only the tip of the cannula may be visualised, and not the whole run of the device inside the vascular bed [12]. The details of implantation will be addressed below.
The major disadvantage, besides the recirculation phenomena, of the double cannula venous system is post-interventional patient management. Since lung function recovery, for example in a bridge to transplant, usually takes 1 to 3 weeks or even more, a bicaval VV ECLS requires bed rest and patients in most circumstances remain sedated and mechanically ventilated, with all its associated inconveniences and complications [12].
Single venovenous cannula for pulmonary ECLS
The aforementioned drawbacks of double venous cannula ECSL were addressed by introduction of the single catheter venovenous ECLS (Avalon© Cannula, Avalon Laboratories, Grand Rapids, Michigan, USA). Although the double venovenous ECLS strategy was adopted and is routinely used as a preferred modality for severe pulmonary refractory failure, the Avalon© cannula should be considered as a first-line strategy for acute respiratory failure and as a bridge to transplantation [14, 15]. Patients may be extubated, mobilised and transferred to normal nursing wards, which facilitates recovery, lung toilet and outcome following lung transplantation [14].
The single venovenous cannula modality of pulmonary ECLS is comprised of one single catheter inserted via the right internal jugular vein (IJV); venous drainage and delivery of oxygenated blood is performed with one single double-lumen device (fig. 1). The Avalon© cannula is a wire-reinforced device with two separate compartments, one for venous drainage and the other for delivery of the oxygenated blood. The delivery segment of the cannula is positioned between the two drainage parts at the inferior third of the device (fig. 1). According to the device structure, the two drainage segments should be positioned in the superior as well in the inferior vena cava, where the deoxygenated blood is drained from the inferior as well from the upper body and is delivered to the oxygenator. The middle segment that is responsible for delivery of the oxygenated blood has to be placed in the right atrium just in front of the tricuspid valve [12]. Positioning of the inflow part of the Avalon cannula in front of the tricuspid valve may be done only by using TEE. Since TEE is obviously an essential fragment in successful implantation, in many centres it was adopted as the sole medium for Avalon© cannula implantation. Unfortunately, TEE as implantation guide may not clearly exclude the looping effect during the positioning of the device [12, 16] and as such does not guarantee a 100% safe implantation.
The commercially available insertion of the Avalon© cannula kit, has a flexible “J tip”, 20 cm, 0.038 inch radiopaque guidewire that is soft and flexible. The soft nature of the wire provides minimal risk of perforation of the heart and/or of the surrounding venous structure, but thus has an affinity to flip out of the correct position and can loop up into the right ventricle or to the right atrium [12, 18, 19]. An undetected looping of the wire, which is the case when only TEE is used as implantation guidance, can direct the dilatator and/or the cannula toward the anterior wall of the right ventricle or toward the lateral wall of the right atrium, with fatal consequences [12, 18, 19]. The incidence of right heart cavity perforation varies between 5% and 15% [15, 18].
To avoid this complication, two strategies may be adopted. If the patient can be transferred into the operating theatre, fluoroscopy can be used as control for cannula positioning in the axis superior vena cava, right atrium and inferior vena cava. In this way, the looping effect can be detected early and safe cannula implantation is assured [12, 15].
In cases when patient transport is not possible, at the bedside, owing to cardiorespiratory instability, salvage VV ECLS is required. In these situations TEE is the only possible guide tool. It is advised that after the flexible wire is implanted a Berenstein directional catheter be placed over the wire. In the next step, the Avalon© wire is removed and a 180 cm, 0.0035” Amplatz Super Stiff guide-wire (Boston Scientific, Natic, MA) is passed in the IVC and subsequently the Avalon© cannula is implanted [12, 17].
Extracorporeal support for cardiac failure
Several options exist for circulatory support in cardiac failure. In an acutely decompensated patient who is under mechanical and/or pharmacological resuscitation as temporary support, ECLS can be a bridge to decision making that may best serve the patient and is the most appropriate therapy strategy. To date there is no evidence comparing different short-term mechanical support devices such as temporary ventricular assist devices, and intra-aortic counterpulsation to AV ECLS for refractory cardiac failure. However, it seems that AV ECLS is the therapy of choice in this setting [19, 20].
The indications for AV ECLS are as follows: unsuccessful weaning from cardiopulmonary bypass, acute myocardial infarction, ischaemic cardiomyopathy, acute myocarditis, acute cardiorespiratory failure (i.e., central pulmonary embolism) and post-partum cardiomyopathy, including acute organ failure following heart transplantation. Successful support as a bridge to heart transplantation has been described as well as support until re-transplantation in the case of refractory allograft failure [21–23]. The reported survival varies between 20% to 60% at experienced centres and is largely dependent on indication, patient selection and mode of employment of this support modality [23]. Basically there are two modalities of the VA ECLS configuration, a central and peripheral cannulation mode.
Central cannulation for cardiac ECLS
In most instances, central ECLS is performed in theatre in the open chest, when weaning off the cardiopulmonary bypass has failed (fig. 2). Rarely, primary sternotomy is performed to establish VA support. In the open chest, the cannulas are inserted into the ascending aorta for delivery of the oxygen and venous drainage is performed by cannulas positioned in the right atrium. This modality of ECLS allows excellent flow conditions, since the venous cannula inserted is not size-restricted (as in the case of a femoral cannulation) and sufficient pump flow for a large body surface area (>2.0 m2) may be achieved. A second advantage of the central perfusion modality is that the central position of the inflow cannula provides an antegrade inlet of oxygenated blood to the aortic arch and its vessels, coronary arteries and other parts of the body. One of the major drawbacks of the central cannulation is that the cannula insertions require a sternotomy and subsequently an open chest. This open chest situation is prone to the very important considerations of bleeding, repeated open wound dressings and to infections. However, devices do exist, such as the Abiomed central cannula (Abiomed, Cardiovascular Inc, Danvers, MA) that may be tunnelled through the abdominal wall, allowing closure of the chest.
Peripheral cannulation for cardiac ECLS
The most common installation of peripheral ECLS is insertion of cannulas in femoral vessels (fig. 2), with a venous cannula inserted into the femoral vein and the arterial cannula into the ispsilateral or contralateral femoral artery. Peripheral ECLS may be considered as rescue option for those cases where the myocardial depression is profound or if the conditions for the cardiac or cardio/respiratory failure are unclear and conditions such as myocarditis are likely. In such a cases, with multiorgan failure and severe coagulopathy, the institution of ECLS may be considered as bridge to decision or even as a successful therapy modality [24]. This is a result of the quick, relatively simple bedside cannulation procedure and installation of full cardiorespiratory support. The major drawback of femoral cannulation is the femoral venous access. This limits the size of the device used, which, as mentioned before, is the major drawback for the flow support achieved. To avoid this, a second venous drainage cannula may be installed in a large vein of the upper body, for example through cannulation of the subclavian vein [24]. The bleeding from the cannulation site may be as high as 10% [23] requiring surgical revision, and the distal limb ischaemia ranges between 13% and 15% [13]. In contrast to central ECLS, peripheral support from femoral vessels leads to the blood mixing in the arch and ascending aorta. In the case of respiratory failure the poorly oxygenated blood will be pumped by the heart to the coronary arteries and the vessels of the arch, while the ECMO circuit is supplying the well-oxygenated blood to the distal segment of the arch. This may result in hypo-oxygenation of the upper body segment as well of the heart. For this reason monitoring the oxygen saturation at the radial artery is necessary, and additionally the ventilation support may not be reduced to rest levels, in order to try to keep the oxygen saturation of blood ejected from the left ventricle above 90%.
|
Table 1: Complications associated with extracorporeal life support in adults. |
|
Complication
|
Incidence (%)
|
| Limb ischaemia and amputation of cannulised limb |
10 to 25 |
| Haemorrhage associated with the anticoagulation regime |
5 to 70 |
| Infections |
15 to 50 |
| Neurological events |
13 to 33 |
| Mechanical complication associated with the circuit |
3 to 22 |
| Inflammation and coagulopathy associated with the circuit |
5 to 8 |
| Damage to the heart and surrounding vessels |
5 to 15 |
| Renal failure |
30 to 50 |
Cannulation technique and pitfalls
The complications related to ECLS in adults are summarised in table 1. Although in many cases the establishment of ECLS circulation is performed in extreme situations, aseptic technique during cannula implantation has to be respected. This includes full sterile preparation, draping, etc., independently of whether the ECLS is performed in theatre, or at the patient’s bedside in the intensive care unit or in the emergency room. Introduction by the Seldinger technique is well-established, and the identification and puncture of the femoral vessels should be done by use of femoral utrasonography. However, in a salvage situation this is not always available. In this case, the vessels may be punctured directly; if after a 3-minute attempt this it is not successful [25], then surgical exposure of the femoral vessels should be performed. Afterwards, the anterior vessel wall is identified and double purse-string sutures (with proline 5-0) are placed to secure the cannula position and to avoid later insertion-site bleeding. In establishment of VA ECLS, in the first attempt the femoral artery should be punctured. After the vessels are successfully punctured and a guide viewer is placed in the femoral artery, heparin (5,000 IU) is given intravenously, aiming for an activated coagulation time (ACT) of above 180 seconds. It should be noted that the guidewire should run smoothly without resistance, and if there is any resistance, we suggest pulling back the wire for a few centimetres and advancing it with a gentle pushing movement into the vessels. The position of the wire may be achieved by rotation of the wire between two fingers. In the next step, the dilatators are advanced into the femoral artery, and at this point we have to be aware that advancing the dilatator may bend the guidewire. If this occurs, subsequent steps for the cannula implantation are almost always impossible and the guidewire has to be changed. To avoid this we propose simultaneous inward movement of the dilatator/cannula while the wire is pulled smoothly; in doing so we get direct feedback regarding the gliding of the wire. Finally, after the arterial cannula is in situ the guidewire may be removed. This procedure is repeated for the introduction of the venous cannula. Here the positioning of the guidewire in the inferior vena cava as well the position of the venous cannula has to be confirmed and checked during the procedure.
The positioning of the arterial cannula in the femoral artery bears the risk of leg ischaemia, which ranges between 10% to 15% [23]. Dual flow arterial cannulas for VA ECLS with sufficient distal flow for the leg have been developed, but unfortunately have never been commercially available [26]. Commonly, there are two strategies that are adopted to avoid leg ischaemia. One is the percutaneous puncture of the distal femoral artery by 10F or 12F cannulas. Normally, the femoral artery is punctured at the mid-thigh under ultrasound guidance, before the venous device is inserted. However, sometimes the percutaneous puncture of the distal femoral artery is not easy. Some institutes use a semi-Seldinger technique distal and proximal to arterial cannula implantation. Here the cannulas are implanted after the surgical exposure of the femoral arteries is completed.
Conclusion
ECLS is a potentially lifesaving procedure in pulmonary, cardiac and cardiopulmonary failure. The usage and the indication for this therapy modality have widened in the past several years, especially after data were provided for VV ECLS in treatment of influenza H1N1 pulmonary failure, which showed benefit over conservative treatment. Although the results for ECLS in cases with pulmonary dysfunction are good, the outcomes in cardiac failure are still modest. At the same time we should remember that introduction of ECLS is invasive, and allied to many complications and pitfalls. From this point of view, it is very important to provide a team approach with good communication together with excellent teamwork among intensivists, cardiac anaesthesiologists, surgeons, perfusionists and other personnel involved.
References
1 Cohn L. Fifty years of open heart surgery 2003 6;107(17):2168–70 Circulation.
2 Norton J. Surgery: Basic science and clinical evidence 2008 Springer New York. p. 1473.
3 MacLaren G, Combes A, Bartlett RH. Contemporary extracorporeal membrane oxygenation for adult respiratory failure: life support in the new era. Intensive Care Med. 2012;38:210–20.
4 Bartlett RH, Gattinoni L. Current status of extracorporeal life support (ECMO) for cardiopulmonary failure. Minerva Anestesiol. 2010;76:534–40.
5 Davies A, Jones D, Bailey M, Beca J, Bellomo R, Blackwell N, et al., Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators: Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888–95.
6 Peek GJ, Mugford M, Tiruvoipati R. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–63.
7 von Segesser LK. Peripheral cannulation for cardiopulmonary bypass. Multimedia Man Cardiothorac Surg. 2006.
8 Jegger D, Tevaearai HT, Mueller XM, Horisberger J, von Segesser LK. Limitations using the vacuum-assist drainage technique during cardiopulmonary bypass procedures. J Extra Corpor Technol. 2003;35:207–21.
9 Berdajs D, Born F, Crosset M, Horisberger J, Künzli A, Ferrari E, et al. Superior venous drainage in the “LifeBox”: a portable extracorporeal oxygenator with a self-expanding venous cannula. Perfusion. 2010;25(4):211–5.
10 Berdajs DA, Bernandi E, Burki M, Hurni M, Tozzi P, Horisberger J, et al. Self-expanding mini-cannula for remote perfusion with pediatric scenarios. Interact Cardiovasc Thorac Surg. 2010;10(6):873–6.
11 von Segesser LK, Siniscalchi G, Kang K, Maunz O, Horisberger J, Ferrari E, et al. Temporary caval stenting improves venous drainage during cardiopulmonary bypass. Interact Cardiovasc Thorac Surg. 2008;7(6):1096–100.
12 Berdajs D Bicaval dual-lumen cannula for venovenous extracorporeal membrane oxygenation: Avalon© cannula in childhood disease. Perfusion. 2014 Jul 28.
13 Sidebotham D, McGeorge A, McGuinness S, Edwards M, Willcox T, Beca J. Extracorporeal membrane oxygenation for treating severe cardiac and respiratory failure in adults: part 2–technical considerations.J Cardiothorac Vasc Anesth. 2010;24(1):164–72.
14 Nosotti M, Rosso L, Tosi D, Palleschi A, Mendogni P, Nataloni I, et al. Extracorporeal membrane oxygenation with spontaneous breathing as a bridge to lung transplantation. Interact CardioVasc Thorac Surg. 2013;61:55–9.
15 Garcia JP, Iacono A, Kon ZN, Griffith BP. Ambulatory extracorporeal membrane oxygenation: a new approach for bridge-to-lung transplantation. J Thorac Cardiovasc Surg. 2010;139:e137–9.
16 Hirose H, Yamane K, Marhefka G, Cavarocchi N. Right ventricular rupture and tamponade caused by malposition of the Avalon cannula for venovenous extracorporeal membrane oxygenation. J Cardiothorac Surg. 2012;7:36.
17 Teman NR, Haft JW, Napolitano LM. Optimal endovascular methods for placement of bicaval dual-lumen cannulae for venovenous extracorporeal membrane oxygenation. ASAIO J. 2013;59: 442–7.
18 Chimot L, Marqué S, Gros A, et al. Avalon© bicaval dual-lumen cannula for venovenous extracorporeal membrane oxygenation: survey of cannula use in France. ASAIO J. 2013;59:157–61.
19 Pagani FD, Lynch W, Swaniker F, Dyke DB, Bartlett R, Koelling T, et al. Extracorporeal life support to left ventricular assist device bridge to heart transplant: A strategy to optimize survival and resource utilization. Circulation. 1999;100:206–10.
20 Pagani FD, Aaronson KD, Swaniker F, Bartlett RH. The use of extracorporeal life support in adult patients with primary cardiac failure as a bridge to implantable left ventricular assist device. Ann Thorac Surg. 2001;71:S82–85.
21 Takayama H, Truby L, Koekort M, Uriel N, Colombo P, Mancini DM, et al. Clinical outcome of mechanical circulatory support for refractory cardiogenic shock in the current era. J Heart Lung Transplant. 2013;32:106–11.
22 Lehmann S, Uhlemann M, Etz CD, Garbade J, Schroeter T, Borger M, et al. Extracorporeal membrane oxygenation: Experience in acute graft failure after heart transplantation. Clin Transplant. 2014;29:12380.
23 Cheng R, Hachamovitch R, Kittleson M, Patel J, Arabia F, Moriguchi J, et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: A meta-analysis of 1,866 adult patients. Ann Thorac Surg. 2014;97:610–6.
24 Brechot N, Luyt CE, Schmidt M, Leprince P, Trouillet JL, Leger P, et al. Venoarterial extracorporeal membrane oxygenation support for refractory cardiovascular dysfunction during severe bacterial septic shock. Crit Care Med. 2013;41:1616–26.
25 Trummer G, Benk C, Klemm R, Biever P, Kalbhenn J, Schmutz A, et al. Short-term heart and lung support: extracorporeal membrane oxygenation and extracorporeal life support Multimed Man Cardiothorac Surg. 2013;2013:mmt008. doi: 10.1093/mmcts/mmt008
26 Berdajs D, Ferrari E, Michalis A, Burki M, Pieterse CW, Horisberger J, et al. New prototype of femoral arterial SmartCannula with anterograde and retrograde flow. Perfusion. 2011;26(4):271–5.




