DOI: https://doi.org/10.4414/smw.2015.14087
At all ages and in all societies infertility has constituted a major health problem. Its incidence is currently thought to be on the rise due to the increasing age of women at childbirth and sequential family planning. As a result of these demographic trends, the demand for medical support is similarly growing, as demonstrated by the widening variety of supporting treatment modalities to alleviate infertility [1]. In addition to conventional in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI), the technology of cryopreservation of gametes and embryos has been improved to such extent that oocytes can now be stored frozen, allowing long term deferral of conception. This strategy may become particularly helpful to young women afflicted by malignant diseases requiring chemotherapy or radiation potentially destructive to their ovaries [2]. For women whose ovarian reserve has declined irreversibly due to ageing or disease, oocytes donated by younger women have now been established as a safe treatment for both donors and acceptors. Transmission of genetic disease to the offspring can be prevented in affected families by the use of preimplantation genetic diagnostics (PGD) or reduced through the sorting of spermatozoa [3]. Both multiple deliveries and OHSS are the most common complications of ART and are associated with high morbidity rates and significant financial burden. Along with the development of new technologies in ART, hormonal stimulation of ovaries has been refined so that premature ovulation as well as OHSS can now be avoided. The elective transfer of one single high quality embryo (eSET) displaying the highest developmental potential has considerably reduced multiple pregnancy rates in various countries [4–6].
In Switzerland the adoption of many of these developments has been hampered by a restrictive and outdated legislation [7], which has been in effect since January 2001 [8] and has remained unchanged since then. Currently, PGD, oocyte donation and the cryopreservation of embryos are prohibited. The number of pronucleate oocytes that may be cultured to the embryonic stage is limited to three. If more than three pronucleate oocytes are available, these must be stored frozen at the pronucleate stage, not at the embryonic stage. The consequences of these legal restrictions are manifold. On the one hand, a rising number of infertile couples seek treatment in surrounding countries; on the other hand, ART continues to induce a variety of otherwise preventable complications, such as the birth of multiples [9] and OHSS.
Despite these restrictions, ART has thrived in Switzerland and is now well embedded in Swiss society. In 1993 the Swiss medical community involved in ART established a central data register that collects detailed information and outcome data of all ART-procedures carried out in Switzerland [10]. This data is transmitted to the Federal Office of Statistics (BfS) in Neuchâtel, to the European IVF-Monitoring Consortium (EIM) of the European Society of Reproduction and Human Embryology (ESHRE) and to the International Committee for Monitoring Assisted Reproductive Technology (ICMART). In Europe, Switzerland belongs to the few countries with complete follow up of all ART data including those of all neonates arising from ART in Switzerland. In response to politicians and lay persons still critical of ART the setting up and pursuing of this registry was motivated by the desire to add transparency to the activities of the Swiss institutions involved in ART.
Twenty years after the initial foundation of FIVNAT-CH a working group of leading experts in ART in Switzerland was assembled over a period of two years to analyse data and to formulate recommendations.
As described earlier [10], data concerning the medical indication for embarking on treatment with ART in both the female and male partners were required, together with details on the treatment protocols for controlled ovarian hyperstimulation, oocyte collection, mode of ART, embryo transfer and luteal support. In addition, information on cryopreservation and thawing of oocytes at the pronucleate stage and embryos was collected together with outcome data including pregnancy, mode of delivery and neonate condition.

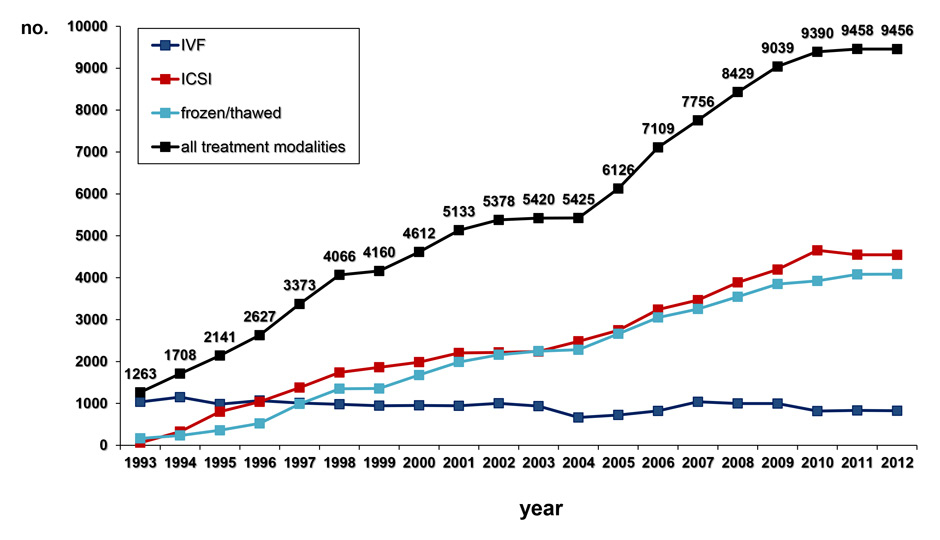
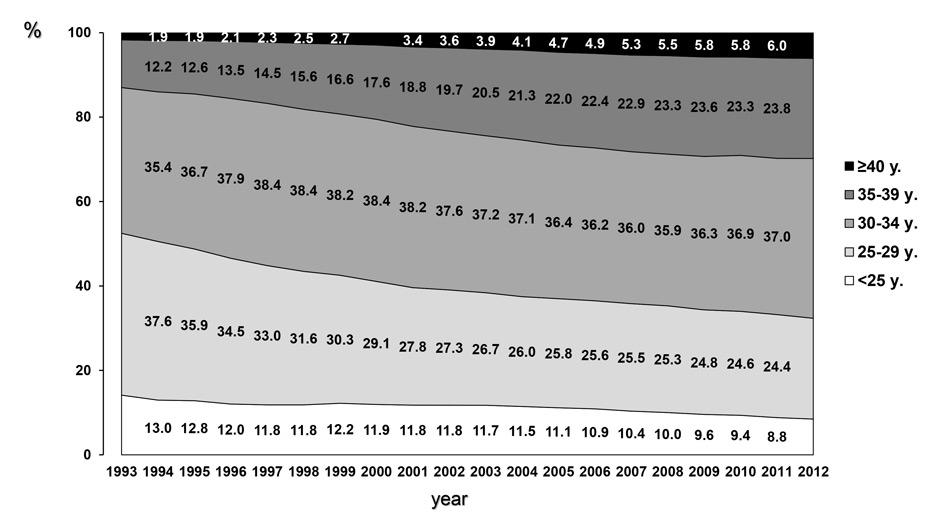
Figure 1
A Age of women giving birth in Switzerland between 1993 and 2012 was extracted from the data base constructed and published by the Swiss Federal Office of Statistics (BfS). During this observation period the proportion of women giving birth at the age of 35 to 39 years or older has nearly doubled, whereas the age of women giving birth at the age of 40 years or older has risen more than threefold.
B During the observation period between 1993 and 2012 the number of treatments with ART, including IVF, ICSI and the transfer of frozen/thawed embryos and oocytes at the pronucleate stage, has risen almost continuously, as evidenced by the Swiss ART data registry, FIVNAT-CH.
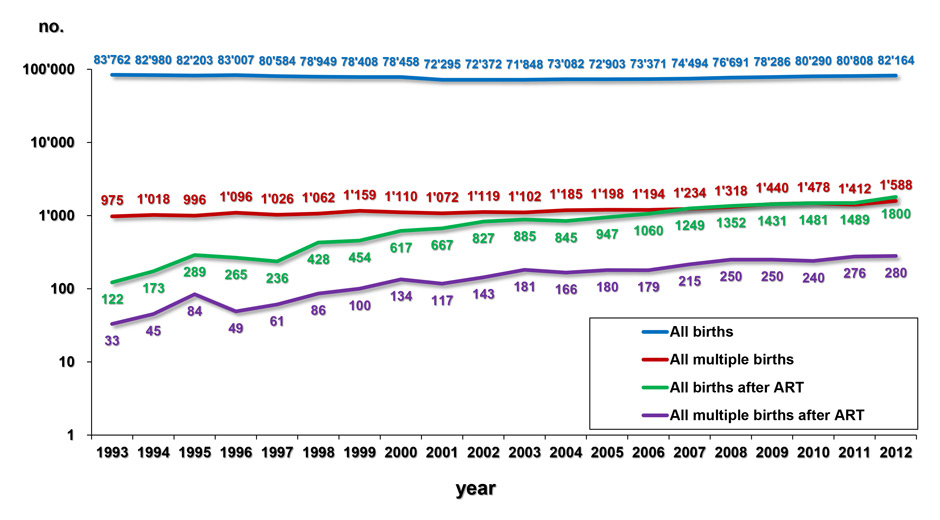
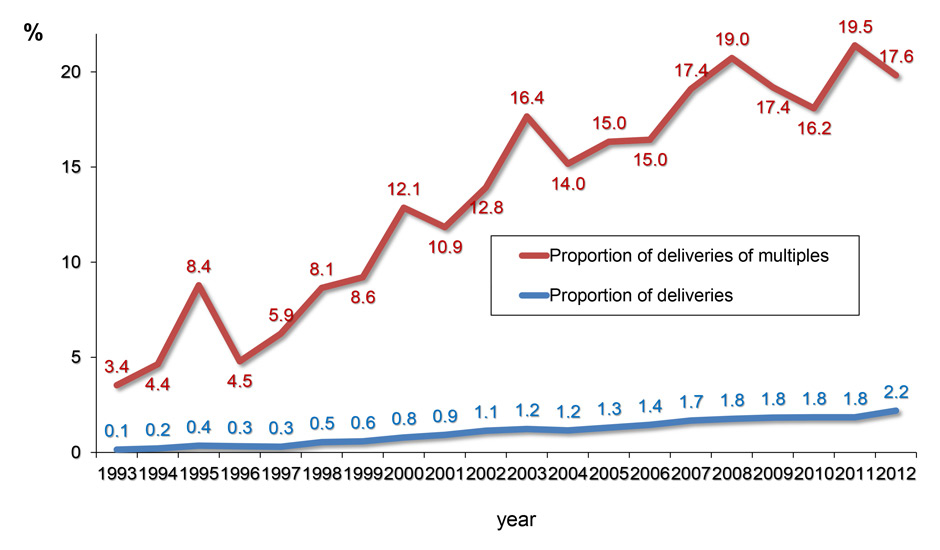
Figure 2
A By putting together the number of all deliveries and the overall number of multiple deliveries (given by the BfS) and the number of deliveries and multiple deliveries after ART (recorded by FIVNAT-CH), the number of births and multiple births generated by ART can be compared with the overall birth data in Switzerland. Since 1993 both the absolute number of births generated by ART and the absolute number of multiple deliveries, mostly of twins, have been rising continuously.
B Since 1993, the proportion of births generated by ART has been rising almost continuously, so that in 2012 2.2% of all deliveries in Switzerland were originated by ART. In addition, the proportion of multiple deliveries generated by ART has risen to 17.6% of all multiple births in Switzerland in 2012.
Data collection was anonymous, but all reports were identified by a registration number given to each participating institution and by an additional identification number given by the institution to each individual couple. The couple’s identification number remained unmodified during repeated treatments in each participating centre. For each treatment cycle started, the centre reported the required data, originally in paper, later by email. The data was reported twice a year to the central office of FIVNAT-CH as treatment data and delivery data were collected separately. The central office verified all incoming data and informed the participating institutions if data were missing or inconsistent. A report was presented during annual meetings of all participating institutions, in which means and trends were evaluated statistically for each institution in comparison to the overall Swiss data. All annual reports were continuously published on the internet website of the Swiss Society of Reproductive Medicine ( http://www.sgrm.org ).
The quality of data reporting was additionally verified by external audits, which were repeated every second year. The external audits were carried out by internationally well known experts, who checked the consistency of a randomly selected set of treatments and a randomly selected set of reported outcome data. They also compared the completeness of the entire reported data set with the local data set. For each audited centre an individual report was written, in which the number of errors in each institution was compared with the others and over time.
Overall demographic data of Switzerland were extracted from a public data base constructed and presented by the Swiss Federal Office of Statistics (BfS, http://www.bfs.ch ). During the time interval spanning 1993 to 2012 the following data sets were downloaded: female age at delivery, overall number of deliveries per year, number of singleton deliveries and number of multiple deliveries per year, incidence of OHSS and number of cancelled treatments.
The data dealing with ART was extracted from the FIVNAT-CH data base by a professional statistician. For all analyses the data of all participating institutions were pooled. Whereas overall outcome data were available since 1993, for detailed analyses the data collected between 2005 and 2012 was combined together. The following outcome parameters were considered: overall number of single and multiple deliveries, the number of single and multiple deliveries after ART, the number of cases with OHSS. For each of these outcome parameters a graphical display with the crude outcome data was given. In addition, differences in the presence or absence of (multiple) deliveries were compared among populations and presented by their odds ratios ± 95% confidence intervals. All differences were assessed using Chi-squared analysis and the Fisher’s exact test. A p-value <0.05 was considered statistically significant.
Since 1993 the proportion of Swiss women giving birth at the age of 35 to 39 years or older nearly doubled, whereas the number of women giving birth at the age of 40 years or older more than tripled to 6% in 2012 (fig. 1A). In parallel, the number of women younger than 30 years giving birth diminished from approximately 50% in 1993 to approximately 33% in 2012. This ongoing demographic trend is accompanied by an almost steadily increasing number of treatments with ART (fig. 1B).
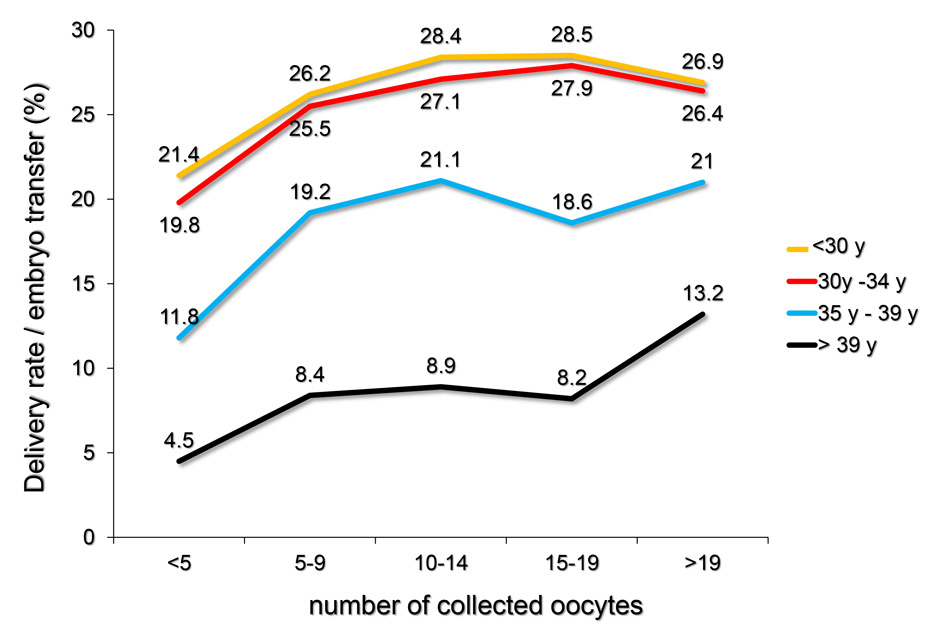
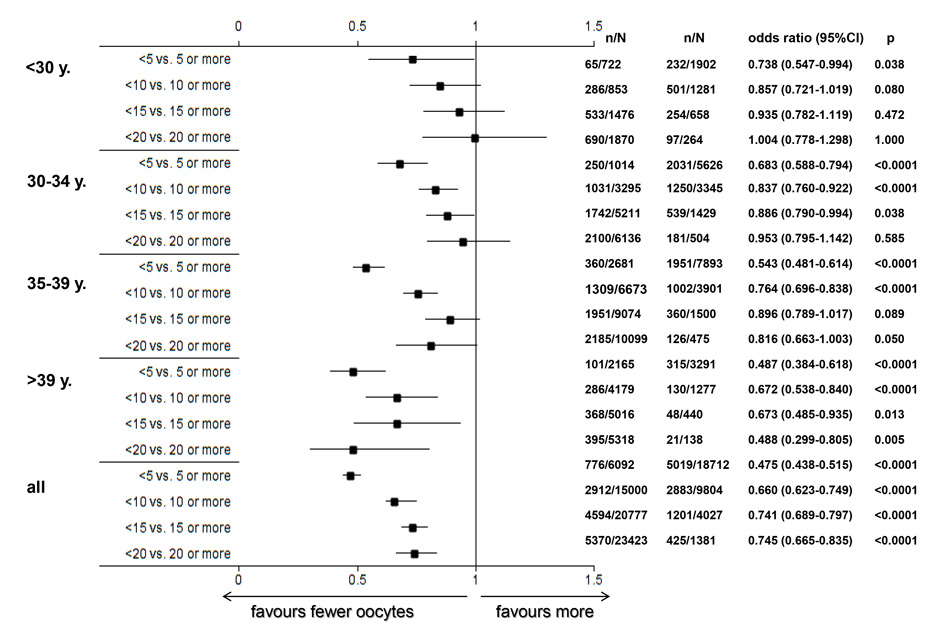
Figure 3
A The yearly delivery rates per embryo transfer (in %) achieved with ART from 2005 to 2012 were computed in relation to the number of collected oocytes and to the age of the infertile women during the treatment. The outcome of ART strongly depends on the age of the treated patient and to a lesser extent on the number of collected oocytes.
B Delivery rates and their relationship with the number of collected oocytes and the age of the treated infertile women at oocyte collection, using the pooled FIVNAT-CH data collected between 2005 and 2012. Among all treated patients the delivery rates per embryo transfer significantly depended on the quantity of retrieved oocytes (p <0.0001), but this dependence was less pronounced among younger patients. In each age category the number of deliveries was significantly lower if fewer than five oocytes were retrieved. In all age categories (except those older than 39 years) the delivery rates were not further improved if more than 15 oocytes were collected.
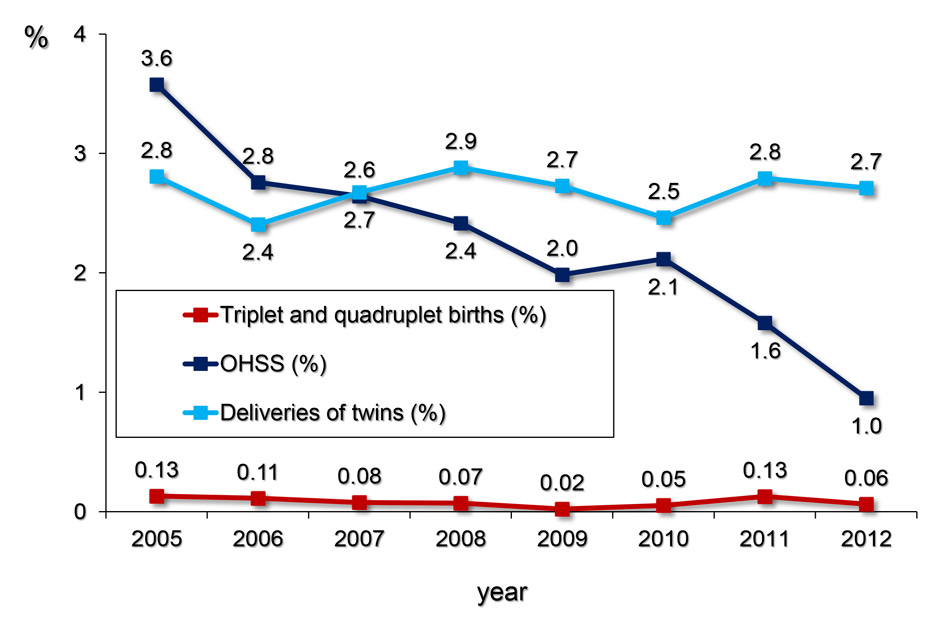
Figure 4
The yearly proportion (%) of twin, triplet and quadruplet deliveries per treatment cycle with ART (including both fresh and thawing cycles) and the proportion of OHSS per freshly stimulated treatment cycles (in %) was calculated from the pooled FIVNAT-CH data collected between 2005 and 2012. While the number of multiple births has remained constant, the incidence of OHSS dropped from 3.6% 1.0%.
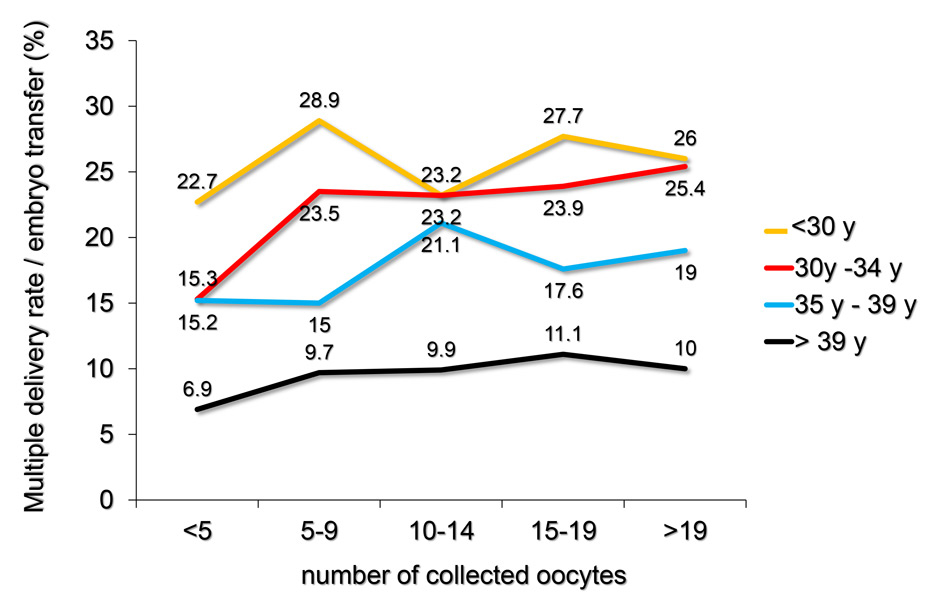
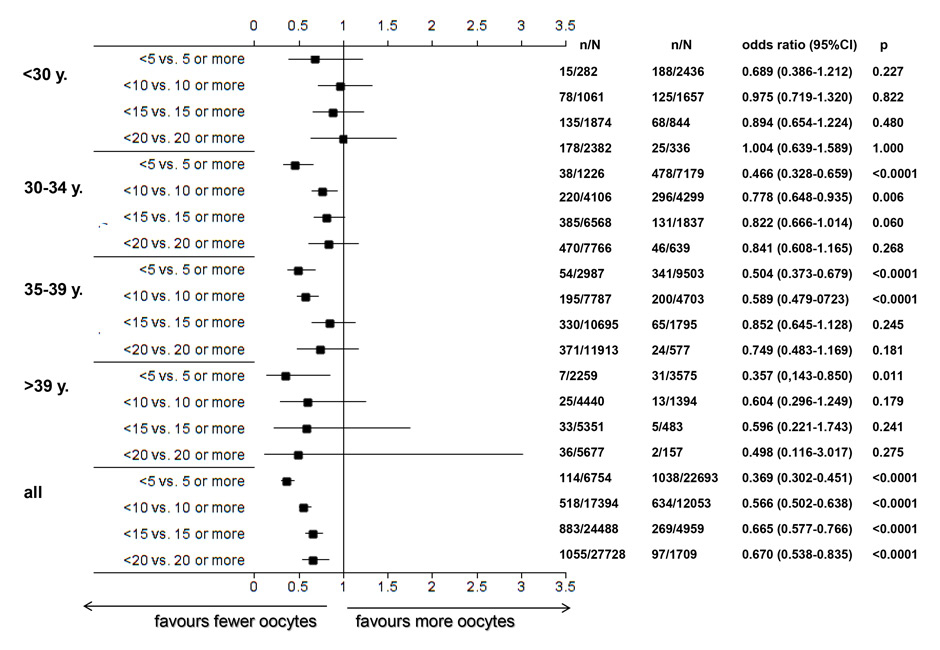
Figure 5
A Using the pooled FIVNAT-CH data, the relationship between the number of retrieved oocytes, the mean age of the treated patients at oocyte collection and the incidence of multiple deliveries was assessed during the observation period from 2005 to 2012. The incidence of multiple deliveries most strongly depended on the age of the treated women and to a lesser extent on the number of collected oocytes.
B Number of multiple deliveries and its relationship with the number of collected oocytes and the age of the treated infertile women at oocyte collection, using the pooled FIVNAT-CH data collected between 2005 and 2012. Whereas the incidence of multiple deliveries per embryo transfer was generally lower among women aged 35 years or older, the incidence of multiple deliveries was very significantly age dependent (p <0.0001) and was lower if fewer than five or ten oocytes had been retrieved. Among women younger than 30 years the incidence of multiple deliveries was not related to the number of retrieved oocytes.
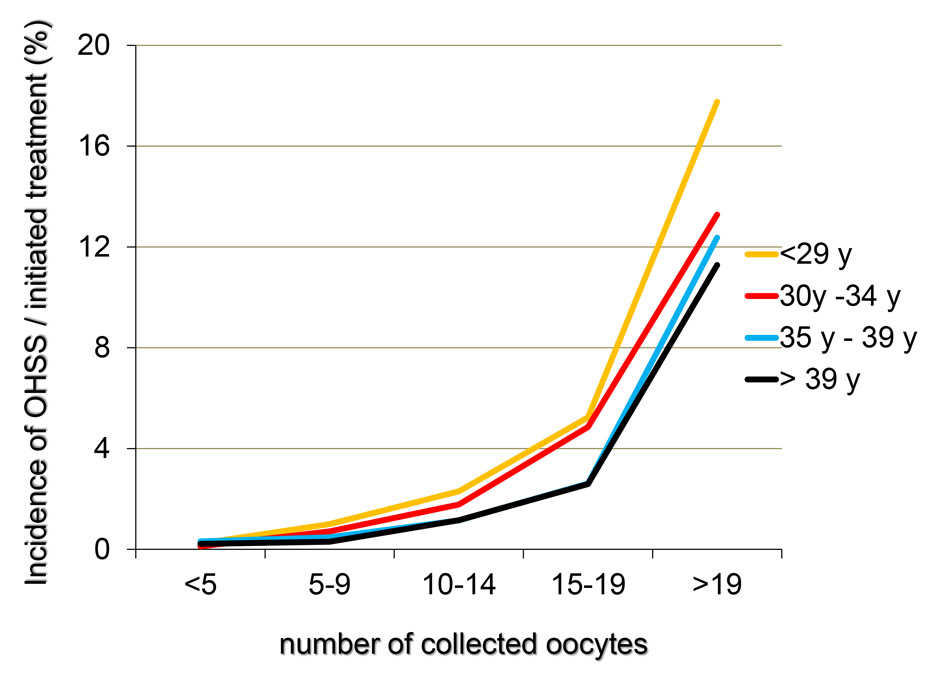
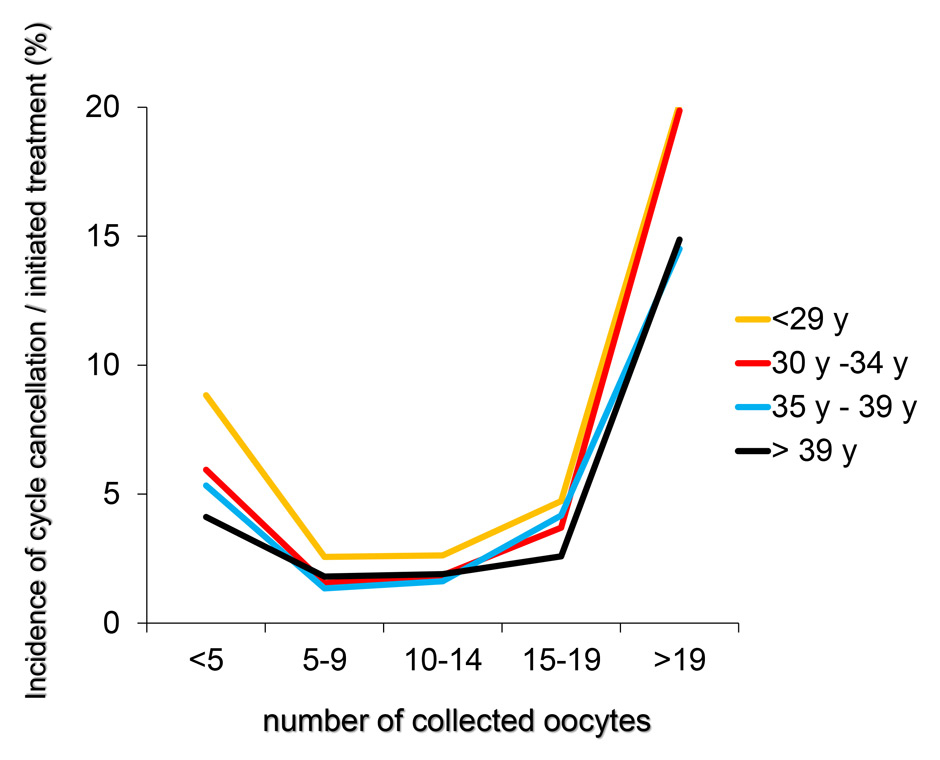
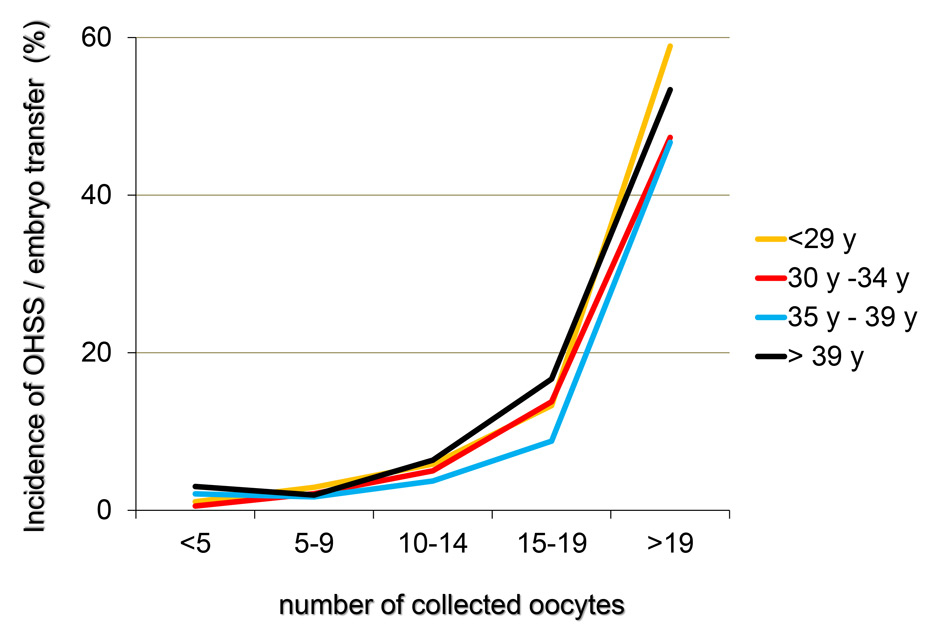
Figures 6
A‒C The incidence of OHSS per initiated controlled ovarian hyperstimulation (in %, panel A), the cancellation rate per initiated cycle and the incidence of OHSS per embryo transfer (in %, panel C) taken from the pooled FIVNAT-CH data collected between 2005 to 2012 were computed in relationship to the number of collected oocytes and the age of the treated infertile patients. The incidence of OHSS rises significantly if more than 14 oocytes are collected. In 17.8% of all treatment cycles in which 19 or more oocytes were collected embryo transfer was cancelled to avoid OHSS. Among all age groups, the cancellation rate was the highest when fewer than five oocytes or 19 or more oocytes were collected (panel B).
Since the first establishment of the FIVNAT-CH data registry in 1993 the absolute number of live births achieved with ART increased nearly constantly (fig. 2A), so that now the proportion of births after ART is approximating 2.2% of all births registered in Switzerland (fig. 2B). Along with the absolute number of deliveries, the absolute number of multiple births rose (fig. 2A). Twin deliveries increasingly contribute to the bulk of multiple births as their numbers rose from 956 in 1993 (1.14%) to 1,559 (1.49%) in 2012. During the same time interval the number of twin deliveries induced by ART and recorded by FIVNAT-CH rose from 28 (2.9% of all twin deliveries) in 1993 to 259 (16.6% of all twin deliveries) in 2012. Therefore, the proportion of multiple deliveries induced by ART increased to a much larger extent than those occurring naturally and now accounts for almost 20% of all multiple births in Switzerland (fig. 2B).
The yearly delivery rates per embryo transfer (in %) achieved with ART from 2005 to 2012 were computed in relation to the number of collected oocytes and to the age of the infertile women during the treatment (fig. 3A). Whereas the delivery rates were generally lower among women aged 35 years or more (2,727 births after 18,757 embryo transfers, 14.5%) as compared to those younger than 35 years (3,068 births after 11,842 embryo transfers, 25.9%), the delivery rates did not vary much among age categories of women younger than 35 years. Among all treated patients the delivery rates per embryo transfer significantly depended on the quantity of retrieved oocytes (p <0.0001), but this dependence was less pronounced among younger patients (fig. 3B). In addition, in each age category the number of deliveries was lower in cases where fewer than five oocytes have been retrieved. In all categories (except those older than 39 years) the delivery rates were not further improved if more than 15 oocytes had been collected (fig. 3B).
The most severe complications of ART are multiple gestations and OHSS. The yearly proportion (in %) of twin, triplet and quadruplet deliveries per treatment cycle with ART (including both fresh and thawing cycles) between 2005 and 2012 together with the proportion of OHSS per freshly stimulated treatment cycles (in %) is depicted in figure 4. Whereas the number of multiple births has remained constant over this observation period, the incidence of OHSS dropped from 3.6% in 2005 to 1.0% in 2012.
In figure 5A the relationship between the number of retrieved oocytes, the mean age of the treated patients during oocyte collection and the incidence of multiple deliveries is assessed during the observation period from 2005 to 2012. Whereas the incidence of multiple deliveries per embryo transfer was lower among women aged 35 years or older (432 multiple births after 2,707 embryo transfers, 16.0%) than women younger than 35 years (719 multiple births after 3,049 embryo transfers, 23.6%), the incidence of multiple deliveries significantly depended on the age of the treated patient (p <0.0001) and was lower, if fewer than five or ten oocytes were retrieved. The incidence of multiple deliveries was unrelated to the number of retrieved oocytes in women younger than 30 years (fig. 5B).
The incidence of OHSS per initiated controlled ovarian hyperstimulation (in %, fig. 6A) and per embryo transfer (in %, fig. 6C) from 2005 to 2012 was computed in relation to the number of collected oocytes and the age of the infertile patients. We also computed the cancellation rate per initiated treatment cycle (fig. 6B). The incidence of OHSS rose significantly if more than 14 oocytes were collected, regardless of the age of the patient. Among all age groups, 17.8% of all initiated treatment cycles embryo transfers were cancelled, if fewer than five but, more frequently, if 19 or more oocytes had been collected (14.5% in women older than 35 years and 20.1% in women younger than 30 years, fig. 6B).
Despite a restrictive legal framework ART has been thriving in Switzerland, as evidenced by the continuous rise of the number of treatments. As a result of this development, the proportion of newborns achieved with ART has now reached 2.2% of all newborns.
Nonetheless, we face several major challenges in ART. Firstly, the age of women seeking ART is constantly rising, which requires personalised and adequate diagnostics and treatment modalities in order to achieve an acceptable outcome. Secondly, the proportion of multiple deliveries has increased disproportionately and ART is now responsible for almost 20% of all multiple births in Switzerland. In the present data set, even in women aged 39 years or more, multiple delivery rates are around 10%, which in this age category is considered high due to the associated significant adverse effects. Thirdly, OHSS has now become a preventable complication of ART and should be avoided accordingly.
Concerned with these issues, a panel of Swiss experts in ART was assembled to elaborate a consensus to increase successful outcome as well as to reduce the incidence of complications of ART and validate these through a statistical review of the Swiss data (FIVNAT-CH data registry 2005–2012).
The results of this review clearly demonstrate the age dependency of both the delivery and the multiple delivery rates after embryo transfer (fig. 3B and 5B). In addition, among women aged 30 years or more, the delivery rate is significantly lower if fewer than five or ten oocytes are retrieved. In most age categories the likelihood of a healthy pregnancy and delivery is not enhanced if more than 15 oocytes are collected. These findings confirm earlier findings in a large cohort of women in the U.K. [11]. The incidence of multiple delivery also correlates both with the age of the patient and the number of retrieved oocytes. Except in women younger than 30 years, the incidence of multiple deliveries is significantly lower, if fewer than ten oocytes are collected (fig. 5B), whereas the risk of OHSS rises in all age categories, if more than 14 oocytes are collected (fig. 6A and B).
Based on these findings, the panel concluded that ovarian stimulation should optimally be designed for the retrieval of 10 to 15 oocytes per treatment cycle. This number of oocytes is associated with the presence of good quality embryos available for embryo transfer but also for the availability of a fair number of pronucleate oocytes for cryopreservation.
As the potency of an individual woman’s ovaries to respond to the ovarian stimulation regimen is highly variable, the panel focused on methods to predict the outcome of controlled ovarian hyperstimulation. Among all available measurements of ovarian reserve, such as the antral follicle count (AFC), the basal FSH concentration and early follicular phase inhibin B concentration, the serum concentration of the anti-Muellerian hormone (AMH) has been shown to correlate best with the number of retrieved oocytes [12–16]. In addition, the AMH serum levels are also helpful for the prediction of low ovarian response as well as of the risk of OHSS [17–18]. A single AMH serum value may guide treatment up to 12 months both for the prediction of poor and excessive ovarian response. However, AMH as one single parameter set alone may not be reliable enough to predict the quality of the oocytes and therefore the final outcome of IVF. As the conditions for the collection of an adequate number of oocytes vary with age, the most adapted gonadotropin dosage should be selected based on age, the AMH serum level and possibly additional parameters. A number of algorithms have been constructed and validated for this purpose, such as the PIVET algorithm [19] and the CONSORT algorithm [20]. Few algorithms have yet been proposed based on AMH [21–23] and large, prospective, multicentre studies are now needed to validate existing nomograms.
Based on these studies the panel concluded that AMH on its own is only of limited value to estimate prospective ovarian response and to reduce the risk of OHSS and that AFC should always be included in the calculation of the expected number of oocytes. Poor assay reproducibility may also account for the limited predictive value of AMH to date [24].
In addition to the fine tuning of the most appropriate dose of gonadotropins to the number of oocytes to be retrieved based on AMH and AFC, the expert panel also recommended the use of GnRH-antagonist based stimulation protocols, as these have been shown to be associated with a significantly lower risk of OHSS despite similar outcome data as compared to the long GnRH-agonistic protocol [25]. The GnRH-antagonistic protocol also offers the opportunity to induce the final maturation of the oocyte with a GnRH-agonist (a process denominated «triggering») thereby avoiding the administration of human chorionic gonadotropin. This helps to avoid the occurrence of OHSS, but necessitates the cryopreservation of all oocytes or oocytes at the pronucleate stage. This freeze-all strategy, demonstrated to be highly effective for the prevention of OHSS in a prospective multicentre study [26], is already widely used by the members of FIVNAT-CH (fig. 6B).
In addition, the expert panel concluded that there is currently no evidence for the superiority of any protocol of ovarian hyperstimulation (short GnRH-agonist, long GnRH-agonist, GnRH-antagonist) with respect to the outcome in low ovarian responders. The incidence of natural cycle IVF in low responders is on the rise, although recent data suggests that it does not benefit genuine poor ovarian responders [27]. Finally, the expert panel concluded that there should be no lower limit for the number of follicles aspirated.
Whereas in neighbouring countries the policy of replacing one single embryo selected from a cohort of embryos is increasingly contributing to a lower incidence of multiple delivery [4–6], the current legal ban on cryopreservation of embryos (except in an emergency situation) and the lack of embryo selection prohibits all progress in this direction [28]. An adaptation of the current restrictive Swiss legislation is urgently required to facilitate the impressive progress in reproductive medicine. This will allow a significant reduction of the major and steadily increasing problem of multiple pregnancies imposing unnecessary health risks and financial burden to Swiss society.
Acknowledgements:The panel of Swiss ART experts (CDG, PF, MvW) was organised by MSD Fertility Switzerland, represented by Dr. med. Isabel M. Gruber, PhD. Protocols were written by RM. The authors are deeply grateful to Maya Weder and to Costanzo Limoni for providing the data from the FIVNAT-CH registry. We thank our Board Members for providing input and fruitful discussions: Bruno Imthurn, Jürgen Weiss, Dorothea Wunder, Felix Häberlin, Ruth Stiller, Susanne Rohner, Gabriel de Candolle and Juan Antonio Garcia Velasco. We express our gratitude to the Swiss ART-institutions that actively participate in the FIVNAT-CH data registry.
1 De Geyter C, De Geyter M, Behre HM. 2009 Assisted Fertilization. Chapter 23 in: “Andrology” (Eds.: E. Nieschlag, H.M. Behre), Third Edition, Springer-Verlag, Berlin, Heidelberg, New York.
2 Von Wolff M, Dian D. Fertility preservation in women with malignant tumors and gonadotoxic treatments. Dtsch Arztebl Int. 2012;109:220–6.
3 De Geyter C, Sterthaus O, Miny P, Wenzel F, Lapaire O, De Geyter M, Sartorius G. First successful pregnancy in Switzerland after prospective sex determination of the embryo through the separation of X-chromosome bearing spermatozoa. Swiss Med Wkly. 2013;142:w13718.
4 Gerris J, De Neubourg D, Mangelschots K, Van Royen E, Vercruyssen M, Barudy-Vasquez J, et al. Elective single day 3 embryo transfer halves the twinning rate without decrease in the ongoing pregnancy rate of an IVF/ICSI programme. Hum Reprod. 2002;17:2626–31.
5 Thurin A, Hausken J, Hillensjö T, Jablonowska B, Pinborg A, Strandell A, Bergh C. Elective single-embryo transfer versus double-embryo transfer in in vitro fertilization. N Engl J Med. 2004;351:2392–402.
6 Van Bensdorp AJ, Slappendel E, Koks C, Oosterhuis J, Hoek A, Hompes P, Broekmans F, Verhoeve H, de Bruin JP, van Weert JM, Traas M, Maas J, Beckers N, Repping S, Mol BW, van der Veen F, van Wely M. The INeS study: prevention of multiple pregnancies: a randomised controlled trial comparing IUI COH versus IVF e SET versus MNC IVF in couples with unexplained or mild male subfertility. BMC Womens Health. 2009;9:35.
7 De Geyter C. (2012) Assisted reproductive medicine in Switzerland. Swiss Med Wkly. 2012;142:w13569.
8 Germond M, Senn A. A law affecting medically assisted procreation is on the way in Switzerland. J Assist Reprod Genet. 1996;16:341–3.
9 Wunder D, Neurohr EM, Faouzi M, Birkhäuser MH. Origin and outcome of multiple pregnancies in Bern, Switzerland, 1995–2006 and the current proposal of the Swiss Parliament to revise the Swiss law of reproductive medicine: Switzerland quo vadis? Swiss Med Wkly. 2013;143:w13864.
10 Van den Bergh M, Hohl MK, De Geyter Ch., Stalberg AM, Limoni C. Ten years of Swiss National IVF Register FIVNAT-CH. Are we making progress? Reprod Biomed Online. 2005;11:632–40.
11 Sunkara SK, Rittenberg V, Raine-Fenning N, Bhattacharya S, Zamora J, Coomarasamy A. Association between the number of eggs and live birth in IVF treatment: an analysis of 400 135 treatment cycles. Hum Reprod. 2011;26:1768–74.
12 La Marca A, Giulini S, Tirelli A, Bertucci E, Marsella T, Xella S, Volpe A. Anti-Müllerian hormone measurement on any day of the menstrual cycle strongly predicts ovarian response in assisted reproductive technology. Hum Reprod. 2007;22:766–71.
13 Andersen AN, Witjes H, Gordon K, Mannaerts B; Xpect investigators. Predictive factors of ovarian response and clinical outcome after IVF/ICSI following a rFSH/GnRH antagonist protocol with or without oral contraceptive pre-treatment. Hum Reprod. 2011;26:3413–23.
14 Weghofer A, Kim A, Barad DH, Gleicher N. Follicle stimulating hormone and anti-Müllerian hormone per oocyte in predicting in vitro fertilization pregnancy in high responders: a cohort study. PLoS One 2012;7:e34290.
15 Anckaert E, Smitz J, Schiettecatte J, Klein BM, Arce JC. The value of anti-Mullerian hormone measurement in the long GnRH agonist protocol: association with ovarian response and gonadotrophin-dose adjustments. Hum Reprod. 2012;27:1829–39.
16 Arce JC, La Marca A, Mirner Klein B, Nyboe Andersen A, Fleming R. Antimüllerian hormone in gonadotropin releasing-hormone antagonist cycles: prediction of ovarian response and cumulative treatment outcome in good-prognosis patients. Fertil Steril. 2013;99:1644–53.
17 Ocal P, Sahmay S, Cetin M, Irez T, Guralp O, Cepni I. Serum anti-Müllerian hormone and antral follicle count as predictive markers of OHSS in ART cycles. J Assist Reprod Genet. 2011;28:1197–203.
18 Lee TH, Liu CH, Huang CC, Wu YL, Shih YT, Ho HN, et al. Serum anti-Müllerian hormone and estradiol levels as predictors of ovarian hyperstimulation syndrome in assisted reproduction technology cycles. Hum Reprod. 2011;23:160–7.
19 Yovich J, Stanger J, Hinchliffe P. Targeted gonadotrophin stimulation using the PIVET algorithm markedly reduces the risk of OHSS. Reprod Biomed Online. 2012;24:281–92.
20 Olivennes F, Howles CM, Borini A, Germond M, Trew G, Wikland M, et al.; CONSORT study group. Individualizing FSH dose for assisted reproduction using a novel algorithm: the CONSORT study. Reprod Biomed Online. 2009;18:195–204.
21 Yates AP, Rustamov O, Roberts SA, Lim HY, Pemberton PW, Smith A, Nardo LG. Anti-Mullerian hormone-tailored stimulation protocols improve outcomes whilst reducing adverse effects and costs of IVF. Hum Reprod. 2011;26:2353–62.
22 La Marca A, Papaleo E, Grisendi V, Argento C, Giulini S, Volpe A. Development of a nomogram based on markers of ovarian reserve for the individualisation of the follicle-stimulating hormone starting dose in in vitro fertilisation cycles. BJOG. 2012;119:1171–9.
23 Oliveira JB, Baruffi RL, Petersen CG, Mauri AL, Nascimento AM, Vagnini L, Ricci J, Cavagna M, Franco JG Jr. A new ovarian response prediction index (ORPI): implications for individualised controlled ovarian stimulation. Reprod Biol Endocrinol. 2012;10:94.
24 Rustamov O, Smith A, Roberts SA, Yates AP, Fitzgerald C, Krishnan M, et al. Anti-Mullerian hormone: poor assay reproducibility in a large cohort of subjects suggests sample instability. Hum Reprod. 2012;27:3085–91.
25 Al-Inany HG, Youssef MA, Aboulghar M, Broekmans F, Sterrenburg M, Smit J, et al. Gonadotrophin-releasing hormone antagonists for assisted reproductive technology. Cochrane Database Syst Rev 2011;CD001750.
26 Griesinger G, Schultz L, Bauer T, Broessner A, Frambach T, Kissler S. Ovarian hyperstimulation syndrome prevention by gonadotropin-releasing hormone agonist triggering of final oocyte maturation in a gonadotropin-releasing hormone antagonist protocol in combination with a “freeze-all” strategy: a prospective multicentre study. Fertil Steril. 2011;95:2029–33.
27 Kedem A, Tsur A, Haas J, Yerushalmi GM, Hourvitz A, Machtinger R, Orvieto R. Is the modified natural in vitro fertilization cycle justified in patients with “genuine” poor response to controlled ovarian hyperstimulation? Fertil Steril. 2014 Mar 25.
28 Fehr P, Nygren KP, De Geyter C. Effect of different embryo transfer strategies on the outcome of assisted reproduction. Ther Umsch. 2009;66:825–9.
Funding / potential competing interests: The data were assembled, analysed and interpreted by a panel of Swiss experts in ART. These activities were organised by MSD Fertility, Switzerland.