
Figure 1
Combined clinical and biomarker assessment for a personalised emergency triage, therapeutic intervention and site of care decisions.
DOI: https://doi.org/10.4414/smw.2015.14079
In the seventies, quality of medical care was considered to be good. Life-expectancy, for example in Switzerland, was around 70 years, less than 3% of the population was more than 80 years old. Average length of hospital stay was around three weeks, fee-for-service was the reimbursement system of choice and healthcare costs consumed around 5% of the gross national domestic product [1].
A minority of patients were admitted through the emergency department (ED) [2]. Community-acquired pneumonia (CAP) worldwide was susceptible to narrow-spectrum antibiotics and generally treated for two weeks or more, allegedly to avoid recurrence and antimicrobial resistance. Clinically suspected acute myocardial infarction (AMI) was confirmed electrocardiographically and by elevated circulating creatine kinase levels, and treated with bed rest, oxygen, rhythm monitoring, sedatives and opioids. “Cancer” was typically considered to be a final diagnosis and pulmonary embolism was frequently diagnosed at autopsy only.
A large fraction of clinical knowledge for internists could be comprehensively summarised in a one volume textbook, such as Harrison’s Principles of Internal Medicine. A doctor’s ruling was considered to be “state of the art”, and legal aspects and malpractice insurance fees were negligible [3].
In 2015, quality of medical care is considered to be good. In Switzerland, life-expectancy is almost 85 years and among the highest in the world, with a doubling of the population older than 80 years as compared with four decades ago. Average length of hospital stay is around seven days, a case-based reimbursement system by diagnosis-related groups (DRGs) has been implemented and healthcare costs consume around 12% of the gross national domestic product [1].
EDs provide 75% or more of medical admissions of usually multimorbid patients with polypharmacy. “Door-to-needle” time has become a dominant benchmark to maximise outcome in an interdisciplinary setting that involves highly-skilled nurses, generalists and specialist physicians. Microbiological cultures are routinely plated to detect multiresistant organisms in patients hospitalised with CAP, who are treated with steroids [269] and antibiotics for a mean of less than 6 days and fewer complications, if procalcitonin (PCT)-guided [4, 5]. For chest pain, standard protocols demand the use of high-sensitivity cardiac troponin rapidly to rule-out or rule-in AMI for early revascularisation and monitoring in costly coronary or intensive care units [6, 7]. Clinical probability assessment and measurement of D-dimer in low- and moderate-risk patients should ensure that suspected pulmonary embolism can be safely ruled out, without the need for additional imaging [8, 9]. “Cancer” is not accepted anymore as a “final” diagnosis. Molecular subtyping on a cellular level, detailed radiographic staging and customised oncological therapy with monoclonal antibodies are routinely performed. This yields, overall, a superior cure and survival rate for “malignant” tumours as compared with diseases previously considered to be more “benign”, such as congestive heart failure (CHF).
Today, as you read this review in PDF format online, it is – at least in part – updated by more timely and widely accessible information on the internet. Expectations of society regarding healthcare have markedly increased. Patient safety drives international efforts to measure and publish the rate of errors and error-related harm. [3] One-third of patients recall a diagnostic error that affected themselves, a family member, or close friend [10] and 55% of surveyed patients listed a diagnostic error as their chief concern when seeing a physician [11]. Malpractice claims against US internists are most frequently due to errors in diagnosis and consequent suboptimal therapy and patient outcome [12].
Apparently, today we perceive a more urgent need for a safe and efficient, but even more personalised, approach of routine medical and nursing care to patients with common complaints. With this aim, laboratory tests can be of help if the indication for ordering is not excessive and unselected but is based on rational criteria. Their performance should be validated in randomised controlled trials in settings appropriate for routine care. The often misinterpreted term “biomarkers” refers to their role as specific markers of biological processes in a given disease state. This is the rational foundation for the belief that they have high potential to individualise clinical care with improved risk stratification, site-of-care decisions, diagnosis and treatment selection. This is not only for selected individuals with neoplastic cases but for common diseases in an everyday patient, therefore providing routine personalised emergency care for all.
The term “personalised medicine” suggests an approach to care that is based on individuals rather than groups. This is not a new conceptper se. Since ancient times, caregivers considered individual characteristics such as age, co-existing conditions, preferences, and beliefs in crafting an personal management strategy. The term personalised medicine became fashionable again with the recent use of individual genomic information in prescribing an expensive and side-effect prone therapy, such as customised monoclonal antibodies in oncology or abacvir for HIV treatment. Thereby, the genetic signature of a patient predicts treatment success or failure or allergic complications and has thus cost-considerations. Testing for human epidermal growth factor receptor type 2 (HER2) helps selection of breast cancer patients who will benefit from trastuzumab [13]. Testing for the KRAS mutation helps selection of patients most likely benefiting from therapies inhibiting the epidermal growth factor receptor [14]. Sequencing of whole genome, the epigenome, transcripts, microRNA, respectively, proteomics or metabolomics have the potential for further improved diagnostics, prognostic assessment, therapeutic targets and stratified treatments for cancer and rare disorders. However, these tools are costly and time-consuming and, therefore, not readily available as point-of-care test for early risk stratification in the emergency setting. In addition, to date individual genomic variants have variable penetrance and minor impact in common illnesses, such as cardiorespiratory diseases. For example, currently available genetic tests add no additional clinical useful information in guiding anticoagulation [15] or cardiovascular risk profiling. For these reasons, the vast majority of acutely ill patients, such as the masses treated in the ED, do currently not benefit from stratification by tests on the genetic or molecular level.

Figure 1
Combined clinical and biomarker assessment for a personalised emergency triage, therapeutic intervention and site of care decisions.
In contrast, blood circulating biomarkers play an important role in the current diagnostic work-up of ED patients. A biomarker may be defined as any protein or other macromolecule that can be objectively measured and evaluated as an indicator of normal biological processes, pathological processes, course of diseases or pharmacological responses to a therapeutic intervention. Readily measurable biomarkers provide important information about aetiology of a disease, and the need for interventions and prognosis. Diagnostic biomarkers confirm the presence or absence of a disease. Thereby, biomarker results need to be interpreted in the context of the pre-test probability and cut-off ranges are to be preferred to dichotomous and overly simplistic cut-offs. A high specificity and a high positive predictive value is required to “rule in” a disease (e.g. a PCT level of 0.5 ng/ml to prescribe antibiotic therapy in respiratory tract infections). Conversely, a high sensitivity and a high negative predictive value is needed to “rule out” a disease (e.g., a PCT level of 0.1 µg/l to withhold antibiotic therapy in infections of the respiratory tract [table 1]). In between there is a grey zone where the biomarker alone does not add sufficient information for a final ruling. Monitoring biomarkers should mirror effectiveness of therapy for the purpose of titration (e.g., BNP in the treatment of congestive heart failure or PCT for antibiotic duration). Surrogate biomarkers should correlate with clinical outcome in the setting of an therapeutic intervention (e.g., HbA1c as surrogate for complications of diabetes). Stratification or staging biomarkers classify diseases based on outcome probability, for example to limit side-effects of aggressive therapies to high-risk patients with poor disease outcome if untreated (e.g. D-dimer to exclude venous thromboembolism in need for anticoagulant therapy in patients with a higher bleeding risk). Companion biomarkers identify patients most likely to benefit from a specific therapy (e.g. toxoplasmosis serology, quantiferon testing in HIV, HER2 positive breast cancer better selects women with breast cancer for treatment with trastuzumab [Herceptin®])
Biomarkers should expedite the correct diagnosis and treatment leading to personalised and tailored therapy [16] (fig. 1). In emergency patients with cardiorespiratory symptoms, patient history, clinical examination, chest radiographs in conjunction with specific biomarker levels provide important information useful for the fast and accurate management of the patient. Point-of-care tests with fast turnaround times can already deliver this information in the prehospital setting including general practices and ambulances which may further improve the management of multimorbid and elderly patients with unspecific complaints [17–20].
Biomarkers, may also improve site-of-care decisions which are pivotal and major cost drivers. Decisions to hospitalise patients admitted to the ED are often based on “gut-feeling” of healthcare providers, patients or relatives rather than objective clinical information. A recent survey involving lower respiratory tract infection (LRTI) patients revealed that, independently of expected mortality based on pneumonia severity index or CURB-65 scoring, most LRTI patients are hospitalised because physicians, nurses, patients and relatives all believe that inpatient management is indicated for medical reasons, particularly fear of severe infection. Nursing reasons and patients’ and relatives’ personal preferences were mentioned to a lesser extent [21].
As students we were taught to collect signs and symptoms and try to fit them in a common physiopathological entity characteristic of a particular disease. This systematic approach with thorough history-taking and physical examination allows one to combine the mosaic fragments of cough, dyspnoea and/or chest pain to frame common clinical diagnoses, such as ACS, pulmonary embolism, acute heart failure (AHF) or CAP. Based on clinical reasoning we initiate more or less specific and targeted therapies, such as revascularisation, thrombolysis, diuretics or anti-microbial drugs. This heuristic strategy, while being efficient, relatively resource-sparing, and generally accurate, is not without fail [22].
Seven decades ago, history-taking alone allowed the allegedly correct diagnosis known these days to be made in 74% of sick patients, usually in later stages of their disease [23]. More recently, medical history and physical examination was presumed to yield a correct final diagnosis in more than half of cases [24]. A Hawthorne bias makes these study figures likely to be overly optimistic. Indeed, a more recent analysis of dyspnoeic patients under ED conditions revealed the correct diagnosis in only 41% after history taking, which was not improved after lung auscultation. Notably, this number varied from 100% (smoke inhalation) to less than 10% (chest wall pain) [25]. In real-life, clinical performance depends largely on skills and experience, and the quality of teaching and supervision of junior doctors [22]. Senior physicians, are given little time or financial incentives for teaching duties and teaching is not an intuitive endeavour for all physicians [26]. Sleep deprivation and circadian rhythm disruption in doctors are strongly associated with human error and harm [27].
The clinical judgment even of experienced doctors can be impaired by cognitive biases, such as availability, anchoring, premature closure and framing. Physicians underestimate their inter-person variability for clinical diagnosis and overestimate their kappa (i.e., the agreement with an independent colleague), even in the presentation of allegedly “easy to diagnose” common diseases. [28] For example, there is a dismal inter-physician agreement about signs of chest examination: the kappas for cyanosis, tachypnoea, and whispered pectoriloquy were only 36%, 25% and 11% (with 100% being perfect agreement) [29].
On average, medical patients are more than 70 years old on hospital admission. Most emergency physicians have not been trained in specific geriatric approaches, and many report being less comfortable when dealing with older patients [30]. Masked (e.g., absence of fever) or unusual presentation of disease in poly-morbid, elderly patients, or limited patient cooperation (dementia, delirium, uncooperativeness or deception) can be contributory [19].
Patterns of presenting complaints of patients vary in cardiorespiratory diseases, including typical local symptoms such as cough, at times productive, dyspnoea, chest pain to systemic moans such as fatigue, anorexia or myalgia [31]. Physical signs are not always present and, if present, may be difficult to elicit when examining the chest [31]. Reports on the validity of such signs have shown considerable disagreement among physicians, leading to unreliable clinical observations in cardiorespiratory diseases [29, 32, 33]. Indeed, clinical signs and symptoms show at best a moderate helpful in the differential-diagnosis in patients with acute dyspnoea [34]. Dullness to percussion, changes in the intensity of breath sounds, and inspiratory crackles may be present in a variety of pulmonary diseases that cause lung stiffening, including AHF, pulmonary fibrosis and obstructive lung disease [25]. Presence of sputum or its dis-colouring, dyspnoea, crackles, fever and increased white blood cell count are insensitive and unspecific parameters for the diagnosis of bacterial LRTI requiring antibiotic therapy [28, 35, 36].
This review focuses on the use of readily available “personalised information” from biomarkers for improved diagnosis and prognosis of common acute diseases, namely cardiovascular and thromboembolic diseases, and infections. Biomarkers for which randomised controlled intervention trials in the emergency setting are available were selected.
Following emergency admission of patients with cardiorespiratory complaints, history taking and physical examination result in a first list of differential diagnoses based on subjective likelihood assumptions. For example, an afebrile elderly patient presenting to the ED with dyspnoea as leading complaint, cough and pressure on the chest (or was it pain?) might suffer from AHF, AMI, CAP with pleuritis or even pulmonary embolism.
In the past, laboratory tests were used to identify organ and system dysfunctions or diseases to confirm initial impressions or rule out alternative diagnoses. While this is still true, testing today is used more and more for prognostication, risk stratification, site-of-care decisions to reduce hospital readmissions [37–40]. Today, most cardiorespiratory diagnoses are not considered final until after laboratory testing is complete [41, 42].
The physicians’ increasing reliance on objective data from diagnostic testing is arguably, at least in part, due to reduced or neglected history and physical examination skills [26, 41]. Importantly, a skilled clinical judgment after focused history-taking and physical examination remains a pre-requisite to minimise testing-related diagnostic errors. Importantly, physicians should not make decisions based upon one isolated finding, but rather on the overall gestalt of the patient’s illness. Thereby, clinical reasoning is essential to determine the pre-test probability of a disease (i.e., prevalence), of treatment failure or complication and for adequate interpretation of laboratory test results. As there is ambiguity about what constitutes disease, more testing will produce more “abnormal” findings, resulting in further testing, which in the end makes patients sicker (and poorer) [43]. It leads to unnecessary worries, procedures, treatments and potentially unnecessary hospitalisations and harm. Conversely, false negative test results lead to underestimation of disease status, delays and possibly worse outcome [41].
The physician’s role in emergency medicine has changed from clinical problem solving by history taking and examination alone to determine the pre- and post-test probabilities essential for the ordering and interpretation of laboratory tests. Misapplication of test results can result from misinterpretation by the clinician – either from misunderstanding the clinical implications of a result or from not understanding the limitations of the test methodology (i.e., statistical variations, performance limitations, or interfering substances) [41]. Therefore, clinical skills of experienced physicians need to be synergised with modern biomarker strategies knowing strengths and limitations of both.
Thanks to advances in quality control, diagnostic errors today are rarely the result of an error in the analytical test itself. Overall, it is estimated that laboratory results are misleadingly wrong in 2%–4% of cases; roughly the same error rates are found in diagnostic radiology [41]. Most laboratory-related errors now originate from the pre-analytical and post-analytical processes, namely issues related to the physician ordering and interpreting of test results. Lapses in the reliable communication of abnormal laboratory and imaging results is a problem, even in systems with advanced electronic medical records [41, 44]. In daily routine the time between the initial clinician-patient encounter, test order, receipt of the sample by the laboratory, the test result availability and clinician action based on results is considered too long by many. Point-of-care testing should minimise testing-related delays provided the quality of the assay, namely sensitivity, is not impaired [45, 46].
There is a true need of evidence-based medicine criteria in laboratory medicine. The challenge to address for scientific investigators is the difficulty to design methodologically rigorous randomised double blind trials without limiting their validity for real-life, namely in the emergency setting. Trials are performed on novel markers rather than established markers. The established markers are mostly indirectly tested as the control arm in studies investigating novel markers. The superiority of the novel markers in such trials may also result from the fact that patients in the biomarker arm are treated and managed by standard operation procedures whereas the control arms are mostly usual care. It may hence be that these studies demonstrate the superiority of patient management by standardized operating procedures (SOP) rather than superiority of patient management by a novel biomarker.
Physicians often disagree about what inappropriate laboratory utilisation is [47]. Unfortunately, many “routine” laboratory tests are being ordered in “bundles” without any impact on diagnostic or therapeutic management. This creates needless traumas of venepuncture and unwarranted prescriptions are a colossal waste of money – an unresolved problem also at our own hospital. Many house staff continue to order recurring laboratory tests (“daily labs”). Multiple teams of physicians often consult on a single case, write multiple sets of phlebotomy and laboratory orders – often duplicated. Blood draws should not be considered innocuous: up to 20% of hospitalised patients with acute myocardial infarction suffered from phlebotomy-related hospital-acquired anaemia with an haemoglobin level of <11 g/dl [48].
The elimination of unnecessary tests and procedures has been identified as a key factor to reduce the trauma and expenses of hospitalisation [49]. The American Board of Internal Medicine’s “Choosing Wisely” campaign to reduce unnecessary and potentially harmful tests is an important first step [50]. Unbundling of tests, supervision by senior physicians, feedback of individual phlebotomy-rates, and warnings in electronic order entry systems lead to a reduction of phlebotomies of 20 to 60% [51, 52]. Unfortunately, these laudable attempts are usually labour intensive and short lived, initially promising effects disappearing shortly after cessation of the educational effort [52]. Efforts incorporating education, requisition design, and funding incentives have demonstrated the most durable effect [43].
| Table 1: Commonly used biomarkers to personalise medicine in the emergency triage with indications and limitations. | |||||||
| Biomarker Assay technique | Common clinical setting | Cut-off ranges (may be assay specific) | Further reading | Rule-in | Caveats in ruling-in (false high, impaired specificity) | Rule-out | Caveats in ruling-out (false low, impaired sensitivity) |
| Proadrenomedullin (ProADM) Fluorescence Immunoassay | Risk stratification in respiratory tract infections in the ED | <0.75 μg/l low risk 0.75–1.5 μg/l intermediate >1.5 μg/l high risk | [39] [261] [262] [263] | More intensified monitoring and treatment | Social and nursing aspects of hospitalisation | Outpatient treatment | Perceptions and preferences of patient and relatives Other important comorbidities |
| D-dimer Highly sensitive rapid ELISA, nephelometric or turbidimetric assays | Venous thromboembolism (VTE) | <500 μg/l: no imaging if low or intermediate (unlikely) clinical probability for VTE Imaging recommended if ≥500 μg/l independent of D-dimer if high VTE probability Patients >50 years: consider age-adapted cut-off ranges: cut-off = age x 10 | [167] [264] | Anticoagulation if VTE ruled in by imaging, | Age ≥80 years, all conditions associated with enhanced fibrin-turnover: e.g., systemic inflammation, vascular dissections, infection, trauma, cancer, pregnancy, inpatients | Consider alternative diagnosis to VTE, e.g., aortic dissection | High clinical probability for VTE, upper-extremity deep vein thrombosis, pregnancy |
| High sensitivity cardiac troponin T or I (hsTp) hsTpT: e.g., electro-chemieluminescent immuno assay (ECLIA) hsTp I: e.g., luminescent oxygen channelling assay (LOCI) | Suspected acute coronary infarction (AMI) | hs-cTnT <14 ng/l low risk 14–52 ng/l intermediate >52 ng/l high risk | [265, 266] | AMI | Myocardial wall stretch and cell necrosis of non-ischaemic origin (e.g.,Tako tsubo, pulmonary embolism, myocarditis), demand ischaemia (e.g. sepsis) persistently elevated level up to 14d, renal failure | Consider alternative diagnosis to AMI, conservative therapy of symptoms and cardiovascular risk factors | Time-lag of 1 to 3 h, rarely circulating antibodies against analyte |
| B-type natriuretic peptide (BNP) or N-terminal fragment of BNP (NT-BNP) or MR-proANP Luminescent oxygen channelling assay (LOCI) | Dyspnoea and / or suspected acute heart failure (AHF) | BNP: <100 ng/l AHF unlikely 100–400 ng/l intermediate >400 ng/l AHF likely NT-BNP: <300 ng/l AHF unlikely 3 age-dependent cut-offs for rule-in of AHF (450, 900, 1800) | [131] [136] | AHF | Age, ACS, myocardial hypertrophy, myocarditis, pulmonary hypertension, stroke NT-BNP: renal failure | Consider alternative diagnosis to AHF | Obesity, flash pulmonary oedema, mitral valve disease, pericardial tamponade or constriction, BNP: limited stability of analyte |
| High sensitive C-reactive protein (hsCRP) Nephelometric assay, latex enhanced turbidimetry and various immunoassays | Cardiovascular risk profiling Inflammatory status | Cardiovascular risk: <1 mg/l: low 1–3 mg/l: intermediate 3.1–10 mg/l: high Inflammatory status <20 mg/l: low 20–50 mg/l: intermediate >50 mg/l: high | [267, 268] | Cardiovascular risk: Statins for primary prevention in the GP setting for antibiotic stewardship in RTIs | Inflammatory acute-phase response of concomitant, non-infectious disease (e.g. SIRS, cancer, thromboembolic disease) | SIRS | Cardiovascular risk: contribution of other cardiovascular risk factors Time-lag to peak response (-72h), steroids, hepatic failure |
| Procalcitonin (PCT) Fluorescence immunoassay | Antibiotic Stewardship in Infections , namely of the respiratory tract in ED and hospital ward antibiotic stewardship; Infectious aetiology of SIRS in the ICU | ED & hospital ward: <(0.1)–0.25 μg/l bacterial infection (very) unlikely; >0.25 (0.5) μg/l ng/ml bacterial infection (very) likely ICU: <(0.25)–0.5 bacterial infection (very) unlikely; >0.5 (1.0) ng/ml bacterial infection(very) likely | [84] [177] [197] | Prescribe antibiotics in LRTI in the ED and hospital –ward setting. In the ICU setting escalation of antibiotic therapy based on serial PCT measurement not recommended | New-borns, children, severe trauma and systemic inflammation malaria, medullary thyroid cancer and paraneoplastic hormone production | Evaluate alternative diagnosis to systemic bacterial infection, discontinue antibiotics in LRTI and sepsis, | Early-course (24h) , sub-acute, and localised infections, insensitive assay, should be applied in conjunction to clinical improvement |
| AB = antibiotic; AHF = acute heart failure; ED = emergency department; GP = general practitioner; ICU = intensive care unit; PCT = procalcitonin; SIRS = systemic inflammatory response syndrome; USA = United States of America; VAP = ventilator-associated pneumonia | |||||||
Hospital EDs are increasingly appreciated by patients for their high-intensity medicine around the clock and are overwhelmed by patients with both urgent and non-urgent problems [53, 54]. This leads to overcrowded ED waiting rooms with long waiting times. As a consequence, patients truly needing urgent care may not be treated in time, whereas patients with non-urgent problems may unnecessarily receive expensive and oversized emergency care. Time to effective treatment is among the key predictors for outcomes across different medical conditions (“time is cure”), including patients with AMI (“time is muscle”) [55], stroke (“time is brain”) [56], CAP [57] and septicaemia (“surviving sepsis campaign”) [58]. In the latter case, early initiation of fluid resuscitation and of appropriate anti-microbial improves outcomes [59–62]. In contrast, other adjunct sepsis therapies such as immuno-modulatory antibodies or tight glucose control have not proven to be beneficial [63, 64].

Figure 2
Systemic multiprofessional risk assessment for improve patient management adapted from [261].
ProADM = proadrenomedullin

Figure 3
CURB-A score combining the traditional CURB-65 criteria with levels of proADM to risk stratify site-of-care decisions in patients with lower respiratory tract infections.
ICU = intensive care unit; ProADM = pro-adrenomedullin; SP = Selbstpflege (self-care); PACD = post-acute care discharge
Emergency medicine is under continuous pressure to improve the value of healthcare services delivered. Physicians have an obligation to their patients and to society in regard to high quality, but also cost-effectiveness. The complexity of increasingly multimorbid patients with acute exacerbations of their chronic diseases is more challenging than ever. Medicine has become more technical and complex leading to system-related errors, such as technical failure, equipment problems and organisational flaws.
For these reasons, an accurate and well-validated triage system in the ED is pivotal for an optimal initial triage of medical patients. ED triage should not only focus on treatment priority, but also on site-of-care decisions (i.e., outpatient versus inpatient management) and early identification and organisation of post-acute care needs. An appropriate risk assessment and subsequent triage is crucial for an optimised patient care and allocation of limited health-care resources. For this purpose, tools have been propagated, namely standardised triage scores for the ED, nursing scores to predict post-acute nursing care needs and biomarkers thought to mirror physiopathological changes and severity of disease (fig. 2).
Many patients prefer outpatient treatment [65]. Admission rates and length of stay are affected by a variety of medical, functional, psychosocial factors including patient and relatives preferences [66–69]. Consecutively, despite a low-risk according to clinical severity scores per se, many patients are still hospitalised for medical co-morbidities and psychosocial reasons [66, 67].
Community-acquired LRTIs include acute bronchitis, acute exacerbations of chronic obstructive pulmonary disease and CAP. They are among the most frequent causes of hospitalisation [70] and direct treatment costs amount to $15 billion in the USA [71]. Inpatient care of CAP is 8 to 20 times more expensive [66, 72] and carries a higher risk of nosocomial infections including Clostridium difficile-associated diarrhoea [73] than outpatient treatment. In the multicentre ProHOSP study, compliance with the PCT guided antibiotic algorithm was 90% [74]. Importantly, only half of the patients with LRTI in the low medical risk groups as determined by PSI or CURB65 were treated as outpatients despite high intensity implementation [74, 75]. Regardless of low observed risk for adverse events, fear of medical complications was the dominant motive for hospitalisation similarly among physicians, nurses, patients and relatives [21]. Thus, clinical severity scores, despite being recommended in guidelines, have been insufficient in clinical application due to complexity, problems memorising, but also because of both relative insensitivity and non-specificity for outcomes [76, 77]. Most deaths in patients with low risk scores are non-CAP-related [76], and most causes of re-admission within 30 days are due to co-morbidities (74%) rather than CAP (20%) [78]. A further limitation of most clinical risk scores for LRTI is that they are only validated for CAP.
Adrenomedullin is a 52 amino acid polypeptide showing sequence homology with other calcitonin-related peptides. It was discovered 20 years ago as a secretion product of a pheochromocytoma [79]. The adrenomedullin gene encodes for a 185 amino acid prepropeptide, post-transcriptionally cleaved to produce the bioactive, amidated adrenomedullin. [80] Adrenomedullin, calcitonin-gene-related peptide (CGRP) and PCT belong to the same calcitonin peptide superfamily and emerge from different CALC genes with teleologically related, yet distinct physiopathological molecular regulation and effects [81–84]. These peptides are prototype “hormokines” which show behaviour of both hormones and cytokines and are ubiquitously hyper-expressed during the host response to bacterial infections [85–87].
Adrenomedullin and other calcitonin-peptides compete for binding to the same receptor family with agonistic and antagonistic properties [83, 88]. They exert their effects via G-coupled seven-transmembrane calcitonin receptor-like receptor in association with RAMP2 or RAMP3 to form the adrenomedullin receptors 1 and 2, respectively [89, 90]. Upregulated by hypoxia, inflammatory cytokines, bacterial products and shear stress, adrenomedullin acts systemically and in autocrine and paracrine fashion [91, 92]. Adrenomedullin is a very potent vasodilating agent believed to play a physiological role in arterial pressure homeostasis with additional immune modulating and metabolic properties [90, 93, 94]. Pleiotropic effects of adrenomedullin have been described: in tumour growth, neovascularisation, endothelium protection, bronchodilation, fertility, and immunity [95–99].
Adrenomedullin is found in plasma in health and at elevated concentrations during various pathologic conditions, including cardiovascular and renal disorders, sepsis, cancer and diabetes [90]. Based on the hormone’s biological importance and effects, the utility of measuring adrenomedullin in blood seems evident. However, abundant binding to peripheral and local receptors, a short half-life, and ex vivo physical characteristics including instability and “stickiness” make circulating adrenomedullin difficult to reliably directly quantify [100, 101]. During adrenomedullin’s processing into a mature hormone, it and an adjoining peptide, mid-regional proadrenomedullin (MR-ProADM; referred to here and elsewhere as proadrenomedullin [ProADM]), are cleaved off of the precursor in a 1:1 ratio. This stoichiometric secretion, the peptide’s apparently minimal if any biological activity and hence, binding, and it’s considerable chemical stability render ProADM an easily and robustly quantifiable surrogate biomarker for adrenomedullin [100, 102, 103]. ProADM has been used as a prognostic marker, alone or in risk stratification with other hormonal propeptides in patients with sepsis and severe pneumonia [82, 104, 105].
Adding prognostic biomarkers to a thorough clinical and interdisciplinary assessment bundles may allow detecting subgroups of patients with higher than anticipated risk requiring expedited assessment, intensified care and monitoring. Conversely, the prediction of a very low risk of adverse events by both interdisciplinary assessment and biomarker embedded in a system of risk-appropriate sites of care (e.g. nurse-led care NLC), structured discharge planning, patient education and immediate follow-up and an anticipated lower risk of healthcare associated infections [106] should provide sufficient confidence for physicians, nurses, patients and relatives in our risk-adapted triage with expedited discharge planning. Also, prognostic markers may help to select specific medical therapies for patients most likely benefiting from them, namely in resource-deprived settings (e.g., busy shifts and/or shortage of ED staffing).
Multiple “promising” prognostic biomarkers have been proposed in observational studies. Yet, the clinical efficacy and safety of their use for prognostication needs to be established in randomised-controlled intervention studies. C-reactive protein (CRP), PCT and other routinely measured markers have at best a moderate prognostic utility in systemic infections and other diseases [105, 107–109]. Thus, they cannot be used for site-of-care decisions. For prognostic purposes, ProADM has proven to be superior to CRP, PCT or any other of the plethora of marker from different biological pathways [105, 110–112]. In patients with CAP and other LRTI, proADM levels measured on admission were significantly associated with disease severity and fatal outcome [93, 111] ProADM had a superior prognostic value for predicting complications in LRTI compared to guideline-recommended clinical severity scores [104, 105, 113–120]. The combination of biomarkers and clinical scores (i.e., CURB65 and PSI) provided superior prognostic accuracy providing an additional margin of safety [105, 116, 117, 119, 121]. Consequently, an improved assessment of LRTI has been proposed based on CURB-65 and ProADM cut-off levels where patients were stratified into three risk classes with estimated mortality rates ranging from 0.65% to 9.8% (fig. 3) [121]. In a recent randomised-controlled pilot study this combined algorithm tended to reduce hospital length of stay without negatively affecting patient outcomes compared to patients stratified according to CURB-65 alone [40]. These results must be interpreted in light of unresolved organisational challenges in discharge planning and availability of beds in nursing homes and other post-acute care settings beds.
In the current society, patients and physicians are risk-averse and appear to prefer objective risk numbers compared to more complex clinical algorithms. Herein, ProADM alone or in combination with a prognostic biomarker bundle has the potential to enhance the interdisciplinary risk assessment and may improve the currently limited compliance with existing guidelines. This may also provide a more individualised and comprehensive risk assessment. Prognostic biomarkers may facilitate selection of patients for whom early discharge is safe and may help to focus resources to the patients in need. Prognostic biomarkers may help physicians to identify patients who would, and would not, benefit from distinct therapies and thus may allow a transition from bundled care to more personalised approaches, which in turn could result in improved patient outcomes.
Acute myocardial infarction (AMI) is the cause of death in more persons worldwide than any other disease [122, 123]. With effective treatment in our hands, accurate and rapid diagnosis is of major medical and economic importance. In the initial assessment, electrocardiography (ECG) is of paramount importance to detect diagnostic ST-elevations which require immediate transfer to a catheter lab. There is no need to wait for the biomarker results. Still, cardiac troponin is measured to use it to 1) determine infarct size and 2) definitely prove that an AMI has occurred. Also, not all ST-elevations are unequivocal, as multiple causes other than STEMI might lead to ST-segment elevations such as left ventricular hypertrophy, myocarditis, takostubo cardiomyopathy, and unknown causes as in healthy young men [124]. In suspected unstable angina and non-ST-elevation myocardial infarction (NSTEMI) cardiac troponins are indeed mandatory in all patients to decrease the diagnostic ambiguity of the clinical diagnosis. In a study setting, experienced primary care physicians correctly classified 88% of low-risk walk-in patients with chest pain as having either an organic or non-organic aetiology solely on the basis of their initial clinical judgment and before ordering any diagnostic tests [125]. In an ED setting with multimorbid patients and a higher pre-test probability for organic disease the ambiguity of clinical findings is much higher, for example >90% in a real-life study under emergency conditions [25]. Therefore, cTn testing is recommended by all current guidelines to improve clinical performance.
With the development of sensitive assays depicting either cTnI or cTnT (the only current biomarkers thought to be unique to the heart) the diagnosis of AMI was revolutionised [123, 126–128]. In a patient presenting with chest pain, a rise in cTn has become a conditio sine qua non for the clinical diagnosis of AMI. Cardiac troponins are our current gold standard for the detection of myocardial necrosis. The more sensitive the cTn assay used, the smaller the number of dying myocardial cells necessary for this signal to get detected. This has enabled patients to be detected even with very small AMIs. It also allows more rapid detection of AMI (usually within 1 or 2h if high-sensitivity cTn assays are used) [127]. Overall, high-sensitivity cTn assays seem to constitute a major medical and economic improvement in clinical practice.
However, the clinical use of these assays has created also challenges and uncertainties: It is unclear, whether the medical benefit observed in previous RCTs for early revascularisation and aggressive antiplatelet therapy also applies to patients with very small AMIs. As these patients still seem to be at an increased risk of death as compared to patients without elevated high-sensitivity cTn levels, the current ESC guidelines encourage us to do so [122, 123, 126–128]. Secondly, myocardial damage is not restricted to AMI, but may also accompany other medical conditions like septic shock, pulmonary embolism, hypertensive heart disease accompanying end-stage kidney disease, or acute heart failure. As we currently lack a biomarker that reliably detects plaque rupture or coronary thrombosis, we are often left with our basic clinical tools including patient history to differentiate AMI from other causes of myocardial damage. The interpretation of cardiac troponin as a quantitative marker of cardiomyocyte damage, where the likelihood for the presence of AMI increases with increasing levels of cardiac troponin, and the use of absolute changes within 1–3h as complimentary information to distinguish causes of chronic elevations such as heart failure (usually not showing relevant short-term changes) from causes leading to acute cardiomyocyte damage such as AMI, which are associated with a rise and/or fall. Third, once a diagnostic test is declared “gold standard”, it becomes practically impossible to definitely rule out false positive test results. This is currently the case with cTn. We strongly think that the heart is invariably the exclusive source of cTn elevations, regardless of the specific patient condition. However, as both the ECG and imaging techniques have by far lower sensitivity for myocardial necrosis as cTn, scientific proof often cannot be delivered in an individual patient. One easy option to exclude analytical false-positive levels is to measure a second cTn assay (e.g. cTnI when initially using cTnT). Fourth, despite the introduction of highly sensitive assays, follow-up measurements after 3 hours are needed to exclude AMI, which may delay time to ED-discharge. The use of assay-specific early algorithms [129], or the combination of cardiac troponin with copeptin, seem to allow an even earlier rule-out [130].
For example, in a meta-analysis identifying the symptoms, signs, and tests most useful in diagnosing acute heart failure (AHF), no single history-taking or physical examination findings provided adequate discrimination [131]. The most discerning features of AHF – such as the presence of paroxysmal nocturnal dyspnoea, an S3 gallop, or jugular venous distention – have such a low incidence (each documented in <50% of patients with AHF) that their sensitivity for the diagnosis of AMF is low. In fact, the best single predictor of AHF was found to be a B-type natriuretic peptide (BNP) value of 250 ng/l with a sensitivity of 89% and a specificity of 81%. Nevertheless, high initial clinical suspicion (based upon the complete history and physical) was the most predictive element in the diagnosis of AHF. These observations highlight the need to interpret BNP levels as quantitative markers of hemodynamic cardiac stress. Low levels (<100 ng/l) are helpful for the rule-out of AHF as the cause of acute dyspnoea, while high levels (>400 ng/l) are helpful for rule-in of AHF. Based on data from several RCTs, natriuretic peptides (BNP, NT-proBNP, or MR-ProANP) should be obtained in all patients presenting with acute dyspnoea to the ED.
Accurate biomarkers of heart failure are highly desirable tools for physicians to either improve their ability make an early and accurate diagnosis or to follow positive or negative changes as a result of therapeutic intervention. The ability of physicians to make earlier diagnoses is valuable because therapeutic interventions are available that can make a significant impact on patient quality of life and cost of care [16, 132–137]. Annual costs of heart failure in Europe and the United States are estimated at $130 billion, 70% of which is due to hospitalisation. Half of heart failure patients are re-admitted within 6 months and 10% are re-admitted twice with heart failure [16, 134]. Fewer re-admissions by guided therapy methods could significantly impact the costs associated with this prevalent disease. All three clinically available natriuretic peptides (BNP, NT-proBNP, or MR-ProANP) seem to have similar diagnostic accuracy in this indication [16, 38, 132–143]. In addition, natriuretic peptides, as quantitative markers of heart failure that summarise the extent of systolic and diastolic left ventricular dysfunction, valvular dysfunction, and right ventricular dysfunction [136], provide valuable information for risk stratification in patients with acute and chronic heart failure [38, 140–143]. Although still under some debate, BNP and NT-proBNP measurements also seem capable of improving the long-term management of patients with chronic HF [141, 142]. Detailed recommendations on how to best apply these biomarkers have recently been provided in this journal [136]. Appropriate cut-off values have been defined in large observational studies, and evidence from large randomised controlled studies confirms both medical and economic benefit from their use.
The use of natriuretic peptides shares one important challenge comparable to that of cTn: Having become the most sensitive test to detect a disorder (heart failure), other current clinically available methods including cardiac imaging have a major limitation in the clarification of unexpected and potential “false positive” elevations of natriuretic peptides. Again, one easy option to exclude analytical false-positive levels is to measure a second natriuretic peptide (e.g. NT-proBNP when initially using BNP).
For many years, the diagnosis or exclusion of acute venous thromboembolism (VTE), defined as deep leg vein thrombosis and/or pulmonary embolism, often relied on invasive and costly imaging techniques, such IV venography and pulmonary angiography. As VTE was confirmed in only about one third of patients in whom it was suspected [144, 145], the development of non-invasive exclusionary tests became necessary. D-dimer, a cross-linked fibrin degradation product, is an indicator of coagulation activation and fibrinolysis and can be measured in both whole blood and plasma [146]. More than 25 years ago, D-dimer testing using quantitative enzyme-linked immunosorbent (ELISA) D-dimer assays was found to be highly sensitive for the detection of VTE (sensitivity ≥95%) [147–149]. Today, a variety of commercial D-dimer assays are available. The main assay categories include quantitative, fully automated rapid ELISA or immunoturbidimetric latex-agglutination assays with a high sensitivity (96%–99%) but a rather low specificity (38%–48%) and qualitative or semi-quantitative latex agglutination and whole-blood point-of-care tests with a moderate sensitivity (69%–85%) but a higher specificity (68%–99%) [146, 150].
Since the 1990s, prospective management studies have demonstrated that it is safe to withhold anticoagulation in patients with a low/intermediate or unlikely clinical probability of VTE based on clinical scores (e.g., Wells scores, revised Geneva score) and a negative highly sensitive D-dimer test (e.g., rapid ELISA), precluding the need for further imaging [151, 152]. If a less sensitive D-dimer test is used (e.g., whole-blood agglutination assay), it is still safe to withhold anticoagulation in patients with a low or unlikely probability of VTE [151, 153–155]. It has been estimated that by assessing patients according to this strategy, at least a 30% decrease in diagnostic imaging can be achieved in outpatients with suspected VTE, resulting in less waiting time and diminished costs of care [154]. These results have been corroborated by randomised clinical trials [156, 157]. Practice guidelines recommend initial D-dimer testing when evaluating patients with either a low (with either a moderately or highly sensitive D-dimer test) or moderate clinical probability (highly sensitive D-dimer only) of VTE [8, 158]. If the D-dimer test is negative, VTE is considered excluded and no further imaging, such as compression sonography or spiral computed tomography, is necessary. A positive D-dimer should be followed by imaging. As it is not clear whether D-dimer tests can safely rule out VTE in patients with a high clinical probability, initial testing with imaging (without D-dimer testing) is recommended. The Choosing Wisely campaign, launched by the American Board of Internal Medicine Foundation to avoid overuse, recommends not to order computed tomography to diagnose pulmonary embolism without initial risk stratification (pre-test probability and D-dimer tests if low probability) [159].
Despite its potential to avoid imaging, D-dimer testing is hampered by several practical limitations. D-dimer tests suffer from a lack of standardisation, results (and cut-off points) are not comparable between different assays. As the ability to exclude VTE depends on test sensitivity, clinicians should be aware of the specific assay used at their institution and its test performance characteristics [146]. A major problem of D-dimer is the lack of specificity for VTE, leading to a high rate of false positive results. Elevated D-dimer levels can be found in any condition associated with enhanced fibrin formation and fibrinolysis, including surgery, trauma, infection, pregnancy, cancer, myocardial infarction, stroke, atrial fibrillation, inflammatory conditions, and advanced age [146]. For instance, while about one third of unselected outpatients with suspected pulmonary embolism have a negative rapid ELISA D-dimer test (conventional cut-off 500 μg/l), this proportion decreases to 5% in patients aged ≥80 years, 11% in patients with cancer, and 4% of inpatients [160–162], which greatly reduces the efficiency and clinical usefulness of D-dimer as an exclusionary test in these populations. The safety of D-dimer testing to exclude VTE in special situations, such as pregnancy or upper extremity deep vein thrombosis, has not been examined in prospective management studies and therefore, the role of D-dimer testing is yet to be determined in such patients.
Although D-dimer testing was originally developed to reduce unnecessary imaging, an increasing use of D-dimer does not decrease or even increases referrals for VTE-related imaging in the real-life setting [163, 164]. Potential underlying reasons are the indiscriminate use of D-dimer because of its easy availability (together with modern imaging techniques, such as multidetector spiral computed tomography) and a declining tolerance to diagnostic uncertainty [154]. Attempts have been made to resolve this issue by better selecting patients requiring D-dimer testing for pulmonary embolism using explicit clinical criteria (PERC rule) [165, 166]. Recently, to increase the specificity of highly sensitive D-dimer assays in patients aged ≥50 years, the utilisation of an age-adjusted cut-off value (cut-off = age x 10) was successfully validated in a large prospective management study [167]. Finally, the clinical value of D-dimer testing in specific populations, such as pregnant women or patients with upper extremity deep vein thrombosis, must be validated in prospective management studies before its use can be recommended in these populations.
Inflammatory toxins, immunogenic antigens, infectious pathogens, and mediators of the host response all stimulate pro- and anti-inflammatory mediators and coordinate recruitment of immune cells to the acute site(s) characteristic for the diseases. Precursors, mature forms and degradation products of mediators involved, penetrate from the original site of action into the circulation. As surrogate blood biomarkers these substances mirror the extent and severity of the insult. Numerous attempts have tried to correlate these different mediators with the presence and course of disease as diagnostic and prognostic markers.
Like in other disease states, ED physicians face the dilemma of ambiguous clinical manifestations of infectious and inflammatory diseases at emergency presentation. Inflammation-related cognitive impairment may worsen the value of history-taking. Multimorbidity further masks typical textbook signs and symptoms of infections or makes their presence ambiguous [168]. Even in patients with typical bacterial CAP the classical sign of fever is absent in almost half of hospitalised patients [28]. A positive culture result of a bacterial pathogen in bodily fluids is considered ultimate diagnostic proof for bacterial infection or sepsis by many. Indeed, the possibility for resistance testing argues for routine sampling of (blood) cultures and is life-saving in a setting with prevalent multiresistance. Clinicians, therefore, order blood cultures liberally if bacteraemia is suspected, for example when patients have fever, chills, leucocytosis, signs of focal infections, indications of sepsis, or suspected endocarditis prior to starting parenteral anti-microbial therapy. Unfortunately, these criteria are unreliable and clinical prediction rules lack external validity and are complex with use of difficult-to-obtain variables [169]. Hence, only a small proportion (4%‒7%) of blood cultures yield true-positive results [169–171]. Of these, as many as half represent contaminants, i.e. false-positives which may unnecessarily expand investigations and extend hospital stay [172]. Hence, ordering blood cultures without appropriate pre-test probability criteria is both wasteful and harmful [173, 174].
In the pre-antibiotic era, mortality from pneumococcal CAP was between 20 and 30% overall, rising to more than 60% for bacteraemic cases [175]. Today with antibiotics, mortality in patients hospitalised for CAP is between 5% to 10%, dependent on co-morbidity [74], and in septic shock rise still up to 50% [87]. Earlier administration of antibiotic therapy improves outcomes in patients with severe sepsis [60]. The current sepsis guidelines, therefore, have a strong recommendation for administering antibiotics within 1 hour of the diagnosis (or suspicion) of severe sepsis. [62] Yet, not all patients with clinical signs of infection – including SIRS criteria – truly have a bacterial infection in need of antibiotic therapy for cure. Beneficial effects of empiric antibiotic therapy must be weighed against their harmful effects particularly in regard to emergence of drug-resistant bacteria and other drug-related side effects [176]. Biomarkers may allow transformation of bundled sepsis care to more personalised patient management [177, 178].
The recognition over 25 years ago that the host response plays an exquisite role in sepsis led to the definition of sepsis that is still the standard today [62]. Unfortunately, the systemic inflammatory response syndrome (SIRS) variables (i.e., body temperature, heart rate, tachypnoea, white blood count) turned out to be ambiguous, lacking sensitivity, specificity and ease of clinical application [179]. Thus, a gold standard to differentiate bacterial from non-bacterial or non-infectious causes in patients with SIRS does not exist, and thus all observational studies are prone to a potential bias. As long as patients with the slightest suspicion of (bacterial) infection are treated with antibiotics “just to be safe”, all observational diagnostic studies are biased due to the lack of independent comparison with a true gold-standard for a relevant bacterial aetiology in true need of anti-microbial therapy. This dilemma can, unfortunately, not be resolved by meta-analysing a selections of inherently flawed observational studies since most patients in those studies were in fact treated with antibiotics despite the low pre-test probability for an infection [180–182]. Indeed, the potential to change clinical decision making is the most important performance measure for a biomarker. Therefore, randomised intervention studies have to be done in which the anti-microbial therapy is guided by a biomarker and in which the primary measure of efficacy is outcome [176]. If the patient recovers without antibiotics, there was obviously no serious bacterial illness in need of antimicrobial therapy. Herein, we discuss the two biomarkers CRP and PCT for which several randomised intervention studies have been published. CRP has become a de facto “universal inflammatory screening marker” in clinical routine (table 1). Considering the greater sensitivity and specificity demonstrated in an impressive body of literature PCT can reduce the limitation of the SIRS staging in sepsis and improve guidance of anti-microbial therapy [177]. Although far from being a perfect marker [180], PCT improves the accuracy of the clinical diagnosis in hospitalised patients with infections of the respiratory tract and sepsis [28, 35, 183]. In contrast to CRP and other biomarkers, circulating PCT levels are not affected by steroid co-medication [184, 269]. Accordingly, it has been included in the PIRO staging system for sepsis, which includes the predisposition, infection, response and organ failure [185].
CRP is an acute phase reactant whose synthesis in the liver is up-regulated by IL-6 in response to inflammation independent of the aetiology. CRP has a role in the clearance of dying and altered cells, and might also have more complex immuno-modulatory functions. Recent evidence suggests that the genetic variants influencing basal CRP level also influence the magnitude of the acute-phase rise in CRP level in active inflammation. These genetic effects might be large enough to directly influence clinical decision-making processes that are based on an interpretation of CRP thresholds [14, 186].
As a biomarker CRP has been used for decades in Europe to screen for the presence of significant inflammatory or infectious disease in the ED [187]. Its advantages include the relatively low pricing in routine labs, widespread availability also for general practitioners as point-of-care tests and high sensitivity even for low grade and chronic systemic inflammation.
Indeed, as a sensitive marker of subtle, sub-acute vascular systemic inflammation, measurements of high-sensitivity CRP (hs-CRP) plasma levels add to both the prognostic information gleaned from assay of plasma lipid risk factors and the risk levels estimated by means of Framingham study– based criteria [188–190]. A large-scale, randomized clinical trial – Justification for the Use of Statins in Primary Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) – demonstrated that potent statin therapy reduces the risk of heart attack and stroke by 50% among men and women with low levels of LDL-cholesterol who are at increased vascular risk mirrored by elevated levels of CRP [191].
Every year there are millions of visits to primary care physicians by adults with a chief of complaint of cough, representing over 5% of all visits to physicians. To determine the aetiology of infections of the respiratory tract history and prescribe antibiotic therapy, history and physical examination are misleading [28, 31, 35], which leads to antibiotic over-prescription “just to be safe”. In acute bronchitis, the antibiotic prescription rate should be zero. Despite years of intensive and internationally concerted efforts, in the US the rate hovers around 70% both in primary care and EDs. [192] Factors such as diagnostic ambiguity about possible bacterial infection, avoidance of potential risks with legal consequences, and patient demands play an important role. Prescription rates of antibiotics was lowered in a study-setting by around 10% by a clinical decision support system [193]. Unfortunately, this effect is only modest and not persistent. Together with measures to enhance communication-skills of general practitioners as point-of-care test CRP reduced antibiotic overuse in mild and usually self-limiting respiratory tract infections by 30 to 40% [194, 195].
Unfortunately, in more poly-morbid and severely ill, and thus more inflamed patients admitted to the ED, CRP tended to increase antibiotic prescriptions for acute cough illness when compared to a clinical algorithm [196]. A low specificity, delayed dynamics and attenuated rise by concomitant steroid medication are important drawbacks of CRP as a biomarker in systemic and more peracute infections (sepsis) especially in the presence of poly-morbidity, which is omnipresent in hospitalised patients [84, 197]. Many physicians use it in hospitalised patients for the follow up of infectious diseases, despite CRPs drawbacks and misleads as marker, for example in LRTI admitted to the ED, [117] hospitalised CAP [28, 105], COPD [198] or sepsis [104].
Despite these limitations in the hospital setting, CRP is bundled in countless “admission labs”. This despite the lack of any guidelines to recommend this (mis-)use and no consequences for the decision-making or therapeutic process. Once established as a “standard of laboratory care” for admission and hospital follow-up, it is almost impossible to not use it in clinical routine. As a contagious laboratory disease artefact this leads directly or indirectly to additional investigations to differentiate the alleged “CRPitis”. This exemplifies the dilemma for biomarkers, that we are rapid in adopting new measures but reluctant to stop measuring them once we have got used to it. Another potential target for the “choosing wisely” of the American College of Physicians campaign?
In systemic infections, calcitonin (CALC) genes are ubiquitously expressed in parenchymal cells and, in essence, the entire body becomes an endocrine organ [90, 199]. PCT, a CALC-I gene product, is stimulated synergistically by the inflammatory mediators of host response (e.g., interleukin (IL)-1β, tumour-necrosis factor (TNF)-α, and IL-6), bacterial products (e.g., [LPS (lipopolysaccharide)], lipotechoic acid) and necrotic body cells [81, 199–202]. This typically occurs following external infection with bacterial micro-organisms. Bacterial translocation triggered across the gut wall by gastrointestinal malperfusion may trigger a similar cascade, which explains why circulating PCT increase both during septic and cardiogenic shock [203, 204].
PCT is the prototype of a hormokine mediator, sharing biological characteristics of both, hormones and cytokines [83, 205]. It circulates as 114 amino acid, lacking the N-terminal dipeptide alanine-proline [90]. Historically, a hormonal function of calcitonin peptides was alluded to calcium homeostasis and bone metabolism [90, 206, 207]. Today, they are thought to adapt metabolism and vascular tone to acute needs in inflammation [81, 93], to combat invading microbes during exogenous infections [208], to modulate migration and phagocytic activity of neutrophils, and to locally increase in pro-inflammatory cytokines and NO [90]. PCT modulates the action of other members of the calcitonin peptide superfamily, including CGRP (calcitonin gene-related peptide), ADM (adrenomedullin) and amylin [88]. The several 100’000–fold increased PCT levels nullify their activities, effects that likely are beneficial in this illness. Accordingly, administration of recombinant PCT protein to septic hamsters with peritonitis doubled their death rate [209]. Conversely, treatment with antiserum reactive against calcitonin precursors increased survival in mono- and polymicrobial sepsis in three animal models (hamster, rats and pigs) [209–212].
Unfortunately, PCT (aka “immune-reactive calcitonin”) was initially propagated as a dichotomous diagnostic and prognostic biomarker for critically-ill patients with pancreatitis [213] and toxic shock syndrome associated with hypocalcaemia, [214] burns and lung injury [215, 216], meningitis in children [217, 218], neonatal infections [219] and adult sepsis in intensive care units [87, 220, 221]. Superior to other biomarkers, PCT appeared to improve the clinical diagnosis of sepsis in critically ill patients [183]. At that time, sepsis was ill-defined as the presence (probable or documented) of infection together with systemic manifestations of inflammation [62, 179]. The consequent ambiguities in interpretation of different cohorts and settings precluded an undisputed sentence on the utility of PCT in the intensive care setting [180, 197, 222, 223]. PCT’s kinetic profile shows a prompt increase within 6–12 hours of infection and circulating PCT levels are cut in half daily when the infection is controlled [224]. PCT correlated with bacterial load [171, 173, 225] and severity of infection and outcome [226–228]. PCT offers additional prognostic information in high-risk patients as an adjunct to existing rules [229]. An increase of PCT suggested early identification of moribund critically ill patients despite being on antibiotic therapy [230]. A subsequent large ICU based multicentre PASS-trial, however, documented deleterious effects when antibiotics and diagnostic measures were escalated based on increasing PCT levels >1 ng/ml in patients for whom infection may not have been the main problem necessitating ICU admission [231]. In this study, measurement PCT was delayed as samples had to be shipped hundreds of kilometres to a central study laboratory. Protocol-driven there were more investigational procedures, increased side-effects of intensified antibiotic treatment and organ-related harm resulting in a prolonged stay in the intensive care unit [231]. The antibiotic escalation strategy like the one used in the PASS-trial can definitely not be recommended in a setting with a high baseline antibiotic exposure, such as an ICU in Denmark [232].
Conversely, a more rational antibiotic de-escalation strategy in critically-ill patients with suspected sepsis syndrome on antimicrobial therapy using adapted cut-off ranges proved to be safe and effective with 25% reduction of antibiotic exposure in the large multicentre PRORATA study in France [233].
To date, the efficacy and safety of PCT protocols to de-escalate antibiotic overuse has been demonstrated in more than 14 randomised, controlled trials in different clinical settings and including infections of varying severity [177, 234, 235]. The respiratory tract is the most common original site of bacterial sepsis [87]. Over 90% of all respiratory infections are initially presumed to be of single or even multiple viral origin [236]. Interestingly, cellular up-regulation of PCT is attenuated by cytokines released in response to viral infections, such as interferon-gamma (INF-γ) [201]. ICU studies including patients with only viral or both viral and bacterial CAP during the H1N1 outbreak found higher PCT levels in the latter group [237–239]. The PCT de-escalation protocols used were similar and all based on the same intuitive concept: recommendations for or against initiation or continuation of antibiotic therapy was based on initial PCT levels, the kinetics of PCT over time, or both, as well as the clinical picture of the patient [74]. The cut-off ranges differed depending on the clinical setting and the acuity of presentation [234]. These protocols proved to be safe and highly effective in terms of lowering antibiotic exposure. In fact, for low-severity patients, such as bronchitis and upper respiratory infections in general practice, prescription rates lowered by 60%–70% were found [177, 234, 235]. In the ED, antibiotic initiation was reduced by almost 50% in severe lower respiratory tract infections and 60% in severe exacerbations of COPD patients with need for hospitalisation [240, 241]. Of note, all these studies were done in settings with a very low antibiotic prescription rate in the control group. Thus, the effect would even more pronounced in countries with higher antibiotic exposure and resistance rates. In higher-severity patients, PCT guidance resulted in a relative reduction in the duration of antibiotic treatment by 40% in CAP and by 25% in the critical care setting [233, 242]. PCT-guide de-escalation of empirical antibiotic therapy resulted in lower medication costs, antibiotic side effects and adverse outcomes [5, 74].
Can these data, mostly obtained in hospitalised patients with respiratory tract infections, be indiscriminately applied to other sites of infection, to all age groups or sepsis in general? Clearly, NO! Community-acquired respiratory tract infections are relatively homogenous; a prerequisite that the procalcitonin algorithm actually worked. Indeed, “sepsis” is merely an ill-defined, heterogeneous clinical syndrome [243]. PCT can merely complement a physician and the clinical judgment on the probable site and source of infection will always remain a fundament for patient care. Therefore, the host response and optimal cut-off ranges for PCT vary with underlying illness, co-morbidity and immune status of the patients as well as the source and virulence of initial infection, such as meningitis, endocarditis, abdominal, urinary tract, catheter related or nosocomial infections [46]. Cut-off ranges have to be calculated by multilevel likelihood ratios and adapted to different settings and types of infection. Rapid and appropriately sensitive assays are to be used [45, 46, 244].
For example, the cut-off used in adults has been shown to be too low for children with a more reactive immune system [245, 246]. In new-borns there is even a physiological rise within the first postnatal days, presumed to be associated with intestinal bacterial colonisation [247, 248]. If the cut-off is adapted accordingly, PCT becomes a reliable marker to diagnose neonatal infections [249]. In a pilot-study from France, a PCT level of <0.5 ng/ml was used to avoid unnecessary antibiotics and hospitalisation in children during an outbreak of viral meningitis [250].
PCT reflects severity of renal lesions in pyelonephritis [251] and bacteremia and bacterial load and in the urosepsis syndrome [225]. Guidelines on the duration of antibiotic therapy are largely based on expert opinion only, therefore infections of the urinary tract are another target for PCT-guided stewardship [252]. Indeed, together with the resolution of pyuria [253], Combined pyuria and PCT guidance led to a 40% reduction of mean antibiotic exposure in a recent randomised pilot study [254].
In patients with suspected bloodstream infections, the aetiology of a presumed “bacterial” cause of fever cannot be detected in 50–80% [171, 173, 174, 255]. Clinicians order blood cultures liberally in patients admitted for CAP in the ED though they are costly and less than 5% of blood cultures yield true-positive results which change anti-microbial therapy [169]. PCT levels on admission accurately predict later blood culture positivity in immunocompetent patients with CAP. A PCT cut-off of 0.1, 0.25 or 0.5 mg/l would enable reduction of the total number of blood cultures by 13, 37 or 52% and still identify 99, 96 or 88%, respectively of positive blood cultures in CAP [171]. These figures are superior to clinical decision rules, which have practical limitations like the need for complex calculations and memorising [256]. As many as half of the positive cultures represent contaminants – organisms (usually coagulase negative staphylococci) inoculated from the skin at the time of sample collection. False-positive blood cultures expose patients to potential harm like additional diagnostic testing (additional blood cultures, echocardiograms, etc.), unnecessary antibiotic administration, missed alternative (infectious or non-infectious) diagnoses and prolonged hospitalisation [169]. PCT also seems to differentiate contaminants from true positive blood cultures [173].
In summary, PCT is the most reliable of the currently known circulating markers of systemic bacterial infections (“sepsis”); it is the only one that correlates well with its presence, course, and outcome in humans. PCT-guided antibiotic de-escalation therapy is considered evidence-based state-of-the-art in respiratory tract infections and recommended by updated guidelines [62, 257]. The hypothesis that hormokines are not only biomarkers but have a pivotal role in the pathophysiology of sepsis is at the least attractive, and at best intuitively obvious.
“With the rapid extension of laboratory tests of greater accuracy, there is a tendency that for reaching a final diagnosis clinicians and students rely more and more on laboratory reports and less on the patients history, the examination and behaviour of the patient, and clinical judgment. While in many cases laboratory findings are invaluable for reaching correct conclusions, the student should never be allowed to forget that it takes a man, not a machine, to understand a man.” A seemingly contemporary statement from 1946! [258].
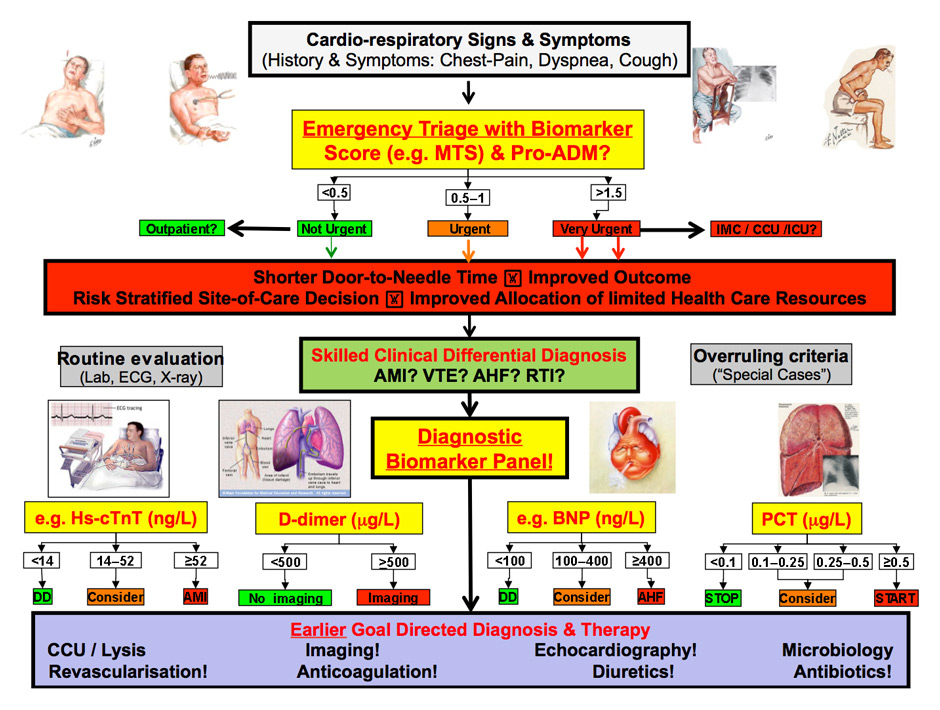
Figure 4
Personalised clinical and biomarker-guided medicine in the emergency department.
POC = point of care; MTS = Manchester Triage Score; ProADM = proadrenomedullin; ICM = intermediate care; CCU = coronary care unit; ICU = intensive care unit; Lab = laboratory analysis of biomarker level; X-ray = chest radiography; ACS = acute coronary syndrome; AHF = acute heart failure; RTI = respiratory tract infections; hs = highly sensitive; DD, = differential diagnosis
Some decry the loss of clinical skills with history taking and physical examination in routine patient care. Others suggest that emphasis on clinical skills is from a bygone era and that the availability of advanced imaging techniques and laboratory tests have supplanted ambiguous history and physical findings [26]. As usual, both extremes are irrational. The allegedly infallible impact of proper history and clinical exams in “the good old days” might appear nostalgic and outdated if applied to the more complex and diversified differential diagnostic spectrum and therapeutic options of modern medicine. Conversely, over-reliance on the new procedures contributes directly to misdiagnosis. Too often, palpably illogical laboratory findings are accepted without question [259]. Technology is an adjunct to clinical judgment and should not become a “gold standard” for diagnosis alone.
Proper risk stratification with biomarkers helps caregivers to more appropriately direct diagnostic, monitoring, or therapeutic interventions. Adverse events due to delays are a major contributor for adverse hospital outcome. In a busy emergency room, however, it is difficult or even impossible to identify patients in whom harm would have been prevented by more aggressive intervention. Substantial evidence exists for many conditions – sepsis for example – showing that earlier and more targeted intervention can improve patient outcomes, especially with the use of protocols or guidelines. Selected diagnostic and prognostic biomarkers should and will have a more prominent role in future emergency triage [260]. More personalised, better-targeted healthcare resource application offers opportunities to improve timeliness, safety, efficacy, and cost-effectiveness of care, as well as quality-of-life of patients and their loved ones. Rationalising for prevention of rationing is the credo!
The future of biomarkers lies in intervention studies across heterogeneous populations in combination with clinical scores and close to “real-life” settings. Comprehensive effectiveness research studies are being carried out aiming to further validate these concepts in “real-world-settings” and thereby improve patient care [261]. Results of these trials may ultimately help to transition from bundled treatment strategies to more individualised patient care thereby providing better, hopefully still empathic, management for the allocation of limited patient and societal resources (fig. 4).
Finally, as physicians we should always be aware that, before and after all, a patients is a human being seeking help. After we have tried to explain to him all the medical progress, fancy biomarkers for personalized diagnosis and novel options for individualised therapy, we might be puzzled by the question of the patient “Personalised medicine, doctor, does this mean that you have more personal time for taking care of me?”
‒ Every medical therapy in emergency care has potential adverse effects and expedited selection of patients most likely to benefit is crucial, making more personalised approaches necessary.
‒ Specific blood biomarkers may allow transition from generalising care bundles to a more tailored management in individual patients thereby reducing the risk for adverse treatment outcomes in patients who – based on their biomarker levels – do not likely benefit from therapy.
‒ Biomarkers measured on admission and during follow-up can support the clinician’s early recognition of cardiorespiratory, inflammatory and infectious diseases, severity assessment with adequate site-of care decisions and most effective therapeutic decisions in individual patients.
‒ Proadrenomedullin (ProADM) is an inflammatory prognostic marker that improves early mortality prediction and might improve site-of-care decisions, as tested in patients with respiratory infections.
‒ Initial D-dimer testing should be done when evaluating patients with either a low (with either a moderately or highly sensitive D-dimer test) or moderate clinical probability (highly sensitive D-dimer only) of venous thromboembolism (VTE). If the D-dimer test is negative (e.g. cut-off <500 μg/l) VTE is excluded, without the need for further imaging.
‒ Cardiac troponin I and T are quantitative markers of cardiomyocyte injury and indispensable tools in the early diagnosis of AMI.
‒ Natriuretic peptides are quantitative markers of hemodynamic cardiac stress and indispensable tools in the early diagnosis of acute heart failure
‒ C-reactive protein (CRP), as marker for more subtle sub-acute inflammation has a role in the risk selection for statin therapy in primary prevention of cardiovascular diseases.
‒ Procalcitonin (PCT) algorithms facilitate assessment of bacterial infection risk and appear to be safe in guiding therapeutic decisions about initiation and duration of anti-microbial therapy in infections with respiratory origin regarding the results from interventional trials.
1 Enderli SK, S. Lorber, C. Sandmeier, H. Gesundheitswesen Schweiz Ausgabe 2014. Basel: Interpharma, 2014.
2 Williams RM. The costs of visits to emergency departments. N Engl J Med. 1996;334(10):642–6.
3 Graber ML. The incidence of diagnostic error in medicine. BMJ quality & safety. 2013;22(Suppl 2):ii21–ii7.
4 Albrich WC, Dusemund F, Bucher B, Meyer S, Thomann R, Kuhn F, et al. Effectiveness and safety of procalcitonin-guided antibiotic therapy in lower respiratory tract infections in “real life”: an international, multicenter poststudy survey (ProREAL). Arch Intern Med. 2012;172(9):715–22.
5 Schuetz P, Mueller B. Biomarker-guided de-escalation of empirical therapy is associated with lower risk for adverse outcomes. Intensive Care Med. 2014;40(1):141.
6 Lipinski MJ, Escarcega RO, D’Ascenzo F, Magalhaes MA, Baker NC, Torguson R, et al. A Systematic Review and Collaborative Meta-Analysis to Determine the Incremental Value of Copeptin for Rapid Rule-Out of Acute Myocardial Infarction. Am J Cardiol. 2014;113(9):1581–91.
7 Raskovalova T, Twerenbold R, Collinson PO, Keller T, Bouvaist H, Folli C, et al. Diagnostic accuracy of combined cardiac troponin and copeptin assessment for early rule-out of myocardial infarction: a systematic review and meta-analysis. Eur Heart J Acute Cardiovasc Care. 2014;3(1):18–27.
8 Torbicki A, Perrier A, Konstantinides S, Agnelli G, Galie N, Pruszczyk P, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J. 2008;29(18):2276–315.
9 Aujesky D, Hayoz D, Yersin B, Perrier A, Barghouth G, Schnyder P, et al. Exclusion of pulmonary embolism using C-reactive protein and D-dimer. A prospective comparison. Thromb Haemost. 2003;90(6):1198–203.
10 Blendon RJ, DesRoches CM, Brodie M, Benson JM, Rosen AB, Schneider E, et al. Views of practicing physicians and the public on medical errors. N Engl J Med. 2002;347(24):1933–40.
11 Kaliniak C. Misdiagnosis is an overlooked and growing patient safety issue and core mission of isabel healthcare. Accessed May 18th.
12 Mangalmurti SS, Harold JG, Parikh PD, Flannery FT, Oetgen WJ. Characteristics of Medical Professional Liability Claims Against Internists. JAMA internal medicine. 2014.
13 Garber AM, Tunis SR. Does comparative-effectiveness research threaten personalized medicine? N Engl J Med. 2009;360(19):1925–7.
14 Paynter NP, Chasman DI, Buring JE, Shiffman D, Cook NR, Ridker PM. Cardiovascular disease risk prediction with and without knowledge of genetic variation at chromosome 9p21.3. Ann Intern Med. 2009;150(2):65–72.
15 Johnson EG, Horne BD, Carlquist JF, Anderson JL. Genotype-based dosing algorithms for warfarin therapy: data review and recommendations. Molecular diagnosis & therapy. 2011;15(5):255–64.
16 Mueller C, Scholer A, Laule-Kilian K, Martina B, Schindler C, Buser P, et al. Use of B-type natriuretic peptide in the evaluation and management of acute dyspnea. N Engl J Med. 2004;350(7):647–54.
17 Wallgren UM, Castren M, Svensson AE, Kurland L. Identification of adult septic patients in the prehospital setting: a comparison of two screening tools and clinical judgment. European journal of emergency medicine: official journal of the European Society for Emergency Medicine. 2013.
18 Heuer JF, Gruschka D, Crozier TA, Bleckmann A, Plock E, Moerer O, et al. Accuracy of prehospital diagnoses by emergency physicians: comparison with discharge diagnosis. European journal of emergency medicine: official journal of the European Society for Emergency Medicine. 2012;19(5):292–6.
19 Misch F, Messmer AS, Nickel CH, Gujan M, Graber A, Blume K, et al. Impact of observation on disposition of elderly patients presenting to emergency departments with non-specific complaints. PLoS One. 2014;9(5):e98097.
20 Nemec M, Koller MT, Nickel CH, Maile S, Winterhalder C, Karrer C, et al. Patients presenting to the emergency department with non-specific complaints: the Basel Non-specific Complaints (BANC) study. Acad Emerg Med. 2010;17(3):284–92.
21 Baehni C, Meier S, Spreiter P, Schild U, Regez K, Bossart R, et al. Which patients with lower respiratory tract infections need inpatient treatment? Perceptions of physicians, nurses, patients and relatives. BMC pulmonary medicine. 2010;10:12.
22 Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165(13):1493–9.
23 Platt R. Manchester University Medical School gazette. 1947;27:139–45.
24 Kirch W, Schafii C. Misdiagnosis at a university hospital in 4 medical eras. Medicine (Baltimore). 1996;75(1):29–40.
25 Leuppi JD, Dieterle T, Koch G, Martina B, Tamm M, Perruchoud AP, et al. Diagnostic value of lung auscultation in an emergency room setting. Swiss Med Wkly. 2005;135(35–36):520–4.
26 Feddock CA. The lost art of clinical skills. Am J Med. 2007;120(4):374–8.
27 Kevat DA, Cameron PA, Davies AR, Landrigan CP, Rajaratnam SW. Safer hours for doctors and improved safety for patients. Med J Aust. 2014;200(7):396–8.
28 Muller B, Harbarth S, Stolz D, Bingisser R, Mueller C, Leuppi J, et al. Diagnostic and prognostic accuracy of clinical and laboratory parameters in community-acquired pneumonia. BMC infectious diseases. 2007;7:10.
29 Spiteri MA, Cook DG, Clarke SW. Reliability of eliciting physical signs in examination of the chest. Lancet. 1988;1(8590):873–5.
30 Samaras N, Chevalley T, Samaras D, Gold G. Older patients in the emergency department: a review. Ann Emerg Med. 2010;56(3):261–9.
31 Metlay JP, Kapoor WN, Fine MJ. Does this patient have community-acquired pneumonia? Diagnosing pneumonia by history and physical examination. Jama. 1997;278(17):1440–5.
32 Koran LM. The reliability of clinical methods, data and judgments (first of two parts). N Engl J Med. 1975 293(13):642–6.
33 Koran LM. The reliability of clinical methods, data and judgments (second of two parts). N Engl J Med. 1975;293(14):695–701.
34 Mueller C, Frana B, Rodriguez D, Laule-Kilian K, Perruchoud AP. Emergency diagnosis of congestive heart failure: impact of signs and symptoms. Can J Cardiol. 2005;21(11):921–4.
35 Stolz D, Christ-Crain M, Gencay MM, Bingisser R, Huber PR, Muller B, et al. Diagnostic value of signs, symptoms and laboratory values in lower respiratory tract infection. Swiss Med Wkly. 2006;136(27–28):434–40.
36 Stolz D, Tamm M. Discriminate use of antibiotics for exacerbation of COPD. Current Opinion in Pulmonary Medicine. 2009;15(2):126–32.
37 Renaud B, Schuetz P, Claessens YE, Labarere J, Albrich W, Mueller B. Proadrenomedullin improves Risk of Early Admission to ICU score for predicting early severe community-acquired pneumonia. Chest. 2012;142(6):1447–54.
38 Bettencourt P, Azevedo A, Pimenta J, Frioes F, Ferreira S, Ferreira A. N-terminal-pro-brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation. 2004;110(15):2168–74.
39 Schuetz P, Litke A, Albrich WC, Mueller B. Blood biomarkers for personalized treatment and patient management decisions in community-acquired pneumonia. Curr Opin Infect Dis. 2013;26(2):159–67.
40 Albrich WC, Ruegger K, Dusemund F, Schuetz P, Arici B, Litke A, et al. Biomarker-enhanced triage in respiratory infections: a proof-of-concept feasibility trial. Eur Respir J. 2013;42(4):1064–75.
41 Epner PL, Gans JE, Graber ML. When diagnostic testing leads to harm: a new outcomes-based approach for laboratory medicine. BMJ quality & safety. 2013;22(Suppl 2):ii6–ii10.
42 Wahner-Roedler DL, Chaliki SS, Bauer BA, Bundrick JB, Bergstrom LR, Lee MC, et al. Who makes the diagnosis? The role of clinical skills and diagnostic test results. J Eval Clin Pract. 2007;13(3):321–5.
43 Hauser RG, Shirts BH. Do We Now Know What Inappropriate Laboratory Utilization Is? An Expanded Systematic Review of Laboratory Clinical Audits. Am J Clin Pathol. 2014;141(6):774–83.
44 Singh H, Arora HS, Vij MS, Rao R, Khan MM, Petersen LA. Communication outcomes of critical imaging results in a computerized notification system. J Am Med Inform Assoc. 2007;14(4):459–66.
45 Nylen E, Muller B, Becker KL, Snider R. The future diagnostic role of procalcitonin levels: the need for improved sensitivity. Clin Infect Dis. 2003;36(6):823–4; author reply 6–7.
46 Muller B, Christ-Crain M, Nylen ES, Snider R, Becker KL. Limits to the use of the procalcitonin level as a diagnostic marker. Clin Infect Dis. 2004;39(12):1867–8.
47 van Walraven C, Naylor CD. Do we know what inappropriate laboratory utilization is? A systematic review of laboratory clinical audits. JAMA. 1998;280(6):550–8.
48 Salisbury AC, Reid KJ, Alexander KP, Masoudi FA, Lai SM, Chan PS, et al. Diagnostic blood loss from phlebotomy and hospital-acquired anemia during acute myocardial infarction. Arch Intern Med. 2011;171(18):1646–53.
49 Detsky AS, Krumholz HM. Reducing the Trauma of Hospitalization. JAMA. 2014.
50 Cassel CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307(17):1801–2.
51 Attali M, Barel Y, Somin M, Beilinson N, Shankman M, Ackerman A, et al. A cost-effective method for reducing the volume of laboratory tests in a university-associated teaching hospital. The Mount Sinai journal of medicine, New York. 2006;73(5):787–94.
52 May TA, Clancy M, Critchfield J, Ebeling F, Enriquez A, Gallagher C, et al. Reducing unnecessary inpatient laboratory testing in a teaching hospital. Am J Clin Pathol. 2006;126(2):200–6.
53 Burt CW, McCaig LF, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2005. Adv Data. 2007;388:1–15.
54 McCaig LF, Burt CW. National Hospital Ambulatory Medical Care Survey: 2002 emergency department summary. Adv Data. 2004;340:1–34.
55 Cantor WJ, Fitchett D, Borgundvaag B, Ducas J, Heffernan M, Cohen EA, et al. Routine early angioplasty after fibrinolysis for acute myocardial infarction. N Engl J Med. 2009;360(26):2705–18.
56 Adams HP, Jr., Effron MB, Torner J, Davalos A, Frayne J, Teal P, et al. Emergency administration of abciximab for treatment of patients with acute ischemic stroke: results of an international phase III trial: Abciximab in Emergency Treatment of Stroke Trial (AbESTT-II). Stroke. 2008;39(1):87–99.
57 Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–96.
58 Puskarich MA, Trzeciak S, Shapiro NI, Heffner AC, Kline JA, Jones AE, et al. Outcomes of patients undergoing early sepsis resuscitation for cryptic shock compared with overt shock. Resuscitation. 2011;82(10):1289–93.
59 Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–77.
60 Kumar A, Haery C, Paladugu B, Kumar A, Symeoneides S, Taiberg L, et al. The duration of hypotension before the initiation of antibiotic treatment is a critical determinant of survival in a murine model of Escherichia coli septic shock: association with serum lactate and inflammatory cytokine levels. J Infect Dis. 2006;193(2):251–8.
61 Puskarich MA, Trzeciak S, Shapiro NI, Arnold RC, Horton JM, Studnek JR, et al. Association between timing of antibiotic administration and mortality from septic shock in patients treated with a quantitative resuscitation protocol. Crit Care Med. 2011;39(9):2066–71.
62 Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165–228.
63 Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358(2):125–39.
64 Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358(2):111–24.
65 Fried BJ, Topping S, Morrissey JP, Ellis AR, Stroup S, Blank M. Comparing provider perceptions of access and utilization management in full-risk and no-risk Medicaid programs for adults with serious mental illness. The journal of behavioral health services & research. 2000;27(1):29–46.
66 Aliyu ZY, Aliyu MH, McCormick K. Determinants for hospitalization in “low-risk” community acquired pneumonia. BMC Infect Dis. 2003;3:11.
67 Labarere J, Stone RA, Scott Obrosky D, Yealy DM, Meehan TP, Auble TE, et al. Factors associated with the hospitalization of low-risk patients with community-acquired pneumonia in a cluster-randomized trial. J Gen Intern Med. 2006;21(7):745–52.
68 Menendez R, Cremades MJ, Martinez-Moragon E, Soler JJ, Reyes S, Perpina M. Duration of length of stay in pneumonia: influence of clinical factors and hospital type. Eur Respir J. 2003;22(4):643–8.
69 McGregor MJ, Fitzgerald JM, Reid RJ, Levy AR, Schulzer M, Jung D, et al. Determinants of hospital length of stay among patients with pneumonia admitted to a large Canadian hospital from 1991 to 2001. Can Respir J. 2005;12(7):365–70.
70 Self WH, Grijalva CG, Zhu Y, McNaughton CD, Barrett TW, Collins SP, et al. Rates of emergency department visits due to pneumonia in the United States, July 2006–June 2009. Acad Emerg Med. 2013;20(9):957–60.
71 Dixon RE. Economic costs of respiratory tract infections in the United States. Am J Med. 1985;78(6B):45–51.
72 Niederman MS, Mandell LA, Anzueto A, Bass JB, Broughton WA, Campbell GD, et al. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med. 2001;163(7):1730–54.
73 Chalmers JD, Al-Khairalla M, Short PM, Fardon TC, Winter JH. Proposed changes to management of lower respiratory tract infections in response to the Clostridium difficile epidemic. J Antimicrob Chemother. 2010;65(4):608–18.
74 Schuetz P, Christ-Crain M, Thomann R, Falconnier C, Wolbers M, Widmer I, et al. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059–66.
75 Yealy DM, Auble TE, Stone RA, Lave JR, Meehan TP, Graff LG, et al. Effect of increasing the intensity of implementing pneumonia guidelines: a randomized, controlled trial. Ann Intern Med. 2005;143(12):881–94.
76 Marrie TJ. Deaths in risk classes I–III: a measure of quality of care in patients hospitalised with CAP? Eur Respir J. 2004;23(1):103–5.
77 Hedlund JU, Ortqvist AB, Kalin ME, Granath F. Factors of importance for the long term prognosis after hospital treated pneumonia. Thorax. 1993;48(8):785–9.
78 Jasti H, Mortensen EM, Obrosky DS, Kapoor WN, Fine MJ. Causes and risk factors for rehospitalization of patients hospitalized with community-acquired pneumonia. Clin Infect Dis. 2008;46(4):550–6.
79 Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, et al. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. BiochemBiophysResCommun. 1993;192(2):553–60.
80 Kitamura K, Sakata J, Kangawa K, Kojima M, Matsuo H, Eto T. Cloning and characterization of cDNA encoding a precursor for human adrenomedullin. Biochem Biophys Res Commun. 1993;194(2):720–5.
81 Linscheid P, Seboek D, Zulewski H, Keller U, Muller B. Autocrine/paracrine role of inflammation-mediated calcitonin gene-related peptide and adrenomedullin expression in human adipose tissue. Endocrinology. 2005;146(6):2699–708.
82 Schuetz P, Christ-Crain M, Muller B. Biomarkers to improve diagnostic and prognostic accuracy in systemic infections. Curr Opin Crit Care. 2007;13(5):578–85.
83 Christ-Crain M, Muller B. Calcitonin peptides – the mediators in sepsis or just another fairy tale? Crit Care Med. 2008;36(5):1684–7.
84 Schuetz P, Christ-Crain M, Muller B. Procalcitonin and other biomarkers to improve assessment and antibiotic stewardship in infections--hope for hype? Swiss Med Wkly. 2009;139(23–24):318–26.
85 Becker KL, Nylen ES, White JC, Muller B, Snider RH, Jr. Clinical review 167: Procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. J Clin Endocrinol Metab. 2004;89(4):1512–25.
86 Becker KL, Snider R, Nylen ES. Procalcitonin assay in systemic inflammation, infection, and sepsis: clinical utility and limitations. Crit Care Med. 2008;36(3):941–52.
87 Muller B, Becker KL, Schachinger H, Rickenbacher PR, Huber PR, Zimmerli W, et al. Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Crit Care Med. 2000;28(4):977–83.
88 Sexton PM, Christopoulos G, Christopoulos A, Nylen ES, Snider RH, Jr., Becker KL. Procalcitonin has bioactivity at calcitonin receptor family complexes: potential mediator implications in sepsis. Crit Care Med. 2008;36(5):1637–40.
89 McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, et al. RAMPs regulate the transport and ligand specificity of the calcitonin- receptor-like receptor. Nature. 1998;393(6683):333–9.
90 Becker KL, Nylen ES, White JC, Muller B, Snider RH, Jr. Procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. J Clin Endocrinol Metab. 2004;89(4):1512–25.
91 Elsasser TH, Kahl S. Adrenomedullin has multiple roles in disease stress: development and remission of the inflammatory response. Microsc Res Tech. 2002;57(2):120–9.
92 Xue Y, Taub P, Iqbal N, Fard A, Clopton P, Maisel A. Mid-region pro-adrenomedullin adds predictive value to clinical predictors and Framingham risk score for long-term mortality in stable outpatients with heart failure. Eur J Heart Fail. 2013;15(12):1343–9.
93 Schuetz P, Christ-Crain M, Morgenthaler NG, Struck J, Bergmann A, Muller B. Circulating precursor levels of endothelin-1 and adrenomedullin, two endothelium-derived, counteracting substances, in sepsis. Endothelium: journal of endothelial cell research. 2007;14(6):345–51.
94 Hoeboer SH, Alberts E, van den Hul I, Tacx AN, Debets-Ossenkopp YJ, Groeneveld AB. Old and new biomarkers for predicting high and low risk microbial infection in critically ill patients with new onset fever: a case for procalcitonin. J Infect. 2012;64(5):484–93.
95 Allaker RP, Grosvenor PW, McAnerney DC, Sheehan BE, Srikanta BH, Pell K, et al. Mechanisms of adrenomedullin antimicrobial action. Peptides. 2006;27(4):661–6.
96 Matsui H, Shimosawa T, Itakura K, Guanqun X, Ando K, Fujita T. Adrenomedullin can protect against pulmonary vascular remodeling induced by hypoxia. Circulation. 2004;109(18):2246–51.
97 Smith JG, Newton-Cheh C, Hedblad B, Struck J, Morgenthaler NG, Bergmann A, et al. Distribution and correlates of midregional proadrenomedullin in the general population. Clinical chemistry. 2009;55(8):1593–5.
98 Temmesfeld-Wollbruck B, Hocke AC, Suttorp N, Hippenstiel S. Adrenomedullin and endothelial barrier function. Thromb Haemost. 2007;98(5):944–51.
99 Stolz D, Kostikas K, Blasi F, Boersma W, Milenkovic B, Lacoma A, et al. Adrenomedullin refines mortality prediction by the BODE index in COPD: the “BODE-A” index. Eur Respir J. 2014;43(2):397–408.
100 Morgenthaler NG, Struck J, Alonso C, Bergmann A. Measurement of midregional proadrenomedullin in plasma with an immunoluminometric assay. Clinical chemistry. 2005;51(10):1823–9.
101 Lewis LK, Smith MW, Yandle TG, Richards AM, Nicholls MG. Adrenomedullin(1–52) measured in human plasma by radioimmunoassay: plasma concentration, adsorption, and storage. Clinical chemistry. 1998;44(3):571–7.
102 Struck J, Tao C, Morgenthaler NG, Bergmann A. Identification of an Adrenomedullin precursor fragment in plasma of sepsis patients. Peptides. 2004;25(8):1369–72.
103 Goode KM, Nicholls R, Pellicori P, Clark AL, Cleland JG. The in vitro stability of novel cardiovascular and sepsis biomarkers at ambient temperature. Clin Chem Lab Med. 2014;52(6):911–8.
104 Christ-Crain M, Morgenthaler NG, Struck J, Harbarth S, Bergmann A, Muller B. Mid-regional pro-adrenomedullin as a prognostic marker in sepsis: an observational study. Crit Care. 2005;9(6):R816–24.
105 Christ-Crain M, Morgenthaler NG, Stolz D, Muller C, Bingisser R, Harbarth S, et al. Pro-adrenomedullin to predict severity and outcome in community-acquired pneumonia [ISRCTN04176397]. Crit Care. 2006;10(3):R96.
106 Chalmers JD, Al-Khairalla M, Short PM, Fardon TC, Winter JH. Proposed changes to management of lower respiratory tract infections in response to the Clostridium difficile epidemic. J Antimicrob Chemother. 2010.
107 Holm A, Pedersen SS, Nexoe J, Obel N, Nielsen LP, Koldkjaer O, et al. Procalcitonin versus C-reactive protein for predicting pneumonia in adults with lower respiratory tract infection in primary care. Br J Gen Pract. 2007;57(540):555–60.
108 Kruger S, Ewig S, Marre R, Papassotiriou J, Richter K, von Baum H, et al. Procalcitonin predicts patients at low risk of death from community-acquired pneumonia across all CRB-65 classes. Eur Respir J. 2008;31(2):349–55.
109 Menendez R, Martinez R, Reyes S, Mensa J, Filella X, Marcos MA, et al. Biomarkers improve mortality prediction by prognostic scales in community-acquired pneumonia. Thorax. 2009;64(7):587–91.
110 Huang DT, Angus DC, Kellum JA, Pugh NA, Weissfeld LA, Struck J, et al. Midregional proadrenomedullin as a prognostic tool in community-acquired pneumonia. Chest. 2009;136(3):823–31.
111 Schuetz P, Wolbers M, Christ-Crain M, Thomann R, Falconnier C, Widmer I, et al. Prohormones for prediction of adverse medical outcome in community-acquired pneumonia and lower respiratory tract infections. Crit Care. 2010;14(3) R106.
112 Stolz D, Christ-Crain M, Morgenthaler NG, Miedinger D, Leuppi J, Muller C, et al. Plasma pro-adrenomedullin but not plasma pro-endothelin predicts survival in exacerbations of COPD. Chest. 2008;134(2):263–72.
113 Christ-Crain M, Breidthardt T, Stolz D, Zobrist K, Bingisser R, Miedinger D, et al. Use of B-type natriuretic peptide in the risk stratification of community-acquired pneumonia. J Intern Med. 2008;264(2):166–76.
114 Christ-Crain M, Schuetz P, Müller B. Biomarkers in the management of pneumonia. Expert Rev Respir Med. 2008;2(5):565–72.
115 Christ-Crain M, Stolz D, Jutla S, Couppis O, Muller C, Bingisser R, et al. Free and total cortisol levels as predictors of severity and outcome in community-acquired pneumonia. Am J Respir Crit Care Med. 2007;176(9):913–20.
116 Muller B, Morgenthaler N, Stolz D, Schuetz P, Muller C, Bingisser R, et al. Circulating levels of copeptin, a novel biomarker, in lower respiratory tract infections. Eur J Clin Invest. 2007;37(2):145–52.
117 Muller B, Suess E, Schuetz P, Muller C, Bingisser R, Bergmann A, et al. Circulating levels of pro-atrial natriuretic peptide in lower respiratory tract infections. J Intern Med. 2006;260(6):568–76.
118 Schuetz P, Muller B, Nusbaumer C, Wieland M, Christ-Crain M. Circulating levels of GH predict mortality and complement prognostic scores in critically ill medical patients. Eur J Endocrinol. 2009;160(2):157–63.
119 Schuetz P, Stolz D, Mueller B, Morgenthaler NG, Struck J, Mueller C, et al. Endothelin-1 precursor peptides correlate with severity of disease and outcome in patients with community acquired pneumonia. BMC infectious diseases. 2008;8:22.
120 Chalmers JD, Singanayagam A, Scally C, Hill AT. Admission D-dimer can identify low-risk patients with community-acquired pneumonia. Ann Emerg Med. 2009;53(5):633–8.
121 Albrich WC, Dusemund F, Ruegger K, Christ-Crain M, Zimmerli W, Bregenzer T, et al. Enhancement of CURB65 score with proadrenomedullin (CURB65–A) for outcome prediction in lower respiratory tract infections: derivation of a clinical algorithm. BMC infectious diseases. 2011;11:112.
122 Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined--a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36(3):959–69.
123 Bertrand ME, Simoons ML, Fox KA, Wallentin LC, Hamm CW, McFadden E, et al. Management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2002;23(23):1809–40.
124 Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. Eur Heart J. 2012;33(20):2551–67.
125 Martina B, Bucheli B, Stotz M, Battegay E, Gyr N. First clinical judgment by primary care physicians distinguishes well between nonorganic and organic causes of abdominal or chest pain. J Gen Intern Med. 1997;12(8):459–65.
126 Hamm CW, Goldmann BU, Heeschen C, Kreymann G, Berger J, Meinertz T. Emergency room triage of patients with acute chest pain by means of rapid testing for cardiac troponin T or troponin I. N Engl J Med. 1997;337(23):1648–53.
127 Mueller C, Neumann FJ, Perruchoud AP, Zeller T, Buettner HJ. Prognostic value of quantitative troponin T measurements in unstable angina/non-ST-segment elevation acute myocardial infarction treated early and predominantly with percutaneous coronary intervention. Am J Med. 2004;117(12):897–902.
128 Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60(16):1581–98.
129 Reichlin T, Schindler C, Drexler B, Twerenbold R, Reiter M, Zellweger C, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med. 2012;172(16):1211–8.
130 Mockel M, Searle J, Hamm C, Slagman A, Blankenberg S, Huber K, et al. Early discharge using single cardiac troponin and copeptin testing in patients with suspected acute coronary syndrome (ACS): a randomized, controlled clinical process study. Eur Heart J. 2014.
131 Wang CS, FitzGerald JM, Schulzer M, Mak E, Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA. 2005;294(15):1944–56.
132 Davis M, Espiner E, Richards G, Billings J, Town I, Neill A, et al. Plasma brain natriuretic peptide in assessment of acute dyspnoea. Lancet. 1994;343(8895):440–4.
133 Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347(3):161–7.
134 Silver MA, Maisel A, Yancy CW, McCullough PA, Burnett JC, Jr., Francis GS, et al. BNP Consensus Panel 2004: A clinical approach for the diagnostic, prognostic, screening, treatment monitoring, and therapeutic roles of natriuretic peptides in cardiovascular diseases. Congest Heart Fail. 2004;10(5 Suppl 3):1–30.
135 Januzzi JL, van Kimmenade R, Lainchbury J, Bayes-Genis A, Ordonez-Llanos J, Santalo-Bel M, et al. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT-proBNP Study. Eur Heart J. 2006;27(3):330–7.
136 Mueller C, Breidthardt T, Laule-Kilian K, Christ M, Perruchoud AP. The integration of BNP and NT-proBNP into clinical medicine. Swiss Med Wkly. 2007;137(1–2):4–12.
137 Ritter M, Laule-Kilian K, Klima T, Christ A, Christ M, Perruchoud A, et al. Gender differences in acute congestive heart failure. Swiss Med Wkly. 2006;136(19–20):311–7.
138 Moe GW, Howlett J, Januzzi JL, Zowall H, Canadian Multicenter Improved Management of Patients With Congestive Heart Failure Study I. N-terminal pro-B-type natriuretic peptide testing improves the management of patients with suspected acute heart failure: primary results of the Canadian prospective randomized multicenter IMPROVE-CHF study. Circulation. 2007;115(24):3103–10.
139 Christ M, Laule-Kilian K, Hochholzer W, Klima T, Breidthardt T, Perruchoud AP, et al. Gender-specific risk stratification with B-type natriuretic peptide levels in patients with acute dyspnea: insights from the B-type natriuretic peptide for acute shortness of breath evaluation study. J Am Coll Cardiol. 2006;48(9):1808–12.
140 Logeart D, Thabut G, Jourdain P, Chavelas C, Beyne P, Beauvais F, et al. Predischarge B-type natriuretic peptide assay for identifying patients at high risk of re-admission after decompensated heart failure. J Am Coll Cardiol. 2004;43(4):635–41.
141 Troughton RW, Frampton CM, Yandle TG, Espiner EA, Nicholls MG, Richards AM. Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (N-BNP) concentrations. Lancet. 2000;355(9210):1126–30.
142 Jourdain P, Jondeau G, Funck F, Gueffet P, Le Helloco A, Donal E, et al. Plasma brain natriuretic peptide-guided therapy to improve outcome in heart failure: the STARS-BNP Multicenter Study. J Am Coll Cardiol. 2007;49(16):1733–9.
143 Richards AM, Doughty R, Nicholls MG, MacMahon S, Sharpe N, Murphy J, et al. Plasma N-terminal pro-brain natriuretic peptide and adrenomedullin: prognostic utility and prediction of benefit from carvedilol in chronic ischemic left ventricular dysfunction. Australia-New Zealand Heart Failure Group. J Am Coll Cardiol. 2001;37(7):1781–7.
144 Investigators P. Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). JAMA: the journal of the American Medical Association. 1990;263(20):2753–9.
145 Cogo A, Lensing AW, Prandoni P, Hirsh J. Distribution of thrombosis in patients with symptomatic deep vein thrombosis. Implications for simplifying the diagnostic process with compression ultrasound. Arch Intern Med. 1993;153(24):2777–80.
146 Bates SM. D-dimer assays in diagnosis and management of thrombotic and bleeding disorders. Semin Thromb Hemost. 2012;38(7):673–82.
147 Rowbotham BJ, Carroll P, Whitaker AN, Bunce IH, Cobcroft RG, Elms MJ, et al. Measurement of crosslinked fibrin derivatives – use in the diagnosis of venous thrombosis. Thromb Haemost. 1987;57(1):59–61.
148 Bounameaux H, Cirafici P, de Moerloose P, Schneider PA, Slosman D, Reber G, et al. Measurement of D-dimer in plasma as diagnostic aid in suspected pulmonary embolism. Lancet. 1991;337(8735):196–200.
149 Stein PD, Hull RD, Patel KC, Olson RE, Ghali WA, Brant R, et al. D-dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review. Ann Intern Med. 2004;140(8):589–602.
150 Di Nisio M, Squizzato A, Rutjes AW, Buller HR, Zwinderman AH, Bossuyt PM. Diagnostic accuracy of D-dimer test for exclusion of venous thromboembolism: a systematic review. Journal of thrombosis and haemostasis: JTH. 2007;5(2):296–304.
151 Fancher TL, White RH, Kravitz RL. Combined use of rapid D-dimer testing and estimation of clinical probability in the diagnosis of deep vein thrombosis: systematic review. BMJ. 2004;329(7470):821.
152 Carrier M, Righini M, Djurabi RK, Huisman MV, Perrier A, Wells PS, et al. VIDAS D-dimer in combination with clinical pre-test probability to rule out pulmonary embolism. A systematic review of management outcome studies. Thromb Haemost. 2009;101(5):886–92.
153 Kruip MJ, Leclercq MG, van der Heul C, Prins MH, Buller HR. Diagnostic strategies for excluding pulmonary embolism in clinical outcome studies. A systematic review. Ann Intern Med. 2003;138(12):941–51.
154 Ten Cate-Hoek AJ, Prins MH. Management studies using a combination of D-dimer test result and clinical probability to rule out venous thromboembolism: a systematic review. Journal of thrombosis and haemostasis: JTH. 2005;3(11):2465–70.
155 Geersing GJ, Zuithoff NP, Kearon C, Anderson DR, Ten Cate-Hoek AJ, Elf JL, et al. Exclusion of deep vein thrombosis using the Wells rule in clinically important subgroups: individual patient data meta-analysis. BMJ. 2014;348:g1340.
156 Wells PS, Anderson DR, Rodger M, Forgie M, Kearon C, Dreyer J, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med. 2003;349(13):1227–35.
157 Kearon C, Ginsberg JS, Douketis J, Turpie AG, Bates SM, Lee AY, et al. An evaluation of D-dimer in the diagnosis of pulmonary embolism: a randomized trial. Ann Intern Med. 2006;144(11):812–21.
158 Bates SM, Jaeschke R, Stevens SM, Goodacre S, Wells PS, Stevenson MD, et al. Diagnosis of DVT: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e351S-418S.
159 Schuur JD, Carney DP, Lyn ET, Raja AS, Michael JA, Ross NG, et al. A top-five list for emergency medicine: a pilot project to improve the value of emergency care. JAMA internal medicine. 2014;174(4):509–15.
160 Righini M, Goehring C, Bounameaux H, Perrier A. Effects of age on the performance of common diagnostic tests for pulmonary embolism. Am J Med. 2000;109(5):357–61.
161 Righini M, Le Gal G, De Lucia S, Roy PM, Meyer G, Aujesky D, et al. Clinical usefulness of D-dimer testing in cancer patients with suspected pulmonary embolism. Thromb Haemost. 2006;95(4):715–9.
162 Miron MJ, Perrier A, Bounameaux H, de Moerloose P, Slosman DO, Didier D, et al. Contribution of noninvasive evaluation to the diagnosis of pulmonary embolism in hospitalized patients. Eur Respir J. 1999;13(6):1365–70.
163 Segard T, Macdonald WB. Changing trends in venous thromboembolism-related imaging in Western Australian teaching hospitals, 2002–2010. Med J Aust. 2013;198(2):100–3.
164 Ingber S, Selby R, Lee J, Geerts W, Brnjac E. Combination pretest probability assessment and D-dimer did not reduce outpatient imaging for venous thromboembolism in a tertiary care hospital emergency department. Cjem. 2014;16(1):53–62.
165 Singh B, Mommer SK, Erwin PJ, Mascarenhas SS, Parsaik AK. Pulmonary embolism rule-out criteria (PERC) in pulmonary embolism--revisited: a systematic review and meta-analysis. Emergency medicine journal: EMJ. 2013;30(9):701–6.
166 Le Gal G, Bounameaux H. Diagnosing pulmonary embolism: running after the decreasing prevalence of cases among suspected patients. Journal of thrombosis and haemostasis: JTH. 2004;2(8):1244–6.
167 Righini M, Van Es J, Den Exter PL, Roy PM, Verschuren F, Ghuysen A, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA. 2014;311(11):1117–24.
168 Sackett DL. The rational clinical examination. A primer on the precision and accuracy of the clinical examination. JAMA. 1992;267(19):2638–44.
169 Coburn B, Morris AM, Tomlinson G, Detsky AS. Does this adult patient with suspected bacteremia require blood cultures? JAMA. 2012;308(5):502–11.
170 Bates DW, Cook EF, Goldman L, Lee TH. Predicting bacteremia in hospitalized patients. A prospectively validated model. Ann Intern Med. 1990;113(7):495–500.
171 Muller F, Christ-Crain M, Bregenzer T, Krause M, Zimmerli W, Mueller B, et al. Procalcitonin levels predict bacteremia in patients with community-acquired pneumonia: a prospective cohort trial. Chest. 2010;138(1):121–9.
172 Bates DW, Goldman L, Lee TH. Contaminant blood cultures and resource utilization. The true consequences of false-positive results. JAMA. 1991;265(3):365–9.
173 Schuetz P, Mueller B, Trampuz A. Serum procalcitonin for discrimination of blood contamination from bloodstream infection due to coagulase-negative staphylococci. Infection. 2007;35(5):352–5.
174 Muller B, Schuetz P, Trampuz A. Circulating biomarkers as surrogates for bloodstream infections. Int J Antimicrob Agents. 2007;30(Suppl 1):S16–23.
175 Bullowa JGM, Wilcox C. Incidence of bacteremia in the pneumonias and its relation to mortality. Arch Intern Med. 1935;55:558–73.
176 Schuetz P, Albrich W, Mueller B. Procalcitonin for diagnosis of infection and guide to antibiotic decisions: past, present and future. BMC medicine. 2011;9(1):107.
177 Schuetz P, Briel M, Mueller B. Clinical outcomes associated with procalcitonin algorithms to guide antibiotic therapy in respiratory tract infections. JAMA. 2013;309(7):717–8.
178 Haubitz S, Mueller B, Schuetz P. Streamlining antibiotic therapy with procalcitonin protocols: consensus and controversies. Expert Rev Respir Med. 2013;7(2):145–57.
179 Vincent JL. Dear SIRS, I’m sorry to say that I don’t like you.. 1997;25(2):372–4.
180 Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis. 2004;39(2):206–17.
181 Tang BM, Eslick GD, Craig JC, McLean AS. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: systematic review and meta-analysis. Lancet Infect Dis. 2007;7(3):210–7.
182 Tang H, Huang T, Jing J, Shen H, Cui W. Effect of procalcitonin-guided treatment in patients with infections: a systematic review and meta-analysis. Infection. 2009;37(6):497–507.
183 Harbarth S, Holeckova K, Froidevaux C, Pittet D, Ricou B, Grau GE, et al. Diagnostic value of procalcitonin, interleukin-6, and interleukin-8 in critically ill patients admitted with suspected sepsis. Am J Respir Crit Care Med. 2001;164(3):396–402.
184 Muller B, Peri G, Doni A, Perruchoud AP, Landmann R, Pasqualini F, et al. High circulating levels of the IL-1 type II decoy receptor in critically ill patients with sepsis: association of high decoy receptor levels with glucocorticoid administration. J Leukoc Biol. 2002;72(4):643–9.
185 Howell MD, Talmor D, Schuetz P, Hunziker S, Jones AE, Shapiro NI. Proof of principle: the predisposition, infection, response, organ failure sepsis staging system. Crit Care Med. 2011;39(2):322–7.
186 Rhodes B, Furnrohr BG, Vyse TJ. C-reactive protein in rheumatology: biology and genetics. Nat Rev Rheumatol. 2011;7(5):282–9.
187 Henriquez-Camacho C, Losa J. Biomarkers for sepsis. BioMed research international. 2014;2014:547818.
188 Libby P, Ridker PM. Inflammation and atherosclerosis: role of C-reactive protein in risk assessment. Am J Med. 2004;116(Suppl 6A):9S–16S.
189 Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–43.
190 Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347(20):1557–65.
191 Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr., Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–207.
192 Barnett ML, Linder JA. Antibiotic prescribing to adults with sore throat in the United States, 1997–2010. JAMA internal medicine. 2014;174(1):138–40.
193 Samore MH, Bateman K, Alder SC, Hannah E, Donnelly S, Stoddard GJ, et al. Clinical decision support and appropriateness of antimicrobial prescribing: a randomized trial. JAMA. 2005;294(18):2305–14.
194 Cals JW, Butler CC, Hopstaken RM, Hood K, Dinant GJ. Effect of point of care testing for C reactive protein and training in communication skills on antibiotic use in lower respiratory tract infections: cluster randomised trial. BMJ. 2009;338:b1374.
195 Cals JW, Schot MJ, de Jong SA, Dinant GJ, Hopstaken RM. Point-of-care C-reactive protein testing and antibiotic prescribing for respiratory tract infections: a randomized controlled trial. Ann Fam Med. 2010;8(2):124–33.
196 Gonzales R, Aagaard EM, Camargo CA, Jr., Ma OJ, Plautz M, Maselli JH, et al. C-reactive protein testing does not decrease antibiotic use for acute cough illness when compared to a clinical algorithm. J Emerg Med. 2011;41(1):1–7.
197 Christ-Crain M, Muller B. Procalcitonin in bacterial infections – hype, hope, more or less? Swiss Med Wkly. 2005;135(31–32):451–60.
198 Stolz D, Christ-Crain M, Morgenthaler NG, Leuppi J, Miedinger D, Bingisser R, et al. Copeptin, C-reactive protein, and procalcitonin as prognostic biomarkers in acute exacerbation of COPD. Chest. 2007;131(4):1058–67.
199 Muller B, White JC, Nylen ES, Snider RH, Becker KL, Habener JF. Ubiquitous expression of the calcitonin-i gene in multiple tissues in response to sepsis. J Clin Endocrinol Metab. 2001;86(1):396–404.
200 Linscheid P, Seboek D, Nylen ES, Langer I, Schlatter M, Becker KL, et al. In vitro and in vivo calcitonin I gene expression in parenchymal cells: a novel product of human adipose tissue. Endocrinology. 2003;144(12):5578–84.
201 Linscheid P, Seboek D, Schaer DJ, Zulewski H, Keller U, Muller B. Expression and secretion of procalcitonin and calcitonin gene-related peptide by adherent monocytes and by macrophage-activated adipocytes. Crit Care Med. 2004;32(8):1715–21.
202 Dandona P, Nix D, Wilson MF, Aljada A, Love J, Assicot M, et al. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab. 1994;79(6):1605–8.
203 de Werra I, Jaccard C, Corradin SB, Chiolero R, Yersin B, Gallati H, et al. Cytokines, nitrite/nitrate, soluble tumor necrosis factor receptors, and procalcitonin concentrations: comparisons in patients with septic shock, cardiogenic shock, and bacterial pneumonia. Crit Care Med. 1997;25(4):607–13.
204 Brunkhorst FM, Clark AL, Forycki ZF, Anker SD. Pyrexia, procalcitonin, immune activation and survival in cardiogenic shock: the potential importance of bacterial translocation. Int J Cardiol. 1999;72(1):3–10.
205 Becker KL, Snider R, Nylen ES. Procalcitonin in sepsis and systemic inflammation: a harmful biomarker and a therapeutic target. Br J Pharmacol. 2010;159(2):253–64.
206 Muller B, Becker KL, Kranzlin M, Schachinger H, Huber PR, Nylen ES, et al. Disordered calcium homeostasis of sepsis: association with calcitonin precursors. Eur J Clin Invest. 2000;30(9):823–31.
207 Habener JF, Schiller AL. Pathogenesis of renal osteodystrophy – a role for calcitonin? 1977;296(19):1112–4.
208 Zudaire E, Portal-Nunez S, Cuttitta F. The central role of adrenomedullin in host defense. J Leukoc Biol. 2006;80(2):237–44.
209 Nylen ES, Whang KT, Snider RH, Jr., Steinwald PM, White JC, Becker KL. Mortality is increased by procalcitonin and decreased by an antiserum reactive to procalcitonin in experimental sepsis. Crit Care Med. 1998;26(6):1001–6.
210 Tavares E, Minano FJ. Immunoneutralization of the aminoprocalcitonin peptide of procalcitonin protects rats from lethal endotoxaemia: neuroendocrine and systemic studies. Clin Sci (Lond). 2010;119(12):519–34.
211 Martinez JM, Wagner KE, Snider RH, Nylen ES, Muller B, Sarani B, et al. Late immunoneutralization of procalcitonin arrests the progression of lethal porcine sepsis. Surgical Inf. 2002;2:193–201.
212 Wagner KE, Martinez JM, Vath SD, Snider RH, Nylen ES, Becker KL, et al. Early immunoneutralization of calcitonin precursors attenuates the adverse physiologic response to sepsis in pigs. Crit Care Med. 2002;30(10):2313–21.
213 Canale DD, Donabedian RK. Hypercalcitoninemia in acute pancreatitis. J Clin Endocrinol Metab. 1975;40(4):738–41.
214 Chesney RW, McCarron DM, Haddad JG, Hawker CD, DiBella FP, Chesney PJ, et al. Pathogenic mechanisms of the hypocalcemia of the staphylococcal toxic- shock syndrome. J Lab Clin Med. 1983;101(4):576–85.
215 Becker K, Silva O, Snider R, Moore C, Geelhood G, Nash D, et al. The pathophysiology of pulmonary calcitonin. In: Becker K, Gazdar A. The Endocrine Lung in Health and Disease. Philadelphia, PA: WB Saunders Co., 1984:277–9.
216 Nylen ES, O'Neill W, Jordan MH, Snider RH, Moore CF, Lewis M, et al. Serum procalcitonin as an index of inhalation injury in burns. Horm Metab Res. 1992;24(9):439–43.
217 Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993;341(8844):515–8.
218 Gendrel D, Raymond J, Assicot M, Moulin F, Iniguez JL, Lebon P, et al. Measurement of procalcitonin levels in children with bacterial or viral meningitis. ClinInfectDis. 1997;24(6):1240–2.
219 Gendrel D, Assicot M, Raymond J, Moulin F, Francoual C, Badoual J, et al. Procalcitonin as a marker for the early diagnosis of neonatal infection. JPediatr. 1996;128(4):570–3.
220 Karzai W, Oberhoffer M, Meier-Hellmann A, Reinhart K. Procalcitonin – a new indicator of the systemic response to severe infections. 1997;25(6):329–34.
221 Ugarte H, Silva E, Mercan D, De Mendonca A, Vincent JL. Procalcitonin used as a marker of infection in the intensive care unit. Crit Care Med. 1999;27(3):498–504.
222 Vincent JL. Procalcitonin: THE marker of sepsis? Crit Care Med. 2000;28(4):1226–8.
223 Ruokonen E, Ilkka L, Niskanen M, Takala J. Procalcitonin and neopterin as indicators of infection in critically ill patients. Acta Anaesthesiol Scand. 2002;46(4):398–404.
224 Brunkhorst FM, Heinz U, Forycki ZF. Kinetics of procalcitonin in iatrogenic sepsis. Intensive Care Med. 1998;24(8):888–9.
225 van Nieuwkoop C, Bonten TN, van’t Wout JW, Kuijper EJ, Groeneveld GH, Becker MJ, et al. Procalcitonin reflects bacteremia and bacterial load in urosepsis syndrome: a prospective observational study. Crit Care. 2010;14(6):R206.
226 Schuetz P, Suter-Widmer I, Chaudri A, Christ-Crain M, Zimmerli W, Mueller B, et al. Prognostic value of procalcitonin in community-acquired pneumonia. Eur Respir J. 2011;37(2):384–92.
227 Haeuptle J, Zaborsky R, Fiumefreddo R, Trampuz A, Steffen I, Frei R, et al. Prognostic value of procalcitonin in Legionella pneumonia. Eur J Clin Microbiol Infect Dis. 2009;28(1):55–60.
228 Schuetz P, Amin DN, Greenwald JL. Role of procalcitonin in managing adult patients with respiratory tract infections. Chest. 2012;141(4):1063–73.
229 Huang D, Weissfeld L, Kellum J, Yealy D, Kong L, Martino M, et al. Risk Prediction With Procalcitonin and Clinical Rules in Community-Acquired Pneumonia. Ann Emerg Med. 2008;52(1):48–58.e2.
230 Jensen JU, Heslet L, Jensen TH, Espersen K, Steffensen P, Tvede M. Procalcitonin increase in early identification of critically ill patients at high risk of mortality. Crit Care Med. 2006;34(10):2596–602.
231 Jensen JU, Hein L, Lundgren B, Bestle MH, Mohr TT, Andersen MH, et al. Procalcitonin-guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomized trial. Crit Care Med. 2011;39(9):2048–58.
232 Johansen ME, Jensen JU, Lundgren JD. Antibiotics in intensive care: too little or too much? Crit Care Med. 2011;39(7):1849–51.
233 Bouadma L, Luyt CE, Tubach F, Cracco C, Alvarez A, Schwebel C, et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 2010;375(9713):463–74.
234 Schuetz P, Chiappa V, Briel M, Greenwald JL. Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Intern Med. 2011;171(15):1322–31.
235 Schuetz P, Muller B, Christ-Crain M, Stolz D, Tamm M, Bouadma L, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012; 9: CD007498.
236 Gencay M, Roth M, Christ-Crain M, Mueller B, Tamm M, Stolz D. Single and multiple viral infections in lower respiratory tract infection. Respiration. 2010;80(6):560–7.
237 Cuquemelle E, Soulis F, Villers D, Roche-Campo F, Ara Somohano C, Fartoukh M, et al. Can procalcitonin help identify associated bacterial infection in patients with severe influenza pneumonia? A multicentre study. Intensive Care Med. 2011;37(5):796–800.
238 Ingram PR, Inglis T, Moxon D, Speers D. Procalcitonin and C-reactive protein in severe 2009 H1N1 influenza infection. Intensive Care Med. 2010;36(3):528–32.
239 Piacentini E, Sanchez B, Arauzo V, Calbo E, Cuchi E, Nava JM. Procalcitonin levels are lower in intensive care unit patients with H1N1 influenza A virus pneumonia than in those with community-acquired bacterial pneumonia. A pilot study. J Crit Care. 2011;26(2):201–5.
240 Christ-Crain M, Jaccard-Stolz D, Bingisser R, Gencay MM, Huber PR, Tamm M, et al. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trial. Lancet. 2004;363(9409):600–7.
241 Stolz D, Christ-Crain M, Bingisser R, Leuppi J, Miedinger D, Muller C, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131(1):9–19.
242 Stolz D, Smyrnios N, Eggimann P, Pargger H, Thakkar N, Siegemund M, et al. Procalcitonin for reduced antibiotic exposure in Ventilator Associated Pneumonia – a randomized study. Eur Respir J. 2009.
243 Carlet J, Cohen J, Calandra T, Opal SM, Masur H. Sepsis: time to reconsider the concept. Crit Care Med. 2008;36(3):964–6.
244 Christ-Crain M, Muller B. Procalictonin – you only find what you look for, and you only look for what you know. J Am Geriatr Soc. 2006;54(3):546; author reply 7–8.
245 Baer G, Baumann P, Buettcher M, Heininger U, Berthet G, Schafer J, et al. Procalcitonin guidance to reduce antibiotic treatment of lower respiratory tract infection in children and adolescents (ProPAED): a randomized controlled trial. PLoS One. 2013;8(8):e68419.
246 Korppi M, Remes S, Heiskanen-Kosma T. Serum procalcitonin concentrations in bacterial pneumonia in children: a negative result in primary healthcare settings. Pediatr Pulmonol. 2003;35(1):56–61.
247 Sachse C, Dressler F, Henkel E. Increased serum procalcitonin in newborn infants without infection. 1998;44(6 Pt 1):1343–4.
248 Assumma M, Signore F, Pacifico L, Rossi N, Osborn JF, Chiesa C. Serum procalcitonin concentrations in term delivering mothers and their healthy offspring: A longitudinal study. Clinical chemistry. 2000;46(10):1583–7.
249 Chiesa C, Panero A, Rossi N, Stegagno M, De Giusti M, Osborn JF, et al. Reliability of procalcitonin concentrations for the diagnosis of sepsis in critically ill neonates. 1998;26(3):664–72.
250 Marc E, Menager C, Moulin F, Stos B, Chalumeau M, Guerin S, et al. Procalcitonin and viral meningitis: reduction of unnecessary antibiotics by measurement during an outbreak. Arch Pediatr. 2002;9(4):358–64.
251 Benador N, Siegrist CA, Gendrel D, Greder C, Benador D, Assicot M, et al. Procalcitonin is a marker of severity of renal lesions in pyelonephritis. Pediatrics. 1998;102(6):1422–5.
252 Drozdov D, Thomer A, Meili M, Schwarz S, Kouegbe RB, Regez K, et al. Procalcitonin, pyuria and proadrenomedullin in the management of urinary tract infections – “triple p in uti”: study protocol for a randomized controlled trial. Trials. 2013;14:84.
253 Ottiger C, Schaer G, Huber AR. Time-course of quantitative urinary leukocytes and bacteria counts during antibiotic therapy in women with symptoms of urinary tract infection. Clinica chimica acta; international journal of clinical chemistry. 2007;379(1–2):36–41.
254 Drozdov D, Schwarz S, Kutz A, Grolimund E, Rast AC, Regez K, et al. A Procalcitonin and Pyuria-based algorithm reduces antibiotic use in urinary tract infections in a randomized controlled trial. In: Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC). Washington, D.C., USA, 2014.
255 Muller B, Prat C. Markers of acute inflammation in assessing and managing lower respiratory tract infections: focus on procalcitonin. Clin Microbiol Infect. 2006;12(Suppl 9):8–16.
256 Shapiro NI, Wolfe RE, Wright SB, Moore R, Bates DW. Who needs a blood culture? A prospectively derived and validated prediction rule. J Emerg Med. 2008;35(3):255–64.
257 Woodhead M, Blasi F, Ewig S, Garau J, Huchon M, Leven M, et al. Guidelines for the management of adult lower respiratory tract infections. Clin Microbiol Infect. 2011;17(Suppl. 6):E1–E59.
258 Allen RB. Medical Education and the Changing Order. The Commonwealth Fund, 1946.
259 Engel GL. Editorial: Are Medical Schools neglecting clinical skills? JAMA. 1976;236(7):861–3.
260 van Zanten AR, Brinkman S, Arbous MS, Abu-Hanna A, Levy MM, de Keizer NF, et al. Guideline bundles adherence and mortality in severe sepsis and septic shock. Crit Care Med. 2014;42(8):1890–8.
261 Schuetz P, Hausfater P, Amin D, Haubitz S, Fassler L, Grolimund E, et al. Optimizing triage and hospitalization in adult general medical emergency patients: the triage project. BMC emergency medicine. 2013;13(1):12.
262 Courtais C, Kuster N, Dupuy AM, Folschveiller M, Jreige R, Bargnoux AS, et al. Proadrenomedullin, a useful tool for risk stratification in high Pneumonia Severity Index score community acquired pneumonia. Am J Emerg Med. 2013;31(1):215–21.
263 Rast AC, Mueller B, Schuetz P. Clinical scores and blood biomarkers for early risk assessment of patients presenting to the emergency department – Critical review. OA Emergency Medicine. 2014;2:1–9.
264 Righini M, Perrier A, De Moerloose P, Bounameaux H. D-Dimer for venous thromboembolism diagnosis: 20 years later. J Thromb Haemost. 2008;6(7):1059–71.
265 Twerenbold R, Reichlin T, Mueller C. Clinical application of sensitive cardiac troponin assays: potential and limitations. Biomark Med. 2010;4(3):395–401.
266 Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361(9):858–67.
267 Salazar J, Martinez MS, Chavez M, Toledo A, Anez R, Torres Y, et al. C-reactive protein: clinical and epidemiological perspectives. Cardiology research and practice. 2014;2014:605810.
268 Ridker PM. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. J Am Coll Cardiol. 2007;49(21):2129–38.
269 Blum CA, Nigro N, Briel M, Schuetz P, Ullmer E, Suter-Widmer I, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia – a randomized, double-blind, placebo-controlled multicenter trial. The Lancet 2015; (in press).
Acknowledgment:We thank Christoph A. Fux, M.D. for critical review and helpful input.
Funding / potential competing interests: PS received support from B•R•A•H•M•S/Thermofisher and bioMérieux to attend meetings, fulfil speaking engagements and for unrestricted research grants. PS is supported by the Swiss National Science Foundation (SNSF Professorship, PP00P3_150531 / 1). DA has received a speaker’s honorarium by bioMérieux. CM has received research support from Abbott, Alere, Beckamn Coulter, B•R•A•H•M•S/Thermofisher, Critical Diagnostics, Roche, Siemens, and Singulex as well as speaker’s/consulting honoraria from Abbott, Astra Zeneca, BG Medicine, bioMérieux, B•R•A•H•M•S/Thermofisher, Cardiorentis, Lilly, Novartis, Roche and Siemens. BM has received research support from Novo-Nordisk, Servier, Mepha, Roche, Hoechst, Bayer, Brahms, Novartis, Pharmacia / Pfizer, Essex, Glaxo-Smith-Kline, Merck, Astra Zeneca as well as consulting fees and speaker’s honoraria from Brahms, Pfizer, Speedel, AMGEN, Eli Lilly, Novartis, Astra-Zeneca, bioMérieux.