Extending resection and preserving function: modern concepts of glioma surgery
DOI: https://doi.org/10.4414/smw.2015.14082
Philippe
Schucht, Juergen
Beck, Kathleen
Seidel, Andreas
Raabe
Summary
Recent studies have demonstrated that the improved prognosis derived from resection of gliomas largely depends on the extent and quality of the resection, making maximum but safe resection the ultimate goal. Simultaneously, technical innovations and refined neurosurgical methods have rapidly improved efficacy and safety. Because gliomas derive from intrinsic brain cells, they often cannot be visually distinguished from the surrounding brain tissue during surgery. In order to appreciate the full extent of their solid compartment, various technologies have recently been introduced. However, radical resection of infiltrative glioma puts neurological function at risk, with potential detrimental consequences for patients’ survival and quality of life. The allocation of various neurological functions within the brain varies in each patient and may undergo additional changes in the presence of a tumour (brain plasticity), making intra-operative localisation of eloquent areas mandatory for preservation of essential brain functions. Combining methods that visually distinguish tumour tissue and detect tissues responsible for critical functions now enables resection of tumours in brain regions that were previously considered off-limits, and benefits patients by enabling a more radical resection, while simultaneously lowering the risk of neurological deficits. Here we review recent and expected developments in microsurgery for glioma and their respective benefits.
Classification of glioma
Gliomas are the most common type of primary brain tumour. Their various degrees of malignancy form the basis of the World Health Organisation (WHO) grading system: pilocytic astrocytoma is the most benign form of glioma (WHO Grade I), followed by a heterogeneous group of so-called “low-grade gliomas” (WHO Grade II) that comprises oligodendroglioma, astrocytoma and oligoastrocytoma. Anaplastic glioma (WHO Grade III) is a malignant and aggressive form of glioma but lacks the malignant characteristics of the glioblastoma (WHO IV), the most aggressive and most common glioma.
What to do when a glioma is detected?
Many gliomas become symptomatic with either seizures or focal neurological deficits and are subsequently detected via MRI. Although MRI can predict a glioma with high accuracy, in most cases a definite diagnosis must be obtained by means of histological analysis.
Treatment options depend on the type of glioma, and patient-specific factors such as location and size of the glioma, patient age, symptoms and neurological status. In addition, three molecular markers – 1p/19q co-deletion, O6-methylguanine methyltransferase (MGMT) promoter methylation and isocitrate dehydrogenase (IDH) 1/2 mutations – are known to have important diagnostic, prognostic and predictive (for treatment efficacy) roles in glioma treatment (for reviews see Tabatabai et al. 2010 [1] and Leu et al. [2]). Treatment may include surgery, radiation therapy and/or chemotherapy. The role of surgery has been debated for decades, as sceptics questioned the rationale of surgery for such an infiltrative disease. However, modern guidelines recommend primary surgical removal for all types of gliomas provided that neurological function is preserved and the tumour appears locally circumscribed on magnetic resonance imaging (MRI).
Surgery for low-grade glioma: recent findings
In order to evaluate the significance of different extents of resections (EOR) in low-grade glioma, we performed a literature review of studies reporting survival after surgery for low-grade glioma over the last 10 years. Three of these studies included volumetric assessment of the EOR (table 1).
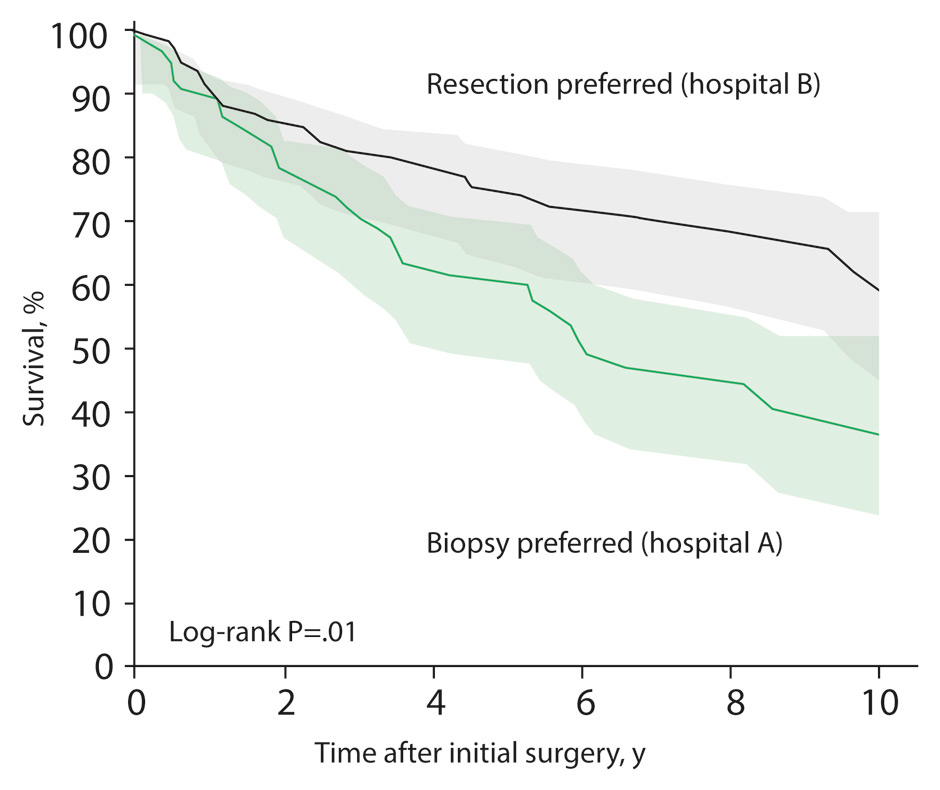
Figure 1
Patient survival according to hospital treatment strategy.
Patients treated at a hospital that favoured a pro-active strategy with early radical resection for low-grade glioma had an improved survival time compared with patients treated in a hospital that followed a “wait-and-see” strategy after biopsy [7].
The largest of these studies in patients with low-grade gliomas, performed by Capelle et al. [5], showed a strong impact of the EOR on survival, especially when a radiological complete resection was obtained [5], and an impact of the preoperative tumour volume (which invariably correlates with the EOR) [6]. Although non-controlled series have a potential selection bias, similar results were found in a recent study with an unusual geographic and medical constellation that essentially eliminated the selection bias: two hospitals in Norway, each taking exclusive care of a large, stable population, followed different strategies for patients with low-grade glioma [7]. One of the hospitals favoured a biopsy followed by a “wait-and-see” strategy, delaying further therapy until malignant progression while the other hospital preferred to perform maximal safe resection whenever possible. Outcome comparison between the two hospitals revealed that patients of the surgery-preferring hospital had a significantly better survival rate, suggesting that a proactive and aggressive treatment plan improves survival of low-grade glioma patients (fig. 1). Moreover, the rate of malignant transformation was twice as high in the “wait-and-see” cohort [7]. Taken together, these findings support a proactive and radical surgical approach for low-grade gliomas rather than a “wait-and-see” strategy.
A recent Cochrane review emphasised the lack of randomised clinical trials or prospective controlled clinical trials on which to base clinical decisions regarding surgery, underscoring the need for further, preferably randomised, trials. The review recommended that physicians approach each case individually and weigh the risks and benefits of an intervention [8]. Surgical possibilities and potential risks should thus be explored soon after radiological diagnosis and certainly before additional tumour growth increases the risks of malignant transformation.
After surgery, low-grade gliomas should be observed by serial MRIs to detect recurrence and progression and may additionally be treated with adjuvant chemotherapy (temozolomide) or radiotherapy. An individual patient’s prognosis depends on a variety of factors. Smaller tumour size, the absence of neurological deficits at diagnosis and oligodendroglial histology have a positive influence on overall survival (for a web-based calculator of prognosis see http://www.eortc.be/tools/lggcalculator ) [6]. Molecular markers are thought to be more accurate than histology for predicting survival; the combination of IDHmut, MGMTmetand 1p/19q co-deletion is associated with the longest survival [2].
|
Table 1:Volumetrically assessed EOR in low grade gliomas and its influence on survival. |
|
Author
|
Number of patients
|
Extent of resection*
|
5-year survival
|
Multivariate p-value
|
| Claus et al. (2005) [3] |
156 |
100%
<100% |
98.2%
92.0% |
<0.05 |
| Smith et al. (2008) [4] |
216 |
>90%
<90% |
97%
76% |
<0.001 |
| Capelle et al. (2013) [5] |
1,091 |
100%
<100% |
100%
77% |
0.0199 |
| *Assessed as the resection percentage of T2, FLAIR and T2/FLAIR signal abnormality on MRI in the reports of Claus et al, Smith et al. and Capelle et al. |
Surgery for glioblastoma: recent findings
For glioblastoma the impact of surgery on progression-free survival and overall survival has also been debated for decades. The lack of adequate assessment of the extent of resection and thus of the success of surgery prior to routine post-operative MRI and volumetric analysis may have contributed to somewhat contradictory results, with institutional series arguing both for and against a survival benefit of resection (for review see Sanai 2012 [9]).
A clearer picture results when the selection of publications is limited to those that provided volumetric assessment of the surgical result based on early post-operative imaging. Three institutional series demonstrated a significant overall survival benefit for radical resection of contrast-enhancing tumour as compared to subtotal resection [10–12], and a fourth series showed a non-significant trend towards improved survival [13].
Moreover, post-hoc analysis of a randomised controlled trial of a surgical adjunct (5–ALA-fluorescence, see below) revealed a significant survival benefit of almost 5 months for gross total resection compared to subtotal resection (16.7 vs 11.8 months; evidence level 2) [14, 15]. This difference in survival time may be due to an increased efficacy of adjuvant treatment after radical resection of the tumour’s solid portion (for review see [16]). A recent report on advanced modelling found a continuous and exponential relationship between the extent of resection and survival, which supports the concept of maximal safe resection of contrast enhancing tumour [17]. To what extent the benefit of resection is influenced by the current recommended use of adjuvant concomitant radio-chemotherapy remains to be determined.
Detecting tumour tissue: how to improve the extent of resection?
In the past, a complete resection of enhancing tumour (CRET) was achieved only in a small minority of glioma surgeries, presumably due to the difficulty of discerning tumour tissue from the surrounding brain tissue during surgery (for high-grade tumours see [18], for low-grade glioma see [19]). In light of the increasingly convincing evidence of the benefit of CRET for survival, new technologies were investigated and implemented for improving tumour detection. Neuronavigation allows correlation of any intra-operative point with the preoperative MRI, and has become standard-of-care at most neurosurgical centres for intra-operative spatial orientation [20–22] (fig. 2). The main limitation of neuronavigation is its stepwise loss of accuracy during surgery [23], which is a source of concern in the crucial final stages of resection. Despite the use of neuronavigation the rates of CRET in resectable tumours are disappointingly low both in low-grade and high-grade gliomas, and range from 20% to 60%. High-grade glioma tissue may be detected thanks to the tumour cells’ altered metabolism: the orally administered drug 5-aminolevulinic acid (5–ALA) leads to fluorescence of tumour cells during surgery, allowing identification and resection of tissue that is otherwise indistinguishable from brain tissue (fig. 3). The use of 5–ALA significantly increases the rate of successful CRET [14], and is thus routinely applied in neurosurgical centres. In addition, a randomised controlled trial showed that use of an intraoperative MRI (iMRI) increases the rate of complete resections [24], a result that can also be obtained using early re-do surgeries for patients whose post-operative MRI reveals a tumour remnant [25]. These various methods are not mutually exclusive; they can be used simultaneously and are thus complementary. The rates of successful complete resection of resectable tumours improve to 96.2% using a combination of these techniques, as shown by our group in a consecutive cohort of patients (the remaining 3.8% of patients had permanent neurological deficits) [26].

Figure 2
Neuronavigation allows correlation of any intra-operative point with the pre-operative MRI.
Intraoperative neuronavigation facilitates spatial orientation during surgery at the crucial interface between a low-grade glioma (hyper-intense), the primary motor cortex (red) and the corticospinal tract (blue).
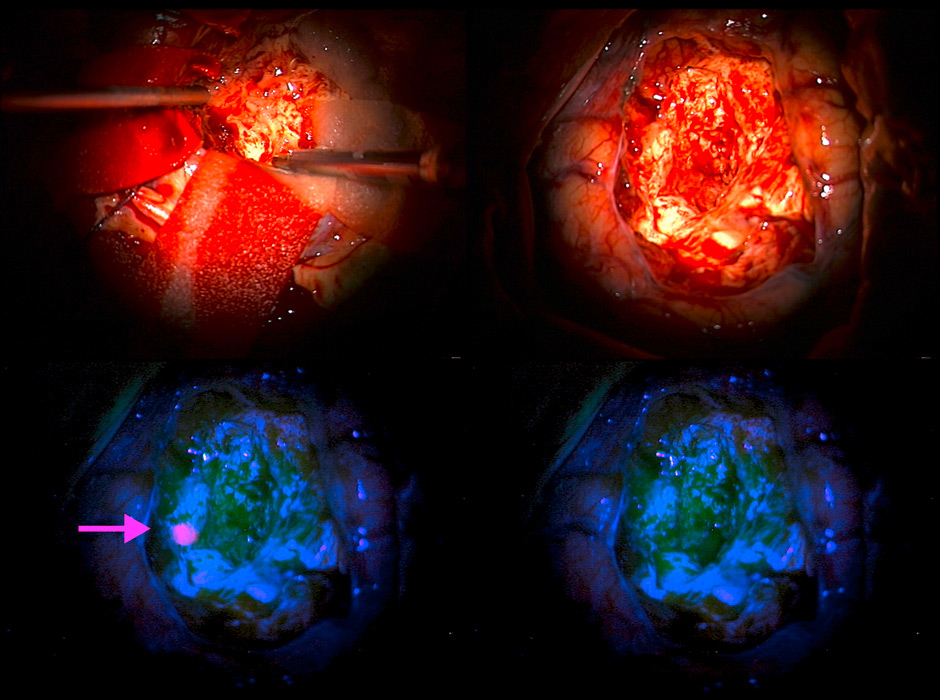
Figure 3
The oral prodrug 5-ALA identifies tumour tissue in surgery for glioblastoma.
Top left: intraoperative view during tumour resection. Top right: surgical site after removal of all tumour tissue as seen in white light microscopy. Bottom left: upon switching to microscopic blue light a small patch of remaining tumour tissue can be seen thanks to 5-ALA fluorescence (arrow). Bottom right: surgical site after removal of all fluorescent tumour.
Protecting brain function while increasing resection: localising functional tissues to increase safety
Oncological benefits derived from extensive resections need to be balanced against the risks of neurological deficits. The ability of the above-mentioned technologies to detect tumour tissue may lead to resections deep into infiltrated brain tissue, potentially endangering neurological function. Stummer et al. showed that 5–ALA guided resections carry a higher risk of post-operative neurological deterioration than conventional resections (26% vs 15%, respectively), even though the difference vanished within weeks [27]. Just as tumour tissue is often indiscernible from normal brain tissue, functionally critical tissues are indistinguishable from tissues with less clinically relevant functions.
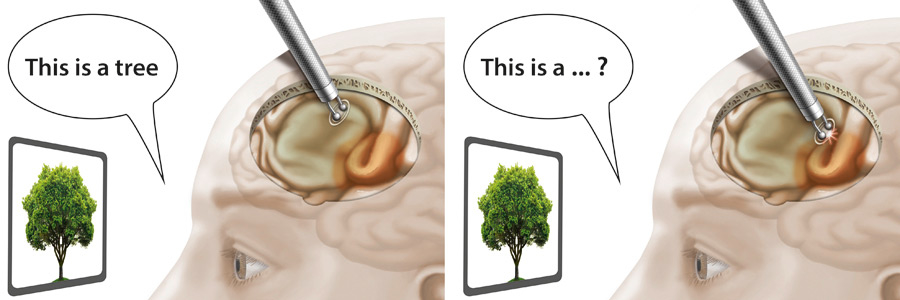
Figure 4
Speech mapping during awake craniotomy.
Left: the patient correctly names items during electrical stimulation of non-eloquent areas and tumour tissue. Right: speech anomalies during stimulation reveal eloquent areas (brown) that need to be spared to avoid postoperative speech deficits.
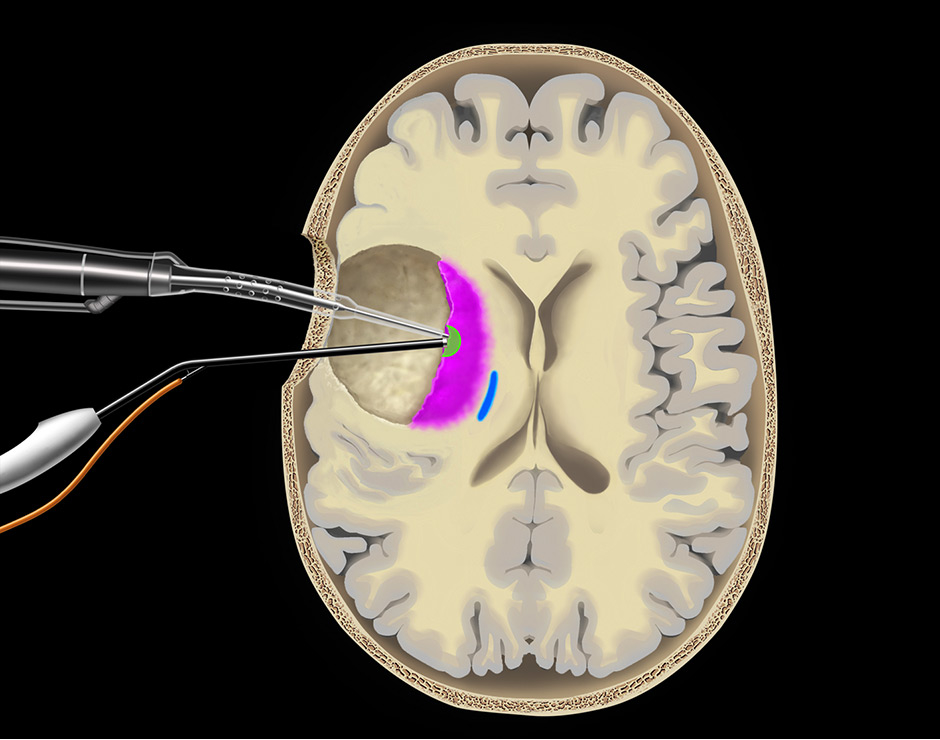
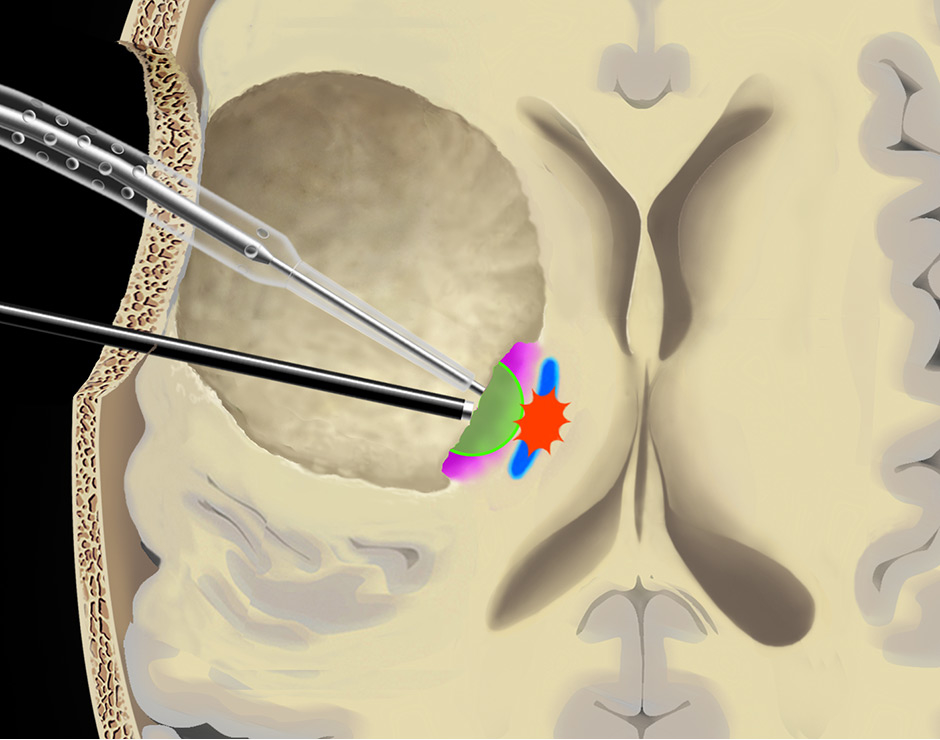
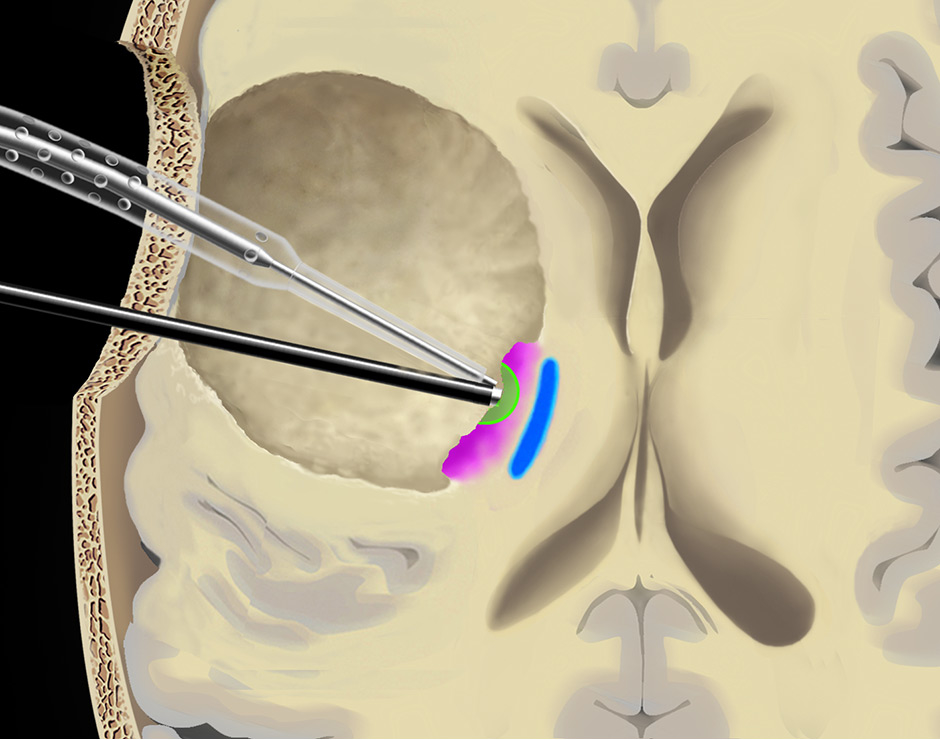
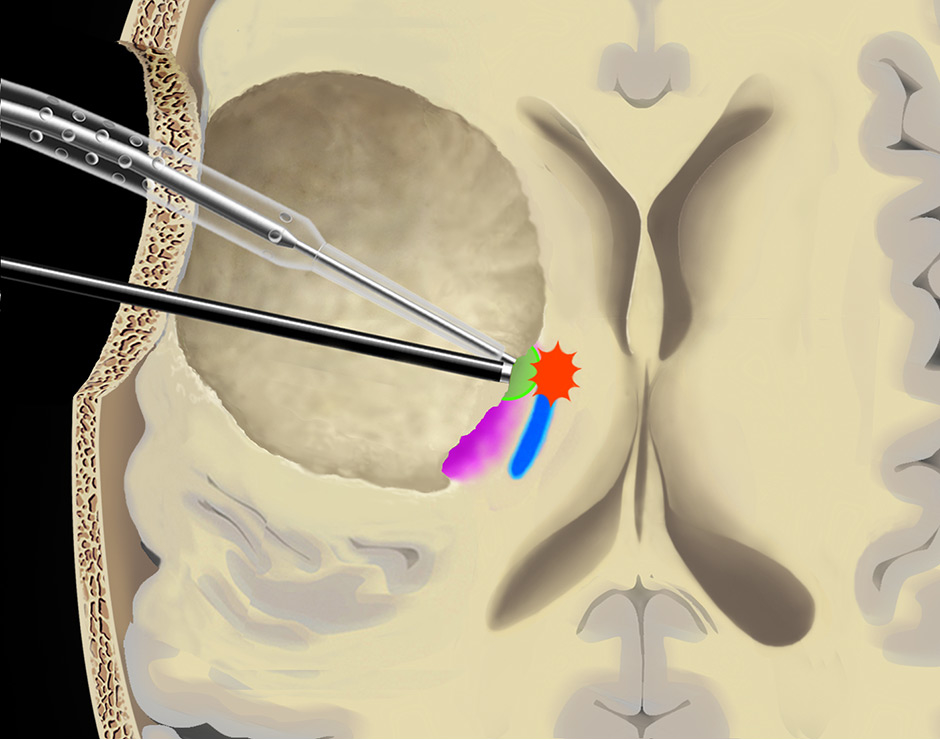
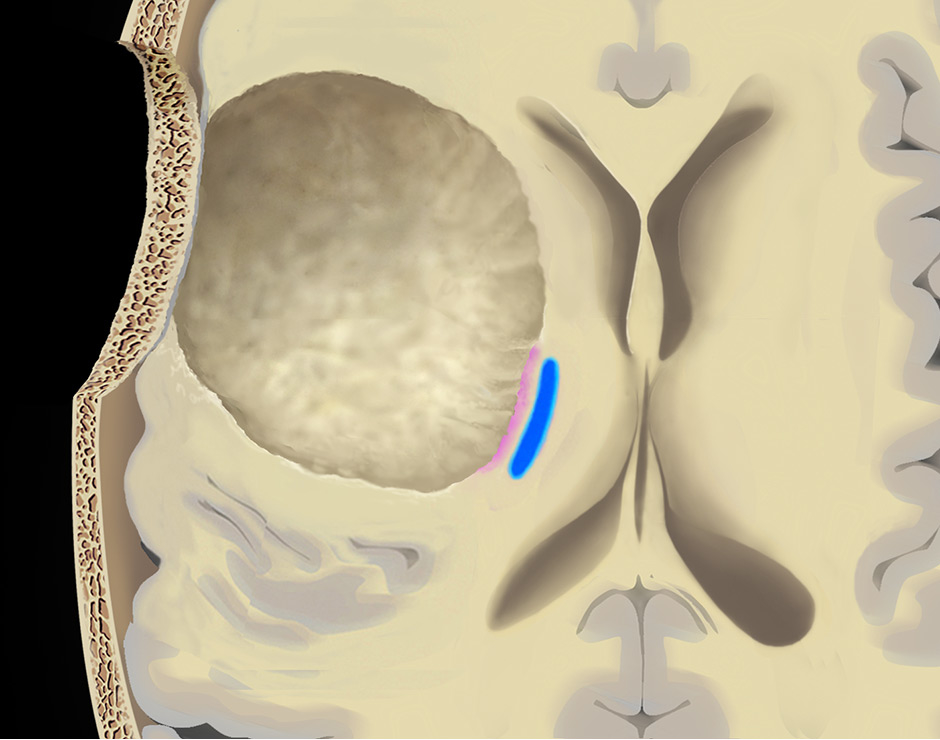
Figure 5
Use of the continuous dynamic mapping device. The continuous dynamic mapping device is used once the surgeon approaches the CST (A). Resection is continued with an initial stimulation intensity of 10–15 mA (corresponding to a distance of 10–15 mm from the CST). The instant an MEP is elicited (B), the surgeon is alerted by the change of the high-pitch negative control sound to a low-pitch alert sound. The stimulation intensity is then reduced by 2 mA, and the resection is continued at sites negative for the set current (C), until again the acoustic switch from the negative control to the alert sound warns the surgeon that the MT has been reached (D). This stepwise approach is continued until a minimal threshold is reached (E). Pink indicates tumor tissue, blue indicates CST fibres, green indicates a region of active resection, and red indicates that an alert sound has occurred. Copyright Andreas Raabe. Published with permission.
Thus, knowing when to stop a resection due to proximity to areas of crucial neurological functions is of obvious and utmost importance. Detailed knowledge of the normal brain anatomy and distribution of function is not sufficient during glioma resection. Interindividual variability and functional relocation (i.e., plasticity) induced by the presence of an infiltrating tumour [28] requires an exact functional brain map at the site of surgery in order to spare areas involved in crucial (so-called eloquent) functions. Preoperative localisation of function, either with functional MRI (fMRI) or navigated transcranial magnetic stimulation (nTMS), provides an approximate map [29, 30]. Furthermore, intra-operative direct cortical and subcortical electrical stimulation (DCS) for functional analysis of the tissue in the tumour’s infiltration zone is required for accurate identification of areas that need to be spared in order to retain the patient’s functional integrity [31, 32]. Motor evoked potentials (MEP) provide real-time information on the integrity of the primary motor cortex and the corticospinal tract [33]. Direct cortical mapping (fig. 4) and phase reversal identify the primary motor and sensory cortices. Subcortical mapping can estimate the distance to the pyramidal tract, acting as guidance close to functionally critical areas [34]. When integrated into the existing surgical tools, continuous and dynamic mapping enables more extensive resection while simultaneously protecting motor function (fig. 5) [35]. Using these techniques and a detailed electrophysiological “Bern-concept”, our group achieved complete motor function protection in 96% of patients with high-risk motor eloquent tumours (fig. 6) [36]. Furthermore, localisation of cortical and subcortical regions relevant to language function is essential for speech preservation during resection of gliomas in proximity to presumed speech areas [31] and requires the patient to be awake during the brain mapping part of surgery (fig. 7). Similarly, intra-operative mapping of visual functions may contribute to increased resections while avoiding tissue essential for vision within the temporal and occipital lobes [37].
Presumed eloquence as a modifiable risk factor for decreased survival
Intraoperative DCS increases the extent of resection by clarifying whether an area preoperatively presumed to be eloquent actually is eloquent, and thus extends the resection right up to the boundary of functional tissue. This concept of presumed eloquence as a modifiable risk factor predictive of decreased survival time due to subtotal resection represents a significant improvement in neurological and oncological risk management. Removing a brain tumour proximal to an eloquent area without mapping and monitoring requires that a considerable safety margin is maintained to spare areas of presumed function, and thereby forfeits the oncological benefit of maximum resection. A comparative study between two series with and without DCS functional mapping led not only to an increase of total resections (from 6% to 25%), but also to a decrease of neurological deficits (from 17% to 6.5%) [19]. Thus, although DCS was initially expected to reduce the extent of resection through identification of unresectable areas, it actually increased the extent of resection in both low-grade glioma and glioblastoma [19, 26, 38].
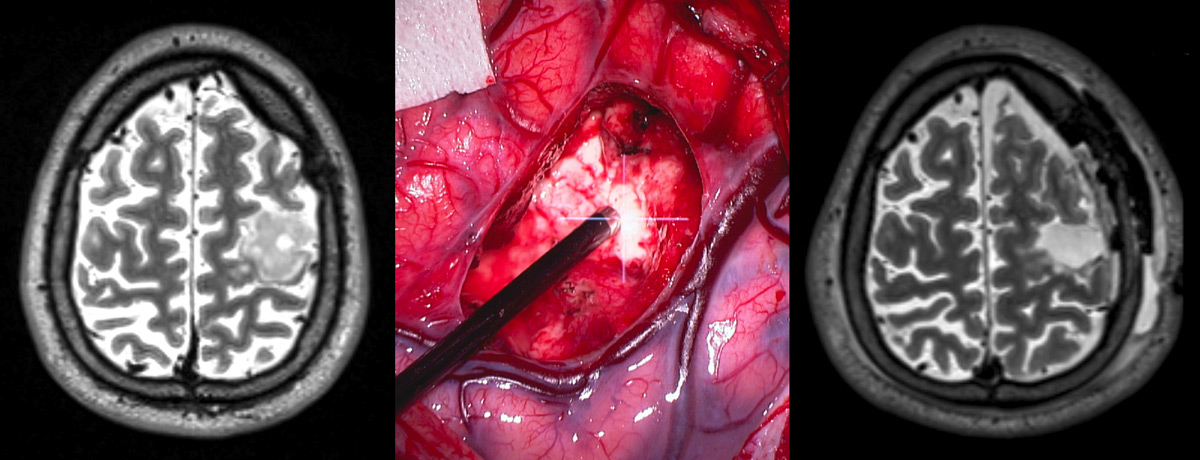
Figure 6
Example of a surgery for a lesion adjacent to eloquent tissue lesion.
Left: Preoperative imaging reveals a glioma within the primary motor cortex. Middle: intraoperative continuous motor mapping guides surgery in proximity to the corticospinal tract. Right: postoperative imaging depicts gross total resection.
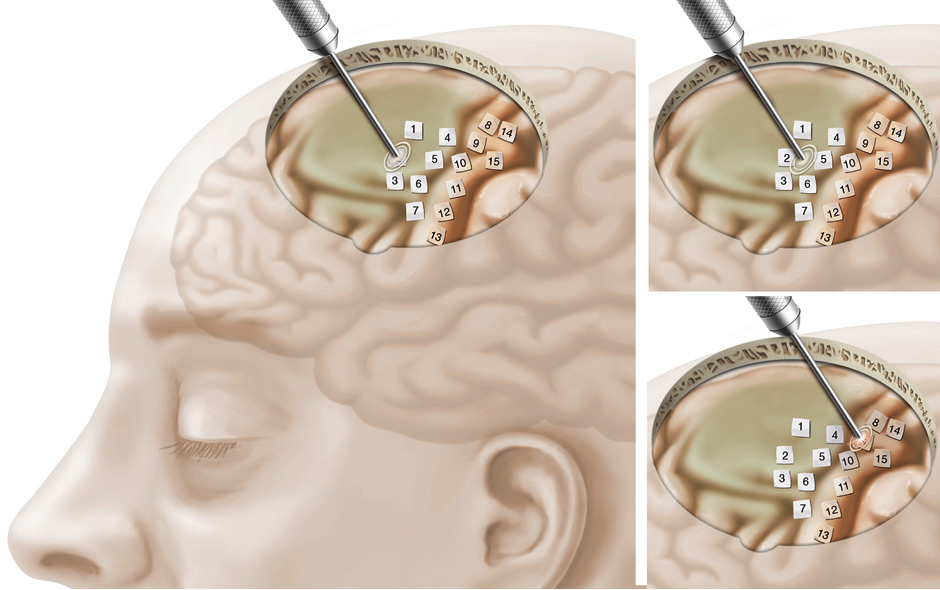
Figure 7
Left: Overview of cortical mapping for motor function.
Top right: Intraoperative cortical mapping detects no motor function at sites labeled 1–7.
Bottom right: Motor function of the hand is detected and spared during surgery at site labeled 8 (further motor function sites detected and spared at sites 9–15).
Any benefit for resection beyond the outer rim of radiological boundaries?
The obvious benefits of extensive, yet tailored and accurate glioma resections are encouraging, but there is room for further improvement. Current resection thresholds are invariably based on radiologically perceived boundaries (fluid-attenuated inversion recovery [FLAIR] MRI for low-grade glioma and gadolinium enhanced T1 MRI for glioblastoma), despite our knowledge that the tumour infiltration goes well beyond these thresholds. Several strategies aim to increase the oncological benefit of surgery by reaching resection thresholds not visible on MRI. Confocal microscopes can be used to identify infiltrating low-grade glioma cells, pushing the resection border into the infiltration zone [39]. Also, supra-total resections of low-grade gliomas are sporadically attempted in non-eloquent areas to improve oncological outcome [40]. 5–ALA, known for its ability to facilitate CRET in high grade gliomas, may lead to resections beyond the margins of gadolinium MRI due to its higher sensitivity for tumour [41]. A preliminary study indicated that extending resection beyond gadolinium and up to the surgical threshold of 5–ALA complete resection provides a survival benefit for patients with glioblastoma [42].
Is there a role for surgery in recurrent glioma?
The recurrence of glioblastoma after initial surgery and adjuvant treatment poses a therapeutic challenge. Evidence for a survival benefit of a second surgery for glioblastoma recurrence is scarce [43] and comes from single centre series in which longer survivals observed in patients with repeated surgeries may have resulted from selection bias, rather than treatment. Higher level evidence is required before re-operation of recurrent glioblastoma can be recommended as standard treatment for defined patient and tumour criteria. Interestingly, many low-grade gliomas are resectable during the second surgery at sites where functionality prevented tumour resection during the initial surgery [44]. This remarkable finding demonstrates the plasticity of the brain and highlights the importance of brain mapping to achieve a maximal but safe resection.
Conclusion
Recent reports confirmed the survival benefit derived from surgery for both low and high grade gliomas. Use of technical adjuncts such as 5–ALA fluorescence and iMRI for identification of tumour remnants help to increase the extent of resection. Intraoperative identification of function further increases the extent of resection by clarifying whether an area preoperatively presumed to be eloquent actually is eloquent, and thus extends the resection right up to the boundaries of functional tissue while avoiding destruction of functional tissue. Thus, the devoted and combined use of the different surgical adjuncts contributes to the goal of maximum safe resection.
References
1 Tabatabai G, Stupp R, van den Bent MJ, Hegi ME, Tonn JC, Wick W, et al. Molecular diagnostics of gliomas: the clinical perspective. Acta Neuropathol. 2010;120(5):585–92.
2 Leu S, von Felten S, Frank S, Vassella E, Vajtai I, Taylor E, et al. IDH/MGMT-driven molecular classification of low-grade glioma is a strong predictor for long-term survival. Neuro Oncol. 2013;15(4):469–79. doi: 10.1093/neuonc/nos317.
3 Claus EB, Horlacher A, Hsu L, Schwartz RB, Dello-IaconoD, Talos F, et al. Survival rates in patients with low-grade glioma after intraoperative magnetic resonance image guidance. Cancer. 2005;103(6):1227–33.
4 Smith JS, Chang EF, Lamborn KR, Chang SM, Prados MD, Cha S, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26(8):1338–45.
5 Capelle L, Fontaine, D, Mandonnet E, Taillandier L, Golmard JL, Bauchet L, et al. Spontaneous and therapeutic prognostic factors in adult hemispheric World Health Organization Grade II gliomas: a series of 1097 cases. J Neurosurg. 2013;118(6):1157–68.
6 Gorlia T1, Wu W, Wang M, Baumert BG, Mehta M, Buckner JC, et al. New validated prognostic models and prognostic calculators in patients with low-grade gliomas diagnosed by central pathology review: a pooled analysis of EORTC/RTOG/NCCTG phase III clinical trials. Neuro Oncol. 2013;15(11):1568–79. doi: 10.1093/neuonc/not117.
7 Jakola A, Mymel KS, Kloster R, Torp SH, Lindal S, Unsgard G. Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. JAMA. 2012;308(18):1881–8.
8 Veeravagu A, Jiang B, Ludwig C, Chang SD, Black KL, Patil CG. Biopsy versus resection for the management of low-grade gliomas. Cochrane Database Syst Rev 2013;155:951–957. doi: 10.1002/14651858.CD009319
9 Sanai N. Emerging operative strategies in neurosurgical oncology. Curr Opin Neurol. 2012;25(6):756–66. doi:10.1097/WCO.0b013e32835a2574
10 Keles GE, Anderson B, Berger MS. The effect of extent of resection on time to tumor progression and survival in patients with glioblastoma multiforme of the cerebral hemisphere. Surg Neurol. 1999;52(4):371–9.
11 Lacroix M, Abi-Said D, Fourney DR, Gokasian ZL, Shi E, DeMonte F, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190–8.
12 Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115(1):3–8.
13 Pope WB, Sayre J, Perlina A, Villablanca JP, Mischel PS, Cloughesy TF. MR imaging correlates of survival in patients with high-grade gliomas. Am J Neuroradiol. 2005;26(10):2466–74.
14 Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ, et al. Fluorescence-guided surgery with 5–aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5):392–401.
15 Stummer W, Reulen HJ, Meinel T, Pichelmeier U, Schumacher W, Tonn JC, et al. Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery. 2008;62(3):564–76.
16 Stummer W, van den Bent MJ, Westphal M. Cytoreductive surgery of glioblastoma as the key to successful adjuvant therapies: new arguments in an old discussion. Acta Neurochir (Wien). 2011;153(6):1211–8. doi: 10.1007/s00701–011–1001–x. Epub 2011 Apr 9.
17 Marko NF1, Weil RJ, Schroeder JL, Lang FF, Suki D, Sawaya RE. Extent of resection of glioblastoma revisited: personalized survival modeling facilitates more accurate survival prediction and supports a maximum-safe-resection approach to surgery. J Clin Oncol. 2014;32(8):774–82. doi: 10.1200/JCO.2013.51.8886.
18 Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen H Jr. Fluorescence-guided resection of glioblastoma multiforme utilizing 5–ALA-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg. 2000;93(6):1003–13
19 Duffau H, Lopes M, Arthuis F, Bitar A, Sichez J-P, Van Effenterre R, et al. Contribution of intraoperative electrical stimulations in surgery of low grade glimas: a comparative study between two series without (1985–1996) and with (1996–2003) functional mapping in the same institutions. J Neurol Neurosurg Psychiatry. 2005;76(6):845–51.
20 Warnke PC. Stereotactic volumetric resection of gliomas. Acta Neurochir Suppl. 2003;88:5–8.
21 Krishnan R, Raabe A, Hattingen E, Szelenyi A, Yahya H, Hermann E, et al. Functional magnetic resonance imaging-integrated neuronavigation: correlation between lesion-to-motor cortex distance and outcome. Neurosurgery. 2004;55(4):904 –14.
22 Willems PW, Taphoorn MJ, Burger H, Berkelbach van der Sprenkel JW, Tulleken CA. Effectiveness of neuronavigation in resecting solitary intracerebral contrast-enhancing tumors: a randomized controlled trial. J Neurosurg. 2006;104(3):360–8.
23 Stieglitz LH, Fichtner J, Andres R, Schucht P, Krähenbühl AK, Raabe A, et al. The Silent Loss of Neuronavigation Accuracy: A Systematic Retrospective Analysis of Factors Influencing the Mismatch of Frameless Stereotactic Systems in Cranial Neurosurgery. Neurosurgery. 2013;72(5):796–807.
24 Senft C, Bink A, Franz K, Vatter H, Gasser T, Seifert V. Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol. 2011;12:997–1003.
25 Schucht P, Murek M, Jilch A, Seidel K, Hewer E, Wiest, R, et al. Early Re-do Surgery for glioblastoma is a feasible and safe strategy to achieve complete resection of enhancing tumor. PLoS One 2013;8(11):e79846.
26 Schucht P, Beck J, Abu-Isa J, Andereggen L, Murek M, Seidel K, et al. Gross total resection rates in contemporary glioblastoma surgery: results of an institutional protocol combining 5–aminolevulinic acid intraoperative fluorescence imaging and brain mapping. Neurosurgery. 2012;71(5):927–36.
27 Stummer W1, Tonn JC, Mehdorn HM, Nestler U, Franz K, Goetz C, et al. ALA-Glioma Study Group. Counterbalancing risks and gains from extended resections in malignant glioma surgery: a supplemental analysis from the randomized 5–aminolevulinic acid glioma resection study. J Neurosurg. 2011;114(3):613–23. doi: 10.3171/2010.3
28 Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J Neurosurg. 1989;71(3):316–26.
29 Seghier ML, Lazeyras F, Pegna AJ, Annoni JM, Zimine I, Mayer E, et al. Variability of fMRI activation during a phonological and semantic language task in healthy subjects. Hum Brain Mapp. 2004;23(3):140–55.
30 Krieg SM, Shiban E, Buchmann N, Gempt J, Foerschler A, Meyer B, et al. Utility of presurgical navigated transcranial magnetic brain stimulation for the resection of tumors in eloquent motor areas. J Neurosurg. 2012;116(5):994–1001. doi: 10.3171/2011.12.JNS111524
31 Duffau H, Capelle L, Sichez N, Denvil D, Lopes M, Sichez JP, et al. Intraoperative mapping of the subcortical language pathways using direct stimulations. An anatomo-functional study. Brain. 2002;125(1):199–214.
32 Duffau H, Capelle L, Denvil D, Sichez N, Gatignol P, Taillandier L, et al. Usefulness of intraoperative electrical subcortical mapping during surgery for low-grade gliomas located within eloquent brain regions: functional results in a consecutive series of 103 patients. J Neurosurg. 2003;98(4):764–78.
33 Seidel K, Beck J, Stieglitz L, Schucht P, Raabe A. The warning-sign hierarchy between quantitative subcortical motor mapping and continuous motor evoked potential monitoring during resection of supratentorial brain tumors. J Neurosurg. 2013;118(2):287–96.
34 Seidel K, Beck J, Stieglitz L, Schucht P, Raabe A. Low Threshold Monopolar Motor Mapping for Resection of Primary Motor Cortex Tumors. Neurosurgery. 2012;71(1):104–14.
35 Raabe A, Beck J, Schucht P, Seidel K. Continuous dynamic mapping of the corticospinal tract during surgery of motor eloquent brain tumors: evaluation of a new method. J Neurosurg. 2014;120(5)1015–24. doi: 10.3171/2014.1.JNS13909.
36 Schucht P, Seidel K. Beck J, Murek M, Jilch A, Wiest R, et al. Intraoperative monopolar mapping during 5-ALA-guided resections of glioblastomas adjacent to motor eloquent areas: evaluation of resection rates and neurological outcome. Neurosurg Focus. 2014;27(6):E16.
37 Gras-Combe G, Moritz-Gasser S, Herbet G, Duffau H. Intraoperative subcortical electrical mapping of optic radiations in awake surgery for glioma involving visual pathways. J Neurosurg. 2012;117(3):466–73.
38 De Witt Hamer PC, Robles SG, Zwinderman AH, Duffar H, Berger MS. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J Clin Oncol. 2012;30(20):2559–65.
39 Sanai N, Snyder LA, Honea NJ, Coons CW, Eschbacher JM, Smith KA, et al. Intraoperative confocal microscopy in the visualization of 5–aminolevulinic acid fluorescence in low-grade gliomas. J Neurosurg. 2011;115(4):740–8.
40 Yordanova YN, Moritz-Gasser S, Duffau H: Awake surgery for WHO Grade II gliomas within “noneloquent” areas in the left dominant hemisphere: toward a “supratotal” resection. J Neurosurg. 2011(115):232–9.
41 Schucht P, Knittel S, Slotboom J, Seidel K, Murek M, Jilch A, et al. 5–ALA complete resections go beyond MR contrast enhancement: shift corrected volumetric analysis of the extent of resection in surgery for glioblastoma. Acta Neurochirurgica. 2014;156(2):305–12.
42 Aldave G, Tejada S, Pay E, Marigil M, Bejarano B, Idoate MA, et al. Prognostic value of residual fluorescent tissue in glioblastoma patients after gross total resection in 5–aminolevulinic Acid-guided surgery. Neurosurgery. 2013;72:915–20; discussion 920–921.
43 Chaichana KL, Zadnik P, Weingart JD, Olivi A, Gallia GL, Blakeley J, et al. Multiple resections for patients with glioblastoma: prolonging survival. J Neurosurg. 2012: DOI: 10.3171/2012.5.JNS12690.
44 Robles SG, Gatignol P, Lehéricy S, Duffau H, et al. Long-term brain plasticity allowing a multistage surgical approach to World Health Organization Grade II gliomas in eloquent areas. J Neurosurg. 2008;109(4):615–24. Doi: 10.3171/JNS/2008/109/10/0615.










