Post-treatment surveillance of head and neck cancer: pitfalls in the interpretation of FDG PET-CT/MRI
DOI: https://doi.org/10.4414/smw.2015.14116
Christian Martin
Meerwein, Marcelo A
Queiroz, Spyros
Kollias, Martin
Hüllner, Patrick
Veit-Haibach, Gerhard Frank
Huber
Summary
QUESTIONS UNDER STUDY: We investigated non-malignancy-associated (18F)fluoro-deoxy-D-glucose (FDG) uptake in the head and neck cancer (HNC) post-treatment follow-up with positron emission tomography – computed tomography / magnetic resonance imaging (PET-CT/MRI). A retrospective study on HNC patients undergoing follow-up or re-staging PET-CT/MRI examinations was performed. Thereby, FDG-positive regions were morphologically correlated to the CT and MRI images and a statement regarding tumour persistence/recurrence.
METHODS: FDG-positive lesions were assessed according to their anatomical localisation and categorised as true positive, true negative, false positive or false negative findings. The gold standard for verification of an FDG-positive lesion was the cytological or histopathological examination of the region of interest. The most likely aetiology was assessed according to the following categories: (1.) physiological uptake (2.) post-surgical, inflammatory uptake, (3.) post-irradiation, inflammatory uptake and (4.) reactive, not otherwise specified.
RESULTS: Tumour recurrence / tumour persistence was found in 14/87 patients (16.1%). A total of 159 non-malignancy-associated FDG-positive lesions were found. Every PET-CT/MRI examination revealed 2.1 ± 1.5 FDG-positive lesions in the head and neck. A total of 107 FDG-positive lesions (67.3%) were categorised as physiological, 52 FDG-positive lesions (32.7%) as inflammatory (post-surgical: n = 14, 8.8%; post-irradiation: n = 9, 5.7%; reactive, not otherwise specified: n = 29, 18.2%). Eight patients (11.8%) underwent invasive diagnostic procedures to clarify indistinct findings.
CONCLUSIONS: Post-treatment follow-up of HNC patients requires interdisciplinary management and familiarity with the patient’s past medical history. Awareness of common confounders of FDG positivity often allows clarification of indistinct lesions. However, a substantial number of approximately 12% of FDG-positive lesions remain unclear unless invasive diagnostic procedures are performed.
Introduction
Post-treatment surveillance of head and neck cancer (HNC) requires periodic clinical and radiological examinations to exclude persistence or recurrence of locoregional disease, distant metastases or secondary tumours. Despite aggressive multimodal therapy regimens, locoregional recurrence appears in up to 40%, whereby most recurrences occur within the first 2 years after primary treatment [1–3]. In recent years, (18F)fluoro-deoxy-D-glucose (FDG) positron emission tomography (PET) – computed tomography (CT) has become a valuable tool in post-treatment surveillance, which provides information on the metabolic activity and the anatomical localisation of the locoregional tumour site and therefore is superior in certain cases to conventional imaging modalities, such as PET, CT or magnetic resonance imaging (MRI) [4–7]. This is especially true for PET-negative conditions, since the negative predictive value has been demonstrated to be consistently high (>90%) and therefore negative periodic clinical examinations may be complemented by PET-CT [8]. However, there are various non-malignant FDG-positive conditions, which may cause increased FDG uptake, resulting in a positive predictive value (PPV) of only 64–77% in detecting locoregional persistence or recurrence [8, 9]. Being confronted with this high number of false-positive results, interpretation of PET-CT is challenging and can result in potentially unnecessary investigations, interventions and patient uncertainty. With the recent introduction of PET-MRI, several of those challenging cases in PET-CT might be solved on the basis of the unmatched soft tissue contrast and partly less susceptibility to dental artefacts [10–12].
The aim of this study was to support treating physicians by providing typical distribution patterns and aetiologies of non-malignancy-associated FDG-uptake in the PET-CT/MRI post-treatment follow-up of HNC.
Material and methods
Patient population
A retrospective study was performed involving consecutive HNC patients undergoing follow-up or re-staging PET-CT/MRI between February 2012 und February 2013. All patients were discussed in our multidisciplinary tumour board and staged according to the latest version of the American Joint Committee on Cancer (AJCC) guidelines [13]. Patients were initially treated with curative intent for primary tumours and had accomplished individual treatment protocols, including surgery and/or radiation therapy (RT) and/or chemotherapy. As part of the post-treatment follow-up programme, patients with a large primary tumour (>T2) and / or multiple regional metastases (>N2a) underwent periodic clinical examinations and PET-CT/MRI examinations at 3, 9 and 18 months post-treatment. Patients with clinically suspected tumour recurrence or persistence after primary therapy received either cyto-/histopathological examinations or further clinical and imaging examinations within shorter time periods.
Imaging
Sequential PET-CT/MRIs (with intravenous contrast media applied in head and neck MRI and PET-CT) were performed on a tri-modality PET-CT/MRI setup (full ring, time-of-flight Discovery PET-CT 690, 3T Discovery MR 750w, both GE Healthcare, Waukesha, WI, USA). For detailed imaging protocols and imaging processing we refer to our previously published paper [11].
Clinical evaluation
Patients’ charts were reviewed for demographic data and tumour characteristics [13], and the patients underwent a clinical examination. Treatment protocols were analysed for surgical resection of the primary tumour, free tissue transfer/local flaps, neck dissection (unilateral vs bilateral), radiation therapy (RT) (unilateral vs bilateral) and chemotherapy. Furthermore, the length of the post-treatment period was assessed.
Statistical analysis
All PET-CT/MRI examinations were reviewed by one dual board-certified radiologist / nuclear medicine physician and one otorhinolaryngology (ENT) surgeon. FDG-positive lesions in the head and neck were assessed according to their anatomical localisation. FDG-positive lesions were graded as true positive, true negative, false positive or false negative, on the basis of further clinical, cytological, histological or ultrasound examinations. The most likely aetiology of FDG positive lesions was categorised as: (1.) physiological uptake (e.g. muscle activity), (2.) post-surgical, inflammatory uptake, (3.) post-irradiation, inflammatory uptake and (4.) reactive, not otherwise specified, inflammatory uptake [14]. In the case of uncertainty regarding the aetiology of a FDG-positive lesion, corresponding MRI examinations were used for better soft-tissue contrast. Of note, for a systematic comparison of PET-CT versus PET-MRI we refer to a previously published study by our group [11]. The gold standard for verification of indistinct FDG-positive lesions was cytological or histopathological examination. Data were expressed as mean ± standard deviation (SD) or median and interquartile range (IQR) if appropriate. Descriptive statistics were made using SPSS for Windows 15.0 (SPSS, Inc., Chicago, IL, USA).
|
Table 1: Patient and tumour characteristics for all 87 patients included. |
| Age at FDG PET/CT-MRI scan: |
|
| Mean ± SD (years) |
63.2 ± 12.0 |
| Range |
24–90 |
| Gender: |
|
| Male |
68 (78.2%) |
| Female |
19 (21.8%) |
| Primary tumour site: |
|
| Oropharynx |
26 (29.9%) |
| Oral cavity |
17 (19.4) |
| Larynx |
14 (16%) |
| Epipharynx |
8 (9.2%) |
| Hypopharynx |
7 (8.1%) |
| Parotid gland |
4 (4.6%) |
| Paranasal sinuses |
3 (3.5%) |
| Skin (BSC, angiosarcoma, melanoma) |
2 (2.3%) |
| Maxilla |
2 (2.3%) |
| Cancer of unknown origin (CUP) |
3 (3.5%) |
| Simultaneous (floor of the mouth/hypopharynx) |
1 (1.2%) |
| Histological workup |
|
| Squamous cell carcinoma |
76 (87.4%) |
| Neuroendocrine carcinoma |
2 (2.3%) |
| Adenocarcinoma |
2 (2.3%) |
| Adenoidcystic carcinoma |
2 (2.3%) |
| Mucoepidermoid carcinoma |
1 (1.2%) |
| Melanoma |
1 (1.2%) |
| Angiosarcoma |
1 (1.2%) |
| Neuroblastoma |
1 (1.2%) |
| Spindle-cell like carcinoma |
1 (1.2%) |
| T-stage: |
|
| T1 |
23 (26.4%) |
| T2 |
29 (33.3%) |
| T3 |
10 (11.5%) |
| T4 |
18 (20.7%) |
| Not otherwise specified |
7 (8.1%) |
| N-stage: |
|
| N0 |
29 (33.3%) |
| N1 |
10 (11.5%) |
| N2 |
40 (46%) |
| N3 |
3 (3.5%) |
| Not otherwise specified |
5 (5.7%) |
| TNM staging: |
|
| Stage I |
6 (6.9%) |
| Stage II |
9 (10.4%) |
| Stage III |
13 (14.9%) |
| Stage IV |
53 (60.9%) |
| Not otherwise specified |
6 (6.9%) |
| BSC = basal cell carcinoma; CT = computed tomography; FDG = (18F)fluoro-deoxy-D-glucose; PET = positron emission tomography |
|
Table 2: Detailed information on all 159 FDG-positive lesions in the subgroup of disease-free patients. |
| Sublingual Space, floor of the mouth |
29 (18.2%) |
| Soft palate |
22 (13.8%) |
| Pharyngeal mucosal space |
17 (10.7%) |
| Palatine tonsils, base of the tongue |
16 (10.1%) |
| Oral cavity, tongue |
10 (6.3%) |
| Hypopharynx, aryepiglottic fold |
10 (6.3%) |
| Larynx |
6 (3.8%) |
| Scalene or sternocleidomastoid muscle |
7 (4.4%) |
| Muscles of mastication |
5 (3.1%) |
| Tracheostoma, voice-prosthesis |
5 (3.1%) |
| Epipharynx |
3 (1.9%) |
| Submandibular gland |
3 (1.9%) |
| Mandible |
2 (1.3%) |
| Parotid Space |
2 (1.3%) |
| Thyroid |
2 (1.3%) |
| Upper oesophagus |
1 (0.6%) |
| Hyoid bone |
1 (0.6%) |
| Mastoid process |
1 (0.6%) |
| Styloid process |
1 (0.6%) |
| Cervical lymph nodes |
|
| Level I |
4 (2.5%) |
| Level II |
9 (5.7%) |
| Level III |
2 (1.3%) |
| Level IV |
– |
| Level V |
1 (0.6%) |
|
Total
|
159 lesions
|
| FDG = (18F)fluoro-deoxy-D-glucose |
Results
Demographic data
In summary, 87 patients were included in this study: 68 male patients (78.2%) vs 19 female patients (21.8%). At the time of PET-CT/MRI examination, the mean age of all patients was 63.4 ± 12.0 years (range 24–90 years). The indication for PET-CT/MRI was routine follow-up in 89.7% (n = 78) and re-staging owing to clinically suspected or histologically confirmed tumour persistence/recurrence in 10.3% (n = 9). Median time between the end of the treatment and PET-CT/MRI examination was 8.8 months (IQR: 3.3–20.5), which coincides with the second follow-up examination, as mentioned above.
Site of primary tumour, TNM staging, histological workup and secondary malignancies
Table 1 shows the initial primary tumour sites, overall TNM staging and histological workup of all patients included. In 9/87 patients (10.3%), analysis of patients’ charts revealed a past medical history of a prior malignancy in the head and neck region, which had already been treated with curative intent before the appearance of the malignancy of interest.
Treatment protocols
In terms of surgical treatment, 45/87 patients (51.7%) had surgical resection of the primary tumour, including 22 patients (25.3%), who received a reconstruction with a local or free flap. Forty-six patients underwent unilateral or bilateral neck dissection (53.9%). With regard to RT, 79/87 patients (90.8%) received RT to the primary tumour site and 68/87 patients (78.2%) to the regional neck.
FDG PET-CT and PET-MRI findings
Overall, tumour recurrence, tumour persistence or regional soft-tissue metastases were found in 14/87 patients (16.1%). In 4/87 (4.6%) patients local tumour recurrence, in 2/87 patients (2.3%) regional tumour recurrence and in 1/87 patient (1.2%) both local and regional tumour recurrence was found. In terms of tumour persistence, 3/87 patients (3.5%) had local and regional persistence and 1/87 patient (1.2%) had regional persistence. Additionally, regional soft-tissue metastases were histologically confirmed in 3/87 patients (3.5%) after neck dissection. Of note, only 5/14 malignant lesions were detected in clinically asymptomatic patients during routine follow-up diagnostic imaging (two local recurrences, one regional persistence, two regional soft-tissue metastases) whilst 9/14 patients underwent PET-CT/MRI owing to clinically or histologically confirmed persistence/recurrence.
Subset of patients without tumour recurrence / persistence / soft-tissue metastases
In 73/87 patients (83.9%) imaging as well as clinical follow-up revealed no tumour persistence, recurrence or soft-tissue metastases. Of these 73 patients, 65 patients (89%) did not require further diagnostic procedures apart from routine clinical examination. In 8/73 (11.7%) patients with a suspicious lesion in PET-CT and PET-MRI, clinical examination could not rule out tumour recurrence/persistence and patients then underwent biopsy (n = 6) or fine needle aspiration (FNA) of regional lymph nodes (n = 2). Three of these eight patients required multiple biopsies to exclude recurrence. Finally, the histological and clinical assessment revealed a radiogenic ulcer in the hypopharynx (n = 1), osteoradionecrosis of the mandible (n = 1), radiogenic chondronecrosis of the corniculate cartilages (n = 1) and unspecific chronic inflammation of the epipharynx, palatine tonsils and base of the tongue respectively (n = 3). The FNA due to indistinct FDG-activity in cervical lymph nodes showed reactive lymphadenopathy in both cases.
A total of 159 FDG-positive lesions with known or unknown anatomic correlates in the head and neck region were found in the 73 patients. On average, every FDG PET-CT and PET-MRI examination revealed 2.1 ± 1.5 FDG-positive lesions in the head and neck region (range: 0–7). Of these 159 lesions, 143 lesions (89.9%) were mainly related to the oral cavity and the oropharynx. Additionally, 16 lesions were related to cervical lymph nodes (10.1%) (table 2).
Review of the most likely aetiology of the lesions showed physiological FDG uptake in 107 lesions (67.3%), in which physiological muscle activity, FDG uptake due to lymphatic tissue and compensatory FDG uptake after contralateral treatment were predominant findings (table 3). Furthermore, 52 FDG-positive lesions (32.7%) were categorised as inflammatory FDG uptake: post-surgical, inflammatory uptake (n = 14, 8.8%) vs post-irradiation, inflammatory uptake (n = 9, 5.7%) vs reactive, not otherwise specified FDG uptake (n = 29, 18.2%) (table 4). Overall, PET-MRI was used for twelve lesions, which were mainly related to the suprahyoid area, to further clarify indistinct FDG accumulation.
|
Table 3: Detailed information on all FDG-positive lesions considered physiological. |
| Floor of the mouth muscles |
27 (25.2%) |
| Soft palate |
21 (19.6%) |
| Muscles of mastication, sternocleidomastoid muscle, scalene muscle |
18 (16.8%) |
| Pharyngeal mucosal space |
16 (15.0%) |
| Palatine tonsils, Waldeyer ring |
8 (7.5%) |
| Larynx, hypopharynx, upper oesophagus |
8 (7.5%) |
| Salivary glands (compensation due to contralateral RT) |
5 (4.7%) |
| Tongue (muscular imbalance due to contralateral resection of the tongue) |
4 (3.7%) |
|
Total
|
107 lesions
|
| FDG = (18F)fluoro-deoxy-D-glucose; RT = radiation therapy |
|
Table 4: Detailed information on all FDG-positive lesions considered to be due to inflammatory processes. |
| Unspecific, reactive |
29 (55.8%) |
| Cervical lymph nodes |
15 |
| Post-surgical |
14 (26.9%) |
| Tracheostoma, voice prosthesis |
5 |
| Oral cavity, tongue |
5 |
| Reconstruction of the mandible (synthetic material) |
1 |
| Hypopharynx (post partial laryngo-pharyngectomy) |
1 |
| Mastoid process |
1 |
| Shifted submandibular gland due to reconstruction |
1 |
| Post-irradiation |
9 (17.3%) |
| Radiogenic ulcer hypopharynx |
1 |
| Osteoradionecrosis (mandible, cricoid, styloid process) |
4 |
| Radiogenic chondronecrosis (corniculate cartilages) |
1 |
| Chronic inflammation (base of the tongue, epipharynx) |
3 |
|
Total
|
52 lesions
|
| FDG = (18F)fluoro-deoxy-D-glucose |
Discussion
In this study of HNC patients in post-treatment follow-up, we demonstrated that non-malignancy-associated FDG-positive side findings are very common. Various physiological and inflammatory conditions may cause increased FDG activity, and management of these findings requires familiarity with patient’s treatment and interdisciplinary discussion of indistinct lesions. Although the clinical examination usually clarifies indistinct findings, invasive examinations may still be necessary in a considerable number of patients.
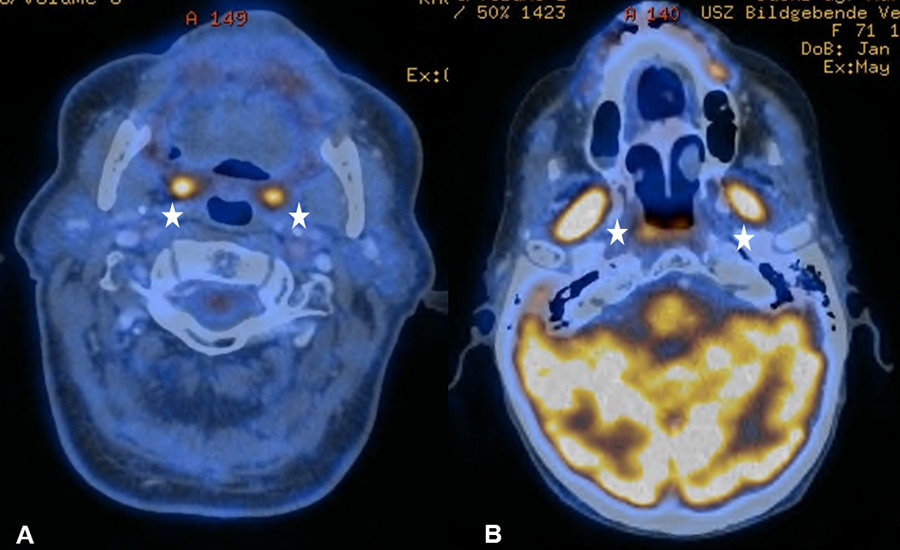
Figure 1
(A) Bilateral physiological uptake in the palatine tonsils in a 83-year old patient with a past medical history of a floor of nose melanoma. (B) Symmetric increased uptake in the muscles of mastication in 71-year old patient treated for tonsillar carcinoma.
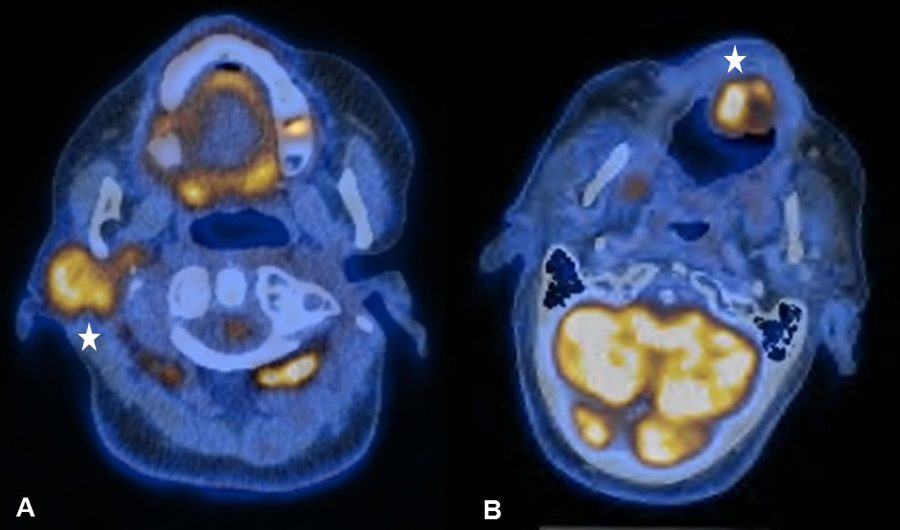
Figure 2
(A) Increased uptake in the right parotid gland in a 56-year old patient with a past medical history of radical parotidectomy and adjuvant radiation therapy (RT) on the left side due to a parotid gland carcinoma. Physiological uptake in the cervical brown fat tissue and the floor of mouth (FOM) muscles. (B) Increased uptake in the left paramedian FOM muscles due to muscular dysbalance in a 69-year old patient with a past medical history of surgical resection and RT of a right-sided base of the tongue carcinoma

Figure 3
(A) Compensatory uptake in the left vocal cord in a 59-year old patient with known right unilateral vocal cord immobility due to a transglottic larynx carcinoma. (B) The corresponding T2-weighted magnetic resonance imaging examination helps to better delineate the immobile right vocal cord by providing superior soft-tissue contrast.
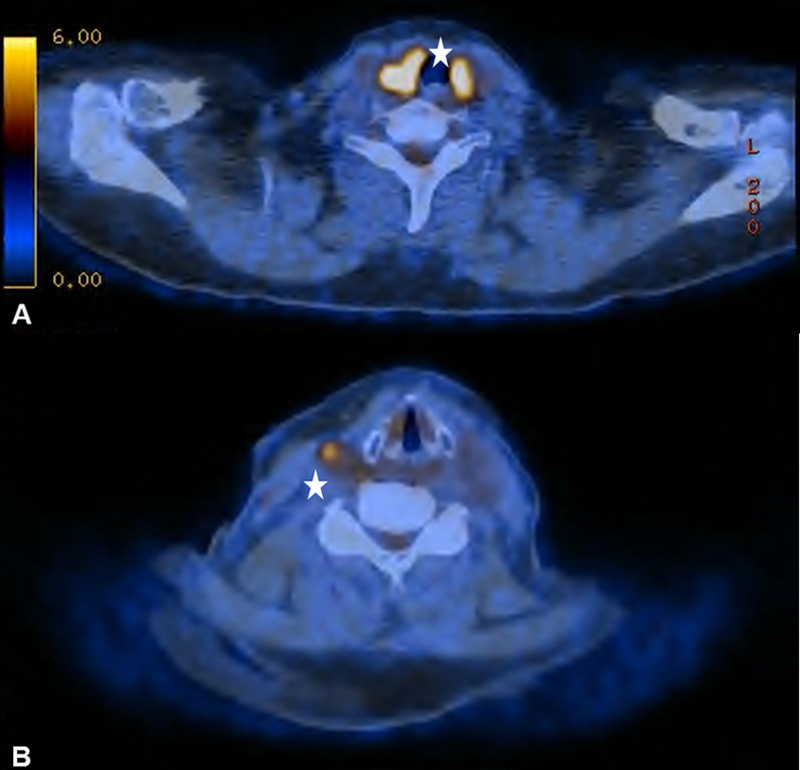
Figure 4
(A) Diffuse bilateral uptake in the thyroid without any morphological correlate in a 67-year old patient with no clinical, ultrasonographical or laboratory signs of thyroid dysfunction. (B) The same patient with corresponding probable reactive lymphadenopathy.
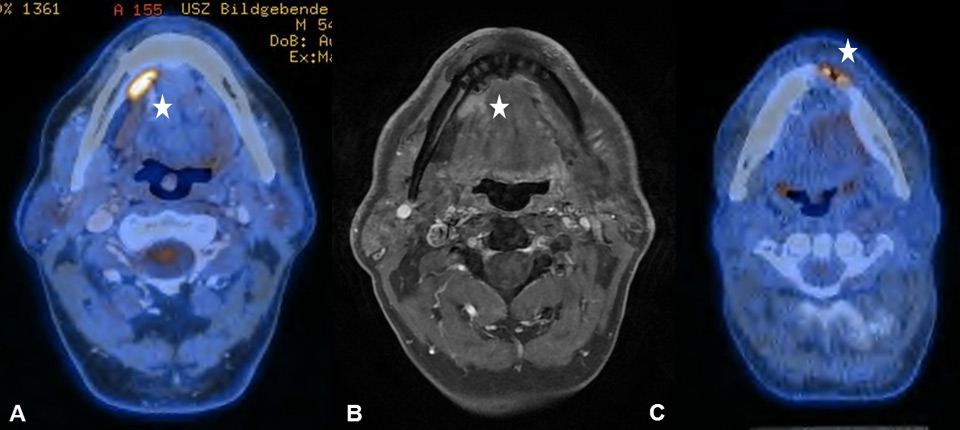
Figure 5
(A) Increased focal uptake in the right submandibular gland in a 54-year old patient, who underwent surgical resection and reconstruction with a pedicled flap owing to a lateral tongue carcinoma. (B) The corresponding T1–weighted magnetic resonance imaging helps to delineate the shifted submandibular gland due to the pectoralis maior myocutaneous flap. (C) Sixty-six year old patient after segmental mandibular resection, mandibular reconstruction and implantation of dental implants due to an anterior floor-of-mouth carcinoma
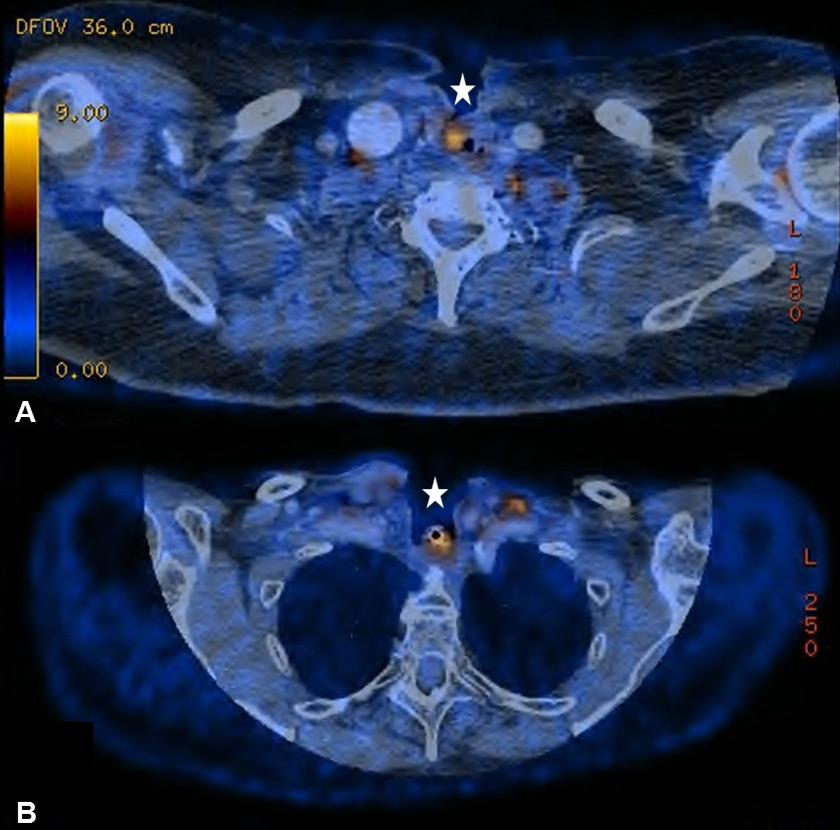
Figure 6
(A) Increased uptake around inserted voice prosthesis in a 74-year old patient with a past medical history of total laryngectomy and radiation therapy (RT) due to a transglottic larynx carcinoma. (B) Seventy-one year old patient after total laryngectomy, partial thyroidectomy and RT with increased uptake around the tracheostoma.
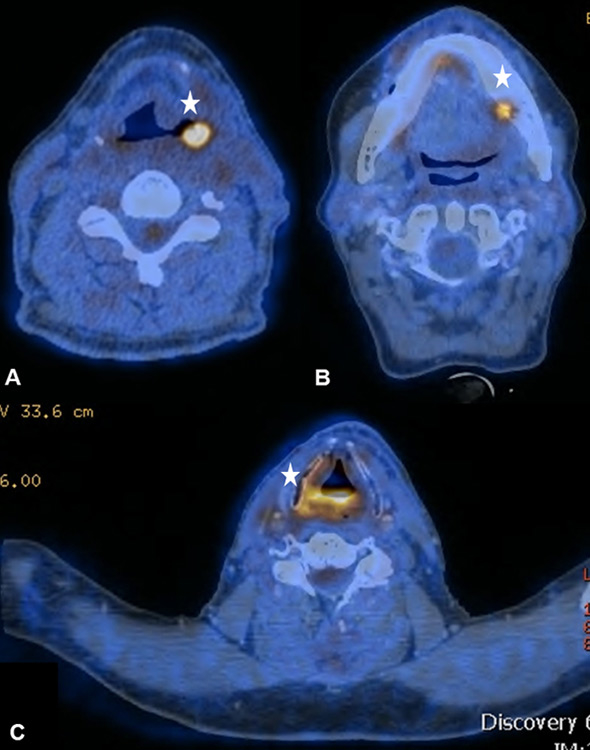
Figure 7
(A) Sixty-two year old patient after curative radio-chemotherapy (RCT) for a supraglottic larynx carcinoma with increased focal uptake in the left hypopharynx due to a radiogenic ulcer. (B) Increased focal uptake of the left mandible due to osteoradionecrosis in a 48-year old patient after curative RCT for an oropharynx carcinoma (C) Radiogenic chondronecrosis of the corniculate cartilages in a 59-year old patient after surgical resection and radiation therapy of a base of tongue carcinoma
It is known that PET-CT is more accurate in detecting HNC recurrence than conventional physical examinations alone and that therefore negative periodic clinical examinations may be complemented by FDG PET-CT [8, 15]. As expected, we observed PET-CT to be a well-established ruling-out test at the expense of a decreased positive predictive value and specificity [7–9]. However, interpretation of indistinct FDG-positive findings remains challenging because of a high number of false-positive lesions and the necessity for potentially unnecessary and invasive diagnostic investigations. As in previous studies, we found typical foci of increased FDG uptake [16, 17]. The pharyngeal mucosa frequently causes physiological FDG uptake, hence the interpretation is usually unproblematic as long as the uptake is located superficially along the mucosal plane in linear configuration [18]. The palatine tonsils and other lymphatic structures of the Waldeyer’s ring typically exhibit FDG uptake, which most likely reflects a so-called “physiological inflammation” of the lymphatic tissue due to confrontation with antigens [18, 19] (fig. 1A). Furthermore, muscles of mastication and intrinsic tongue muscles were shown to be highly sensitive to exogenous confounders such as chewing gum during examination and therefore a good quality of PET-CT/MRI clearly depends on the compliance of the patient [20, 21] (fig. 1B). With regard to the floor of mouth (FOM) muscles, a recent study investigating the effect of a supine versus a sitting position on physiological FDG accumulation, did not find any alterations and concluded that there is no trick to avoid or reduce this kind of disturbing FDG uptake [22]. The correlation between muscular activity and increased FDG uptake was also shown for the larynx, since talking can cause FDG uptake in the muscles of phonation as well as in the vocal cords [18, 23]. In terms of FDG uptake in the salivary glands, the parotid, submandibular and sublingual glands may all reveal mild to moderate symmetric or asymmetric uptake, although these findings underlie a marked inter-individual variability [16, 17]. As there is a close relationship between reduction in FDG uptake into the salivary glands and increasing doses of RT, we found five patients with asymmetric salivary gland FDG uptake due to surgical treatment and RT to the contralateral side [24] (fig. 2A). Similarly, we observed four patients with asymmetric FDG uptake in the tongue and FOM as a consequence of muscular imbalance due to contralateral surgical treatment (fig. 2B). Another well-known phenomenon is the increased FDG uptake in the contralateral vocal cord if a recurrent laryngeal nerve palsy is present (fig. 3AB).
Our case series is typical for a tertiary referral centre, with squamous cell cancer being the most common disease and the oral cavity / oropharynx being the most common primary tumour sites. The high proportion of multimodal treatment protocols in our cohort reflects the fact that most patients were treated for advanced disease. Our current practice is to refer patients with small primary tumours and/or limited regional lymphatic spread for clinical post-treatment surveillance combined with non-metabolic imaging (CT, US), whereas patients with a large primary tumour (>T2) and/or multiple regional metastases (>N2a) routinely undergo periodic clinical and PET-CT or now partly PET-MRI examinations. In this particular subgroup of patients, as mentioned before, the risk of distant metastases is markedly increased and the whole-body imaging multimodality provides superior information, when compared with MRI or CT alone. The first post-treatment baseline PET-CT/MRI should usually be scheduled at least 2 months post-treatment in order to reduce the rate of false-positive findings due to treatment-related inflammatory changes [25, 26]. In the subgroup of tumour-free patients, non-malignancy-associated FDG lesions could be managed by means of clinical examination in 83.9% of all patients. However, even though the timing for the PET-CT/MRI examination appears to be appropriate, eight patients (11.7%) required invasive diagnostic procedures to clarify indistinct lesions.
One of the most common causes of false-positive FDG findings in the post-treatment setting is increased FDG uptake due to benign conditions, such as inflammation, infections or granulomatous processes [27, 28]. Thus, literally any inflammatory process in the head and neck region can cause increased FDG activity [27]. In our study, 32.7% (52/179) of all FDG-positive lesions were categorised as inflammatory FDG uptake, of which a distinct attribution to an anatomical substrate or a treatment-modality was possible in only 44.2% (23/52). In 55.8% (29/52), FDG-positive findings were categorised as reactive, unspecific lesions; 15/29 of these lesions were related to cervical lymph nodes. Thereby, we often assumed multi-aetiological reasons of the FDG-positive lesion with treatment-related and non-treatment-related contributors [15]. Reactive lymphoid hyperplasia arising from inflammatory or infectious processes frequently causes FDG accumulation in cervical lymph nodes and often requires a clinical and ultrasonographic re-assessment [29]. However, in our case series invasive diagnostic procedures such as FNA were necessary in only two patients, indicating that those indistinct lesions in cervical lymph nodes are manageable in a reasonable way. Liu et al. investigated the clinical significance of diffuse thyroid uptake on PET-CT and usually considered FDG uptake into the thyroid to be benign and very likely secondary to thyroiditis and/or hypothyroidism [30] (fig. 4AB).
Typical post-surgical findings were inflammatory FDG uptake of the tongue and the oral cavity due to transoral surgical resections or a shifted submandibular gland owing to a pectoralis major myocutaneous flap (fig. 5AB). Patients after laryngectomy, formation of a tracheostoma and insertion of voice prosthesis frequently showed increased FDG-activity around the voice prosthesis (fig. 6AB). Implantation of synthetic materials in the head and neck region, such as a voice prosthesis, can cause increased mucus production and tracheal wall damage with accompanying immunological cell accumulation and then increased FDG uptake [31]. Lastly, dental implants or non-removable bridgework can also cause artefacts in PET-CT [32]. However, as it was previously shown by our group, those artefacts might partly be reduced in PET-MRI [11, 33]. Therefore, PET-MRI seems to be an additional tool to complement PET-CT, especially when evaluating the oropharynx and oral cavity, since synthetic material typically causes artefacts in this region.
Irradiation-related inflammatory FDG uptake was observed because of a radiogenic ulcer in the hypopharynx, osteoradionecrosis, radiogenic chondronecrosis and chronic mucosal inflammation (fig. 7ABC). The occurrence of false-positive FDG uptake caused by osteoradionecrosis in HNC patients after RT is a known difficulty and well-reported for nasopharyngeal carcinoma [34, 35]. Management of these lesions is challenging since the clinical examination can be inconclusive and definitive exclusion of malignancy often requires multiple biopsies. Re-evaluation by a multidisciplinary tumour board plays a pivotal role in adequate management of these challenging patients: surgeons, nuclear medicine physicians, radiation oncologists, oncologists and pathologists need to carefully review indistinct findings and to determine individual diagnostic algorithms.
Without doubt, biopsy is the gold standard for clarifying indistinct FDG-positive lesions. We are aware of the fact that in this educational study, grading of indistinct lesions as true positive, true negative, false positive or false negative findings was mainly based on clinical and radiological examinations and the further course of disease. However, the aim of this paper is to support the treating physicians and to help avoiding potential unnecessary investigations and complications. As a result of the high number of false-positive FDG PET-CT findings, invasive procedures should always be carefully considered in a multidisciplinary setting and build the last step in the diagnostic algorithm.
Conclusion
Post-treatment follow-up of HNC patients requires familiarity with the patient’s medical history, especially in terms of prior treatment modalities. PET-CT/MRI provides high sensitivity at the expense of decreased specificity. Hence, non-malignancy-associated FDG-positive lesions are common side-findings of routine follow-up examinations. Awareness of common physiological and treatment-related confounders of FDG-positivity often allows clarification of indistinct lesions. However, despite interdisciplinary discussion of unclear FDG-positive lesions, further invasive diagnostic procedures are necessary in a non-negligible proportion of asymptomatic patients.
References
1 Ang KK, Trotti A, Brown BW, Garden AS, Foote RL, Morrison WH, et al. Randomized trial addressing risk features and time factors of surgery plus radiotherapy in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;51(3):571–8.
2 Leemans CR, Tiwari R, Nauta JJ, van der Waal I, Snow GB. Recurrence at the primary site in head and neck cancer and the significance of neck lymph node metastases as a prognostic factor. Cancer. 1994;73(1):187–90.
3 F.P. Kuhn MH, S.S. Kollias, G.K. Von Schulthess, P. Veit‑Haibach. Comparison of contrast-enhanced PET/MRI and contrast-enhanced PET/CT in patients with head and neck cancers Volume ECR Abstracts, Insights Imaging 2013;4(Suppl 1):S145–S3842013.
4 Adams S, Baum RP, Stuckensen T, Bitter K, Hor G. Prospective comparison of 18F-FDG PET with conventional imaging modalities (CT, MRI, US) in lymph node staging of head and neck cancer. Eur J Nucl Med. 1998;25(9):1255–60.
5 Kitagawa Y, Nishizawa S, Sano K, Ogasawara T, Nakamura M, Sadato N, et al. Prospective comparison of 18F-FDG PET with conventional imaging modalities (MRI, CT, and 67Ga scintigraphy) in assessment of combined intraarterial chemotherapy and radiotherapy for head and neck carcinoma. J Nucl Med. 2003;44(2):198–206.
6 Kunkel M, Forster GJ, Reichert TE, Jeong JH, Benz P, Bartenstein P, et al. Detection of recurrent oral squamous cell carcinoma by [18F]-2–fluorodeoxyglucose-positron emission tomography: implications for prognosis and patient management. Cancer. 2003;98(10):2257–65.
7 Wong RJ, Lin DT, Schoder H, Patel SG, Gonen M, Wolden S, et al. Diagnostic and prognostic value of [(18)F]fluorodeoxyglucose positron emission tomography for recurrent head and neck squamous cell carcinoma. J Clin Oncol. 2002;20(20):4199–208.
8 Abgral R, Querellou S, Potard G, Le Roux PY, Le Duc-Pennec A, Marianovski R, et al. Does 18F-FDG PET/CT improve the detection of posttreatment recurrence of head and neck squamous cell carcinoma in patients negative for disease on clinical follow-up? J Nucl Med. 2009;50(1):24–9.
9 Ryan WR, Fee WE, Jr., Le QT, Pinto HA. Positron-emission tomography for surveillance of head and neck cancer. Laryngoscope. 2005;115(4):645–50.
10 F.P. Kuhn MH, S.S. Kollias, G.K. Von Schulthess, P. Veit‑Haibach. Comparison of contrast-enhanced PET/MRI and contrast-enhanced PET/CT in patients with head and neck cancers (ECR Abstracts). Insights Imaging. 2013;4(Suppl):S 145–S 384.
11 Queiroz MA, Hüllner M, Kuhn F, Huber G, Meerwein C, Kollias S, et al. PET/MRI and PET/CT in follow-up of head and neck cancer patients. Eur J Nucl Med Mol Imaging 2014.
12 Ghanooni R, Delpierre I, Magremanne M, Vervaet C, Dumarey N, Remmelink M, et al. ¹⁸F-FDG PET/CT and MRI in the follow-up of head and neck squamous cell carcinoma. Contrast Media Mol Imaging. 2011;6(4):260–6.
13 Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Springer; 2010.
14 Bhargava P, Rahman S, Wendt J. Atlas of confounding factors in head and neck PET/CT imaging. Clin Nucl Med. 2011;36(5):e20–9.
15 Krabbe CA, Pruim J, Dijkstra PU, Balink H, van der Laan BF, de Visscher JG, Roodenburg JL. 18F-FDG PET as a routine posttreatment surveillance tool in oral and oropharyngeal squamous cell carcinoma: a prospective study. J Nucl Med. 2009;50(12):1940–7.
16 Nakamoto Y, Tatsumi M, Hammoud D, Cohade C, Osman MM, Wahl RL. Normal FDG distribution patterns in the head and neck: PET/CT evaluation. Radiology. 2005;234(3):879–85.
17 Abouzied MM, Crawford ES, Nabi HA. 18F-FDG imaging: pitfalls and artifacts. J Nucl Med Technol. 2005;33(3):145–55; quiz 162–3.
18 Blodgett TM, Fukui MB, Snyderman CH, Branstetter BFt, McCook BM, Townsend DW, Meltzer CC. Combined PET-CT in the head and neck: part 1. Physiologic, altered physiologic, and artifactual FDG uptake. Radiographics. 2005;25(4):897–912.
19 Kawabe J, Okamura T, Shakudo M, Koyama K, Sakamoto H, Ohachi Y, et al. Physiological FDG uptake in the palatine tonsils. Ann Nucl Med. 2001;15(3):297–300.
20 Jackson RS, Schlarman TC, Hubble WL, Osman MM. Prevalence and patterns of physiologic muscle uptake detected with whole-body 18F-FDG PET. J Nucl Med Technol. 2006;34(1):29–33.
21 Rikimaru H, Kikuchi M, Itoh M, Tashiro M, Watanabe M. Mapping energy metabolism in jaw and tongue muscles during chewing. J Dent Res. 2001;80(9):1849–53.
22 Haerle SK, Hany TF, Ahmad N, Burger I, Huber GF, Schmid DT. Physiologic [18F]fluorodeoxyglucose uptake of floor of mouth muscles in PET/CT imaging: a problem of body position during FDG uptake? Cancer Imaging. 2013;13(1):1–7.
23 Kamel EM, Goerres GW, Burger C, von Schulthess GK, Steinert HC. Recurrent laryngeal nerve palsy in patients with lung cancer: detection with PET-CT image fusion – report of six cases. Radiology. 2002;224(1):153–6.
24 Roach MC, Turkington TG, Higgins KA, Hawk TC, Hoang JK, Brizel DM. FDG-PET assessment of the effect of head and neck radiotherapy on parotid gland glucose metabolism. Int J Radiat Oncol Biol Phys. 2012;82(1):321–6.
25 Greven KM, Williams DW, 3rd, McGuirt WF, Sr., Harkness BA, D’Agostino RB, Jr., Keyes JW, Jr., Watson NE, Jr. Serial positron emission tomography scans following radiation therapy of patients with head and neck cancer. Head Neck. 2001;23(11):942–6.
26 Greven KM, Williams DW, 3rd, Keyes JW, Jr., McGuirt WF, Watson NE, Jr., Randall ME, Raben M, Geisinger KR, Cappellari JO. Positron emission tomography of patients with head and neck carcinoma before and after high dose irradiation. Cancer. 1994;74(4):1355–9.
27 Fukui MB, Blodgett TM, Snyderman CH, Johnson JJ, Myers EN, Townsend DW, et al. Combined PET-CT in the head and neck: part 2. Diagnostic uses and pitfalls of oncologic imaging. Radiographics. 2005;25(4):913–30.
28 Zhuang H, Yu JQ, Alavi A. Applications of fluorodeoxyglucose-PET imaging in the detection of infection and inflammation and other benign disorders. Radiol Clin North Am. 2005;43(1):121–34.
29 King KG, Kositwattanarerk A, Genden E, Kao J, Som PM, Kostakoglu L. Cancers of the oral cavity and oropharynx: FDG PET with contrast-enhanced CT in the posttreatment setting. Radiographics. 2011;31(2):355–73.
30 Liu Y. Clinical significance of thyroid uptake on F18–fluorodeoxyglucose positron emission tomography. Ann Nucl Med. 2009;23(1):17–23.
31 Stokkel MP, Bongers V, Hordijk GJ, van Rijk PP. FDG positron emission tomography in head and neck cancer: pitfall or pathology? Clin Nucl Med. 1999;24(12):950–4.
32 Goerres GW, Hany TF, Kamel E, von Schulthess GK, Buck A. Head and neck imaging with PET and PET/CT: artefacts from dental metallic implants. Eur J Nucl Med Mol Imaging. 2002;29(3):367–70.
33 Delso G, Wollenweber S, Lonn A, Wiesinger F, Veit-Haibach P. MR-driven metal artifact reduction in PET/CT. Phys Med Biol. 2013;58(7):2267–80.
34 Wang CH, Liang JA, Yen KY, Hsieh TC, Sun SS, Wu YC, Lin YY, Kao CH. Tl-201 SPECT in clarifying false positive FDG PET findings caused by osteoradionecrosis in a case of nasopharyngeal carcinoma. Clin Nucl Med. 2009;34(8):515–7.
35 Hung GU, Tsai SC, Lin WY. Extraordinarily high F-18 FDG uptake caused by radiation necrosis in a patient with nasopharyngeal carcinoma. Clin Nucl Med. 2005;30(8):558–9.






