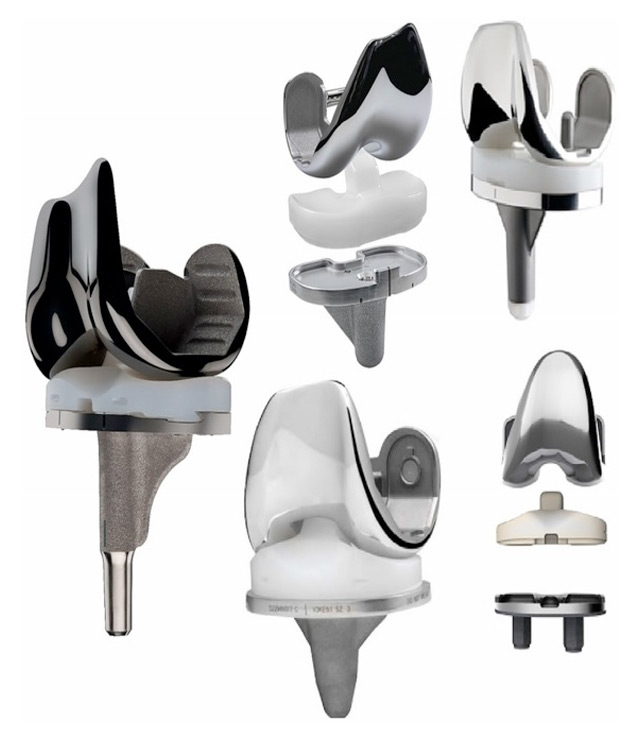
Figure 1
Illustration of a few of the currently available total knee arthroplasty designs.
DOI: https://doi.org/10.4414/smw.2015.14096
Abbreviations
ACL anterior cruciate ligament
CAS computer-assisted surgery
CRR cumulative revision rates
OA osteoarthritis
PCL posterior cruciate ligament
PFJ patellofemoral joint
PPF periprosthetic fractures
PSI patient Specific Instrumentation
SKAR Swedish arthroplasty register
TKA total knee arthroplasty
UKA unicondylar knee arthroplasty
Osteoarthritis (OA) is a term commonly used to describe the clinical and pathological outcomes of an active process that involves cartilage destruction, subchondral bone thickening and new bone formation, and results in the structural and functional failure of a joint [1, 2]. The functional disability associated with OA is a clinical burden, with over 25% of people aged over 55 years suffering knee pain on most days of the month [3]. Several risk factors that significantly increase the risk of knee OA have been identified, including obesity, previous knee injury, previous knee surgery and occupations involving bending or lifting [4].

Figure 1
Illustration of a few of the currently available total knee arthroplasty designs.
OA is managed on an individual basis, and management is optimised in line with the therapeutic response. A stepwise approach based on the severity of symptoms is recommended, beginning with preventive approaches involving alignment correction and cartilage regenerative procedures as well as pharmacological pain controlling strategies, and ending with joint resurfacing and arthroplasty in advanced stages of disease [5].
The history of total knee arthroplasty (TKA) began in the 1860s, when Fergusson reported the first resection for knee OA [6]. Interpositional arthroplasties, using materials such as joint capsules, muscle, fascia and free fascial grafts, were attempted over subsequent years, but were ultimately unsuccessful [7]. The first reports of total joint replacement were made by Thermestocles Gluck in the 1880s, who used an ivory hinged design fixed with a cement made from plaster of Paris, pumice and colophony [8, 9].
Condylar knee designs, in which the femoral and tibial load-bearing surfaces are replaced with unconnected artificial components, were first investigated in the late 1960s [8]. The Canadian surgeon Frank Gunston subsequently implanted the first polycentric knee prosthesis in 1971 [10]. The idea of introducing separate medial and lateral femoral components was novel at the time, and began of the era of the condylar knee prosthesis. Incremental improvements in component materials, geometry and fixation continued throughout the subsequent decades [9]. However, it was only after marked improvements in our understanding of the structure of the knee and the complexities of knee kinematics that the durable and highly functional implants that we use today (fig. 1) could be produced.
The primary indication for knee arthroplasty is significant and disabling pain due to severe OA [11]. However, the impact of knee dysfunction on a patient’s quality of life should be considered carefully [12]. The procedure is generally indicated in older patients with modest daily activities, but younger patients may befound to be suitable candidates after critical evaluation [13]. Depending on the location and severity of OA within the knee joint, several options may be considered, including partial and total joint arthroplasty.
Isolated patellofemoral joint (PFJ) arthritis accounts for approximately 10% of knee arthritis cases, and is most common in younger females [14]. A variety of surgical treatment options are available if nonoperative measures fail to achieve satisfying results [15, 16]. Isolated lesions within the PFJ may be treated with alignment procedures, chondrocyte implantation, microfracture or partial lateral facetectomy, with the aim of delaying the necessity for TKA. Joint replacement procedures are considered once both the patellar and femoral sides of the PFJ are involved [17, 18].
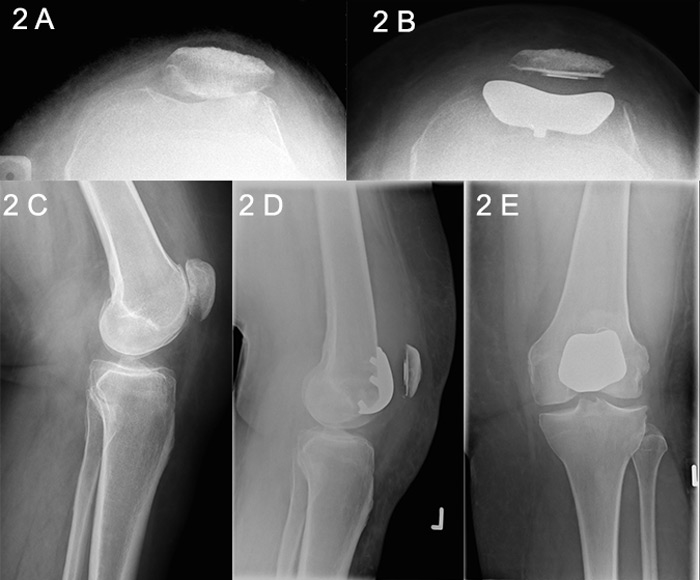
Figure 2
Patellofemoral joint (PFJ) arthritis.
A. Skyline X-Ray showing PFJ osteoarthritis. B. Skyline X-ray after PFJ replacement. C. Lateral X-ray showing PFJ osteoarthritis. D. Postoperative lateral X-ray after PFJ arthroplasty. E. Anteroposterior X-ray after PFJ arthroplasty.
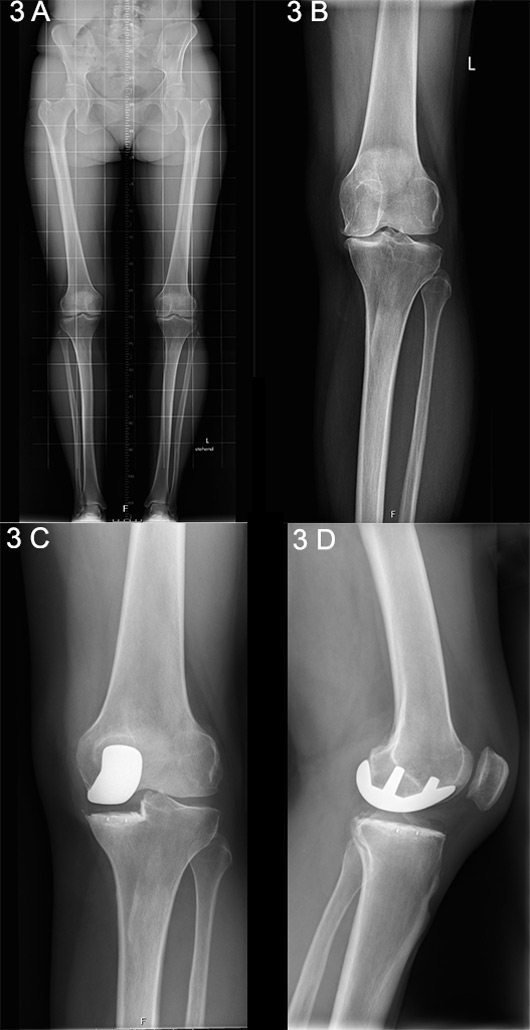
Figure 3
A. Long-standing X-ray showing unicompartmental medial knee osteoarthritis. B. Anteroposterior X-ray showing unicompartmental medial knee osteoarthritis. C. Postoperative anteroposterior X-ray following unicondylar knee arthroplasty (UKA). D. Lateral X-ray following UKA.
The first patellar prosthesis was described by McKeever in 1955, followed by the first femoral component 24 years later, as described separately by Lubinus and Blazina [19, 20]. Enhanced second-generation prostheses with a broad symmetrical trochlear flange evolved in the 1990s, and the design has been refined and updated continually in the intervening years (fig. 2 A‒E) [21]. The Cochrane-registered Warwick trial was designed to determine whether there is a difference in functional knee scores and quality-of-life outcomes, as well as complication rates, between patellofemoral arthroplasty and TKA in patients with severe PFJ arthritis. Reports of the trial are currently awaited [22].
Unicompartmental, or unicondylar, knee arthroplasty (UKA) is the preferred choice when the intention is to preserve the intrinsic joint stabilising structures, as well as healthy joint compartments [23] (fig. 3 A‒D) [24]. The general indication for consideration of a UKA procedure is based an isolated involvement of either the lateral or medial tibiofemoral compartment, identified upon clinical and radiographic examination [25, 26]. Silent preoperative radiographic signs of patellofemoral joint disease can be ignored and are not considered a contraindication for UKA [27, 28]. There is only evidence to suggest that, despite a good outcome, patients with lateral patellofemoral OA receiving medial UKA have slightly inferior results than those without, as has been reported by the Oxford Group [29].
An intact anterior cruciate ligament (ACL) is an important prerequisite for UKA, as the altered knee kinematics and contact stresses would otherwise increase failure rates [30]. There are concerns over an increased failure rate with UKA versus TKA due to aseptic loosening and the progression of arthritis in the contralateral compartment [25]. Technique-associated factors, mainly the achievement of correct alignment during surgery, have been proven predictive for increased polyethylene wear and contralateral progression of OA due to undesirable peak loads [31, 32]. If correctly indicated and performed, UKA can provide long-lasting successful results [33, 34].
The first prosthesis designed to replace all three knee compartments was introduced in 1972 by John Insall. Described as the “total condylar prosthesis”, it achieved very good outcomes and had a huge impact on condylar arthroplasty [35, 36]. Since then, there has been a remarkable increase in the number of TKAs performed annually. In the USA alone, over 700,000 TKAs were performed in 2011, a figure that is expected to increase by more than 600% by 2030 [37]. Figure 4 shows a radiograph of an arthritic knee before and after knee replacement.
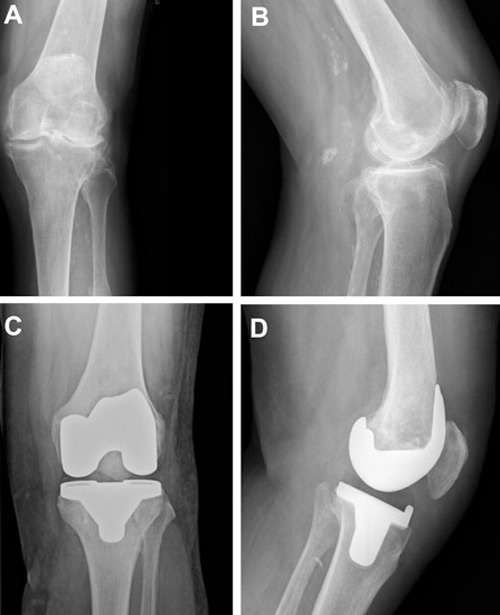
Figure 4
A. Anteroposterior X-ray of tricompartmental osteoarthritis. B. Lateral X-ray of tricompartmental osteoarthritis. C. Anteroposterior X-ray following total knee arthroplasty (TKA). D. Lateral X-ray following TKA.
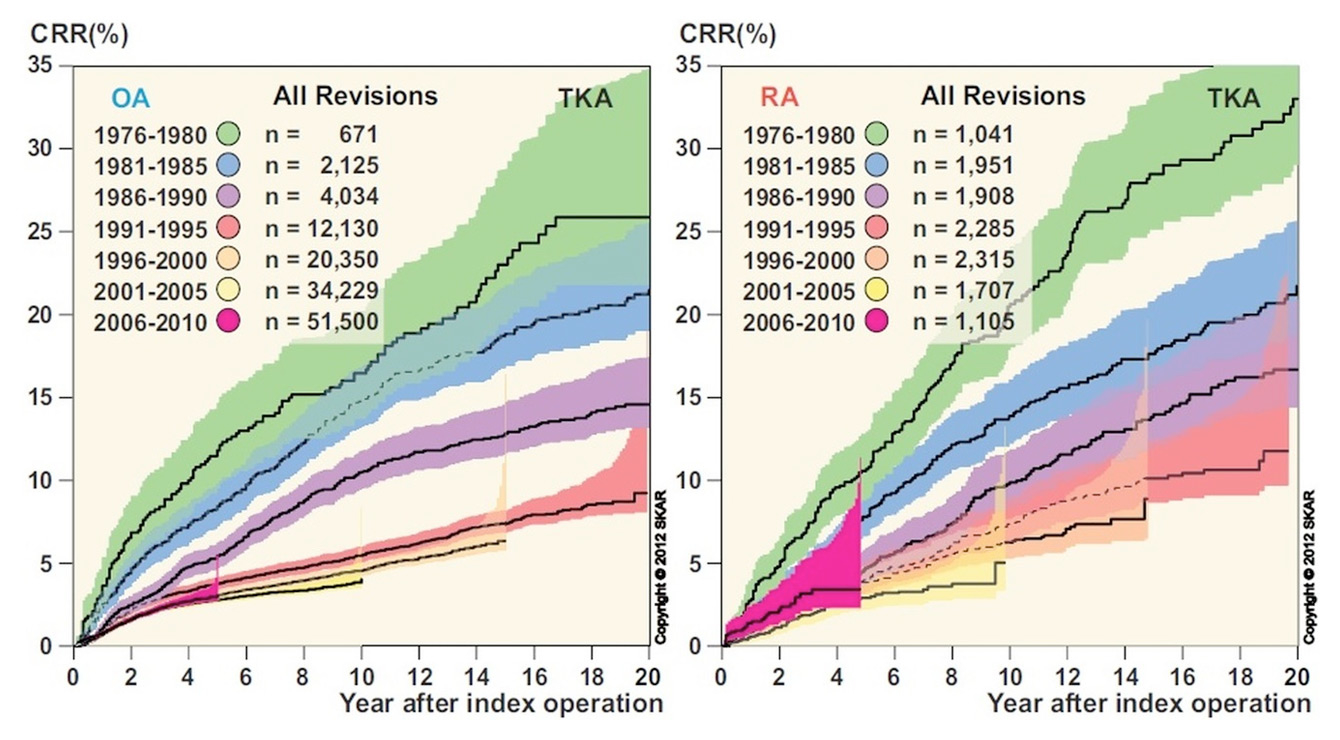
Figure 5
Cumulative revision rates (CRR) after total knee arthroplasty, with separate graphs for osteoarthritis (OA) and rheumatoid arthritis (RA). Reproduced with the kind permission of the Swedish Knee Arthroplasty Register.
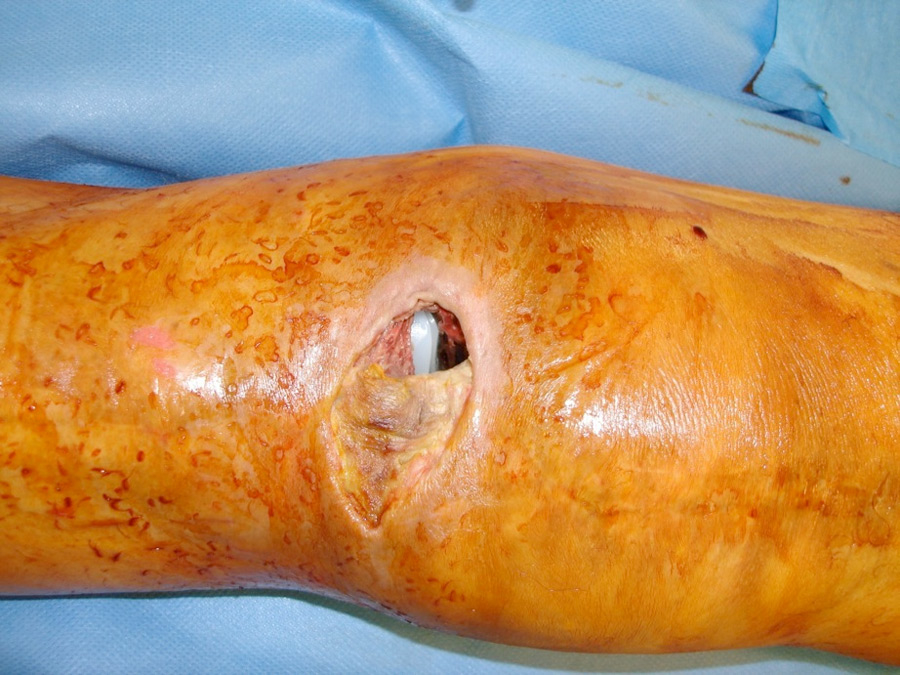
Figure 6
A worst-case scenario of a chronic periprosthetic infection with severe soft tissue damage.
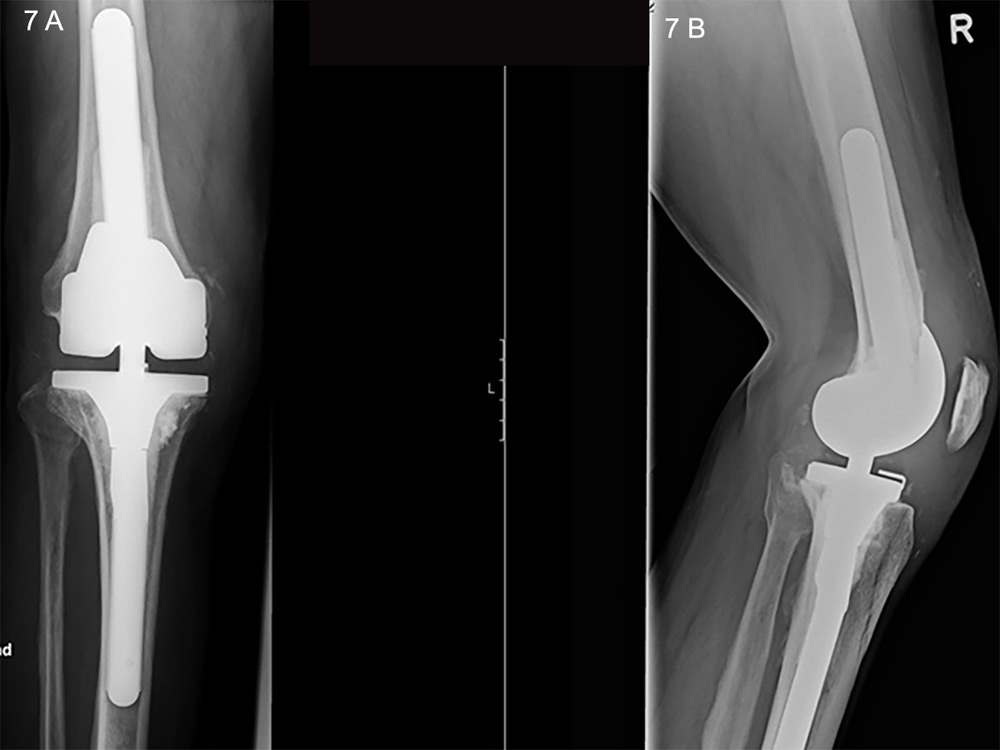
Figure 7
A. Anteroposterior X-ray of an hinged revision prosthesis. B. Lateral view in the same joint.
There are a number of types of TKA implant designs, which are intended to offer the surgeon options for individual patients. The various choices imply that each specific problem has a corresponding implant that provides a reliable solution. However, the results of comparative studies into many of the designs have not demonstrated, aside from a few instances, the marked improvements in outcomes that were expected. Until the current date, it has not been possible to produce a prosthetic design fully restoring the complex knee joint kinematics. The most common types of TKA design are discussed below.
Although the ACL is routinely sacrificed during TKA, the posterior cruciate ligament (PCL) can be sacrificed or preserved. A corresponding prosthetic design, with or without a tibial intercondular prominence, is implanted accordingly [38]. There is no evidence favouring preservation of the PCL over its substitution [39].
Originating from Fred Buechel’s “floating socket” philosophy, mobile bearing in TKA was introduced by the two Johns ‒ Goodfellow and O’Connor ‒ who described the theoretical principle of decreasing polyethylene wear by increasing implant conformity, and surface area for distribution of forces thereby reducing unidirectional stresses [40, 41]. The theoretical advantages could not, however, be substantiated by evidence, since a recent meta-analysis showed no difference in incidence of aseptic loosening or revision rates between fixed or mobile bearing designs, nor, especially, that the quality of polyethylene material has improved greatly [42].
High flexion, defined as a flexion angle >120º, may be necessary for patients in Asian countries and requires refinement of the prosthesis design and surgical technique [43, 44]. However, currently available randomised clinical trials (RCTs) suggest that clinical rating scores are not significantly higher than those achieved by posterior stabilised TKA designs [45].
There has been a great deal of debate as to the effect of gender, due to gender-related anatomic variability, on the results of TKA [46, 47]. The distal femur tends to be narrower in females for any given anteroposterior dimension [48], and a female-specific system was released (GenderSolutions™, Zimmer Inc., Warsaw, IN, USA). There are, however, larger differences in femur dimensions between races than between genders, which complicate the matter [49, 50]. Studies have failed to show any benefits with gender-specific implants, and the use of implants for the “wrong” gender has led to court cases [51]. Currently, several manufactures provide narrow femoral component designs without a gender specific nomenclature.
In patients with fixed valgus or varus deformity, constrained devices, such as constrained condylar knee and hinge types, may achieve a satisfactory balance. The level of constraint can be adapted to the individual, with higher constraints reserved for more severe cases [52]. Patients with a grade III fixed lateral collateral ligament deformity, in which medial stabilisers are no longer functional, benefit from additional constraint; for less severe grade I and II deformities however, there is no evidence of additional benefit of constrained devices over cruciate retaining or posterior stabilised designs [53–55]. It is, however, important to note that alongside the stability achieved through increased constraint, the forces on the component-bone interface also increase: this is to be kept in mind given the risk of loosening [56]. In general, the level of constraint can be categorised as follows starting from the least to the most constrained designs: (1.) cruciate retaining with minimal constraint (as mentioned above); (2.) cruciate substituting designs with a prominent tibial intercondylar prominence, a femoral cam and a deep articulating surface to decrease tibial translation [57]; (3.) unlinked varus-valgus constraint which possesses a tall tibial post and a deep femoral box, which account for coronal plane stability in the case of insufficient collateral ligaments [58]; (4.) rotating hinge implants, where the tibial and femoral components are linked with an axle restricting varus-valgus and translational movements. The choice of the correct degree of constraint is based on the ligamentous and bony condition; if sufficient, a primary posterior stabilised design would provide a good option, as it has been shown in literature reports [59]. In the case of insufficient (but not absent) collaterals, a semi-constrained design would be ideal provided that there is minimum bone loss [60, 61]. It is important to consider augmentation techniques using cement, bone grafts or augments to compensate for bone loss, before deciding on further constraint [62]. Hinged prosthesis should be preserved for cases of severe ligament disruption and bone loss [63, 64].
Several further designs have also been introduced with the intention of improving knee kinematics, including asymmetric tibial trays to enhance tibial coverage. Although these designs have been shown to provide for increased tibial coverage, they are also associated with increased posterolateral and posteriomedial overhang, with a subsequently increased likelihood of impingement [65]. Fully anatomic prosthesis were introduced but subsequently withdrawn owing to unfavourable outcome regarding iliotibial band traction syndrome resulting from increased rotation in a knee lacking cruciate ligaments [66]. The philosophy of less sacrifice of intrinsic stabilising structures led to more effort in development of bicruciate-retaining prosthetic designs were both cruciate ligaments along with the collateral ligaments are preserved, thus preserving the intrinsic knee stabilisers; such knee designs are now available, the results of which are to be awaited [67].
TKA is an established procedure that provides good or excellent results in the majority cases, notwithstanding the challenges. Spatial considerations such as soft-tissue balancing, alignment of the leg and restoration of the joint line are crucial for achieving good long-term outcomes. Up to 25% of all prosthetic failures have been attributed to malpositioning or malalignment of the implant [68]. Setting reference points and ensuring correct alignment during surgical implantation are an important part of every knee arthroplasty procedure. Surgical options available for component positioning are discussed below.
The debate over whether there is a difference in outcome and prosthesis survival between cemented and cementless implants can be attributed to early articles reporting inferior survival with cementless implants [69, 70]. Surveys clearly demonstrate survival benefit, since the cumulative revision rate has been shown to be higher in cementless impants [71].
Although anterior pain reduction after TKA was achieved with tricompartmental arthroplasties, difficult-to-manage patella complications arose [72, 73]. A recent meta-analysis of RCTs revealed no difference in outcomes between patella resurfacing and non-resurfacing, including for knee pain [74]. However, resurfaced patients require less additional surgery [74]. The indications for patella resurfacing are currently centre- or surgeon-dependent and, in some countries, politically based, particularly when the definition of total joint replacement centres on tricompartmental arthroplasty.
The use of CAS and associated medical imaging modalities have garnered increased interest over the past few decades as a method of enhancing the accuracy of alignment during knee arthroplasty [75]. If one imagines the alignment achieved by surgeons as a bell curve, CAS aims to decrease the extremes by reducing the number of ‘outliers’. The actual long-term benefits for the majority of patients are yet to be established, however [76, 77].
It is important to note that there are currently no routinely available patient-specific implants, and custom-made prosthesis are reserved for specific cases. However, patient-specific cutting-blocks have emerged in recent years, providing unique, customisable blocks that conform to each individual patient's knee shape. After magnetic resonance imaging and X-ray scans, patient data are typically uploaded to the company website and the blocks manufactured [76]. PSI is an attractive idea for optimising accuracy in bone preparation [76], and minimising complications arising from various knee anatomies [68]. The technology remains in its early stages, with only limited number of studies, and the first challenges in terms of the feasibility of the logistical process have been reported [78]. Further drawbacks are the need of a preoperative CT scan as well as accuracy issues that have recently been attributed to CT-based patient-specific cutting blocks [79]. Despite these difficulties, however, PSI remains an attractive option, as it reduces surgical time and sterilisation costs [80].
The most comprehensive assessment of real-world arthroplasty outcomes has come from the numerous arthroplasty registers that have been set up around the globe, the first of which was established in Sweden in 1975 [81]. Since then, a number of countries have launched nationwide registers to gather information on the survival rates of available implants [82]. In 2009, a common database for Scandinavian countries – the Nordic Arthroplasty Register Association – was inaugurated. It brought together data on 151,814 knee arthroplasties performed in Denmark, Norway and Sweden, with Finland joining the project in 2010 [83].
Registries usually publish detailed yearly reports, with statistical frequency distributions and survival analysis of primary and revision knees prosthetic implants since the registry began. As the oldest, the Swedish arthroplasty register (SKAR) represents a benchmark. Its 2012 annual report revealed cumulative revision rates (CRR) of 25% at 20 years for TKAs performed in patients with OA between 1976 and 1980. During this period, the 10-year CRR dropped from 15% (TKAs implanted between 1976 and 1980) to 4% (TKAs implanted between 1996 and 2000) [84]. Higher 10-year CRRs were observed for prostheses implanted in patients with rheumatoid arthritis between 1976 and 1980 (20%) and between 1996 and 2000 (6%) (fig. 5) [84].
Alongside implant survival, an important measure of outcome following knee arthroplasty is patient satisfaction, including improvements in physical function and health-related quality of life. Registries, therefore, began to integrate such patient-reported outcomes. The SKAR conducted a survey in 1997 of patient satisfaction and unreported revisions, which had a remarkably high response rate of 95%. The results indicated that 8% of patients were dissatisfied 2–17 years after their knee replacement. This percentage doubled among patients who had undergone revision arthroplasty [85].
A number of instruments for the functional assessment of knee arthroplasty have also been developed, with the aim of standardising postoperative evaluation. These are either completed by clinicians, such as the Hospital for Special Surgery Knee Score and the Knee Society Score, or by patients, such as the Western Ontario and McMaster Universities Osteoarthritis Index. Clinical studies investigating long-term postoperative functional outcome after TKA demonstrate effect sizes of over 2.5, indicating that functional performance more than doubled after the procedure [86].
Although TKA is a very successful treatment option for OA, complications do occur. Potential problems include infection, instability, misalignment, osteolysis, fracture, anterior knee pain and allergy to one or more of the prosthetic components [62]. The increasing number of TKA procedures performed annually has served to increase the number of TKA complications and revisions. Revision arthroplasty can be challenging owing to bone defects, ligament instability and difficulties with fixation [62]. The complications associated with TKA are discussed below.
An important, but previously often overlooked, aspect of TKA success is patient satisfaction. Clinical reports indicate that up to 20% of primary TKA patients are dissatisfied with the outcome of the procedure over the long term [87]. Failure to meet patient expectations is by far the most important factor for lowered postoperative outcomes, with others including the severity of preoperative arthritic pain and postoperative complications requiring hospital readmission [87]. Satisfaction following knee arthroplasty is significantly associated with domains related to pain and function [88]. As these are the major reasons for undergoing TKA in the first place, dissatisfaction is clearly a problem of failing to meet expectations. Early attempts to set patients’ expectations in context should therefore be undertaken, ideally during the first visit, as this can have a considerable impact on postoperative outcomes [89].
Loosening is the leading cause of TKA failure, accounting for 30% of primary revisions [90–92]. Particulate wear debris, such as polyethylene, poly(methyl-methacrylate) cement or metal, results in a foreign-body inflammatory response, which causes osteolysis – a leading cause of late reoperations [93]. The extent of the inflammatory response is largely determined by the shape, size, type and concentration of the particles, with smaller particles inducing increased biological activity, thus increasing the rate of osteolysis [93]. The probability of revision caused by component wear increases from 10.5% up to 2 years postoperatively to 33.3% over 2 years postoperatively [94]. The presentation of instability includes tenderness over the pes anserinus bursa, instability on stair descent and a positive posterior drawer test [95].
Periprosthetic infections are a devastating problem and account for 20% of all primary knee revisions (fig. 6) [84]. They can be classified as early (<3 months after surgery), delayed (3–24 months after surgery) or late “chronic” (>24 months after surgery). Early and delayed infections are typically acquired during surgical implantation, whereas late infections are the result of haematogenous seeding [96]. There is controversy over the classical definition of a chronic infection being related to the passage of time, as the pathological mechanism is dependent on the formation of a biofilm. A reliable, increasingly used parameter is the interval from symptom onset, where a 3-week cut-off has been used to define a chronic infection [97]. It is important to note that only an open tissue biopsy is sufficient for confirmation of diagnosis, the sensitivity of which by far overweighs that of joint fluid aspiration [96]. Once the diagnosis of an infection is made, a radical surgical intervention that takes into account the nature of the infection and the microorganism involved has to be planned; antibiotic treatment is an adjunct but does not have solitary role. The intervention could take the form either of a radical debridement and exchange of the polyethylene inlay in the case of acute infection, or a single-stage procedure, in which the implant is removed and replaced by a second after radical debridement, or a two-stage procedure, with implantation of an antibiotic-augmented cement spacer to establish aseptic conditions before final revision, the latter of which is the gold-standard for chronic infections [98, 99]. Figure 7 shows an implanted revision prosthesis.
This is a common postoperative complication following TKA. As little as 3º varus or valgus angulation of the tibial tray may cause significant variations in the pressure distribution across the medial and lateral compartments [100]. Femoral component malrotation, on the other hand, is the major cause of patellofemoral complications [101–105]. These complications are likely to require revision, during which it is important to achieve a stable, balanced knee with optimal function through restoration of the joint line and soft tissue balancing [106].
Intraoperative fractures may occur at any stage of a TKA, and have an estimated incidence of 0.4% (107). Another important considered is late periprosthetic fracture (PPF). The incidence of PFF is between 0.3% and 2.5%, and primarily follows minor trauma [108–111]. Risk factors for PPFs include osteoporosis, anterior femoral notching, rheumatoid arthritis, steroid therapy, neurological disease, previous revision arthroplasty, local osteolysis and infection [112]. New-onset regional pain in a patient at high risk for PPF should therefore be further investigated to rule out a possible fracture.
Persistent knee pain and inadequate range of motion are amongst the common complications following TKA, and range in prevalence from approximately 7%–12% to as much as 54%–60% [113–115]. Overstuffing of the components or elevation of the joint line should be avoided by following the correct procedural approach. One of the underlying causes of persistent knee pain is complex regional pain syndrome (CRPS). CPRS is a subentity of persistent chronic pain, which is defined as pain after a surgical procedure lasting for at least 2 months, and is experienced by approximately 20% of TKA patients [116, 117]. The typical presentation following TKA is constant pain unrelated to physical activity, with limited flexion, and cutaneous hypersensitivity without radiographic signs of infection or lucency [118]. If these complications do occur, radiographs, bone scans, laboratory tests and local analgesic infiltration should be performed to identify the cause [114].
As noted above, chronic inflammation resulting from particulate wear is a leading cause of implant failure following TKA. Metals and acrylic cement undergo wear and corrosion when in contact with biological fluids, and ions released by this process may interact with the immune system, inducing a delayed-type hypersensitivity reaction [119]. The presence of positive skin reactions to at least one hapten is associated with a 4–fold increased likelihood of TKA failure [120]. The clinical significance of hypersensitivity is therefore clearly an important, but apparently underestimated, cause of failure. However, there is a discrepancy between skin and deep-tissue sensitivity [119]. A practical, specific and sensitive universal tool to enable testing for, or diagnosis of, sensitivity to implant materials is therefore required. The authors recommend the use of titanium or oxidised zirconium implants in the case of suspected allergy or patient request due to the reduced potential of causing hypersensitivity [121, 122].
One of the most important challenges in revision TKA is the management of bone deficiency, which causes difficulties in implant alignment and the achievement of a stable bone-implant interface [62]. Successful management of bone deficiency could be achieved through the use of cement, morselised or formed allografts, metal wedges and augmentors together with stem supplements including offsets and cones that help decrease stresses on the implant-bone interface [62, 123]. The selection of implants is primarily based on the status of ligamentous and stabilising structures [59].
As discussed, the considerable advances in the understanding of knee kinematics during the past few decades has led to improvements in the design of prosthetic knee implants and an explosion in the number of options for specific patient scenarios. However, there has also been a steep increase in the number of procedures performed annually, a trend that is set to continue in the coming decades. This is being accompanied by a rise in the number of complications encountered, some of which present the surgeon with considerable difficulties. The challenge of increasing patient expectations and the high rate of patient dissatisfaction and failures compared with arthroplasty of the hip, are increasingly gaining attention by the orthopaedic community. There is growing emphasis on careful patient selection and joint-preserving surgery. Current trends show an upward shift in timing of arthroplasty, alongside focus on joint preserving approaches, and improvement of prosthetic designs with increasing respect for intrinsic joint stabilising structures, along with finding the correct balance between anatomical design and kinematic functionality.
1 Nuki G. Osteoarthritis: a problem of joint failure. Z Rheumatol. 1999;58(3):142–7.
2 Peat GM, Moores L, Goldingay S, Hunter M. Pain management program follow-ups. a national survey of current practice in the United Kingdom. J Pain Symptom Manage. 2001;21(3):218–26.
3 Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis. 2001;60(2):91–7.
4 Felson DT. Clinical practice. Osteoarthritis of the knee. N Engl J Med. 2006;354(8):841–8.
5 Hunter DJ, Felson DT. Osteoarthritis. BMJ. 2006;332(7542):639–42.
6 Gordon-Taylor G. Sir William Fergusson, 1808–1877. Medical history. 1961;5:1–14.
7 Ranawat CS. History of total knee replacement. J South Orthop Assoc. 2002;11(4):218–26.
8 Amendola L, Tigani D, Fosco M, Dallari D. History of Condylar Total Knee Arthroplasty. In: Fokter S, editor. Recent Advances in Hip and Knee Arthroplasty. Rijeka: InTech; 2012.
9 Williams D, Garbuz D, Masri B. Total knee arthroplasty: Techniques and results. BC Medical Journal. 2010;52(9):447–54.
10 Gunston FH. Polycentric knee arthroplasty. Prosthetic simulation of normal knee movement. J Bone Joint Surg Br. 1971;53(2):272–7.
11 Mancuso CA, Ranawat CS, Esdaile JM, Johanson NA, Charlson ME. Indications for total hip and total knee arthroplasties. Results of orthopaedic surveys. J Arthroplasty. 1996;11(1):34–46.
12 Hawker GA, Wright JG, Coyte PC, Williams JI, Harvey B, Glazier R, et al. Determining the need for hip and knee arthroplasty: the role of clinical severity and patients’ preferences. Medical Care. 2001;39(3):206–16.
13 Diduch DR, Insall JN, Scott WN, Scuderi GR, Font-Rodriguez D. Total knee replacement in young, active patients. Long-term follow-up and functional outcome. J Bone Joint Surg Am. 1997;79(4):575–82.
14 Davies AP, Vince AS, Shepstone L, Donell ST, Glasgow MM. The radiologic prevalence of patellofemoral osteoarthritis. Clin Orthop Relat Res. 2002(402):206–12.
15 Fulkerson JP. Alternatives to patellofemoral arthroplasty. Clin Orthop Relat Res. 2005(436):76–80.
16 Saleh KJ, Arendt EA, Eldridge J, Fulkerson JP, Minas T, Mulhall KJ. Symposium. Operative treatment of patellofemoral arthritis. J Bone Joint Surg Am. 2005;87(3):659–71.
17 Leadbetter WB. Patellofemoral arthroplasty in the treatment of patellofemoral arthritis: rationale and outcomes in younger patients. Orthop Clin North Am. 2008;39(3):363–80, vii.
18 Leadbetter WB, Seyler TM, Ragland PS, Mont MA. Indications, contraindications, and pitfalls of patellofemoral arthroplasty. J Bone Joint Surg Am. 2006;88(Suppl 4):122–37.
19 Lubinus HH. Patella glide bearing total replacement. Orthopedics. 1979;2(2):119–27.
20 Blazina ME, Fox JM, Del Pizzo W, Broukhim B, Ivey FM. Patellofemoral replacement. Clin Orthop Relat Res. 1979(144):98–102.
21 Ackroyd CE, Chir B. Development and early results of a new patellofemoral arthroplasty. Clin Orthop Relat Res. 2005(436):7–13.
22 Odumenya M, McGuinness K, Achten J, Parsons N, Spalding T, Costa M. The Warwick patellofemoral arthroplasty trial: a randomised clinical trial of total knee arthroplasty versus patellofemoral arthroplasty in patients with severe arthritis of the patellofemoral joint. BMC Musculoskelet Disord. 2011;12:265.
23 Scott RD, Santore RF. Unicondylar unicompartmental replacement for osteoarthritis of the knee. J Bone Joint Surg Am. 1981;63(4):536–44.
24 Tinius M, Hepp P, Becker R. Combined unicompartmental knee arthroplasty and anterior cruciate ligament reconstruction. Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA. 2012;20(1):81–7.
25 Berger RA, Nedeff DD, Barden RM, Sheinkop MM, Jacobs JJ, Rosenberg AG, et al. Unicompartmental knee arthroplasty. Clinical experience at 6– to 10–year followup. Clin Orthop Relat Res. 1999(367):50–60.
26 Ashraf T, Newman JH, Evans RL, Ackroyd CE. Lateral unicompartmental knee replacement survivorship and clinical experience over 21 years. J Bone Joint Surg Br. 2002;84(8):1126–30.
27 Berend KR, Lombardi AV, Jr., Morris MJ, Hurst JM, Kavolus JJ. Does preoperative patellofemoral joint state affect medial unicompartmental arthroplasty survival? Orthopedics. 2011;34(9):e494–6.
28 Hauptmann SM, Kreul U, Mazoochian F, C VS-P, Jansson V, Muller PE. [Influence of patellofemoral osteoarthritis on functional outcome after unicondylar knee arthroplasty]. Der Orthopade. 2005;34(11):1088, 90–3.
29 Beard DJ, Pandit H, Ostlere S, Jenkins C, Dodd CA, Murray DW. Pre-operative clinical and radiological assessment of the patellofemoral joint in unicompartmental knee replacement and its influence on outcome. J Bone Joint Surg Br. 2007;89(12):1602–7.
30 Suero EM, Citak M, Cross MB, Bosscher MR, Ranawat AS, Pearle AD. Effects of tibial slope changes in the stability of fixed bearing medial unicompartmental arthroplasty in anterior cruciate ligament deficient knees. The Knee. 2012;19(4):365–9.
31 Argenson JN, Parratte S. The unicompartmental knee: design and technical considerations in minimizing wear. Clin Orthop Relat Res. 2006;452:137–42.
32 Hernigou P, Deschamps G. Alignment influences wear in the knee after medial unicompartmental arthroplasty. Clin Orthop Relat Res. 2004(423):161–5.
33 Bruni D, Gagliardi M, Akkawi I, Raspugli GF, Bignozzi S, Marko T, et al. Good survivorship of all-polyethylene tibial component UKA at long-term follow-up. Knee Surg Sports Traumatol Arthrosc. 2014.
34 Schlueter-Brust K, Kugland K, Stein G, Henckel J, Christ H, Eysel P, et al. Ten year survivorship after cemented and uncemented medial Uniglide(R) unicompartmental knee arthroplasties. Knee. 2014;21(5):964–70.
35 Insall JN, Hood RW, Flawn LB, Sullivan DJ. The total condylar knee prosthesis in gonarthrosis. A five to nine-year follow-up of the first one hundred consecutive replacements. J Bone Joint Surg Am. 1983;65(5):619–28.
36 Insall JN, Kelly M. The total condylar prosthesis. Clin Orthop Relat Res. 1986(205):43–8.
37 Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780–5.
38 Banks SA, Markovich GD, Hodge WA. In vivo kinematics of cruciate-retaining and -substituting knee arthroplasties. J Arthroplasty. 1997;12(3):297–304.
39 Jacobs WC, Clement DJ, Wymenga AB. Retention versus removal of the posterior cruciate ligament in total knee replacement: a systematic literature review within the Cochrane framework. Acta Orthop. 2005;76(6):757–68.
40 Buechel FF, Pappas MJ, DePalma AF. “Floating-socket” total shoulder replacement: anatomical, biomechanical, and surgical rationale. J Biomed Mater Res. 1978;12(1):89–114.
41 Goodfellow J, O’Connor J. The mechanics of the knee and prosthesis design. J Bone Joint Surg Br. 1978;60–b(3):358–69.
42 van der Voort P, Pijls BG, Nouta KA, Valstar ER, Jacobs WC, Nelissen RG. A systematic review and meta-regression of mobile-bearing versus fixed-bearing total knee replacement in 41 studies. Bone Joint J. 2013;95–b(9):1209–16.
43 Argenson JN, Scuderi GR, Komistek RD, Scott WN, Kelly MA, Aubaniac JM. In vivo kinematic evaluation and design considerations related to high flexion in total knee arthroplasty. J Biomech. 2005;38(2):277–84.
44 Delp SL, Kocmond JH, Stern SH. Tradeoffs between motion and stability in posterior substituting knee arthroplasty design. J Biomech. 1995;28(10):1155–66.
45 McCalden RW, MacDonald SJ, Bourne RB, Marr JT. A randomized controlled trial comparing “high-flex” vs “standard” posterior cruciate substituting polyethylene tibial inserts in total knee arthroplasty. J Arthroplasty. 2009;24(6 Suppl):33–8.
46 Barrett WP. The need for gender-specific prostheses in TKA: does size make a difference? Orthopedics. 2006;29(9 Suppl):S53–5.
47 Booth RE, Jr. Sex and the total knee: gender-sensitive designs. Orthopedics. 2006;29(9):836–8.
48 Hitt K, Shurman JR, 2nd, Greene K, McCarthy J, Moskal J, Hoeman T, et al. Anthropometric measurements of the human knee: correlation to the sizing of current knee arthroplasty systems. J Bone Joint Surg Am. 2003;85–A Suppl 4:115–22.
49 Ho WP, Cheng CK, Liau JJ. Morphometrical measurements of resected surface of femurs in Chinese knees: correlation to the sizing of current femoral implants. Knee. 2006;13(1):12–4.
50 Karlson EW, Daltroy LH, Liang MH, Eaton HE, Katz JN. Gender differences in patient preferences may underlie differential utilization of elective surgery. Am J Med. 1997;102(6):524–30.
51 Greene KA. Gender-specific design in total knee arthroplasty. J Arthroplasty. 2007;22(7 Suppl 3):27–31.
52 Girard J, Amzallag M, Pasquier G, Mulliez A, Brosset T, Gougeon F, et al. Total knee arthroplasty in valgus knees: predictive preoperative parameters influencing a constrained design selection. Orthop Traumatol Surg Res. 2009;95(4):260–6.
53 Ranawat AS, Ranawat CS, Elkus M, Rasquinha VJ, Rossi R, Babhulkar S. Total knee arthroplasty for severe valgus deformity. J Bone Joint Surg Am. 2005;87(Suppl 1)(Pt 2):271–84.
54 Rossi R, Rosso F, Cottino U, Dettoni F, Bonasia DE, Bruzzone M. Total knee arthroplasty in the valgus knee. Int Orthop. 2014;38(2):273–83.
55 Pang HN, Yeo SJ, Chong HC, Chin PL, Chia SL, Lo NN. Joint line changes and outcomes in constrained versus unconstrained total knee arthroplasty for the type II valgus knee. Knee Surg Sports Traumatol Arthrosc. 2013;21(10):2363–9.
56 Cameron HU, Hunter GA. Failure in total knee arthroplasty: mechanisms, revisions, and results. Clin Orthop Relat Res. 1982(170):141–6.
57 Ritter MA, Davis KE, Farris A, Keating EM, Faris PM. The Surgeon’s Role in Relative Success of PCL-Retaining and PCL-Substituting Total Knee Arthroplasty. HSSJ. 2014;10(2):107–15.
58 Naudie DD, Rorabeck CH. Managing instability in total knee arthroplasty with constrained and linked implants. Instr Course Lect. 2004;53:207–15.
59 Vasso M, Beaufils P, Schiavone Panni A. Constraint choice in revision knee arthroplasty. Int Orthop. 2013;37(7):1279–84.
60 Morgan H, Battista V, Leopold SS. Constraint in primary total knee arthroplasty. J Am Acad Orthop Surg. 2005;13(8):515–24.
61 Lachiewicz PF, Soileau ES. Results of a second-generation constrained condylar prosthesis in primary total knee arthroplasty. J Arthroplasty. 2011;26(8):1228–31.
62 Radnay CS, Scuderi GR. Management of bone loss: augments, cones, offset stems. Clin Orthop Relat Res. 2006;446:83–92.
63 Meijer MF, Reininga IH, Boerboom AL, Stevens M, Bulstra SK. Poorer survival after a primary implant during revision total knee arthroplasty. Int Orthop. 2013;37(3):415–9.
64 Yang JH, Yoon JR, Oh CH, Kim TS. Primary total knee arthroplasty using rotating-hinge prosthesis in severely affected knees. Knee Surg Sports Traumatol Arthrosc. 2012;20(3):517–23.
65 Wernecke GC, Harris IA, Houang MT, Seeto BG, Chen DB, MacDessi SJ. Comparison of tibial bone coverage of 6 knee prostheses: a magnetic resonance imaging study with controlled rotation. J Orthop Surg. (Hong Kong). 2012;20(2):143–7.
66 Luyckx L, Luyckx T, Bellemans J, Victor J. Iliotibial band traction syndrome in guided motion TKA. A new clinical entity after TKA. Acta Orthop Belg. 2010;76(4):507–12.
67 Cherian JJ, Kapadia BH, Banerjee S, Jauregui JJ, Harwin SF, Mont MA. Bicruciate-retaining total knee arthroplasty: a review. J Knee Surg. 2014;27(3):199–205.
68 Tibesku CO, Innocenti B, Wong P, Salehi A, Labey L. Can CT-based patient-matched instrumentation achieve consistent rotational alignment in knee arthroplasty? Arch Orthop Trauma Surg. 2012;132(2):171–7.
69 Chockalingam S, Scott G. The outcome of cemented vs. cementless fixation of a femoral component in total knee replacement (TKR) with the identification of radiological signs for the prediction of failure. Knee. 2000;7(4):233–8.
70 Rorabeck CH, Bourne RB, Nott L. The cemented kinematic-II and the non-cemented porous-coated anatomic prostheses for total knee replacement. A prospective evaluation. J Bone Joint Surg Am. 1988;70(4):483–90.
71 Lutz MJ, Halliday BR. Survey of current cementing techniques in total knee replacement. ANZ J Surg. 2002;72(6):437–9.
72 Barrack RL, Wolfe MW, Waldman DA, Milicic M, Bertot AJ, Myers L. Resurfacing of the patella in total knee arthroplasty. A prospective, randomized, double-blind study. J Bone Joint Surg Am. 1997;79(8):1121–31.
73 Mayman D, Bourne RB, Rorabeck CH, Vaz M, Kramer J. Resurfacing versus not resurfacing the patella in total knee arthroplasty: 8– to 10–year results. J Arthroplasty. 2003;18(5):541–5.
74 Pilling RW, Moulder E, Allgar V, Messner J, Sun Z, Mohsen A. Patellar resurfacing in primary total knee replacement: a meta-analysis. J Bone Joint Surg Am. 2012;94(24):2270–8.
75 Mason JB, Fehring TK, Estok R, Banel D, Fahrbach K. Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty. 2007;22(8):1097–106.
76 Nunley RM, Ellison BS, Zhu J, Ruh EL, Howell SM, Barrack RL. Do patient-specific guides improve coronal alignment in total knee arthroplasty? Clin Orthop Relat Res. 2012;470(3):895–902.
77 Sparmann M, Wolke B, Czupalla H, Banzer D, Zink A. Positioning of total knee arthroplasty with and without navigation support. A prospective, randomised study. J Bone Joint Surg Br. 2003;85(6):830–5.
78 Koskinen E, Eskelinen A, Paavolainen P, Pulkkinen P, Remes V. Comparison of survival and cost-effectiveness between unicondylar arthroplasty and total knee arthroplasty in patients with primary osteoarthritis: a follow-up study of 50,493 knee replacements from the Finnish Arthroplasty Register. Acta Orthop. 2008;79(4):499–507.
79 Lustig S, Scholes CJ, Oussedik SI, Kinzel V, Coolican MR, Parker DA. Unsatisfactory accuracy as determined by computer navigation of VISIONAIRE patient-specific instrumentation for total knee arthroplasty. J Arthroplasty. 2013;28(3):469–73.
80 Boonen B, Schotanus MG, Kort NP. Preliminary experience with the patient-specific templating total knee arthroplasty. Acta Orthop. 2012;83(4):387–93.
81 Bulstrode C. Total hip replacement: the way forward. Ann R Coll Surg Engl. 1996;78(2):129–32.
82 Kolling C, Simmen BR, Labek G, Goldhahn J. Key factors for a successful National Arthroplasty Register. J Bone Joint Surg Br. 2007;89(12):1567–73.
83 Havelin LI, Robertsson O, Fenstad AM, Overgaard S, Garellick G, Furnes O. A Scandinavian experience of register collaboration: the Nordic Arthroplasty Register Association (NARA). J Bone Joint Surg Am. 2011;93(Suppl 3):13–9.
84 Swedish Knee Arthroplasty Register. Annual Report 20122012 [cited 1 May 2013]. Available from: http://www.knee.nko.se/english/online/uploadedFiles/117_SKAR_2012_Engl_1.0.pdf.
85 Robertsson O, Dunbar M, Pehrsson T, Knutson K, Lidgren L. Patient satisfaction after knee arthroplasty: a report on 27,372 knees operated on between 1981 and 1995 in Sweden. Acta Orthop Scand. 2000;71(3):262–7.
86 Kane RL, Saleh KJ, Wilt TJ, Bershadsky B. The functional outcomes of total knee arthroplasty. J Bone Joint Surg Am. 2005;87(8):1719–24.
87 Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57–63.
88 Robertsson O, Dunbar MJ. Patient satisfaction compared with general health and disease-specific questionnaires in knee arthroplasty patients. J Arthroplasty. 2001;16(4):476–82.
89 Piscitelli P, Iolascon G, Innocenti M, Civinini R, Rubinacci A, Muratore M, et al. Painful prosthesis: approaching the patient with persistent pain following total hip and knee arthroplasty. Clinical cases in mineral and bone metabolism: the official journal of the Italian Society of Osteoporosis, Mineral Metabolism, and Skeletal Diseases. 2013;10(2):97–110.
90 Clarke HD, Scott WN. Knee: axial instability. Orthop Clin North Am. 2001;32(4):627–37, viii.
91 Fehring TK, Valadie AL. Knee instability after total knee arthroplasty. Clin Orthop Relat Res. 1994(299):157–62.
92 Lonner JH, Siliski JM, Scott RD. Prodromes of failure in total knee arthroplasty. J Arthroplasty. 1999;14(4):488–92.
93 Gupta SK, Chu A, Ranawat AS, Slamin J, Ranawat CS. Osteolysis after total knee arthroplasty. J Arthroplasty. 2007;22(6):787–99.
94 Fennema P, Heyse TJ, Uyl-de Groot CA. Cost-effectiveness and clinical implications of advanced bearings in total knee arthroplasty: a long-term modeling analysis. Int J Technol Assess Health Care. 2014;30(2):218–25.
95 Vince KG, Abdeen A, Sugimori T. The unstable total knee arthroplasty: causes and cures. J Arthroplasty. 2006;21(4 Suppl 1):44–9.
96 Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351(16):1645–54.
97 Ahmad SS, Huber K, Evangelopoulos DS, Kleer B, Kohlhof H, Schar M, et al. The cement prosthesis-like spacer: an intermediate halt on the road to healing. ScientificWorldJournal. 2013;2013:763434.
98 Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350(14):1422–9.
99 Romano CL, Gala L, Logoluso N, Romano D, Drago L. Two-stage revision of septic knee prosthesis with articulating knee spacers yields better infection eradication rate than one-stage or two-stage revision with static spacers. Knee Surg Sports Traumatol Arthrosc. 2012;20(12):2445–53.
100 Werner FW, Ayers DC, Maletsky LP, Rullkoetter PJ. The effect of valgus/varus malalignment on load distribution in total knee replacements. J Biomech. 2005;38(2):349–55.
101 Aglietti P, Buzzi R, Gaudenzi A. Patellofemoral functional results and complications with the posterior stabilized total condylar knee prosthesis. J Arthroplasty. 1988;3(1):17–25.
102 Clayton ML, Thirupathi R. Patellar complications after total condylar arthroplasty. Clin Orthop Relat Res. 1982(170):152–5.
103 Huo MH, Sculco TP. Complications in primary total knee arthroplasty. Orthopaedic review. 1990;19(9):781–8.
104 Lynch AF, Rorabeck CH, Bourne RB. Extensor mechanism complications following total knee arthroplasty. J Arthroplasty. 1987;2(2):135–40.
105 Webster DA, Murray DG. Complications of Variable Axis total knee arthroplasty. Clin Orthop Relat Res. 1985(193):160–7.
106 Mihalko WM, Krackow KA. Flexion and extension gap balancing in revision total knee arthroplasty. Clin Orthop Relat Res. 2006;446:121–6.
107 Alden KJ, Duncan WH, Trousdale RT, Pagnano MW, Haidukewych GJ. Intraoperative fracture during primary total knee arthroplasty. Clin Orthop Relat Res.2010;468(1):90–5.
108 Culp RW, Schmidt RG, Hanks G, Mak A, Esterhai JL, Jr., Heppenstall RB. Supracondylar fracture of the femur following prosthetic knee arthroplasty. Clin Orthop Relat Res. 1987(222):212–22.
109 Delport PH, Van Audekercke R, Martens M, Mulier JC. Conservative treatment of ipsilateral supracondylar femoral fracture after total knee arthroplasty. J Trauma. 1984;24(9):846–9.
110 Figgie MP, Goldberg VM, Figgie HE, 3rd, Sobel M. The results of treatment of supracondylar fracture above total knee arthroplasty. J Arthroplasty. 1990;5(3):267–76.
111 Rorabeck CH, Taylor JW. Periprosthetic fractures of the femur complicating total knee arthroplasty. Orthop Clin North Am. 1999;30(2):265–77.
112 McGraw P, Kumar A. Periprosthetic fractures of the femur after total knee arthroplasty. Journal of orthopaedics and traumatology: official journal of the Italian Society of Orthopaedics and Traumatology. 2010;11(3):135–41.
113 Daluga D, Lombardi AV, Jr., Mallory TH, Vaughn BK. Knee manipulation following total knee arthroplasty. Analysis of prognostic variables. J Arthroplasty. 1991;6(2):119–28.
114 Ritter MA. Postoperative pain after total knee arthroplasty. J Arthroplasty. 1997;12(3):337–9.
115 Shoji H, Yoshino S, Komagamine M. Improved range of motion with the Y/S total knee arthroplasty system. Clin Orthop Relat Res. 1987(218):150–63.
116 Beswick AD, Wylde V, Gooberman-Hill R, Blom A, Dieppe P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. Brit Med J open. 2012;2(1):e000435.
117 Macrae WA. Chronic post-surgical pain: 10 years on. Br J Anaesth. 2008;101(1):77–86.
118 Katz MM, Hungerford DS, Krackow KA, Lennox DW. Reflex sympathetic dystrophy as a cause of poor results after total knee arthroplasty. J Arthroplasty. 1986;1(2):117–24.
119 Hallab N, Merritt K, Jacobs JJ. Metal sensitivity in patients with orthopaedic implants. J Bone Joint Surg Am. 2001;83–A(3):428–36.
120 Granchi D, Cenni E, Tigani D, Trisolino G, Baldini N, Giunti A. Sensitivity to implant materials in patients with total knee arthroplasties. Biomaterials. 2008;29(10):1494–500.
121 DesJardins JD, Burnikel B, LaBerge M. UHMWPE wear against roughened oxidized zirconium and CoCr femoral knee components during force-controlled simulation. Wear. 2008;264(3–4):245–56.
122 Lee JK, Maruthainar K, Wardle N, Haddad F, Blunn GW. Increased force simulator wear testing of a zirconium oxide total knee arthroplasty. Knee. 2009;16(4):269–74.
123 Tsukada S, Wakui M, Matsueda M. Metal block augmentation for bone defects of the medial tibia during primary total knee arthroplasty. J Orthop Surg Res. 2013;8:36.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.