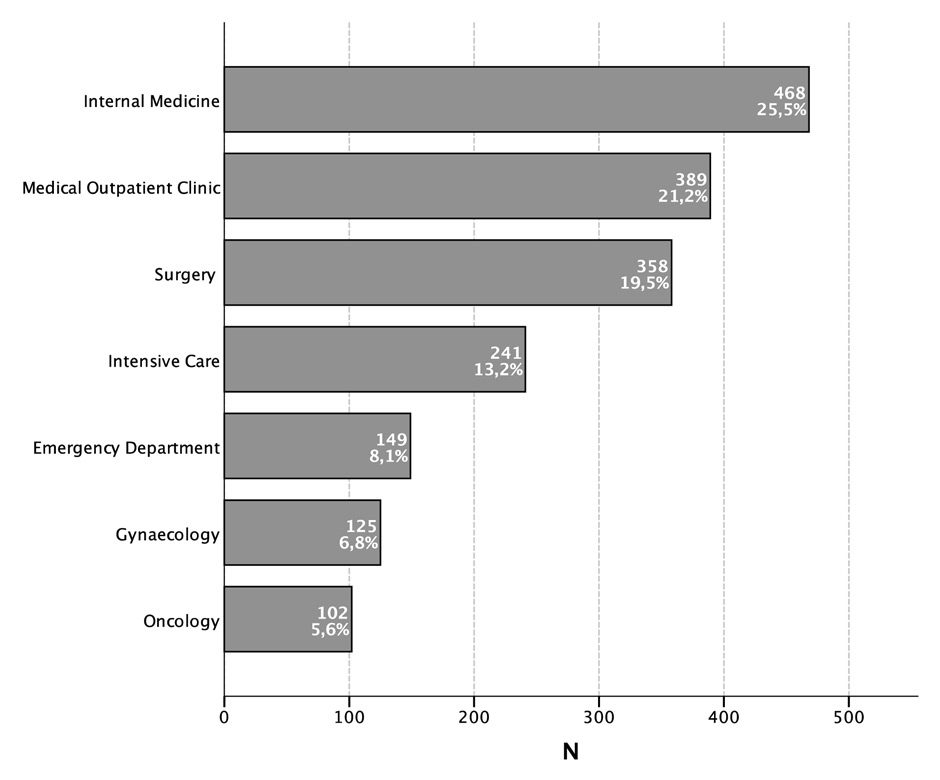
Figure 1
Distribution of all red blood cell units transfused in a period of 15 months.
DOI: https://doi.org/10.4414/smw.2015.14084
In the developed world, transfusion of blood or blood components is one of the most common medical procedures performed in hospitals. Whether to transfuse or not is a complex medical decision. The potential harm and benefit of a blood transfusion have to be considered. Substantial efforts have been made to increase the safety of red blood cell concentrates. There is a careful donor selection by questionnaires, which excludes a range of potential donors. Additionally, there is testing for a wide range of viral infections in donors, leukodepletion of red cell concentrates and sophisticated pre-transfusion testing in patients and blood products. Furthermore, the surveillance of adverse events has been tightly regulated. In Switzerland every hospital is kept under surveillance by a governmental organisation (Swissmedic) and the local Red Cross centres, which have the exclusive right to provide the blood components. These efforts have led to ever increasing prices of blood components and to the shortage of available blood products during public holidays due to the lack of suitable blood donors at these times. It has been shown that liberal transfusion strategies are associated with adverse outcomes in the setting of intensive care units [1], in children [2], hospital wards [3] and in patients with upper gastrointestinal bleeding [4]. Despite the availability of transfusion guidelines from the AABB (formerly the American Association of Blood Banks) [5], a Cochrane Review [6] and European guidelines [7, 8], the American Society of Hematology adapted as one of their contributions to the Choosing Wisely® initiative a recommendation concerning red cell blood transfusion thresholds. They refer to European and American guidelines published in 2013 to limit red cell blood transfusions to return to a safe haemoglobin level of 7–8 g/dl in stable non cardiac patients, or to limit symptoms of anaemia. Furthermore, administration of 2 RBC units should be avoided when 1 unit would be sufficient [9].
The primary aim of this observational study was to determine whether we act according to these guidelines in our hospital. Secondly, we wanted to identify differences within specific patient groups or hospital departments which require different transfusion strategies.
Between January 2012 and March 2013, all red blood cell (RBC) transfusions were analysed in a Swiss hospital providing a population of 160,000 and treating annually 10,000 inpatients and 40,000 outpatients. The surgical department provides orthopaedic, abdominal, urologic and trauma but no cardiovascular surgery. We analysed the last haemoglobin level measured before the red blood cell transfusion. The measurement of the haemoglobin concentration was performed within 24 hours before the RBC units were ordered. The transfusion was within 48 hours after ordering at the discretion of the responsible physician. Haemoglobin levels, haematocrit and blood group of patients and RBC products were recorded centrally in our laboratory. All transfusion activities were grouped into units such as the internal medicine ward, the department of surgery (ward and operating room), the emergency room, the intensive care unit, the gynaecology ward, the medical outpatient clinic and the oncology outpatient clinic.
We performed a review of the patient charts to assess co-morbidities and indications of transfusions in these patients such as gastrointestinal bleeding, procedure associated bleeding in the operating room, postpartal bleeding or transfusions due to a blood disease. Classification of co-morbidities was performed when the diagnosis was stated in the medical history of the patient. Acute coronary syndrome was defined by the medical record and with a troponin measurement twice the upper normal level. Finally, acute bleeding was present if on-going bleeding could be verified by means of endoscopy, melaena, intraoperative blood loss or with high clinical suspicion (such as a decrease in haemoglobin without evidence of haemolysis). Acute coronary syndrome was defined as elevated troponin levels within 48 hours of transfusion.
Glomerular filtration rate was estimated using the MDRD (Modification of Diet in Renal Disease [10]) or the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration [11]) method.
For subsequently performed red blood cell transfusions (e.g., haemorrhagic shock, planned transfusions for patients with haemoglobinopathies etc.), only the first haemoglobin level was registered.
Haemoglobin and haematocrit were measured using a UniCel® DxH™ 800 Coulter® Cellular Analysis System from Beckman Coulter. In emergency situations, haemoglobin determination was performed using the blood gas analyst Radiometer ABL 700®.
All blood components are provided by one blood bank. The cost for one unit of red blood cells was 212.50 CHF [12] (approximately 176 €).
Continuous data are presented as mean (±SD = standard deviation), and categorical data are presented as numbers (%). To compare continuous variables, Student’s two sided and unpaired t-test was used, whereas Chi-square was used to compare categorical variables. When calculating p-values for continuous variables distributed into the different departments, one-way analysis of variance (ANOVA) was conducted, and Tukey post-hoc testing was performed to compare the individual group means. A p-value inferior to 0.05 was considered as statistically significant for all tests. All data analysis was performed using SPSS® Statistics (version 22 for Mac, © 2011, IBM).
| Table 1: Characteristics, co-morbidities and indications for transfusions within the departments. | ||||||||
| Internal medicine | Gynaecology | Oncology | Emergency department | Surgery | Intensive care | Medical outpatient clinic | All | |
| n = 468 | n = 125 | n = 102 | n = 149 | n = 358 | n = 241 | n = 389 | n = 1,832 | |
| Characteristics | ||||||||
| Male sex | 278 (59.4%) | 1 (0.8%) | 16 (15.7%) | 69 (46.3%) | 154 (42.8%) | 149 (61.8%) | 305 (78.4%) | 972 (53.0%) |
| Age (years) | 75.7 (±12.7) | 47.1 (±19.4) | 60.0 (±7.1) | 77.5 (±13.8) | 78.7 (±11.7) | 70.7(±13.2) | 48.0 (±23.3) | 67.0 (±20.4) |
| Mean haemoglobin before transfusion (g/dl) | 7.30 (±1.0) | 7.40 (±1.6) | 7.43 (±0.7) | 7.49 (±1.4) | 7.73 (±1.0) | 7.82 (±0.9) | 7.92 (±1.1) | 7.61 (±1.1) |
| Mean haematocrit before transfusion (%) | 22.0 (±3.0) | 21.6 (±4.6) | 22.4 (±2.3) | 22.4 (±4.6) | 22.6 (±3.8) | 23.6 (±3.1) | 23.4 (±3.3) | 22.7 (±3.5) |
| Co-morbidities | ||||||||
| Coronary artery disease Acute coronary syndrome | 175 (37.4%) 32 (6.7%) | 3 (2.4%) 0 (0%) | 8 (7.8%) 0 (0%) | 43 (28.9%) 10 (6.7%) | 84 (23.5%) 13 (3.6%) | 104 (43.2%) 48 (19.9%) | 52 (13.4%) 0 (0%) | 469 (25.6%) 103 (5.6%) |
| Diabetes | 111 (23.7%) | 7 (5.6%) | 4 (3.9%) | 22 (14.8%) | 31 (8.6%) | 48 (19.9%) | 100 (25.7%) | 323 (17.6%) |
| PAOD | 44 (9.4%) | 2 (1.6%) | 0 (0%) | 11 (7.4%) | 11 (3.1%) | 32 (13.3%) | 11 (2.8%) | 111 (6.1%) |
| Cerebrovascular disease | 51 (10.9%) | 0 (0%) | 68 (66.7%) | 19 (12.8%) | 21 (5.8%) | 28 (11.6%) | 4 (1.3%) | 191 (10.4%) |
| Renal clearance (ml/min) | 60.4 (±33.9) | 96.2 (±35.2) | 109.7 (±26.2) | 64.0 (±34.6) | 69.8 (±30.6) | 73.3 (±46.5) | 99.3 (±40.2) | 77.1 (±40.1) |
| COPD | 40 (8.5%) | 1 (0.8%) | 2 (2.0%) | 1 (0.7%) | 4 (1.1%) | 17 (7.1%) | 31 (8.0%) | 96 (5.2%) |
| Malignancy | 115 (24.6%) | 27 (21.6%) | 92 (90.2%) | 32 (21.5%) | 94 (26.1%) | 40 (16.6%) | 22 (5.7%) | 422 (23.0%) |
| Indications for transfusion | ||||||||
| Upper GI-bleeding | 138 (29.5%) | 0 (0%) | 70 (68.6%) | 48 (32.2%) | 50 (13.9%) | 47 (19.5%) | 14 (3.6%) | 367 (20.0%) |
| Lower GI-bleeding | 72 (15.4%) | 0 (0%) | 0 (0%) | 28 (18.8%) | 0 (0%) | 5 (2.1%) | 8 (2.1%) | 113 (6.2%) |
| Postpartum bleeding | 0 (0%) | 52 (41.6%) | 0 (0%) | 3 (2.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 55 (3.0%) |
| Haematological disorder | 89 (19.0%) | 2 (1.6%) | 10 (9.8%) | 32 (21.5%) | 13 (3.6%) | 8 (3.3%) | 335 (86.1%) | 480 (26.2%) |
| Operation related transfusion | 30 (6.4%) | 94 (75.2%) | 2 (2.0%) | 45 (30.2%) | 286 (79.4%) | 120 (49.8%) | 0 (0%) | 577 (31.5%) |
| Acute bleeding | 123 (26.3%) | 73 (58.4%) | 0 (0%) | 59 (39.6%) | 89 (24.7%) | 59 (24.5%) | 4 (1.0%) | 407 (22.2%) |
| Continuous variables are presented as mean (±SD), categorical variables as n (%). PAOD = Peripheral artery occlusive disease; COPD = Chronic obstructive pulmonary disease; GI = gastro-intestinal. | ||||||||
In total, 53% of all patients (n = 972) were male. All transfusions but one (n = 124, 99.2%) were performed in female patients on the gynaecology ward. One transfusion was due to neonatal anaemia in a male child. One female cancer patient with chronic bleeding due to Osler’s disease was treated with nearly weekly transfusions in the department of oncology. This has led to a bias in the proportion of female patients (n = 86, 84.3%) in this group. A male predominance was found in transfusions administered in the medical outpatient department (n = 305, 78.4%).

Figure 1
Distribution of all red blood cell units transfused in a period of 15 months.
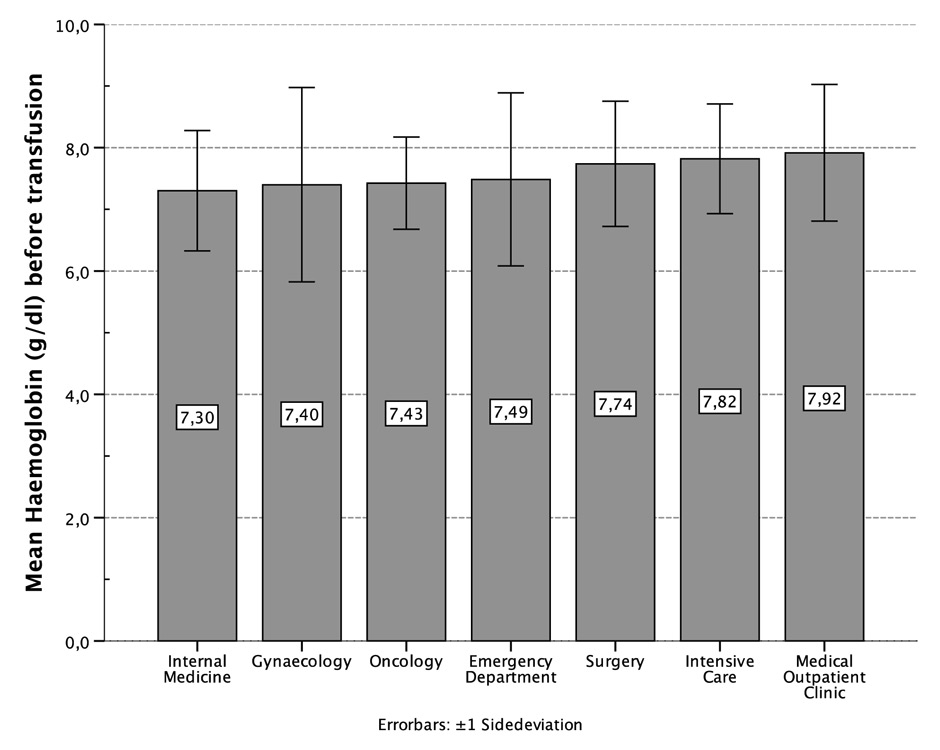
Figure 2
Mean haemoglobin (g/dl) before transfusion, distributed by departments.
The mean age in all patients was 67.0 years (±20.4). Due to transfusion activities in the maternity ward, patients in gynaecology were younger with a mean of 47.1 years (±19.4). Similarly, the mean age of patients in the outpatient medical department was 48.0 years (±23.3). The oldest patients were found in the department of surgery (78.7 years, ± 11.7).
469 transfusions (25.6%) were performed in patients with coronary artery disease (CAD). Most of these patients were treated in the intensive care unit (n = 104, 43.2%) and the internal medicine ward (n = 111, 23.7%). Acute coronary syndrome (ACS) was present in 103 transfusion activities (5.6%), with the highest proportion in the intensive care unit (n = 48, 19.9%). Similar rates were found in the emergency department (n = 10, 6.7%) and the internal medicine ward (n = 32, 6.7%). Patients with cancer received 422 transfusions (23.0%), and diabetes was present in 323 transfusion activities (17.6%). Other co-morbidities and their distribution between the departments are presented in table 1.
The predominant indications for transfusions overall were surgery related blood loss (n = 577, 31.5%), transfusions for haematological disorders (n = 480, 26.2%) and upper gastrointestinal bleeding (n = 367, 20.0%). Other indications were lower gastrointestinal bleeding (n = 113, 6.2%) and post-partum bleeding (n = 55, 3.0%). In 242 transfusions (13.1%) another bleeding site (e.g., ruptured aortic aneurysm) was present, or no clear indication could be identified retrospectively.
In total, 1,832 RBC products were transfused during the 15 month observational period. Most of them were administered in the internal medicine ward (n = 468, 25.5%), followed by the medical outpatient department (n = 389, 21.2%), the department of surgery (n = 358, 19.5%) and the intensive care unit (n = 241, 13.1%). Others were administered in the emergency department (n = 149, 8.1%), the gynaecology department (n = 125, 6.8%) and the oncology ambulatory (n = 102, 5.6%, fig. 1). In the year 2012, 29 RBC units were administered per 1,000 patients treated in our hospital. This was 8.5% less than in 2010 when 32 units per 1,000 patients were given. In 2012 in the internal medicine ward (including intensive care unit) 189 units were given per 1,000 patients, followed by 66 units in the surgical ward (including operating room), 48 units in the medical outpatient department and 41 units in the gynaecology ward.
In 1,127 units (61.7%), pre-transfusion measurement of haemoglobin was recorded. Other transfusion activities (n = 705, 38.3%) consisted of subsequent transfusions without haemoglobin measurement in the interval (e.g., haemorrhagic shock, on-going surgical blood loss, planned transfusion for haematological patients). The mean haemoglobin and haematocrit before transfusion were 7.61 g/dl (±1.1) and 22.7% (±3.5) respectively. Pre-transfusion levels of haemoglobin differed significantly between the 7 departments (p <0.001). The internal medicine ward had the lowest transfusion threshold with 7.30 g/dl (±1.0). Compared to this department, the surgery ward and operating room had higher transfusion thresholds (7.73 g/dl ± 1.0, p <0.001), similar to the intensive care unit (7.82 g/dl ± 0.9, p <0.001) and the medical outpatient clinic (7.92 g/dl ± 1.1, p <0.001). The latter had the highest transfusion threshold. When compared to the medical outpatient clinic, significantly lower thresholds were found in the gynaecology department (7.40 g/dl ± 1.6, p = 0.015), the oncology clinic (7.43 g/dl ± 0.7, p = 0.040) and the emergency room (7.49 g/dl ± 1.4, p = 0.029, fig. 2).
In general, mean haemoglobin levels before transfusion did not differ significantly between patients with CAD and patients without (7.64 g/dl ± 1.0 vs 7.59 g/dl ± 1.1, p = 0.48). However, patients treated in the emergency room (8.26 g/dl ± 1.0 vs 7.12 g/dl ± 1.4, p <0.001) as well as in the department of surgery (7.95 g/dl ± 0.9 vs 7.65 g/dl ± 1.1, p = 0.03) were transfused at a higher transfusion threshold when CAD was present. In contrast, patients without CAD who were treated in the medical outpatient department had a much higher pre-transfusion level of haemoglobin than patients with CAD (8.09 g/dl ± 1.0 vs 6.91 g/dl ± 1.0, p <0.001). In transfusions for patients with acute coronary syndrome (ACS), a tendency to a higher transfusion threshold (7.84 g/dl ± 0.7) than in other CAD patients (7.58 g/dl ± 1.0) could be found (p = 0.05).
In patients with acute bleeding, lower mean haemoglobin was present before transfusion in comparison to patients with chronic blood loss (7.43 g/dl ± 1.2 vs 7.65 g/dl ± 1.1, p = 0.01). When reviewing the different departments, this finding only remained significant in the department of gynaecology (6.84 g/dl ± 1.5 vs 7.89 g/dl ± 1.5, p = 0.007), all other departments did not show a significant difference. Patients who received a RBC transfusion during surgery had significantly higher haemoglobin levels prior to transfusion when compared to all patients who underwent surgery (8.40 g/dl ± 1.4 vs 7.77 g/dl ± 1.1, p = 0.002). Finally, patients with haematological disorders (table 2)were transfused at a higher threshold when compared to patients without (7.78 g/dl ± 1.1 vs 7.56 g/dl ± 1.1, p = 0.006).
Most patients were A positive (n = 667, 36.4%), followed by 0 positive (n = 597, 32.6%) and A negative (n = 187, 10.2%). Distributions of other blood groups are shown in table 3, and are similar to the blood group prevalence in the Swiss population [13]. One patient was excluded from this analysis due to neonatal anaemia without a known blood group. An 0 negative split product was transfused in that child. Patients with blood groups 0 negative, 0 positive, A negative and A positive most often received RBC products with identical blood groups. For all other blood groups, a high proportion of other compatible blood groups were transfused. In patients with rare blood groups such as AB positive and AB negative, identical blood groups were transfused in only 2 cases (4.4%) and 2 cases (11.1%) respectively. 0 negative RBC products were administered for patients within all blood groups, and were mostly transfused in the medical outpatient clinic (n = 72, 33%), followed by the internal medicine ward (n = 56, 25.7%), the department of surgery (n = 39, 17.9%) and the emergency department (n = 24, 11.0%).
| Table 2: Haematological disorders. | |
| Haematological disorders | n = 480 |
| Myelodysplastic syndrome | 198 (41.2%) |
| Thalassaemia | 159 (33.1%) |
| Osteomyelofibrosis* | 58 (12.2%) |
| Other** | 65 (13.5%) |
| * WHO Classification of tumours of haematopoietic and lymphoid tissues, 2008. **Such as sickle cell anaemia, acute leukaemia, lymphomas. | |
| Table 3: Distribution of blood groups (BG) within patients and RBC products. | ||||||||||
| BG of RBC product | ||||||||||
| 0 neg | 0 pos | A neg | A pos | AB neg | AB pos | B neg | B pos | |||
| 0 neg | 162 (100%) | – | – | – | – | – | – | – | 162 (8.8%) | |
| 0 pos | 11 (1.8%) | 586 (98.2%) | – | – | – | – | – | – | 597 (32.6%) | |
| BG of patient | A neg | 18 (9.6%) | – | 169 (90.4%) | – | – | – | – | – | 187 (10.2%) |
| A pos | 4 (0.6%) | 27 (4.0%) | 11 (1.6%) | 625 (93.7%) | – | – | – | – | 667 (36.4%) | |
| AB neg | 3 (16.7%) | – | 13 (72.2%) | – | 2 (11.1%) | – | – | – | 18 (1.0%) | |
| AB pos | 2 (4.4%) | 2 (4.4%) | – | 38 (84.4%) | 1 (2.2%) | 2 (4.4%) | – | – | 45 (2.5%) | |
| B neg | 13 (56.5%) | – | – | – | – | – | 10 (43.5%) | – | 23 (1.3%) | |
| B pos | 4 (3.0%) | 89 (67.4%) | – | – | – | – | – | 39 (29.5%) | 132 (7.2%) | |
| 218 (11.9%) | 704 (38.4%) | 193 (10.5%) | 663 (36.2%) | 3 (0.2%) | 2 (0.1%) | 10 (0.5%) | 39 (2.1%) | 1,831 (100%)* | ||
| * 1 Patient was excluded due to neonatal anaemia without known blood group, 0 neg split product was transfused in that case. | ||||||||||
This study investigated the current transfusion practices in a Swiss community hospital and whether restrictive transfusion strategies are applied. There is only limited high quality evidence available to produce stringent protocols to support transfusion guidelines. The evidence that a higher haemoglobin level might be associated with a worse outcome has been shown in different circumstances for instance in gastrointestinal bleeding or during ICU stay [13]. There is agreement that no transfusions should be performed when the haemoglobin is 10 g/dl or greater. Usually RBC transfusion is considered between haemoglobin levels of 7 and 8 g/dl. There is good evidence from high quality trials to consider transfusions in stable and hospitalised patients with haemoglobin levels between 7 and 8 g/dl. There is somewhat weaker evidence to transfuse below 8 g/dl in patients with chest pain, orthostatic hypotension, acute coronary syndrome or congestive heart failure [5]. There is some consideration about other targets in sepsis patents or patients after hip surgery at least in the first 6 hours of treatment [7, 14]. In ICU patients without acute coronary syndrome, after the first 6 hours, a haemoglobin level of 7 or 8 g/dl respectively, is to consider [1, 13]. Even in active bleeding such as gastrointestinal bleeding a restrictive transfusion strategy was associated with a superior outcome [4].
In all departments, the average transfusion threshold has lain between the suggested haemoglobin level of 7 to 8 g/dl which might reflect an awareness of a restrictive transfusion strategy in our hospital. This finding was somehow surprising since no clear transfusion guidelines have been implemented. The lowest haemoglobin trigger could be observed in the internal medicine ward, which might be due to a higher awareness of negative transfusion effects (e.g., volume overload). A higher threshold could be found in the intensive care unit, which might reflect the high amount of patients with ACS (19.9%) and the physicians’ fear of worsening instability in the presence of severe anaemia. As stated above, no high quality evidence is provided to guide transfusion activities in these patients to date but a restrictive strategy seems to be equivalent to a liberal strategy in terms of outcome [1, 13, 15, 16].
In the medical outpatient department, a high proportion of transfusions (86.1% of units transfused in that department) were given to patients with haematological diseases such a transfusion dependent thalassaemia, patients with myelodysplastic syndroms, sickle cell patients and rarer diseases such as myelofibrosis, which require regular transfusion throughout the year. Transfusion thresholds in these patients differ from the above mentioned. In these patients other transfusion thresholds apply due to the regular interval of their transfusions, their co-morbidities, the specific diseases or social circumstances such as work or school. So it is clear that the median haemoglobin level was the highest in this group compared with all the other departments.
To our surprise the transfusion rate already decreased of 8.5% within 2 years, with a transfusion rate dropping from 32 units per 1,000 patients treated in 2010, 30 units per 1,000 patients in 2011 to 29 per 1,000 patients in 2012. There were no other changes observed in this time period such a severity of disease or introduction of new transfusion guidelines. This observation is in accordance with an overall decline in transfusion requirements in other hospitals of this size in our country [17]. This might be due to an acceptance of a restricted transfusion strategy in these hospitals or the awareness of the ever-increasing costs for one unit of red cell concentrates. The rate of transfused units was significantly higher in the medical ward and the intensive care unit than the surgical ward including the operating room. This might be explained due to the higher amount of patients with ACS and gastrointestinal bleeding treated in the internal ward and in the intensive care unit compared to the surgical ward. This was observed despite the higher age in the patients treated by the surgeons. However, we did not count the red cell units donated by patients before elective hip or knee surgery and therefore we might underestimate the red cell concentrates used in the operating room.
No significant difference between transfusion triggers for patients with CAD or without could be found and only a tendency towards a higher transfusion threshold could be found when ACS was present. However, presence of symptoms could not be assessed retrospectively in these patients. Although present guidelines suggest RBC transfusion below a haemoglobin level of 8 g/dl [5], conclusive evidence of benefit in mortality is still lacking [13, 15]. The difference in the medical outpatient clinic in haemoglobin in patients with and without CAD is due to the small group of patients with CAD in this group (n = 52, 13.4%). Most of the patients are young and have therefore a higher haemoglobin value.
Every year we have to give a detailed report to our transfusion centre, concerning all blood products transfused in relation to the blood groups of the patients. We use all blood products in accordance with the transfusion. Since in Switzerland the transfusion services give themselves the right to set the price, we have to pay extra for all rhesus negative products. Therefore we use all the products carefully and with good consideration in order not to waste any product (as shown in table 3).
We analysed the red cell transfusion in a large community hospital in order to assess the need to implicate one of the Choosing Wisely® initiatives of the American society of Hospital Medicine. All departments in our hospital act in accordance to the current recommendations despite different patient groups treated and therefore no new guidelines need to be implemented and we can concentrate on other initiatives of the Swiss society of internal medicine in “Smarter Medicine” [18].
1 Hébert PC, Wells G, Blajchman MA, et al. A Multicenter, Randomized, Controlled Clinical Trial of Transfusion Requirements in Critical Care. N Engl J Med. 1999;340:409–17.
2 Lacroix J, Hebert PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356:1609–19.
3 Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453–62.
4 Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21.
5 Carson JL, Grossman BJ, Kleinman S, et al. Red blood cell transfusion: a clinical practice guideline from the AABB*. Ann Intern Med. 2012;157:49–58.
6 Carson JL, Carless PA, Hebert PC. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. The Cochrane database of systematic reviews 2012;4:Cd002042.
7 Retter A, Wyncoll D, Pearse R, et al. Guidelines on the management of anaemia and red cell transfusion in adult critically ill patients. Br J Haematol. 2013;160:445–64.
8 Guidelines for the clinical use of red cell transfusions. Br J Haematol. 2001;113:24–31.
9 Hicks LK, Bering H, Carson KR, et al. The ASH Choosing Wisely(R) campaign: five hematologic tests and treatments to question. Blood. 2013;122:3879–83.
10 Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70.
11 Levey AS, Stevens LA, Schmid CH, et al. A New Equation to Estimate Glomerular Filtration Rate. Ann Intern Med. 2009;150:604–12.
12 http://www.zhbsd.ch/Media/File/Preisliste_Version%202_2013_mAGB.pdf. (Accessed 19.12.2013)
13 Wu WC, Rathore SS, Wang Y, Radford MJ, Krumholz HM. Blood transfusion in elderly patients with acute myocardial infarction. N Engl J Med. 2001;345:1230–6.
14 Carson JL, Terrin ML, Noveck H, et al. Liberal or Restrictive Transfusion in High-Risk Patients after Hip Surgery. N Engl J Med. 2011;365:2453–62.
15 Carson JL, Brooks MM, Abbott JD, et al. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. Am Heart J. 2013;165:964–71 e1.
16 Corwin HL, Gettinger A, Pearl RG, et al. The CRIT Study: Anemia and blood transfusion in the critically ill – current clinical practice in the United States. Crit Care Med. 2004;32:39–52.
17 http://www.srk-zuerich.ch/srk/pdf/Wer_wir_sind/jahresbericht/2013–Jahresbericht.pdf. (Accessed 27.05.2014)
18 http://www.smartermedicine.ch . (Accessed 27.05.2014)
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article were reported.
Authors’ contribution: BS and AB contributed equally to this work