Figure 1
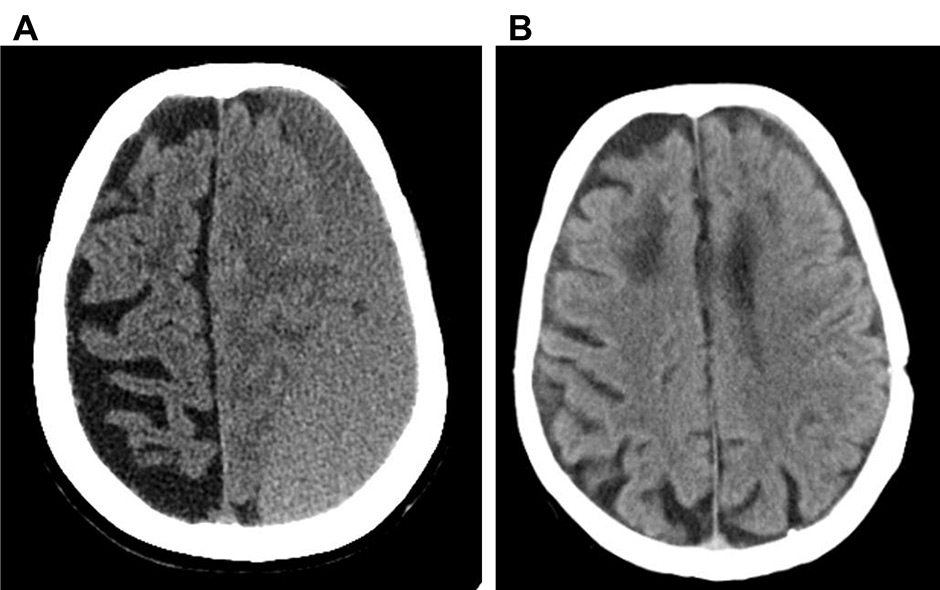
Representative CT scan image of a left fronto-temporo-parietal-occipital cSDH, before surgery (A) and after three month-follow-up during apixaban treatment (B).
DOI: https://doi.org/10.4414/smw.2015.14048
We report the case of a 79-yearold Caucasian woman with a medical history of paroxysmal-permanent non-valvular atrial fibrillation (FANV), hypertension, previous stroke and heart failure, who developed, during anticoagulant treatment with warfarin, a chronic subdural haematoma (cSDH) of the left cerebral hemisphere. She was admitted to another hospital where she underwent a craniotomy for evacuation of the cSDH. At discharge, the low-molecular-weight heparin (LMWH) enoxaparin was prescribed owing to recent neurosurgical intervention and to balance the high thromboembolic risk (CHADS2VASC score 8) with the risk of bleeding (HASBLED 4). This therapy was discontinued thirty days later because of the onset of an allergic skin reaction. Considering the safety profile of apixaban (2.5 mg b.i.d) [1, 2] in patients at high risk of bleeding and of thromboembolic events (creatinine >1.5 mg/dl, age >80 years old, weight <60 kg), the patient was treated with this drug 12 hours after the last enoxaparin dose. Other LMWH or fondaparinux were previously tested but not tolerated. No major bleeding complications or other side effects were observed. The patient was admitted to our department thirty days later because of heart failure due to atrial fibrillation with rapid ventricular response (120 b.p.m). During the hospitalisation, the patient also presented a prolonged QTc interval in association with serious hypomagnesaemia and hypokalaemia that were promptly corrected. Trans-oesophageal echocardiography (EET) revealed no left auricular thrombosis. a CT scan was repeated and did not show recurrence of cSDH (fig. 1). Electrical cardioversion was then performed obtaining the restoration of normal sinus rhythm, and treatment with apixaban was continued. During follow-up, the patient presented recurrences of atrial fibrillation leading to acute heart failure, which required new hospitalisation. Owing to the difficult heart rate control despite therapy with beta blockers and digitalis (amiodarone was not used because of prolonged QTc and electrolyte disorders, and verapamil was not tolerated), and owing to the persistence of symptoms and signs of heart failure (Class NYHA II–III at discharge), a cardiac ablation and pacemaker implantation strategy was adopted. Apixaban was interrupted 48 hours before the intervention and it was resumed within 12 hours after surgery, without bridging with LMWH due to the previous adverse reaction. The patient was discharged three days after the procedure. For a total follow-up period of 210 days (seven months after cSDH and three months after implantation), no thromboembolic or bleeding complications occured.

Figure 1
Representative CT scan image of a left fronto-temporo-parietal-occipital cSDH, before surgery (A) and after three month-follow-up during apixaban treatment (B).
Apixaban is a direct and competitive inhibitor of factor Xa with fast onset of action, short offset of action, rapid absorption and no food interaction. In patients with non-valvular atrial fibrillation and at least one additional risk factor for stroke, apixaban (5 mg b.i.d) significantly reduced the risk of stroke and systemic embolism, major bleeding, intracranial bleeding and death as compared with warfarin [1]. Despite a study population totally different from the present case, the same apixaban regimen was shown to reduce the risk of stroke without any difference in the rates of major bleeding as compared with low dose aspirin [2]. Patients maintained on oral anticoagulant (OAC) therapy are at increased risk for cSDH, although the mechanism for this increased risk is incompletely understood. After cSDH surgery physicians have a relevant dilemma in the management of OAC because of either the increased risk of thromboembolic complications (due to a prolonged discontinuation) or of the bleeding risk (due to early reintroduction) [3]. Generally patients such as the one described in our case report are excluded from clinical trials to determine more complex clinical management.
Considering the safety profile of apixaban 2.5 mg b.i.d in patients with a high risk of bleeding [1, 2], we prescribed this dosage to our patient in order to reduce bleeding risk and to prevent thromboembolic events. Usually, adequate anticoagulation therapy with warfarin for three weeks before cardioversion is recommended [4]. In a post-hoc retrospective subgroup analysis from the ARISTOTLE trial, it was shown that stroke or systemic embolism rates after electrical cardioversion were comparable with apixaban and warfarin. In this trial, a total of 743 cardioversions were performed in 540 patients: Importantly, 75% of the cardioversions occurred in one year [5]. However, this study presented some important limitations, such as the number of patients; it was underpowered to draw meaningful conclusions [5]. In our case electrical cardioversion was performed earlier, after only 30 days of apixaban pre-treatment, so we might speculate as a hypothesis requiring additional pathophysiological argumentation, that the more rapid use of effective anticoagulants may shorten the pre-treatment time before cardioversion. More data are necessary to test apixaban safety and efficacy in this setting. It is known that 12%–45% of patients undergoing device implantation are on OAC [6] and perioperative management of these subjects may be considered a common clinical problem. Interrupting OAC might promote thromboembolic events and maintenance of therapeutic OAC may increase the risk of bleeding. Although uninterrupted OAC therapy with warfarin appears to be safe, recent reports have also shown that heparin bridging therapy is often associated with bleeding events [6, 7]. Therefore, there is an interest in taking advantage of the newer and shorter-acting oral anticoagulants around the time of catheter ablation for AF and devices implantation. To our knowledge there are no available data about the peri-interventional use of FXa inhibitors, such as apixaban [8].
In conclusion, our report over the observation period of 210 days showed that apixaban (2.5 mg b.i.d) might be safe and effective in the context of a recent cSDH, electric cardioversion of FANV, cardiac ablation and pacemaker implantation procedures. Additional studies, including case series, are needed to support any findings from this case report.
1 Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al.; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–92.
2 Connolly SJ1, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, et al.; AVERROES Steering Committee and Investigators. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364(9):806–17.
3 Ducruet AF, Grobelny BT, Zacharia BE, Hickman ZL, DeRosa PL, Anderson K, et al. The surgical management of chronic subdural hematoma. Neurosurg Rev. 2012;35(2):155–69.
4 European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery, Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, et al.; ESC Committee for Practice Guidelines. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace. 2010;12(10):1360–420.
5 Flaker G, Lopes RD, Al-Khatib SM, Hermosillo AG, Hohnloser SH, Tinga B, et al.; ARISTOTLE Committees and Investigators. Efficacy and safety of apixaban in patients after cardioversion for atrial fibrillation: insights from the ARISTOTLE Trial (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation). J Am Coll Cardiol. 2014;63(11):1082–7.
6 Marquie C, De Geeter G, Klug D, Kouakam C, Brigadeau F, Jabourek O, et al. Post-operative use of heparin increases morbidity of pacemaker implantation. Europace. 2006;8(4):283–7.
7 Airaksinen KE, Korkeila P, Lund J, Ylitalo A, Karjalainen P, Virtanen V, et al. Safety of pacemaker and implantable cardioverter-defibrillator implantation during uninterrupted warfarin treatment – the FinPAC study. Int J Cardiol. 2013;168(4):3679–82.
8 Bhave PD, Knight BP. Optimal strategies including use of newer anticoagulants for prevention of stroke and bleeding complications before, during, and after catheter ablation of atrial fibrillation and atrial flutter. Curr Treat Options Cardiovasc Med. 2013;15(4):450–66.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.