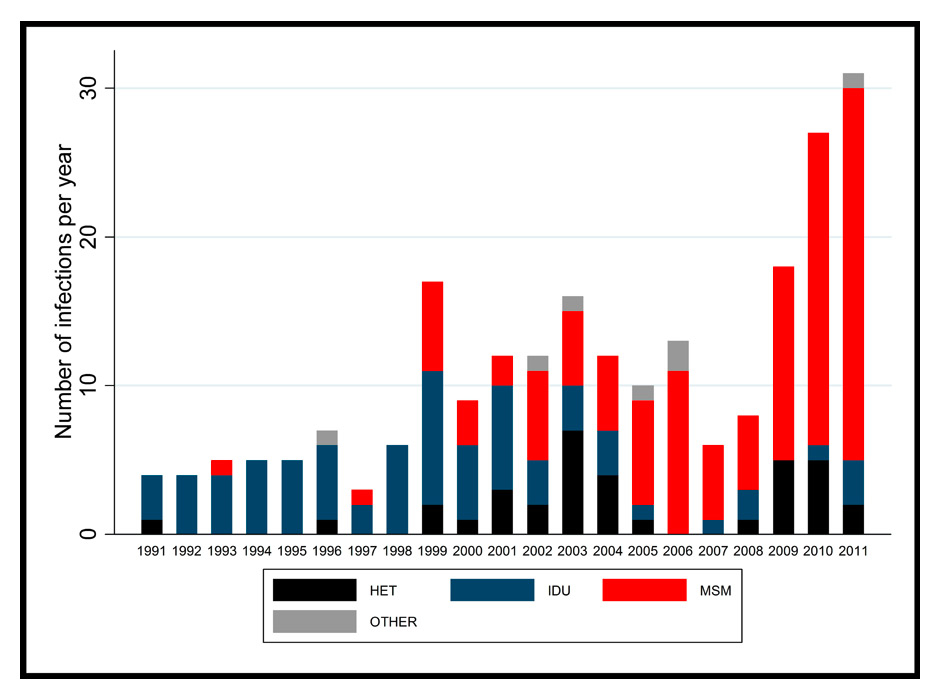
Figure 1
Incident HCV infections by transmission route in the Swiss HIV Cohort Study.
HET = heterosexual; IDU = injection drug use; MSM = men who have sex with men (refer to most likely mode of HIV transmission).
DOI: https://doi.org/10.4414/smw.2015.14093
The hepatitis C virus (HCV) was discovered in 1989, after having been first described as “non-A, non-B hepatitis”, a cause of chronic hepatitis in transfusion recipients in the 1970s [1, 2]. Since then, HCV infection has been increasingly recognised as a major source of liver disease, causing approximately one quarter of all cases of liver cirrhosis and cancer worldwide, and accounting for 500,000 deaths per year [3]. In 2005, over 185 million individuals (2.8% of the world population) were estimated to be infected with chronic HCV infection, determined as a single positive anti-HCV antibody test [4]. The highest prevalence is found in East and Central Asia, as well as in North Africa, whereas Western Europe is considered to have a moderate prevalence (1.5%–3.5%). A recent report showed that, by 2007, HCV infection had even superseded HIV infection as a cause of death in the United States [5].
Approximately 75% of acutely infected patients develop chronic HCV infection, of whom 20% develop liver cirrhosis during the two decades after infection if left untreated [6, 7]. The rate of fibrotic progression is influenced by several risk factors, of which age at infection, sex, hepatitis B virus (HBV)-coinfection, alcohol intake, chronic immune suppression as well as genetic factors are most important [8]. In a recent genome-wide association study including data from the Swiss Hepatitis C Cohort Study (www.swisshcv.ch), several genetic polymorphisms linked to genes regulating apoptosis were found to be associated with the development of liver fibrosis [9]. Coinfection with HIV, which shares routes of transmission with HCV, has a profound impact on the natural history of HCV-related liver disease: compared with HCV-monoinfected individuals, coinfected ones are less likely to experience spontaneous HCV clearance in the early phase of infection, have higher viral loads and an accelerated progression to liver fibrosis, cirrhosis, hepatocellular carcinoma and death [10–13].
In industrialised countries, the burden of HCV infection affected mainly persons who inject drugs (PWID) until the first cases of sexually transmitted infections in HIV-infected men who have sex with men (MSM) were described 10 years ago (table 1) [14–16]. These reports were followed by the description of localised epidemics in large European cities [17–19] and, finally, nationwide epidemics among HIV-infected MSM populations, such as the one in the Swiss HIV Cohort Study [20]. Besides the importance of specific risk groups, the age of infected individuals, the viral genotype distribution, as well as the rate of progression to end-stage liver disease varies widely across settings [21]. Thus, the epidemiological patterns of HCV infection must be analysed and interpreted separately for the different regions of the world.
For many years, treatment of HCV infection consisted of weekly injections of pegylated interferon (IFN) alpha and daily doses of ribavirin (RBV), generally for one year. In the context of its duration, many contraindications, relatively poor success and high price as well as the many related side-effects, HCV therapy uptake has been consistently poor [22–24]. However, in the last two years, novel therapeutic approaches have emerged with the use of direct acting antivirals (DAA), improving the tolerability and success rate of HCV treatment and creating a new paradigm in the management of HCV infection [25]. The perspective to have all-oral, once-daily, pan-genotypic, 2- to 3-month treatment courses without major side effects or drug interactions and rates of sustained virological responses (SVR) above 90%, offers hope for the eradication of HCV infection [26]. However, this goal will only be reached if important barriers, such as the poor uptake of HCV screening and the high cost of these new HCV therapies are addressed.
In this review, we summarise the most important aspects and recent trends in HCV epidemiology, and describe the impact of new management strategies at the individual and population level, before discussing the ultimate goal of HCV eradication and related challenges.
| Table 1: Differences between classic and new HCV epidemics in Switzerland. | ||
| Classic epidemic | New epidemic | |
| Predominant transmission group | PWID (~60%)* | MSM (>80%)* |
| Role of HIV infection | Small (<10% coinfected with HIV) | Important (>90% coinfected with HIV) |
| Peak HCV transmissions | 1980s | 2005 onwards |
| Estimated number of individuals in Switzerland* | 80,000 | 200 |
| Estimated treatment uptake** | 10% | 75% |
| Re-infection incidence | Low (0.8–4.7 per 100 py)*** | High (8.0–15.2 per 100 py)**** |
| Preventive measures | Needle syringe programmes, opioid substitution treatment | Condom use, sexual behaviour campaigns |
| Impact on liver disease burden at the population level& | Large | Small |
| Transmission rate per infected individual | Relatively small in countries with well-established drug substitution programmes | Relatively large due to continuous risk behaviour |
| MSM = men who have sex with men; PWID = persons who inject drugs * from www.swisshcv.ch and Wandeler et al. [20] ** From Wandeler et al. [60] and Dore et al. [21] *** from Grady et al. [61] **** from Martin et al. [62] and Lambers et al. [63] & Number of patients with hepatic decompensation, HCC and liver-related deaths | ||
Percutaneous exposure to HCV is the main transmission route for HCV infection worldwide. Despite well-described epidemics in haemophiliacs and dialysis patients in the 1980s, PWID represent the highest proportion of the HCV epidemic in Switzerland (table 1). In Europe, the majority of PWID are infected with HCV: according to a recent systematic review, the global HCV prevalence among this population group was 67% and over 80% in 12 countries. Globally, around 10 million active PWID are currently infected with HCV with more than a million living each in China, Russia as well as in the USA [27]. According to a recent analysis of data from the national HCV notification system and the Swiss hepatitis C cohort study, the birth year distribution of HCV infections in Switzerland peaks in individuals born between 1955 and 1974, which correlates with the overall pattern of injection drug use (IDU) in the Swiss population [28]. Harm-reduction strategies for PWID such as needle syringe programmes and opioid substitution treatment have been very successful for the prevention of HIV infection [29], but have had more limited impact for HCV prevention, where a combination of interventions is often needed [30]. Aging cohorts of HCV-infected patients constitute an increasing pool of patients at high risk to develop end-stage liver disease and to die of liver disease.
Eastern Europe and Asia have the largest populations of PWID infected with HCV [27]. Many countries in these regions face alarming increases in the incidence of new HCV infections in these vulnerable populations, partially because of stigmatising and criminalising laws against PWID, and due to insufficient resources and the lack of political will for the implementation of prevention strategies. Despite the scientific evidence in favour of harm reduction strategies, punitive instead of therapeutic approaches for PWID are still frequent in many low- and middle-income countries and represent a major hurdle in the fight against the global HCV epidemic.
It has been clearly shown that PWID can be successfully treated for HCV even in the context of on-going injection drug use [31]. Nevertheless, HCV treatment uptake in this population has been consistently low in most settings. As an example, between 1999 and 2011, the proportion of HCV-infected PWID from a representative Australian sample who reported ever having received an HCV therapy increased slightly, but remained below 10% [32]. The situation has generally been similar in HIV-infected PWID: In a single centre of the Swiss HIV Cohort Study, less than 10% of HIV/HCV-coinfected patients started an HCV treatment during active follow-up, mainly because of contraindications related to other comorbidities and uncontrolled addictions [22]. The poor uptake of HCV therapy in PWID is due to a wide range of barriers, including patient refusal, comorbidities, continuous injection drug use and alcohol consumption and the anticipated side-effects of IFN-based therapy. Furthermore, medical practitioner’s reluctance to treat PWID as well as health systems failures also contribute to the poor access to treatment in many settings. Finally, the suitability of treatment settings and models of care also seem to play an important role in the acceptance of HCV treatment for PWID [33].
The possibility of sexual transmission of HCV infection was raised in several reports in the early 1990’s [34, 35]. In a large sero-survey of over 1,000 patients attending a genitourinary clinic in London, Tedder and colleagues were among the first to link the transmission of HCV infection to MSM sexual practices and the occurrence of other sexually transmitted infections (STI) [34]. These first epidemiological studies were followed by further evidence including reports from resource-limited countries, which highlighted the association between HCV and MSM, but the overall number of infections remained low or the association disappeared after adjusting the estimates for injection drug use [36, 37]. It was only a few years later that concerns were raised about the potential increase in the number of HCV infections in HIV-infected MSM populations who did not have a history of injection drug use [14–16]. As an example, in a case note review of a sexual health clinic in London, the incidence of new HCV seroconversions in HIV-infected MSM significantly increased between 1997 and 2002, in parallel to the increase in other sexually transmitted infections (STI) such as syphilis [14]. As the number of incident HCV infections remained low, these analyses were reproduced in larger cohorts and collaborative multi-cohort studies [18, 38]. Small epidemics localised in large European cities were described in groups of patients with high risk sexual behaviour, including traumatic anal intercourse, fisting and the use of sex toys [39]. Several networks of HCV transmission specific to such high-risk groups could be shown in England, Germany and the Netherlands, with transmission chains fuelled by permucosal infections [17, 18, 40]. Furthermore, phylogenetic analyses identified several transmission clusters in Europe and Australia within a large international HCV transmission network [41].

Figure 1
Incident HCV infections by transmission route in the Swiss HIV Cohort Study.
HET = heterosexual; IDU = injection drug use; MSM = men who have sex with men (refer to most likely mode of HIV transmission).
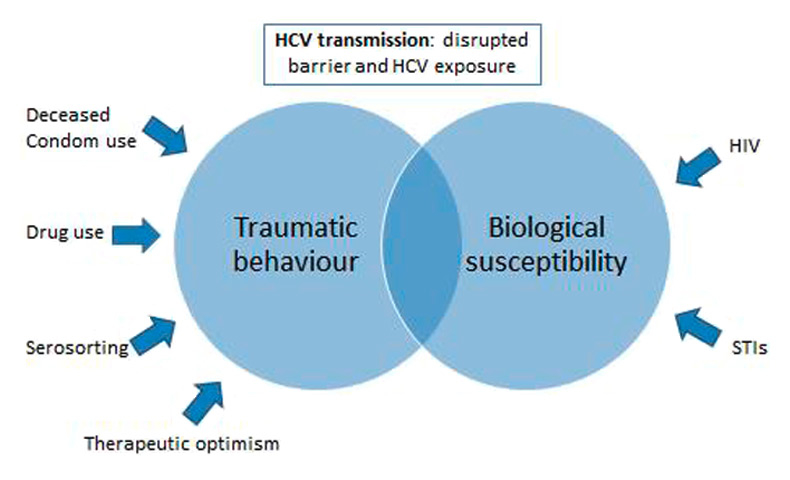
Figure 2
Factors associated with the transmission of HCV in HIV-infected MSM (adapted from Danta et al. [70]).
In 2012, a comprehensive analysis of HCV seroconversions in the Swiss HIV Cohort Study (SHCS) showed an 18–fold increase in HCV incidence in HIV-infected MSM between 1998 and 2011, whereas the incidence in PWID decreased steadily (table 1) [20]. This report was based on the systematic screening of HCV infection for all SHCS participants since 1998 and was the first study to report HCV incidence in a nationwide and representative HIV-infected population. Figure 1 shows the number of incident HCV infections by calendar year and transmission group in the SHCS. Incident HCV infections were associated with unsafe sex and co-infections with syphilis and hepatitis B. Several biological and behavioural factors seem to play a role in this new epidemiological trend (fig. 2). More than separate risk factors, it is probably the interplay between a number of these that has an impact on the sexual transmission of HCV in HIV-infected MSM. In addition to the increased biological susceptibility to HCV induced by HIV-infection and other STI, the consumption of alcohol and recreational drugs as well as the engagement in unprotected high risk sexual practices lead to the high vulnerability of certain HIV-infected MSM. Phylogenetic analyses among MSM in the SHCS revealed that patients with closely related HIV viruses were more likely to acquire HCV infection if their partner was HCV-positive [42]. This finding underscores the role of domestic HCV transmission linked to sexual risk behaviour. Seroadaptive behaviour (‘serosorting’) is increasingly common in HIV-infected MSM, leading to the broader uptake of unprotected, traumatic sexual practices [43]. Importantly, Hasse et al. showed that, in Switzerland, condom use had decreased among HIV-infected individuals with an undetectable HIV viral load during anti-retroviral therapy in recent years, further increasing the proportion of individuals at risk of HCV infection [44]. Kouyos and colleagues found a broad HIV transmission bottleneck in HCV-infected MSM [45]. As a broad bottleneck is consistent with direct viral transmission through traumatic events, this finding supports the role of traumatic sex for HCV transmission among HIV-infected MSM (fig. 2).
According to the Global Burden of Diseases Project (GBD 2010), the prevalence of people with anti-HCV has increased from 2.3% to 2.8% between 1990 and 2005 globally [4]. Even though trends in HCV incidence vary across countries, the main drivers of HCV infection in the general population have remained fairly stable over the past years [46]. Injection drug use remains the main mode of transmission in industrialised countries, whereas unsterile tattoos and piercings in non-professional settings might play an increasing role in young populations [47]. Risk factors for HCV infection in resource-limited settings remain ill-defined, even though unsafe therapeutic injection practices and blood transfusions seem to be the main routes of infection [46].
In Switzerland, there were an estimated 82,000 viraemic HCV infections in 2013 [48]. According to this study, the viraemic population peaked in 1996 and was projected to decrease to 63,000 by 2013 due to intensified treatment and reduced transmissions. However, the proportion of advanced liver fibrosis or hepatic decompensations was projected to increase and account for more than 20% of total infections by 2030. This indicates that the disease burden caused by HCV infections will continue to rise in the next decades if treatment uptake and efficacy do not increase substantially. Bruggmann and colleagues estimated that, if treatment uptake increases from currently 1,100 to 5,300 per year, test rate increased from 1,050 to 3,490 per year, and if treatment efficacy rises to 90% using the new DAA regimens, HCV-related mortality would decline by 70% and 1,640 liver-related deaths would be averted by 2030 in Switzerland. This indicates that treatment interventions could indeed have a substantial impact on the morbidity and mortality at the population level.
The landscape of HCV epidemiology in HIV-infected patients has changed in recent years due to the increasing role played by its sexual transmission in MSM and the decreasing incidence in injection drug use in PWID. In the context of successful harm reduction strategies for PWID in Switzerland, the number of HCV-infected patients entering HIV care has decreased in recent years, whereas the number of incident HCV infections during follow-up has increased. The new epidemic of HCV infections in HIV-infected MSM and the potentially increased sexual transmission of HCV reported in cohorts of HIV-infected women [49] raised the question of whether sexual transmissions of HCV could also occur in HIV-uninfected individuals.
Among HIV-uninfected heterosexual people in long-term monogamous relationships, the risk of sexual transmission of HCV is extremely low. Most spousal transmissions seem to be explained by the common practice of sharing syringes [50]. However, the probability of acquiring HCV infection has been shown to increase with the number of sexual partners [51]. A recent systematic review on the sexual transmission of HCV underlined the importance of confounding by other risk factors in the majority of these epidemiological studies [52]. Most reports showing an association between heterosexual contact and the acquisition of HCV infection relied on self-reports as ascertainment of injection drug use and exposure to other sources of infection including sharp objects could not be excluded. HIV-uninfected MSM do not seem to be at higher risk of acquiring HCV either. In a recent serological survey among over 800 HIV-uninfected MSM with a median of 6 sexual partners a year in Zurich, the prevalence of anti-HCV was 0.4% and only one patient had a replicating HCV infection [53]. Thus, HCV prevalence in HIV-uninfected MSM in Switzerland does not seem to be higher than in the general population despite the large proportion of these men reporting unprotected anal intercourse and traumatic sexual practices. These results are comparable to most other evaluations in European, Australian and American cities and are in line with a recent systematic review which came to the conclusion that systematic screening of HCV infection in HIV-uninfected MSM was not recommended [54]. Taken together, the literature available to date does not place sexual transmission as an important mode of transmission of HCV in HIV-uninfected individuals.
The field of HCV infection is facing an unprecedented situation in terms of treatment perspectives. The better understanding of the HCV life cycle as well as important efforts in drug development, have led to the discovery of several direct-acting antiviral agents (DAA) [25]. Within a few years, we have moved from lengthy courses of IFN-based treatment with many side-effects and poor treatment success to shorter treatments with well-tolerated and more potent oral drugs. Within the next two years, most HCV-infected patients, including those with cirrhosis, HIV-infection and those who failed previous therapies, will benefit from atwo to three-month course of all-oral treatment regimens with over 90% chances of experiencing a sustained virological response. As successful antiviral therapy is associated with a decreased progression to end-stage liver disease and mortality, as well as with an improved quality of life, it is now clear that much of the HCV-related morbidity can be controlled with treatment [55]. The new treatment options have moved the discussion from individual treatment efficacy to the ambitious goal of global elimination (reduction of incidence to zero but requiring preventive measures to prevent re-establishment, as with measles) or even eradication (permanent reduction to zero cases, as with smallpox) [56].
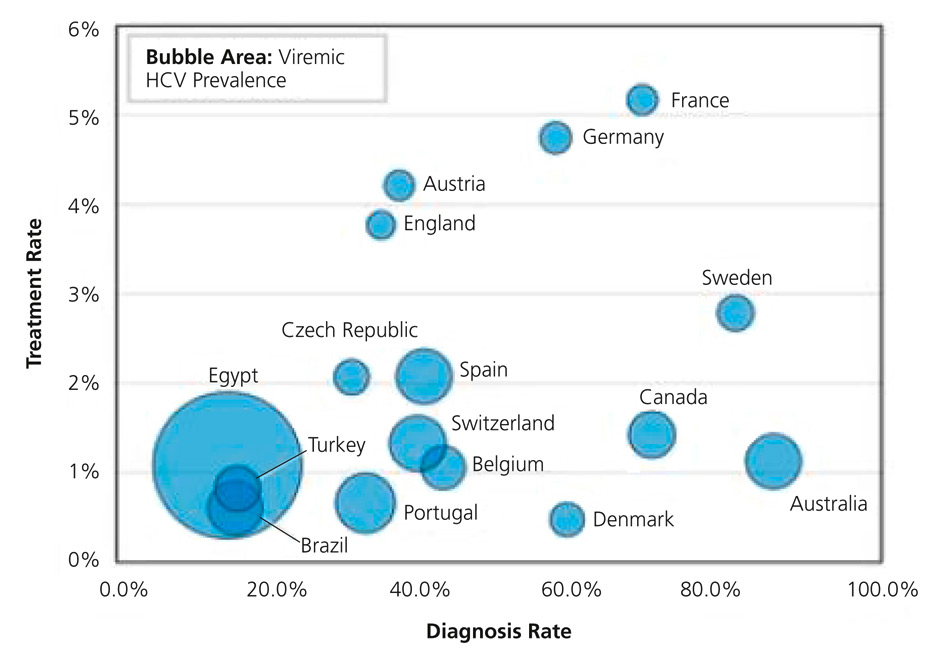
Figure 3
HCV prevalence, diagnosis and treatment rates in 2013 (from: Dore GJ, Ward J, Thursz M. Hepatitis C disease burden and strategies to manage the burden. J Viral Hepat. 2014;21(Suppl 1):1–4, reprint with permission from John Wiley and Sons).
The control of HCV infection implies primary prevention measures, such as the testing of blood supplies and ensuring safe injections and medical procedures, combined with the management of existing infections. As the pool of existing infections is extremely large, increasing the uptake of HCV testing and treatment is a necessity and remains the main challenge on the road to HCV eradication. Lack of HCV awareness and access to health care for those at highest risk are the main barriers to overcome. At present, there are wide differences in testing and treatment uptake between countries, as shown in figure 3 for Europe. In the United States, more than half of HCV-infected individuals are unaware of their infection [57], and this proportion is even much higher in resource-limited settings. Sound HCV testing strategies focused on specific age groups and high-risk populations have been implemented in several countries. These strategies need to be adapted to the local epidemiologic situation, and to go along with interventions to improve access to care and treatment for those in need. The slowly increasing availability of point-of-care anti-HCV tests should also have an impact on the uptake of HCV screening, especially in low-income settings.
In all regions of the world, only a small minority of HCV-infected patients have ever received IFN-alpha-based antiviral therapy. In low and middle income countries, HCV treatment is not accessible for almost all affected persons and treatment uptake rates remain below 1%. By the end of 2005, treatment uptake in European countries was estimated to range between 16% in France and 1% in Romania and Russia [58]. These numbers have not increased after the availability of the first generation of DAA including boceprevir and telaprevir. Only 12% of the patients with a chronic HCV infection in 2011 in the Swiss HIV Cohort Study started HCV therapy with one of the new protease inhibitors during follow-up [59]. However, recent trends in HCV epidemiology already had an impact on treatment uptake during acute HCV infection, as shown in a recent analysis in HIV-infected patients in Switzerland [60]. Treatment uptake in incident HCV infections has increased from 33% before 2005 to 75% after 2005. These results were mainly driven by the large proportion of MSM accounting for incident HCV infections in the SHCS after this date. However, similar trends can be expected for PWID in the future, as it is increasingly recognised that the willingness for treatment is high and that it is effective and cost-efficient in this population [56].
Another challenge for HCV eradication is the high frequency of HCV re-infections in certain patient populations. Whereas this does not seem to be a major issue in PWID, where re-infections are rare even in the context of on-going injection drug use [61], early reports from HIV-infected MSM cohorts are of greater concern. Among 145 HIV-infected MSM with a documented primary HCV infection at a large hospital in London, the re-infection rate was 8.0 per 100 person-years over a median follow-up time of 2.1 years [62]. In another study from Amsterdam, the incidence of re-infection was even higher, with an estimated 15.2 per 100 person-years [63]. These results underline the need for more extensive risk behaviour counselling and prevention in certain high-risk groups such as HIV-infected MSM in large European cities.
The soon-to-be available interferon-free, pangenotypic, single-tablet antiviral combinations for HCV infection will undoubtedly increase the overall treatment uptake provided its accessibility is improved through cost-reductions. One of the greatest assets of these newer generations of antiviral treatment is their broad applicability in general clinical practices or in resource-limited settings due to their safety and relative simplicity in management and monitoring [64]. In analogy to the long-lasting fight for affordable anti-retroviral therapy for HIV infection in Africa, similar efforts will have to be made for HCV therapies in order to expect an impact of these new treatment modalities on the global HCV epidemic [65]. In Switzerland, the high cost of the new HCV drugs has limited their promising potential in terms of treatment uptake as their prescription is currently restricted to patients with advanced liver disease.
The new therapeutic strategies have also led experts to advocate HCV treatment as prevention, in analogy to recent developments in the field of HIV infection [66]. In contrast to HIV infection, HCV treatment is finite in time and often leads to cure. Thus, it is obvious that a combination of enhanced HCV treatment efficacy as well as improved diagnosis and treatment uptake would have a huge impact on population-level disease burden. Furthermore, treatment as prevention strategies could also be a way to address re-infections in high-risk groups. Mathematical modelling work has given important insights into the feasibility of treatment as prevention. The introduction of new DAAs will only have a modest impact on projected advanced liver disease burden, mortality and the number of new infections if treatment uptake remains at current levels [21, 67]. However, according to a recently published modelling study adapted for PWID populations in Scotland, Canada and Australia, realistic increases in treatment uptake of DAA-based antiviral therapies could halve the HCV prevalence within the next 15 years [68]. Finally, in a similar model adapted to the HCV epidemic in HIV-infected MSM in Switzerland, condom use and HCV treatment uptake were the most important factors in curbing the epidemic in this population [A. Rauch, SSI Annual Meeting Aarau, August 2014].
The HCV epidemic has evolved over the years: besides the constant burden seen in PWID, smaller epidemics of sexually transmitted HCV infections have recently emerged in HIV-infected MSM. After two decades of slow and incremental improvements in HCV therapy, a new era has started with the availability of new generations of oral, direct-acting antiviral drugs, allowing new perspectives in terms of HCV control. HCV infection has many of the attributes needed to be a good candidate for eradication: it can be cleared from the host through treatment, there is no non-human reservoir and transmission chains can be broken. However, the poor uptake of HCV testing, the high cost of the new antiviral therapies as well as the frequent re-infections after successful treatments are some of the challenges that will need to be addressed for the better control of the HCV epidemic. Furthermore, the eradication of an infectious agent needs strong financial support as well as political and societal will. Although these factors have not always been present in the past years, the call to develop a comprehensive approach to control chronic hepatitis during the 2010 World Health Assembly [69] leaves hope for a better control of HCV worldwide.
Acknowledgment:We thank all patients, doctors, and nurses associated with the Swiss HIV Cohort Study (SHCS), and with the Swiss Hepatitis C Cohort Study, supported by the Swiss National Science Foundation.
1 Rakela J, Redeker AG. Chronic liver disease after acute non-A, non-B viral hepatitis. Gastroenterology. 1979;77(6):1200–2.
2 Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244(4902):359–62.
3 Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet.2012;380(9859):2095–128.
4 Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57(4):1333–42.
5 Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156(4):271–8.
6 Grebely J, Page K, Sacks-Davis R, et al. The effects of female sex, viral genotype, and IL28B genotype on spontaneous clearance of acute hepatitis C virus infection. Hepatology. 2014;59(1):109–20.
7 Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48(2):418–31.
8 McCaughan GW, George J. Fibrosis progression in chronic hepatitis C virus infection. Gut. 2004;53(3):318–21.
9 Patin E, Kutalik Z, Guergnon J, et al. Genome-wide association study identifies variants associated with progression of liver fibrosis from HCV infection. Gastroenterology. 2012;143(5):1244–52 e1–12.
10 Sulkowski MS, Mehta SH, Torbenson MS, et al. Rapid fibrosis progression among HIV/hepatitis C virus-co-infected adults. AIDS. 2007;21(16):2209–16.
11 Graham CS, Baden LR, Yu E, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33(4):562–9.
12 Merchante N, Giron-Gonzalez JA, Gonzalez-Serrano M, et al. Survival and prognostic factors of HIV-infected patients with HCV-related end-stage liver disease. AIDS. 2006;20(1):49–57.
13 Grebely J, Prins M, Hellard M, et al. Hepatitis C virus clearance, reinfection, and persistence, with insights from studies of injecting drug users: towards a vaccine. Lancet Infect Dis. 2012;12(5):408–14.
14 Browne R, Asboe D, Gilleece Y, et al. Increased numbers of acute hepatitis C infections in HIV positive homosexual men; is sexual transmission feeding the increase? Sex Transm Infect. 2004;80(4):326–7.
15 Gotz HM, van Doornum G, Niesters HG, den Hollander JG, Thio HB, de Zwart O. A cluster of acute hepatitis C virus infection among men who have sex with men – results from contact tracing and public health implications. AIDS. 2005;19(9):969–74.
16 Gambotti L, Batisse D, Colin-de-Verdiere N, et al. Acute hepatitis C infection in HIV positive men who have sex with men in Paris, France, 2001–2004. Euro surveillance: bulletin europeen sur les maladies transmissibles = European communicable disease bulletin. 2005;10(5):115–7.
17 Danta M, Brown D, Bhagani S, et al. Recent epidemic of acute hepatitis C virus in HIV-positive men who have sex with men linked to high-risk sexual behaviours. AIDS. 2007;21(8):983–91.
18 van de Laar TJ, van der Bij AK, Prins M, et al. Increase in HCV incidence among men who have sex with men in Amsterdam most likely caused by sexual transmission. J Infect Dis. 2007;196(2):230–8.
19 Vogel M, Deterding K, Wiegand J, et al. Initial presentation of acute hepatitis C virus (HCV) infection among HIV-negative and HIV-positive individuals-experience from 2 large German networks on the study of acute HCV infection. Clin Infect Dis. 2009;49(2):317–9; author reply 9.
20 Wandeler G, Gsponer T, Bregenzer A, et al. Hepatitis C Virus Infections in the Swiss HIV Cohort Study: A Rapidly Evolving Epidemic. Clin Infect Dis. 2012;55(10):1408–16.
21 Dore GJ, Ward J, Thursz M. Hepatitis C disease burden and strategies to manage the burden (Guest Editors Mark Thursz, Gregory Dore and John Ward). J Viral Hepat. 2014;21(Suppl 1):1–4.
22 Rauch A, Egger M, Reichen J, Furrer H. Chronic hepatitis C in HIV-infected patients: low eligibility and applicability of therapy with pegylated interferon-alpha plus ribavirin. J Acquir Immune Defic Syndr. 2005;38(2):238–40.
23 Grint D, Peters L, Schwarze-Zander C, et al. Temporal changes and regional differences in treatment uptake of hepatitis C therapy in EuroSIDA. HIV Med. 2013;14(10):614–23.
24 Clark PJ, Thompson AJ, Patel K, Muir AJ, Volk ML. Trends in hepatitis C treatment uptake in the United States. Hepatology. 2014 Jun 18.
25 Pawlotsky JM. New hepatitis C therapies: the toolbox, strategies, and challenges. Gastroenterology. 2014;146(5):1176–92.
26 Chung RT, Baumert TF. Curing chronic hepatitis C – the arc of a medical triumph. N Engl J Med. 2014;370(17):1576–8.
27 Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378(9791):571–83.
28 Bruggmann P, Richard JL. Birth year distribution in reported hepatitis C cases in Switzerland. Eur J Public Health. 2014 Jul 23.
29 Degenhardt L, Mathers B, Vickerman P, Rhodes T, Latkin C, Hickman M. Prevention of HIV infection for people who inject drugs: why individual, structural, and combination approaches are needed. Lancet. 2010;376(9737):285–301.
30 Hagan H, Pouget ER, Des Jarlais DC. A systematic review and meta-analysis of interventions to prevent hepatitis C virus infection in people who inject drugs. J Infect Dis. 2011;204(1):74–83.
31 Grebely J, Bruggmann P, Backmund M, Dore GJ. Moving the agenda forward: the prevention and management of hepatitis C virus infection among people who inject drugs. Clin Infect Dis. 2013;57(Suppl 2):S29–31.
32 Iversen J, Grebely J, Topp L, Wand H, Dore G, Maher L. Uptake of hepatitis C treatment among people who inject drugs attending Needle and Syringe Programs in Australia, 1999–2011. J Viral Hepat. 2014;21(3):198–207.
33 Bruggmann P, Litwin AH. Models of care for the management of hepatitis C virus among people who inject drugs: one size does not fit all. Clin Infect Dis. 2013;57(Suppl 2):S56–61.
34 Tedder RS, Gilson RJ, Briggs M, et al. Hepatitis C virus: evidence for sexual transmission. BMJ. 1991;302(6788):1299–302.
35 Buchbinder SP, Katz MH, Hessol NA, Liu J, O’Malley PM, Alter MJ. Hepatitis C virus infection in sexually active homosexual men. J Infect. 1994;29(3):263–9.
36 Marx MA, Murugavel KG, Tarwater PM, et al. Association of hepatitis C virus infection with sexual exposure in southern India. Clin Infect Dis. 2003;37(4):514–20.
37 Bodsworth NJ, Cunningham P, Kaldor J, Donovan B. Hepatitis C virus infection in a large cohort of homosexually active men: independent associations with HIV-1 infection and injecting drug use but not sexual behaviour. Genitourinary medicine. 1996;72(2):118–22.
38 van der Helm JJ, Prins M, del Amo J, et al. The hepatitis C epidemic among HIV-positive MSM: incidence estimates from 1990 to 2007. AIDS. 2011;25(8):1083–91.
39 Schmidt AJ, Rockstroh JK, Vogel M, et al. Trouble with bleeding: risk factors for acute hepatitis C among HIV-positive gay men from Germany – a case-control study. PLoS One. 2011;6(3):e17781.
40 Vogel M, van de Laar T, Kupfer B, et al. Phylogenetic analysis of acute hepatitis C virus genotype 4 infections among human immunodeficiency virus-positive men who have sex with men in Germany. Liver Int. 2010;30(8):1169–72.
41 van de Laar T, Pybus O, Bruisten S, et al. Evidence of a large, international network of HCV transmission in HIV-positive men who have sex with men. Gastroenterology. 2009;136(5):1609–17.
42 Kouyos RD, Rauch A, Boni J, et al. Clustering of HCV coinfections on HIV phylogeny indicates domestic and sexual transmission of HCV. Int J Epidemiol. 2014 Jan 22.
43 Snowden JM, Raymond HF, McFarland W. Seroadaptive behaviours among men who have sex with men in San Francisco: the situation in 2008. Sex Transm Infect. 2011;87(2):162–4.
44 Hasse B, Ledergerber B, Hirschel B, et al. Frequency and determinants of unprotected sex among HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis. 2010;51(11):1314–22.
45 Kouyos RD, Rauch A, Braun DL, et al. Higher Risk of Incident Hepatitis C Virus Coinfection Among Men Who Have Sex With Men, in Whom the HIV Genetic Bottleneck at Transmission Was Wide. J Infect Dis. 2014 Jun 18.
46 Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5(9):558–67.
47 Tohme RA, Holmberg SD. Transmission of hepatitis C virus infection through tattooing and piercing: a critical review. Clin Infect Dis. 2012;54(8):1167–78.
48 Bruggmann P, Negro F, Bihl F, et al. The disease burden of chronic hepatitis C virus (HCV) infection in Switzerland. The International Liver Congress 2014, London, Great Britain. P1285.
49 Frederick T, Burian P, Terrault N, et al. Factors associated with prevalent hepatitis C infection among HIV-infected women with no reported history of injection drug use: the Women’s Interagency HIV Study (WIHS). AIDS patient care and STDs. 2009;23(11):915–23.
50 Stroffolini T, Lorenzoni U, Menniti-Ippolito F, Infantolino D, Chiaramonte M. Hepatitis C virus infection in spouses: sexual transmission or common exposure to the same risk factors? Am J Gastroenterol. 2001;96(11):3138–41.
51 Salleras L, Bruguera M, Vidal J, et al. Importance of sexual transmission of hepatitis C virus in seropositive pregnant women: a case-control study. J Med Virol. 1997;52(2):164–7.
52 Tohme RA, Holmberg SD. Is sexual contact a major mode of hepatitis C virus transmission? Hepatology. 2010;52(4):1497–505.
53 Schmidt AJ, Falcato L, Zahno B, et al. Prevalence of hepatitis C in a Swiss sample of men who have sex with men: whom to screen for HCV infection? BMC Public Health. 2014;14:3.
54 Yaphe S, Bozinoff N, Kyle R, Shivkumar S, Pai NP, Klein M. Incidence of acute hepatitis C virus infection among men who have sex with men with and without HIV infection: a systematic review. Sex Transm Infect. 2012;88(7):558–64.
55 Thomas DL. Global control of hepatitis C: where challenge meets opportunity. Nature medicine. 2013;19(7):850–8.
56 Grebely J, Dore GJ. Can hepatitis C virus infection be eradicated in people who inject drugs? Antiviral Res. 2014;104:62–72.
57 Denniston MM, Klevens RM, McQuillan GM, Jiles RB. Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001–2008. Hepatology. 2012;55(6):1652–61.
58 Lettmeier B, Muhlberger N, Schwarzer R, et al. Market uptake of new antiviral drugs for the treatment of hepatitis C. J Hepatol. 2008;49(4):528–36.
59 Haubitz S SV, Ambrosioni J, Cavassini M, Stöckle M, Schmid P, Decosterd L, Metzner K, Fehr J, Rauch A Protease Inhibitors To Treat Hepatitis C in the Swiss HIV Cohort Study: High Efficacy But Low Uptake. 21st Conference on Retroviruses and Opportunistic Infections, March 3–6, 2014; abstract 658.
60 Wandeler G RJ, Metzner KJ, Fehr J, Stöckle M, Cavassini M, Ambrosioni J, Keiser O, Furrer H, Rauch A and the Swiss HIV Cohort Study. Incident HCV Infections in the Swiss HIV Cohort Study: Natural History and Treatment Outcomes. Conference on Retroviruses and Opportunistic Infections (CROI), Boston, 3.–6. March 2014.
61 Grady BP, Schinkel J, Thomas XV, Dalgard O. Hepatitis C virus reinfection following treatment among people who use drugs. Clin Infect Dis. 2013;57(Suppl 2):S105–10.
62 Martin TC, Martin NK, Hickman M, et al. Hepatitis C virus reinfection incidence and treatment outcome among HIV-positive MSM. AIDS. 2013;27(16):2551–7.
63 Lambers FA, Prins M, Thomas X, et al. Alarming incidence of hepatitis C virus re-infection after treatment of sexually acquired acute hepatitis C virus infection in HIV-infected MSM. AIDS. 2011;25(17):F21–7.
64 Jayasekera CR, Barry M, Roberts LR, Nguyen MH. Treating hepatitis C in lower-income countries. N Engl J Med. 2014;370(20):1869–71.
65 Ford N, Singh K, Cooke GS, et al. Expanding access to treatment for hepatitis C in resource-limited settings: lessons from HIV/AIDS. Clin Infect Dis. 2012;54(10):1465–72.
66 Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505.
67 Grebely J, Matthews GV, Lloyd AR, Dore GJ. Elimination of hepatitis C virus infection among people who inject drugs through treatment as prevention: feasibility and future requirements. Clin Infect Dis. 2013;57(7):1014–20.
68 Martin NK, Vickerman P, Grebely J, et al. Hepatitis C virus treatment for prevention among people who inject drugs: Modeling treatment scale-up in the age of direct-acting antivirals. Hepatology. 2013;58(5):1598–609.
69 Sixty-third World Health Assembly closes after passing multiple resolutions. Available from http://www.who.int/mediacentre/news/releases/2010/wha_closes_20100521/en/index/html.2010 http://www.who.int/mediacentre/news/releases/2010/wha_closes_20100521/en/ ., 2010.
70 Danta M, Rodger AJ. Transmission of HCV in HIV-positive populations. Curr Opin HIV AIDS. 2011;6(6):451–8.
Funding / potential competing interests: We declare no competing interests.