Impact of local vascular lesions assessed with optical coherence tomography and ablation points on blood pressure reduction after renal denervation
DOI: https://doi.org/10.4414/smw.2015.14102
Jelena-Rima
Ghadri, Roman
Gaehwiler, Milosz
Jaguszewski, Isabella
Sudano, Julia
Osipova, Renate
Schoenenberger-Berzins, Paul
Erne, Thomas F.
Lüscher, Christian
Templin
Summary
Local vascular injury is detectable with optical coherence tomography (OCT) after catheter-based renal denervation (RDN). However, it is unclear whether the number and type of vascular lesions or the number of ablation points could affect blood pressure (BP) reduction.
The aim of the study was to assess the impact of vascular injury induced by RDN detected with OCT and the number of ablation points on BP response after 1, 3 and 6 months.
METHODS: RDN was either performed with a Simplicity® catheter or an EnligHTNTM multielectrode basket followed by OCT. BP was recorded prospectively as office measurement and 24-hour ambulatory blood pressure monitoring (24-h ABPM) at each time point. Correlations between type and number of vascular lesions, as well as ablation points, on BP reduction were performed.
RESULTS: Out of 16 patients, two were lost to BP follow-up. We documented a BP reduction at 1, 3 and 6 months in both office and 24-h ABPM. The Δmean office systolic BP (SBP) reduction was –18.75 ± 24.55 mm Hg, –20.58 ± 16.92 mm Hg and –18.75 ± 29.39 mm Hg, respectively, and the Δmean 24h-ABPM SBP reduction was –6.50 ± 23.45 mm Hg, –16.88 ± 26.64 mm Hg and –13.89 ± 21.20 mm Hg, respectively. The number of vascular lesions did not correlate with office and 24h-SBP and diastolic BP reduction. However, there was a correlation between ablation points and office Δmean SBP reduction at 6 months (p <0.02).
CONCLUSIONS: Our study demonstrates for the first time that the number and type of vascular lesions as assessed with OCT did not predict the success of BP reduction after RDN. However, we observed a substantial decrease in office SBP in relation to the number of ablation points at 6 months.
Introduction
Renal sympathetic denervation (RDN) is a catheter-based procedure which has been established after first randomised trials as a treatment option for patients with resistant hypertension [1]. Different percutaneous RDN systems are currently available using different ablation techniques [2].
Given the conflicting results from previous studies and SYMPLICITY HTN-3 trial the predicted response and success after RDN with respect to blood pressure (BP) reduction is still unknown [3–5].
In this context, there are no data as to whether the number or type of vascular lesions, irrespective of ablation points, could potentially affect the results for BP reduction. Furthermore, it is unclear if the amount of ablation points could affect BP reduction. The Simplicity HTN-2 and HTN-3 study, which assessed the effectiveness and safety of RDN with the Simplicity® catheter, did not show any vascular abnormalities at any sites of radiofrequency delivery on angiography [1]. However, we have recently shown with optical coherence tomography (OCT) that renal vascular injury is present after RDN with either the Simplicity® catheter or the EnligHTNTM multielectrode basket [6, 7]. These vascular tissue lesions specifically include vessel wall oedema, thrombus formation and dissections, which are not visible with classic coronary angiography. OCT offers a technique with the immense advantage of creating high-resolution images allowing accurate tissue characterisation, and the spatial resolution is approximately 10-fold higher than intravascular ultrasound (IVUS) [8]. Vascular damage such as vessel wall oedema, a common side effect of RDN, may reflect the effectiveness of renal denervation in successfully reducing BP [6]. Furthermore, extensive tissue damage including all detectable vascular lesions could lead to a more pronounced decrease in BP. Therefore, the aim of the present study was to evaluate the BP response in relation to vascular tissue damage and number of ablation points after RDN.
Methods
Patient population and catheter-based renal denervation
This was a sub-analysis of a prospective comparative RDN OCT study at two cardiology sites in patients with resistant hypertension [6]. Patient recruitment and inclusion criteria have been already published elsewhere [6]. Briefly, patients were included with resistant hypertension, which was defined as primary or idiopathic with systolic blood pressure (SBP) values >160 mm Hg values and ineffective BP treatment. Patients were excluded if they had a glomerular filtration rate <45 ml/min or allergies to contrast medium.
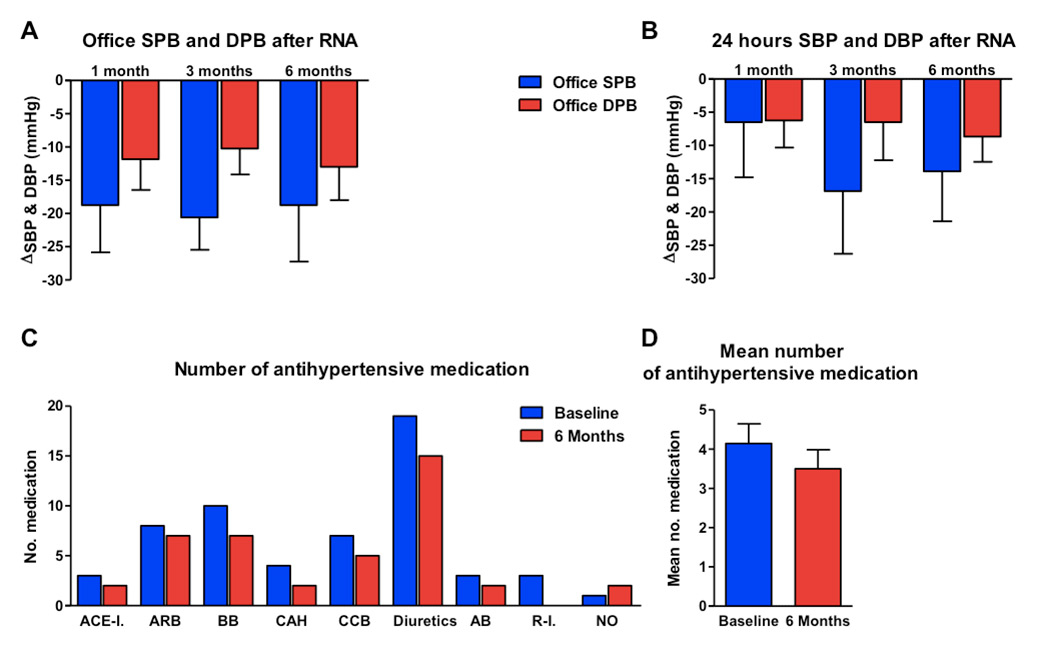
Figure 1
Blood pressure changes from baseline at 1 month, 3 months and 6 months as determined by office measurement (A) and 24-hour ambulatory blood pressure measurements (B). Mean antihypertensive medication intake at baseline and at 6 months of follow-up (C and D).
AB = alpha-blocker; ACE-I = angiotensin converting-enzyme inhibitor; ARB = angiotensin receptor blocker; BB = beta-blocker; CAH = centrally acting antihypertensive; CCB =calcium channel blocker; DBP = diastolic blood pressure; NO = nitrovasodilator; R-I = renin inhibitor; RNA = renal nerve ablation; SBP = systolic blood pressure
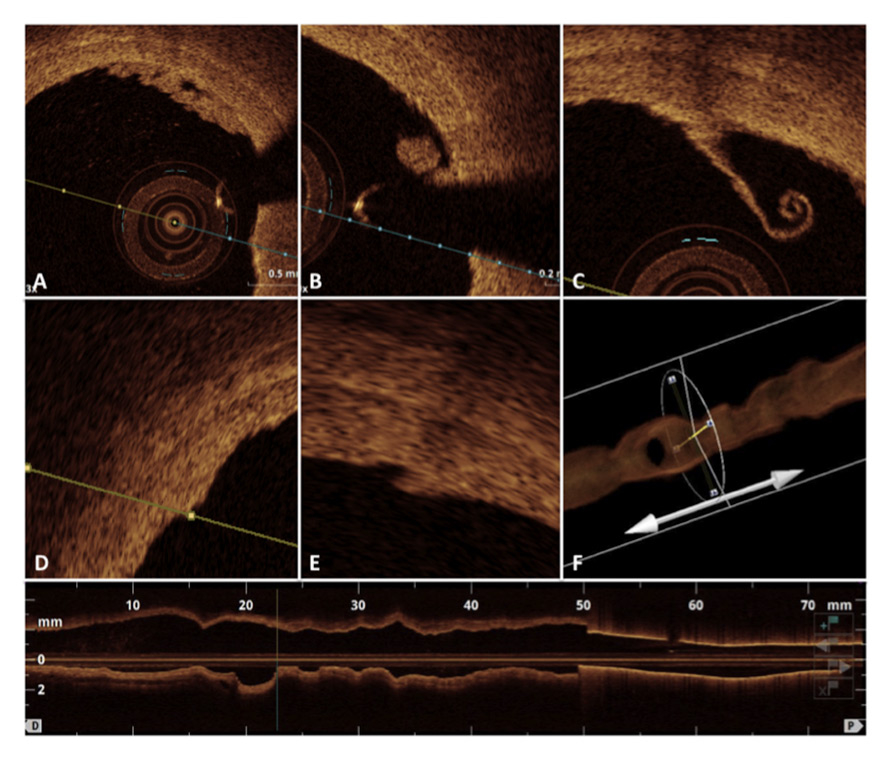
Figure 2
Intraluminal local morphological defects in the acute phase just after the procedure of renal denervation including intraluminal thrombus formations (A and B), endothelial detachment (C), vessel wall oedema (D), dissection (E) and massive vascular vasospasm as detected by the 3D optical coherence tomography reconstruction.
BP was sequentially recorded at 1, 3 and 6 months after RDN. RDN was either performed with the Simplicity® catheter (Medtronic-Adrian) or the EnligHTNTM multielectrode (St. Jude Medical).
Postdischarge information was obtained as part of our hospital quality assurance using a standardised clinical questionnaire.
Optical coherence tomography
In all patients OCT was performed using the C7–XR imaging system (Light-Lab Imaging, Inc., Westford, USA) before and after renal denervation, as previously reported [6].
Vascular lesion characterisation
Oedema was characterized as a significant endothelial-intimal notch identified on OCT at the luminal wall surface. Furthermore, vasospasm and vessel dissections, as well as thrombus formation (protruding mass with a diameter of ≥5 mm in cross sections), were noted if present [6].
Clinical examination of arterial blood pressure
Blood pressure and heart frequency monitoring was performed in an ambulant setting using an inflatable digital electrical sphygmomanometer (Microlife® or Omron® devices) in accordance with the current guidelines. At intervals of 1, 3, and 6 months after RDN, BP was recorded and compared with baseline data. All patients also underwent digital 24-hour ambulatory blood pressure monitoring (24–h ABPM) (Spacelab® devices) to analyse daily and nocturnal variations of the BP profile. In this regard, participants were requested not to change daily habits and not to move their arm during measurements. Furthermore, every patient was requested to document bed and waking time for adequate interpretation of the protocol.
Statistical analysis
Continuous variables are given as mean ± standard error of the mean (SEM) or standard deviation (SD) if appropriate. Differences between groups were tested using two-sided t-tests for continuous endpoints when normally distributed (Shapiro-Wilk test) or Wilcoxon rank-sum test for non-normally distributed continuous endpoints. Correlation analysis was used to determine the association between ablation points as well as lesions and BP reduction. SPSS software (Chicago, Illinois; Version 20.0) and Graph pad (Version 5.0) were applied for statistical analysis. A p-value <0.05 was considered as statistical significant.
|
Table 1:Baseline Characteristics (n = 14). |
|
Age, years |
57.8 ± 9.9 |
|
Gender, male |
8 (57) |
|
Cardiovascular history
|
|
| Known CAD or stroke |
4 (29) |
|
Cardiovascular risk factors
|
|
| Hypertension |
14 (100) |
| Hyperlipidaemia |
9 (64) |
| Smoking, current |
6 (43) |
| Obesity |
7 (50) |
| Diabetes mellitus |
3 (21) |
|
Medication on admission
|
|
| Aspirin |
4 (29) |
| Clopidogrel |
1 (7) |
| Statin |
7 (50) |
| Beta-blocker |
10 (71) |
| ACEI/AT-II |
11 (79) |
| Diuretics |
11 (79) |
| CCB |
7 (50) |
| Depicted are counts, n, incidence (%) or mean ± SD
ACEI = angiotensin converting-enzyme inhibitors; AT-II = angiotensin-receptor blocking agents; CAD = coronary artery disease; CCB = calcium-channel blocker |
Results
Sixteen patients were enrolled in the study and two patients were lost to blood pressure follow-up. Baseline characteristics are shown in table 1. The mean baseline office systolic BP was 168.50 ± 18.29 mm Hg and 24-h ABPM was 159.29 ± 16.37 mm Hg. During repeat BP measurement at 1, 3 and 6 months the mean office systolic BP was 150.83 ± 19.14 mm Hg, 146.50 ± 15.58 mm Hg and 147.42 ± 18.42 mm Hg, respectively. The mean systolic 24-h ABPM was 155.25 ± 16.15 mm Hg, 138.25 ± 13.33 mm Hg and 141.00 ± 15.91 mm Hg, respectively. We documented an essential Δmean BP reduction at all time-points in both office and 24-h ABPM (fig. 1A and B, table 2). At 1, 3 and 6 months, the office Δmean systolic BP (SBP) reduction was –18.75 ± 24.55 mm Hg, –20.58 ± 16.92 mm Hg and –18.75 ± 29.39 mm Hg, respectively, and the Δmean 24-h ABPM SBP reduction was –6.50 ± 23.45 mm Hg, 16.88 ± 26.64 mm Hg and 13.89 ± 21.20 mm Hg, respectively.
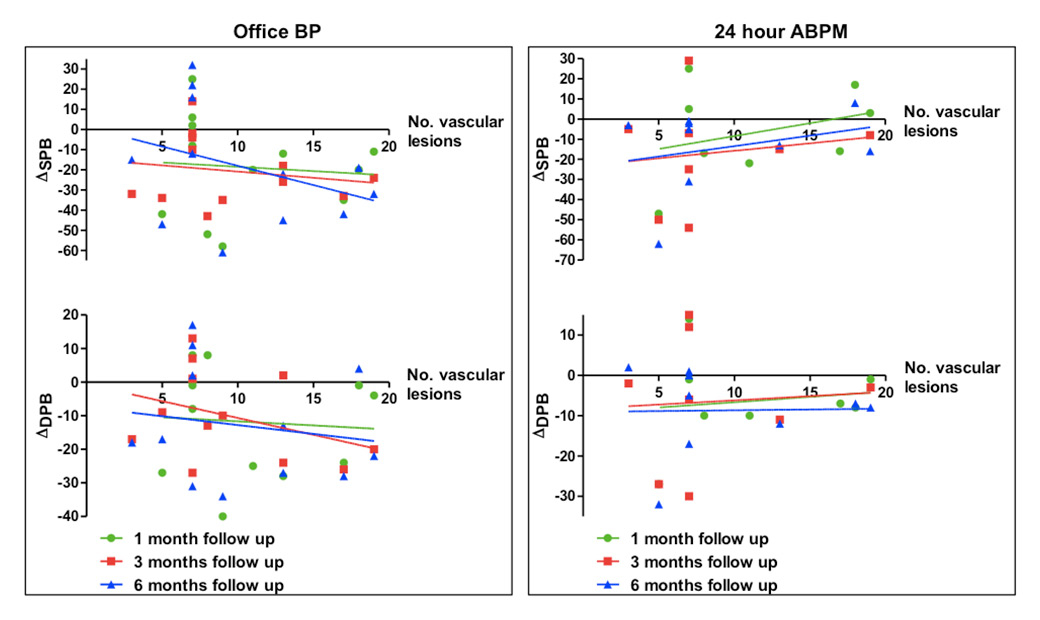
Figure 3
Correlation between blood pressure (BP) reduction (Δmean office and 24-hour ambulant blood pressure monitoring [ABPM]) and vascular lesions.
DBP = diastolic blood pressure; SBP = systolic blood pressure monitoring
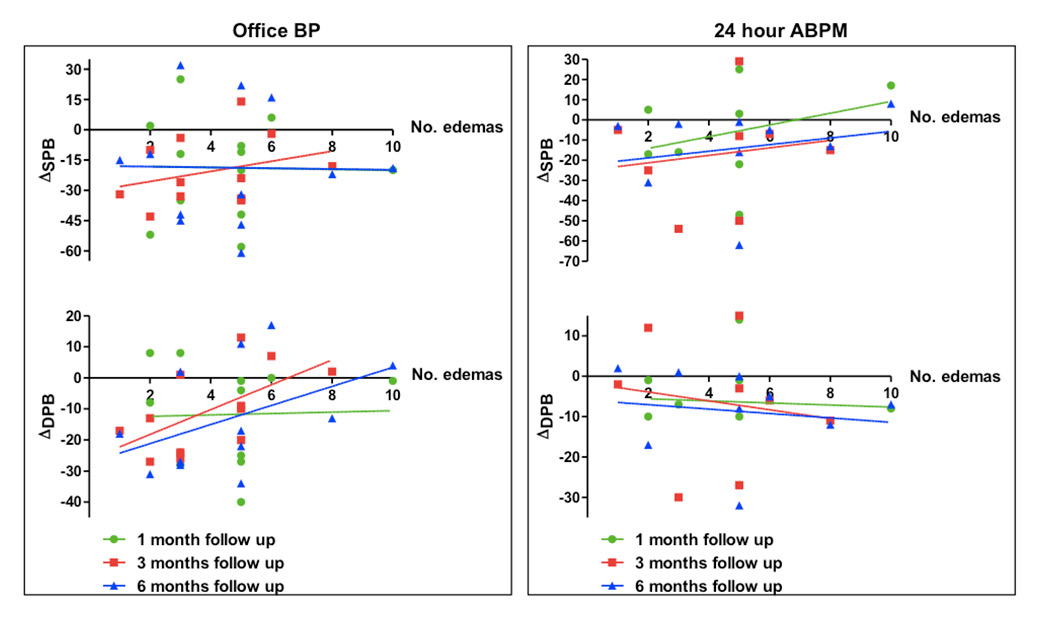
Figure 4
Correlation between blood pressure (BP) reduction (Δmean office and 24-hour ambulant blood pressure monitoring [ABPM]) and oedema.
DBP = diastolic blood pressure; SBP = systolic blood pressure monitoring
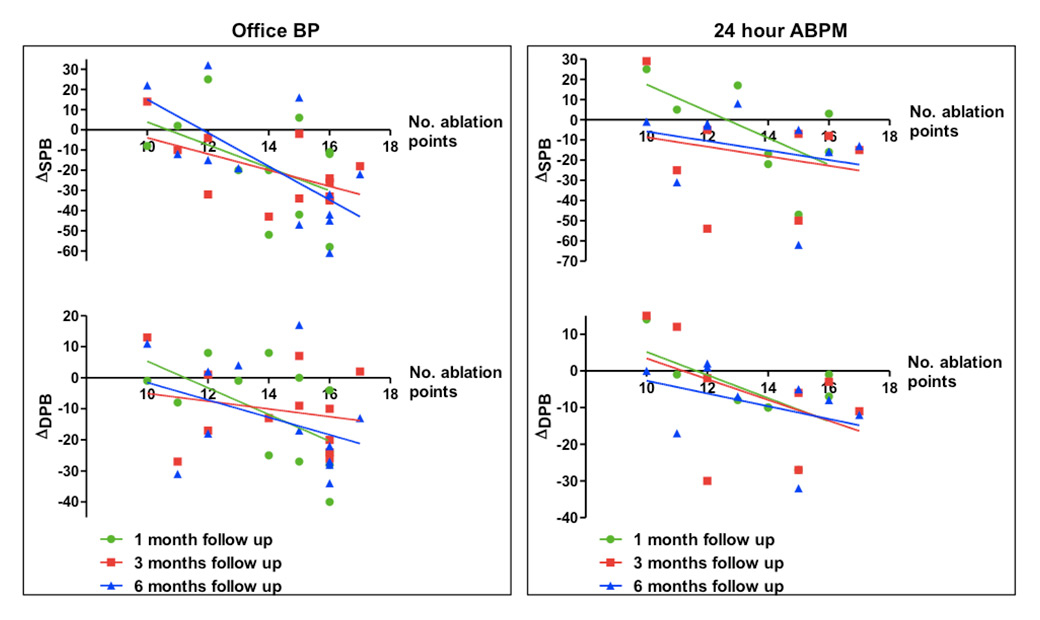
Figure 5
Correlation between blood pressure reduction (BP) (Δmean office and 24-hour ambulant blood pressure monitoring [ABPM]) and ablation points.
DBP = diastolic blood pressure; SBP = systolic blood pressure monitoring
By 6 months, patients were able to reduce antihypertensive therapy from 4.14 ± 1.88 drugs at baseline to 3.00 ± 2.00 at follow-up (fig. 1C and D).
During renal denervation procedures, 197 ablation points were performed in 28 renal arteries. Patients received an average of 7.0 ± 1.3 ablations per renal artery (range 4 to 11). There were no major clinical complications related to the RDN procedure as previously reported.
The OCT pullbacks performed before and after each procedure documented in total 141 different local acute morphological vascular changes. In detail, 60 areas of prominent oedema, 57 intraluminal thrombi, 21 vessel vasospasms and 3 wall dissections were observed (fig. 2). The total amount of all vascular lesions including the combination of oedema, thrombus formation, dissections and spasms did not reveal any correlation to BP reduction at 1, 3 and 6 months follow-up (office BP and 24-h ABPM) (fig. 3). Furthermore, no correlation between oedema and BP reduction was documented at any time of follow-up (fig. 4).
Interestingly, at 6 months of follow-up there was a correlation between the total number of ablation points and systolic BP reduction, although no correlation was observed at earlier follow-up time-points (1 and 3 months) (r = –0.66, p <0.02) (fig. 5). Complete correlation analysis is given in table 2.
|
Table 2: Correlations between optical coherence tomography findings and blood pressure (BP) in follow up (n = 14). |
|
|
Office BP
|
24-hour ambulatory BP monitoring
|
|
Systolic BP
|
Diastolic BP
|
Systolic BP
|
Diastolic BP
|
|
Follow up
|
1 month |
3 months |
6 months |
1 month |
3 months |
6 months |
1 month |
3 months |
6 months |
1 month |
3 months |
6 months |
|
Δmean
|
–18.750 |
–20.580 |
–18.750 |
–11.830 |
–10.250 |
–13.000 |
–6.500 |
–16.880 |
–13.890 |
–6.250 |
–6.500 |
–8.667 |
|
SD
|
24.550 |
16.920 |
29.390 |
16.080 |
13.420 |
17.320 |
23.450 |
26.640 |
21.200 |
11.510 |
16.170 |
10.750 |
|
Ablation points
|
| p-value |
0.113 |
0.063 |
0.019
|
0.060 |
0.499 |
0.221 |
0.100 |
0.589 |
0.489 |
0.114 |
0.266 |
0.301 |
| r |
–0.482 |
–0.551 |
0.664 |
–0.558 |
–0.217 |
–0.382 |
–0.622 |
–0.227 |
–0.266 |
–0.602 |
–0.448 |
–0.389 |
|
Lesions (overall)
|
| p-value |
0.792 |
0.581 |
0.262 |
0.819 |
0.246 |
0.610 |
0.458 |
0.735 |
0.463 |
0.760 |
0.877 |
0.957 |
| r |
–0.085 |
–0.178 |
–0.352 |
–0.074 |
–0.363 |
–0.164 |
0.308 |
0.144 |
0.282 |
0.130 |
0.066 |
0.021 |
|
Oedemas
|
| p-value |
0.948 |
0.351 |
0.958 |
0.924 |
0.041 |
0.143 |
0.443 |
0.709 |
0.572 |
0.893 |
0.716 |
0.712 |
| r |
–0.021 |
0.295 |
–0.017 |
0.031 |
0.596 |
0.450 |
0.318 |
0.158 |
0.219 |
–0.057 |
–0.154 |
–0.144 |
Discussion
Although previously the effectiveness of RDN in reducing blood pressure in patients with resistant hypertension has been documented by different investigations and randomised trials [1, 3, 9–11], the SYMPLICITY HTN-3 trial failed to confirm these results [5].
Conflicting data on RDN efficacy in resistant hypertension could be caused by many factors including different study design, lack of statistical power, effectiveness of the RDN procedure, mixed medication therapy, and different patient populations [12].
To date, no harmful long-term effects of RDN have been observed and the safety profile [13] has been studied by repeated renal angiograms and magnetic resonance imaging (MRI) [9]. Recently, we have shown that, despite the above mentioned safety issues, local tissue damage at the ablation sites such as oedema, dissection and thrombus formation can occur after RDN, and that OCT has the ability to visualise vascular lesions, which are primarily not apparent on angiography [6]. The present study explored the impact of endothelial damage after RDN on blood pressure reduction in order to identify a potential marker in terms of morphological tissue damage that may predict the response to RDN; this would be of major interest also potentially to explain non-responsiveness, which is estimated to affect between 8–16% of patients [9, 14]. Although it is speculated that non-responsiveness might be attributed to a different mechanism of hypertension, which is not caused by sympathetic hyperactivity, non-responsiveness may also be caused by technical aspects, i.e. in patients with dual renal arteries and/or accessory arteries not accessible for RDN and advanced vascular remodelling [15].
In line with previously published trials [3, 9, 10, 14, 16–21], we documented a reduction in systolic and diastolic BP as assessed by office and 24-h ABPM at 1, 3 and 6 months of follow up.
Furthermore, we also show that the total amount of local vascular lesions could not predict the success of blood pressure reduction in patients with resistant hypertension. Specifically, vessel wall oedema which is well defined by OCT and histologically corresponds to cellular swelling and connective tissue coagulation within the medial and adventitial layers [22] failed to show an effect on blood pressure reduction. Although preclinical studies have demonstrated that 17% of the artery wall circumference is affected by a transmural injury after RDN affecting particularly the media and adventitia [22], it is conceivable that most observed vascular wall oedema represents only a surface response to RDN, and that most of the sympathetic nerve fibres in the adventitial layer are impacted by OCT, with invisible damage caused by the applied high-frequency energy. In this respect, OCT has a very high spatial resolution, but the penetration depth is limited, particularly in large vessels, such as renal arteries. This is in line with the fact that not all applied ablation points correspond to vessel wall oedema. In this regard, it has been shown that newer-generation RDN devices such the One Shot balloon from Covidien did not result in any vascular lesion [23], although the clinical response on BP reduction has been shown in previous studies [20]. Therefore, it seems that local vascular lesions induced at the vascular surface are not able to predict the success of RDN in terms of BP reduction. It is more likely that deeper damage in the vessel wall (e.g. in the adventitial layer) is responsible for the success of RDN; however, these lesions are undetectable with OCT.
Recently, Vogel et al. demonstrated no impact of applied ablation points on BP reduction [24]. In contrast to these results, in the present study we demonstrated a correlation between ablation points and systolic BP reduction at 6 months follow up. However, future studies are needed to confirm this preliminary observation in a larger patient population.
Study limitations
The number of patients studied is still small to draw final conclusions, but this is the first study that demonstrated with OCT that vascular lesions apparently do not influence the blood pressure outcome. Larger studies are warranted to assess carefully patients who will benefit from RDN and to define solid predictors for the success of RDN. Since OCT has a limited penetration depth, renal nerves localised in the adventitial layer of the arterial wall cannot be directly visualised [25]. Vascular lesions are contact points between ablation electrode and arterial wall. However, these points should be related to the nerve ablation points localized in adventitia.
Conclusions
This study for the first time indicates that the magnitude and type of vascular lesions induced by RDN as assessed by OCT could not predict the success of blood pressure reduction in patients with resistant hypertension. In addition, we observed a significant correlation between the number of applied ablation points and SBP reduction after 6 months of follow up. Therefore, not the vascular lesions but the ablation points could serve as an important predictor for the success of blood pressure reduction.
References
1 Symplicity HTNI, Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903–9.
2 Bunte MC, Infante de Oliveira E, Shishehbor MH. Endovascular treatment of resistant and uncontrolled hypertension: therapies on the horizon. JACC Cardiovasc Interv. 2013;6:1–9.
3 Mahfoud F, Ukena C, Schmieder RE, Cremers B, Rump LC, Vonend O, et al. Ambulatory blood pressure changes after renal sympathetic denervation in patients with resistant hypertension. Circulation. 2013;128:132–40.
4 Mahfoud F, Lüscher TF, Andersson B, Baumgartner I, Cifkova R, Dimario C, et al. Expert consensus document from the European Society of Cardiology on catheter-based renal denervation. Eur Heart J. 2013;34:2149–57.
5 Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, et al. and Investigators SH-. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–401.
6 Templin C, Jaguszewski M, Ghadri JR, Sudano I, Gaehwiler R, Hellermann JP, et al. Vascular lesions induced by renal nerve ablation as assessed by optical coherence tomography: pre- and post-procedural comparison with the Simplicity (R) catheter system and the EnligHTN (TM) multi-electrode renal denervation catheter. Eur Heart J. 2013;34:2141–8.
7 Jaguszewski M, Ghadri JR, Lüscher TF, Templin C. Optical coherence tomography to reveal vascular lesions after catheter-based renal nerve ablation with a novel multi-electrode EnligHTN system. Kardiol Pol. 2013;71:775.
8 Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. 1991;254:1178–81.
9 Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–81.
10 Esler MD, Krum H, Schlaich M, Schmieder RE, Bohm M, Sobotka PA. Renal sympathetic denervation for treatment of drug-resistant hypertension: one-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation. 2012;126:2976–82.
11 Ott C, Mahfoud F, Schmid A, Ditting T, Sobotka PA, Veelken R, et al. Renal denervation in moderate treatment-resistant hypertension. J Am Coll Cardiol. 2013;62:1880–6.
12 Lüscher TF, Mahfoud F. Renal nerve ablation after SYMPLICITY HTN-3: confused at the higher level? Eur Heart J. 2014;35:1706–11.
13 Dorr O, Liebetrau C, Mollmann H, Achenbach S, Sedding D, Szardien S, et al. Renal sympathetic denervation does not aggravate functional or structural renal damage. J Am Coll Cardiol. 2013;61:479–80.
14 Symplicity HTN-1 Investigators. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension. 2011;57:911–7.
15 Schlaich MP, Schmieder RE, Bakris G, Blankestijn PJ, Bohm M, Campese VM, et al. International expert consensus statement: Percutaneous transluminal renal denervation for the treatment of resistant hypertension. J Am Coll Cardiol. 2013;62:2031–45.
16 Mahfoud F, Cremers B, Janker J, Link B, Vonend O, Ukena C, et al. Renal hemodynamics and renal function after catheter-based renal sympathetic denervation in patients with resistant hypertension. Hypertension. 2012;60:419–24.
17 Hering D, Mahfoud F, Walton AS, Krum H, Lambert GW, Lambert EA, et al. Renal denervation in moderate to severe CKD. J Am Soc Nephrol. 2012;23:1250–7.
18 Ukena C, Mahfoud F, Kindermann I, Barth C, Lenski M, Kindermann M, et al. Cardiorespiratory response to exercise after renal sympathetic denervation in patients with resistant hypertension. J Am Coll Cardiol. 2011;58:1176–82.
19 Ott C, Janka R, Schmid A, Titze S, Ditting T, Sobotka PA, et al. Vascular and renal hemodynamic changes after renal denervation. Clin J Am Soc Nephrol. 2013;8:1195–201.
20 Ormiston JA, Watson T, van Pelt N, Stewart R, Stewart JT, White JM, et al. Renal denervation for resistant hypertension using an irrigated radiofrequency balloon: 12–month results from the Renal Hypertension Ablation System (RHAS) trial. EuroIntervention. 2013;9:70–4.
21 Worthley SG, Tsioufis CP, Worthley MI, Sinhal A, Chew DP, Meredith IT, et al. Safety and efficacy of a multi-electrode renal sympathetic denervation system in resistant hypertension: the EnligHTN I trial. Eur Heart J. 2013;34:2132–40.
22 Steigerwald K, Titova A, Malle C, Kennerknecht E, Jilek C, Hausleiter J, et al. Morphological assessment of renal arteries after radiofrequency catheter-based sympathetic denervation in a porcine model. J Hypertens. 2012;30:2230–9.
23 Stabile E, Ambrosini V, Squarcia R, Salemme L, Popusoi G, Esposito G, et al. Percutaneous sympathectomy of the renal arteries: the OneShot Renal Denervation System is not associated with significant vessel wall injury. EuroIntervention. 2013;9:694–9.
24 Vogel B, Kirchberger M, Zeier M, Stoll F, Meder B, Saure D, et al. Renal sympathetic denervation therapy in the real world: results from the Heidelberg registry. Clinical research in cardiology: official journal of the German Cardiac Society. 2014;103:117–24.
25 Prati F, Guagliumi G, Mintz GS, Costa M, Regar E, Akasaka T, et al. and Expert’s OCTRD. Expert review document part 2: methodology, terminology and clinical applications of optical coherence tomography for the assessment of interventional procedures. Eur Heart J. 2012;33:2513–20.




