Subclinical hypothyroidism: summary of evidence in 2014
DOI: https://doi.org/10.4414/smw.2014.14058
Christine
Baumgartner, Manuel Raphael
Blum, Nicolas
Rodondi
Summary
Subclinical hypothyroidism, which is defined as elevated thyroid-stimulating hormone (TSH) levels with free thyroxine concentrations within the reference range, is a common disorder that increases with age and affects up to 18% of the elderly, with a higher prevalence in women compared to men. Prospective data have shown an increased risk of coronary heart disease events, heart failure, and cardiovascular mortality among affected adults. Conflicting results have been found on the association between subclinical hypothyroidism and cognitive impairment, depression and the risk of fractures. Management strategies including screening and treatment of subclinical hypothyroidism are still controversial, while the ongoing European randomised controlled trial “TRUST” targets to solve these uncertainties. This narrative review aims to assess current evidence on the clinical aspects, as well as screening and treatment recommendations in adults with subclinical hypothyroidism.
List of abbreviations
CHD coronary heart disease
CI confidence interval
fT4 free thyroxine
GP general practitioner
HR hazard ratio
NYHA New York Heart Association
RCT randomised controlled trial
TSH thyroid-stimulating hormone
Subclinical hypothyroidism
Subclinical hypothyroidism is biochemically defined as elevated levels of thyroid-stimulating hormone (TSH) with normal levels of free thyroxine (fT4) [1], while controversies exist on the limits of the TSH reference range [2]. The prevalence of subclinical hypothyroidism is large and ranges between 3% and 18% in the adult population, with women, elderly persons, and iodine sufficient populations being affected more often [3]. An international survey has demonstrated that 94% of the general practitioners (GPs) have diagnosed subclinical hypothyroidism in a patient during the past year [4, 5]. Despite the large prevalence, evidence on screening and the benefits and risks of treatment is still controversial [2].
The most common cause of subclinical hypothyroidism is chronic autoimmune thyroiditis associated with antithyroid peroxidase antibodies (Hashimoto’s thyroiditis) [6]. Individuals with subclinical hypothyroidism are often asymptomatic, but clinical manifestations can include non-specific complaints or symptoms similar to those seen in overt hypothyroidism, such as fatigue, weakness, weight gain, cold intolerance, and constipation [6].
Adverse clinical effects of overt thyroid disorders are well known, and given the multiple actions of thyroid hormones on the heart, the vessels, bones and brain, long-term adverse outcomes could be suspected even in subclinical dysfunction. Table 1 provides an overview of the level of evidence on the association between subclinical hypothyroidism and various clinical conditions.
With the aim to assess current evidence on the clinical aspects of subclinical hypothyroidism as well as the risks and benefits of its screening and treatment, we have performed a narrative review based on already conducted systematic reviews [7, 8] and other recently published non-systematic reviews [6, 9], with additional information retrieved from systematic reviews on risks of subclinical hypothyroidism [10, 11]. The evidence has been updated by a Pubmed search on the risks and treatment of subclinical hypothyroidism of most recent articles published until 2014.
|
Table 1:Level of evidence in 2014 on the risks of treatment and the benefits of subclinical hypothyroidism. |
|
Clinical condition
|
Strength of association
|
Benefits of treatment
|
| |
TSH 4.5–9.9 mIU/l
|
TSH ≥10 mIU/l
|
|
| Progression to overt hypothyroidism |
Good |
Stronger |
Effective, especially if TSH ≥10 mU/l |
| Elevation in serum total cholesterol and LDL |
Fair |
Stronger |
Insufficient |
| Risk of coronary heart disease |
Insufficient |
Stronger |
No evidence |
| Risk of congestive heart failure |
Insufficient |
Stronger |
No evidence |
| Cardiac dysfunction |
Insufficient |
Insufficient |
Insufficient |
| Systemic symptoms of hypothyroidism |
Insufficient |
Insufficient |
Insufficient |
| Neuropsychiatric symptoms (e.g., depression, cognitive dysfunction) |
Insufficient |
Insufficient |
Insufficient |
| Muscle strength |
Insufficient |
Insufficient |
Insufficient |
| Fatigue |
Insufficient |
Insufficient |
Insufficient |
|
|
|
Risks of treatment
|
| Development of subclinical hyperthyroidism |
|
|
14%–21% |
| Adapted according to [59]
LDL = low-density lipoprotein cholesterol; TSH = thyroid-stimulating hormone |
Natural course and progression to overt hyperthyroidism
Individuals with subclinical hypothyroidism are at risk for progression to overt thyroid dysfunction with an average yearly progression rate of 2% to 6% and an increased risk in females, individuals with higher levels of TSH, and in the presence of antithyroid peroxidase antibodies, although those without antithyroid peroxidase antibodies have also a higher risk of progression [12]. In contrast, TSH levels normalise in 15% to 65% of those with a single elevated TSH without treatment, over follow-up periods going from 1 to 6 years, and the likelihood of spontaneous recovery is higher with TSH levels <10 mIU/l [13].
TSH levels vary throughout the day and are highest at night, and considerable variations within the same person over months can occur that can be accentuated by sleep deprivation or exercise [14]. Serum TSH is known to increase with age, while this increase might not be associated with mortality [15] and might even be associated with longevity as suggested by the Leiden 85+ Study that prospectively followed 599 individuals from age 85 years through age 89 years [16]. Other reasons for persistent or transient TSH elevation with normal fT4 levels in the absence of thyroid pathologies include the recovery phase after major illness (euthyroid sick syndrome), obesity, adrenal insufficiency, TSH-assay problems (i.e., presence of heterophilic antibodies), medications (i.e., amiodarone, sertraline, lithium, tyrosine-kinase inhibitors, etc.), chronic renal failure, central hypothyroidism, or in rare cases mutations leading to the inactivation of the TSH receptor [9].
Based on the population mean and the finding in the Wickham survey that already TSH levels above 2 mIU/l have an increased risk of developing hypothyroidism in the future [12], the upper limit of the TSH reference range is still under debate [14]. The fact that millions of people would be identified with subclinical hypothyroidism especially in the light of raising TSH levels over the lifetime and given the lack of evidence on adverse outcomes and therapeutic consequences in individuals with high normal TSH levels speak against narrowing the TSH reference level [14].
Cardiovascular risk and heart failure
Thyroid hormones are well known to act on the heart and vasculature, and the impact of subclinical thyroid dysfunction on the cardiovascular system has been an important topic of research in recent years. Subclinical hypothyroidism can lead to impaired systolic and diastolic cardiac function [17] as well as vascular dysfunction with increased vascular stiffness and endothelial dysfunction [18]. A pooled analysis of individual participant data has found an increase in heart failure events in individuals with a TSH of 10 mIU/l and higher compared to euthyroid controls with a hazard ratio (HR) 1.86 (95% confidence interval [CI] 1.27–2.72) [11] (table 2). Subclinical hypothyroidism has also been associated with an increased risk of fatal and non-fatal coronary heart disease (CHD) events [10]. An individual participant data analysis including more than 55,000 participants showed an age and sex-adjusted HR of 1.89 (95% CI 1.28–2.80) for CHD events in persons with TSH levels above 10 mIU/l [10] compared with euthyroid individuals, and a corresponding HR of 1.58 (95% CI 1.10–2.27) for CHD mortality (table 2). Risks were not increased for participants with TSH levels <7 mIU/l, and there was a significant trend for the risk of CHD events and mortality at higher TSH levels. Mortality from any cause was not increased. In line with the results from the individual participant data analysis, another study that had been published later did not show an association between subclinical hypothyroidism and overall death in the oldest old [15]. In contrast, the Leiden 85+ Study showed a reduced risk of cardiovascular and overall mortality in subclinically hypothyroid individuals aged 85years [16]. These findings might suggest an interaction of age on the effects of thyroid hormones, which could not be demonstrated in the individual participant data analysis that assessed outcomes in people of all ages [10].
The increased cardiovascular risk that is primarily observed with TSH levels of 10 mIU/l and above can be explained by several mechanisms. TSH has known effects on the endocrine system, and studies have shown elevated total cholesterol and a higher prevalence of dyslipidaemia in individuals with subclinical hypothyroidism [19]. A systematic review including 13 heterogeneous studies concluded that thyroxine treatment leads to a reduction in serum total cholesterol and LDL cholesterol in persons with subclinical hypothyroidism [20]; this finding has been confirmed in subsequent randomised controlled trials (RCTs) [18]. Evidence on the association of subclinical hypothyroidism with increased blood pressure is controversial. In a cross-sectional study, Liu et al. found an increased blood pressure in these individuals [21], while this finding could not be confirmed in other studies [22]. An interventional study including 56 women with subclinical hypothyroidism found an elevated systolic and diastolic blood pressure, serum cholesterol and homocystein levels compared to healthy controls, with normalisation of these factors after 18 months of levothyroxine therapy [23]. One prospective cohort study found a higher prevalence but not incidence of metabolic syndrome in adults with subclinical hypothyroidism [24], while another large cohort study could not find a difference in weight change in these individuals [25]. Other possible explanations for the increased cardiovascular risk in persons with subclinical hypothyroidism include increased carotid intima-media thickness [19], hypercoagulability, insulin resistance, oxidative stress [6], and endothelial dysfunction [18]. A reduction in carotid intima-media thickness [19] and improvement of brachial artery endothelial function following thyroxine replacement in individuals with subclinical hypothyroidism has been shown [18].
In a small RCT, normalisation of TSH levels by thyroxine replacement therapy led to an improvement in cardiac function [17], and recent findings based on retrospective administrative data suggested that thyroxine treatment leads to a reduction in ischaemic heart disease in younger individuals, but not in persons aged 70 years or older [26]. Adequately powered RCTs examining a potential reduction of cardiovascular events through restoration of euthyroidism are lacking.
Thyroid hormones have known effects on heart rate and cardiac excitability. In contrast to the increased risk of atrial fibrillation in patients with excess thyroid hormones [27], evidence on this association in individuals with subclinical hypothyroidism is conflicting, while recent observational data have suggested a protective effect of subclinical hypothyroidism on the development of atrial fibrillation [28].
|
Table 2:Risk for Coronary Heart Disease (CHD) mortality and events and heart failure events according to thyroid-stimulating hormone levels. |
|
TSH level
|
HRs for CHD mortality (95% CI)
|
HRs for CHD events (95% CI)
|
HRs for heart failure events (95% CI)
|
| TSH 10.0–19.9 mIU/l |
1.58 (1.10–2.27) |
1.89 (1.28–2.80) |
1.86 (1.27–2.72) |
| TSH 7.0–9.9 mIU/l |
1.42 (1.03–1.95) |
1.17 (0.96–1.43) |
1.65 (0.84–3.23) |
| TSH 4.5‒6.9 mIU/l |
1.09 (0.91–1.30) |
1.00 (0.86–1.18) |
1.01 (0.81–1.26) |
| TSH 0.45–4.49 mIU/l* |
1.00 (reference) |
1.00 (reference) |
1.00 (reference) |
| TSH 0.10–0.44 mIU/l |
1.24 (0.96–1.61) |
1.27 (1.03–1.58) |
1.31 (0.88–1.95) |
| TSH <0.10 mIU/l |
1.84 (1.12–3.00) |
1.08 (0.69–1.69) |
1.94 (1.01–3.72) |
| Adapted according to [10, 11, 27].
CHD = coronary heart disease; CI = confidence interval; HR = hazard ratio (age and gender-adjusted); TSH = thyroid-stimulating hormone.* TSH 0.5–4.49 mIU/l for association between subclinical hypothyroidism and CHD mortality and CHD events. |
Musculoskeletal system and functional capacity
Persons with subclinical hypothyroidism more often suffer from weakness and myalgia [29], and reduced muscle strength has been shown in these individuals [30]. Confirming this hypothesis, beneficial effects of levothyroxine replacement on strength measurements [30] and cardiopulmonary exercise performance [31] have been demonstrated. A possible mechanism for the lower exercise capacity could be higher oxygen requirements during exercise in people with subclinical hypothyroidism [31] as well as a possible association with anaemia [32]. On the other hand, large cohort studies did not find a reduction in functional mobility or functional capacity assessed in elderly individuals with subclinical hypothyroidism [33, 34], and persons with only mild elevations of TSH even revealed a slightly better mobility than euthyroid controls [33].
High TSH levels as seen in hypothyroidism have been shown to directly affect bone metabolism through inhibition of osteoclast formation and survival, osteoblast differentiation and expression of collagen type 1 [35]. For subclinical hypothyroidism, an increased risk for hip fractures in men (multivariable-adjusted HR 2.31 [95% CI 1.25–4.27]) but not in women was demonstrated in a prospective cohort study including 3,567 participants with a median follow-up of 13 years [36]. In contrast, another prospective study of 25,205 individuals from Norway did not show a significant association with hip or forearm fractures when the results were adjusted for age, BMI and smoking status except in a subgroup of women with TSH >4.0 mIU/l and negative antithyroid peroxidase antibodies, where the HR for hip fracture was 1.75 (95% CI 1.24–2.46) [37].
Neuropsychiatric symptoms
An association between subclinical hypothyroidism and mood disorders including depression and increased anxiety [38], as well as a reduced quality of life [18] have been suggested, whereas other studies did not confirm these findings [39]. Treatment failure for depression has been more commonly observed in patients with subclinical hypothyroidism [40]. Evidence of the association between cognitive dysfunction and subclinical hypothyroidism is conflicting [16, 41], and the most recent population-based cross-sectional study examining 2,050 participants including 141 individuals with subclinical hypothyroidism did not show an association with mild cognitive impairment, which represents the earliest detectable clinical stage of cognitive impairment [42]. In the currently largest RCT evaluating the impact of thyroxine replacement on cognitive function with inclusion of 94 elderly participants with subclinical hypothyroidism, treatment did not lead to an improvement of cognitive function after a follow-up of 12 months [43].
Other clinical implications
The influence of overt hypothyroidism on gastrointestinal mobility with symptoms such as constipation are well known. A recent study demonstrated impaired gastric motility and consecutive symptoms in premenopausal women with subclinical hypothyroidism [44]. Given the suggested associations between subclinical hypothyroidism and the metabolic syndrome, Chung et al. performed a cross-sectional study and found a dose-dependent relation between TSH levels and non-alcoholic fatty liver diseases in individuals with subclinical and overt hypothyroidism [45].
To date, there is no strong evidence on the association between subclinical hypothyroidism and anaemia: persons with subclinical hypothyroidism had higher mean haemoglobin levels compared to their euthyroid counterparts in one cohort study, but this finding was not significant [46]. However, two RCTs have concluded that the addition of levothyroxine in subclinically hypothyroid patients with iron-deficiency anaemia lead to a more pronounced increase in haemoglobin and ferritin than treatment with iron salt alone [32, 47], whereas the restoration of euthyroidism has not led to a change in haemoglobin in another RCT including 66 women with subclinical hypothyroidism [48].
Subclinical hypothyroidism and pregnancy
For pregnant women, lower trimester-specific TSH reference ranges should be used due to the changes in thyroid physiology during pregnancy. Thyroid hormones are crucial for the normal foetal maturation and brain development, and the foetus relies on placental passage of maternal thyroid hormones during the first trimester of pregnancy due to the immaturity of the foetal thyroid gland and the consecutive inability to produce sufficient thyroid hormones. Foetal consequences of maternal overt hypothyroidism including perinatal morbidity and mortality and neurological impairment are widely known, and subclinical hypothyroidism has also been associated with adverse outcomes during pregnancy. Subclinical hypothyroidism affects 0.5% to 2.5% of women during reproductive age and can lead to higher rates of placental abruption [49], pregnancy loss [50], gestational hypertension, and severe preeclampsia [51]. An increased risk of preterm delivery [49] has been found in subclinically hypothyroid women and might explain some of the neonatal complications seen in neonates born to those mothers, including neonatal respiratory distress and an increased risk of neonatal death, with however conflicting data among different studies [49, 50]. Whether maternal subclinical hypothyroidism leads to impaired cognitive development in children is uncertain [52].
|
Table 3: Recommendations for screening of subclinical hypothyroidism in asymptomatic adults. |
|
Organisation
|
Screening recommendation
|
| American Thyroid Association, American Association of Clinical Endocrinologists and The Endocrine Society |
Routine examination in all adults, including pregnant women or women wishing to become pregnant, especially if symptoms/signs compatible with thyroid dysfunction |
| College of American Pathologists |
Women ≥50 years consulting a physician, all geriatric patients admitted to a hospital and at least every five years |
| American Academy of Family Physicians |
Patients ≥60 years |
| American College of Obstetrics and Gynecology |
High risk patients (autoimmune disorder, family history for thyroid disease) |
| American College of Physicians |
Women >50 years with recent occurrence of symptoms compatible with thyroid disorder |
| Royal College of Physicians |
No screening indicated |
| U.S. Preventive Services Task Force |
Insufficient evidence for or against screening |
| Swiss Society of Endocrinology and Diabetes [55] |
Women, age ≥40 years, unspecific complaints, geriatric patients, patients at high risk: after therapy of overt hyperthyroidism, combined autoimmune syndrome, smokers |
| Adapted according to [59] |
Recommendations for screening and treatment
Due to the lack of large-scale RCTs examining relevant clinical outcomes, current screening and treatment recommendations are principally based on observational data, small clinical trials with short follow-up durations and expert opinions. Screening recommendations vary widely across different medical societies and expert groups (table 3) [6]. Overall, screening of the general population cannot be recommended and should likely be restricted to high risk individuals including patients with autoimmune disorders, a personal or family history of thyroid disease, or those with potential symptoms (table 3). Also in asymptomatic pregnant women evidence for universal screening is equivocal, and most professional societies recommend a targeted screening strategy, e.g., in women coming from iodine insufficient areas or in those with morbid obesity, diabetes type 1, a family or personal history of thyroid disease or a history of abortion or preterm delivery [53]. Because of the lack of large RCTs addressing thyroid screening, the exact group that would benefit from screening remains unclear.
The central question in medical care of patients with subclinical hypothyroidism is whether these patients should be treated or not. Several interventional placebo-controlled trials have examined the effect of levothyroxine replacement in individuals with subclinical hypothyroidism with assessment of various outcomes, such as surrogate markers of cardiovascular risk (carotid intima-media thickness, serum lipid levels, body mass index) [19], cardiac function [17], mood disorders, symptoms [54], and cognition [43], with major limitations including small sample sizes (up to 110 participants) and short follow-up durations. A Cochrane Systematic Review published in 2007 concluded that evidence suggests a beneficial effect of thyroid hormone replacement on surrogate markers for cardiovascular risk, such as improved serum cholesterol levels and cardiac function, but the impact on clinical outcomes could not be assessed given the current data. Therefore, the authors concluded that no clear recommendation concerning efficacy and safety of thyroid hormone replacement in individuals with subclinical hypothyroidism could be stated and that the decision whether or not to treat should be individualised [7]. Table 1 summarises the evidence on treatment benefits in various clinical conditions.
Most experts and societies suggest treatment of subclinical hypothyroidism if TSH levels are >10 mIU/l based on the available evidence [2], even though long-time risks and benefits of treatment in this population are not known. For persons with moderately elevated TSH concentrations between 4.5–10 mIU/l, TSH levels should be monitored every 6 to 12 months [2] and treatment outside of a clinical trial is currently not recommended [1]. Current guidelines from the Swiss Society of Endocrinology and Diabetes recommend treatment of individuals with subclinical hypothyroidism according to a risk stratification taking into account a TSH level >10 mIU/l, the presence of goitre, antithyroid antibodies, cardiovascular risk factors or prevalent CHD, smoking, dyslipidaemia, clinical symptoms, ovulatory dysfunction or infertility, and pregnancy [55]. In pregnant women with TSH levels above the trimester-specific TSH cutoffs and in women with infertility or wishing to become pregnant with TSH values of 2.5 mIU/l or higher, initiation of thyroxine replacement therapy is recommended [2, 53]. All these recommendations are based on expert opinion in the absence of randomised clinical trials on the treatment effects of subclinical hypothyroidism on clinical outcomes. The Swiss guidelines recommend an initial treatment dose of 25 ug daily in persons aged above 50 years or with known CHD; in all other individuals, levothyroxine should be dosed at 50 to 75 µg daily at the beginning of treatment with the goal of reducing the TSH level into the reference range [55]. The European Thyroid Association recommends similar thyroxine starting doses, with 25–50 ug daily in elderly individuals or those with cardiac disease, and weight-adjusted starting doses of approximately 1.5 µg/kg daily in all other persons [56]. Before treatment is initiated, a follow-up blood test should be ordered after three to six months of the initial diagnosis given the high rate of spontaneous normalisation of elevated TSH levels (see differential diagnoses of TSH elevations above in chapter “Natural course and progression to overt hyperthyroidism”). Risks of treatment have been mainly associated with overtreatment, which is reported in 14% to 21% of subclinically hypothyroid individuals on thyroxine replacement therapy [3]. Possible adverse effects from thyroxine include atrial fibrillation, angina pectoris, congestive heart failure, and symptoms associated with excess thyroid hormone such as nervousness and palpitations [1]. Overtreatment can result in a decrease of bone mineral density and an increase in fracture risk [57].
Despite this lack of evidence, levothyroxine is a widely prescribed drug, and prescription rates are rising. Management strategies regarding the initiation and dosing of thyroxine substitution differ significantly between different countries with treatment initiation rates varying from 35% in The Netherlands to 75% in Germany [4]. 54% of the Swiss GPs reported to start a thyroxine replacement therapy in patients with subclinical hypothyroidism [4]. These variations in treatment strategies and the existing controversy in guidelines concerning screening and treatment in individuals with subclinical hypothyroidism reflect the current uncertainty. Several experts have called for a large RCT to end this uncertainty and controversy about management strategies of these persons [1, 2, 7].
Conclusion and perspectives: the TRUST trial
With the aim to resolve these uncertainties regarding the risks and benefits of treatment on relevant clinical outcomes in elderly persons with subclinical hypothyroidism, the first adequately powered RCT has been initiated [58]. The TRUST Study (Thyroid hormone Replacement for Untreated older adults with Subclinical hypothyroidism: a randomised placebo-controlled Trial) is a European multicentre study that is conducted in five centres in four European countries including Switzerland, Scotland, Ireland and The Netherlands, and examines thyroid hormone replacement therapy versus placebo in elderly individuals with persisting subclinical hypothyroidism (www.trustthyroidtrial.com). A total of 3,000 participants will be enrolled in all sites. Participating centres in Switzerland include the Inselspital Bern and the University Hospital of Lausanne that are both supported by the local institutes of family medicine, BIHAM (Berner Institut für Hausarztmedizin) and IUMG (Institut universitaire de médecine générale) in Lausanne, with a large number of participants recruited in general practices (fig. 1). A broad population of community-dwelling individuals aged 65 years or older with persistent subclinical hypothyroidism, defined as a TSH level 4.6 to 19.9 mIU/l measured on two or more occasions at least three months apart with normal fT4 levels, are included in the study to ensure a good generalisability of the study results and allow direct conclusions for clinical decisions that need to be taken by GPs. To meet this aim, only few exclusion criteria have been defined: these include patients on thyroid-altering medication, recent thyroid surgery or radio-iodine therapy within the last year, grade IV NYHA heart failure, recent hospitalisation for major illness to exclude patients with euthyroid sick syndrome, a prior clinical diagnosis of dementia, and acute coronary syndrome within the last four weeks. The participants are randomised to either placebo or levothyroxine with a starting dose of 50 µg daily (25 µg in patients with a weight <50 kg or a history of CHD) and with subsequent dose adjustments according to the serum TSH level. Participants are followed for one to four years to reach the target number of cardiovascular outcomes, and a number of relevant multimodal clinical outcomes are assessed: primary outcomes include fatal and non-fatal cardiovascular events, and disease-specific quality of life, secondary outcomes are muscle strength, blood pressure, cognitive function, functional capacity, general quality of life, and mortality.
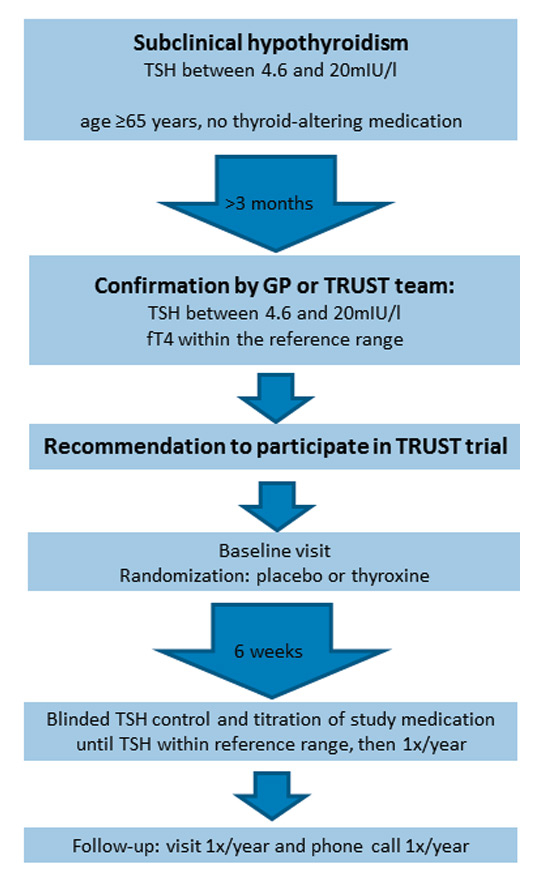
Figure 1
TRUST trial. Process for inclusion in the TRUST trial: Confirmation of subclinical hypothyroidism and patient visits during the course of the trial. See chapter “Conclusion and perspectives: The TRUST trial” for more details. Figure: adapted according to [59].
fT4 = free thyroxine; GP = general practitioner; TSH = thyroid-stimulating hormone
Due to the current lack of evidence regarding the optimal treatment strategy in individuals with subclinical hypothyroidism, to date the best management of such persons is the inclusion in ongoing trials. For persons who cannot be included in the trial, we do not recommend initiating treatment in individuals with TSH levels <10 mIU/l, but to follow serum TSH values [2]. If TSH levels exceed 10 mIU/l, levothyroxine substitution might be considered, but even in these persons, there is no direct evidence from RCTs justifying treatment [8].
References
1 Helfand M. Screening for subclinical thyroid dysfunction in nonpregnant adults: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2004;140(2):128–41. Epub 2004/01/22. PubMed PMID: 14734337.
2 Surks MI, Ortiz E, Daniels GH, Sawin CT, Col NF, Cobin RH, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA: the journal of the American Medical Association. 2004;291(2):228–38. Epub 2004/01/15. doi: 10.1001/jama.291.2.228. PubMed PMID: 14722150.
3 Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160(4):526–34. Epub 2000/03/01. PubMed PMID: 10695693.
4 Virgini V, Baumgartner C, Bischoff T, Haller DM, Frey P, Rosemann T, et al. Comment les médecins de famille prennent-ils en charge l’hypthyroïdie infraclinique? Revue medicale suisse. 2014;10(420):526–9. Epub 2014/04/08. PubMed PMID: 24701670.
5 den Elzen WP, Lefèbre-van de Fliert AA, Virgini V, Frey P, Mooijaart SP, Kearney P, et al. International variation in GP treatment strategies for subclinical hypothyroidism in older adults. Br J Gen Pract. In press.
6 Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29(1):76–131. Epub 2007/11/10. doi: 10.1210/er.2006–0043. PubMed PMID: 17991805.
7 Villar HC, Saconato H, Valente O, Atallah AN. Thyroid hormone replacement for subclinical hypothyroidism. The Cochrane database of systematic reviews. 2007(3):CD003419. Epub 2007/07/20. doi: 10.1002/14651858.CD003419.pub2. PubMed PMID: 17636722.
8 Rugge B, Balshem H, Sehgal R, Relevo R, Gorman P, Helfand M. Screening and treatment of subclinical hypothyroidism or hyperthyroidism. Comparative Effectiveness Reviews, No.24. Rockville (MD): Agency for Healthcare Research and Quality (US). 2011. http://effectivehealthcare.ahrq.gov/ehc/products/129/750/Hypo-Hyper-Thyroid_CER24_20111114.pdf http://effectivehealthcare.ahrq.gov/ehc/products/129/750/Hypo-Hyper-Thyroid_CER24_20111114.pd .
9 Cooper DS, Biondi B. Subclinical thyroid disease. Lancet. 2012;379(9821):1142–54.
10 Rodondi N, den Elzen WP, Bauer DC, Cappola AR, Razvi S, Walsh JP, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA: the journal of the American Medical Association. 2010;304(12):1365–74. Epub 2010/09/23. doi: 10.1001/jama.2010.1361. PubMed PMID: 20858880; PubMed Central PMCID: PMC3923470.
11 Gencer B, Collet TH, Virgini V, Bauer DC, Gussekloo J, Cappola AR, et al. Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation. 2012;126(9):1040–9. Epub 2012/07/24. doi: 10.1161/CIRCULATIONAHA.112.096024. PubMed PMID: 22821943; PubMed Central PMCID: PMC3884576.
12 Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clinical endocrinology. 1995;43(1):55–68. Epub 1995/07/01. PubMed PMID: 7641412.
13 Somwaru LL, Rariy CM, Arnold AM, Cappola AR. The natural history of subclinical hypothyroidism in the elderly: the cardiovascular health study. The Journal of clinical endocrinology and metabolism. 2012;97(6):1962–9. Epub 2012/03/23. doi: 10.1210/jc.2011–3047. PubMed PMID: 22438233; PubMed Central PMCID: PMC3387427.
14 Surks MI, Goswami G, Daniels GH. The thyrotropin reference range should remain unchanged. J Clin Endocrinol Metab. 2005;90(9):5489–96.
15 Waring AC, Arnold AM, Newman AB, Buzkova P, Hirsch C, Cappola AR. Longitudinal changes in thyroid function in the oldest old and survival: the cardiovascular health study all-stars study. J Clin Endocrinol Metab. 2012;97(11):3944–50.
16 Gussekloo J, van Exel E, de Craen AJ, Meinders AE, Frolich M, Westendorp RG. Thyroid status, disability and cognitive function, and survival in old age. JAMA: the journal of the American Medical Association. 2004;292(21):2591–9. Epub 2004/12/02. doi: 10.1001/jama.292.21.2591. PubMed PMID: 15572717.
17 Monzani F, Di Bello V, Caraccio N, Bertini A, Giorgi D, Giusti C, et al. Effect of levothyroxine on cardiac function and structure in subclinical hypothyroidism: a double blind, placebo-controlled study. J Clin Endocrinol Metab. 2001;86(3):1110–5.
18 Razvi S, Ingoe L, Keeka G, Oates C, McMillan C, Weaver JU. The beneficial effect of L-thyroxine on cardiovascular risk factors, endothelial function, and quality of life in subclinical hypothyroidism: randomized, crossover trial. J Clin Endocrinol Metab. 2007;92(5):1715–23.
19 Monzani F, Caraccio N, Kozakowa M, Dardano A, Vittone F, Virdis A, et al. Effect of levothyroxine replacement on lipid profile and intima-media thickness in subclinical hypothyroidism: a double-blind, placebo- controlled study. J Clin Endocrinol Metab. 2004;89(5):2099–106. Epub 2004/05/06. doi: 10.1210/jc.2003–031669. PubMed PMID: 15126526.
20 Danese MD, Ladenson PW, Meinert CL, Powe NR. Clinical review 115: effect of thyroxine therapy on serum lipoproteins in patients with mild thyroid failure: a quantitative review of the literature. J Clin Endocrinol Metab. 2000;85(9):2993–3001.
21 Liu D, Jiang F, Shan Z, Wang B, Wang J, Lai Y, et al. A cross-sectional survey of relationship between serum TSH level and blood pressure. J Hum Hypertens. 2010;24(2):134–8.
22 Walsh JP, Bremner AP, Bulsara MK, O’Leary P, Leedman PJ, Feddema P, et al. Subclinical thyroid dysfunction and blood pressure: a community-based study. Clin Endocrinol. 2006;65(4):486–91. Epub 2006/09/21. doi: 10.1111/j.1365–2265.2006.02619.x. PubMed PMID: 16984241.
23 Adrees M, Gibney J, El-Saeity N, Boran G. Effects of 18 months of L-T4 replacement in women with subclinical hypothyroidism. Clin Endocrinol. 2009;71(2):298–303.
24 Waring AC, Rodondi N, Harrison S, Kanaya AM, Simonsick EM, Miljkovic I, et al. Thyroid function and prevalent and incident metabolic syndrome in older adults: the Health, Ageing and Body Composition Study. Clin Endocrinol. 2012;76(6):911–8. Epub 2011/12/23. doi: 10.1111/j.1365–2265.2011.04328.x. PubMed PMID: 22187968; PubMed Central PMCID: PMC3334430.
25 Garin MC, Arnold AM, Lee JS, Tracy RP, Cappola AR. Subclinical hypothyroidism, weight change, and body composition in the elderly: the Cardiovascular Health Study. J Clin Endocrinol Metab. 2014;99(4):1220–6. Epub 2014/01/18. doi: 10.1210/jc.2013–3591. PubMed PMID: 24432998; PubMed Central PMCID: PMC3973778.
26 Razvi S, Weaver JU, Butler TJ, Pearce SH. Levothyroxine treatment of subclinical hypothyroidism, fatal and nonfatal cardiovascular events, and mortality. Arch Intern Med. 2012;172(10):811–7.
27 Collet TH, Gussekloo J, Bauer DC, den Elzen WP, Cappola AR, Balmer P, et al. Subclinical hyperthyroidism and the risk of coronary heart disease and mortality. Arch Intern Med. 2012;172(10):799–809.
28 Selmer C, Olesen JB, Hansen ML, Lindhardsen J, Olsen AM, Madsen JC, et al. The spectrum of thyroid disease and risk of new onset atrial fibrillation: a large population cohort study. BMJ. 2012;345:e7895. Epub 2012/11/29. doi: 10.1136/bmj.e7895. PubMed PMID: 23186910; PubMed Central PMCID: PMC3508199.
29 Reuters VS, Teixeira Pde F, Vigario PS, Almeida CP, Buescu A, Ferreira MM, et al. Functional capacity and muscular abnormalities in subclinical hypothyroidism. Am J Med Sci. 2009;338(4):259–63.
30 Brennan MD, Powell C, Kaufman KR, Sun PC, Bahn RS, Nair KS. The impact of overt and subclinical hyperthyroidism on skeletal muscle. Thyroid. 2006;16(4):375–80.
31 Mainenti MR, Vigario PS, Teixeira PF, Maia MD, Oliveira FP, Vaisman M. Effect of levothyroxine replacement on exercise performance in subclinical hypothyroidism. J Endocrinol Invest. 2009;32(5):470–3.
32 Ravanbod M, Asadipooya K, Kalantarhormozi M, Nabipour I, Omrani GR. Treatment of iron-deficiency anemia in patients with subclinical hypothyroidism. Am J Med. 2013;126(5):420–4.
33 Simonsick EM, Newman AB, Ferrucci L, Satterfield S, Harris TB, Rodondi N, et al. Subclinical hypothyroidism and functional mobility in older adults. Arch Intern Med. 2009;169(21):2011–7. Epub 2009/11/26. doi: 10.1001/archinternmed.2009.392. PubMed PMID: 19933964; PubMed Central PMCID: PMC2879334.
34 Virgini VS, Wijsman LW, Rodondi N, Bauer DC, Kearney PM, Gussekloo J, et al. Subclinical thyroid dysfunction and functional capacity among elderly. Thyroid. 2014;24(2):208–14.
35 Abe E, Marians RC, Yu W, Wu XB, Ando T, Li Y, et al. TSH is a negative regulator of skeletal remodeling. Cell. 2003;115(2):151–62.
36 Lee JS, Buzkova P, Fink HA, Vu J, Carbone L, Chen Z, et al. Subclinical thyroid dysfunction and incident hip fracture in older adults. Arch Intern Med. 2010;170(21):1876–83. Epub 2010/11/26. doi: 10.1001/archinternmed.2010.424. PubMed PMID: 21098345.
37 Svare A, Nilsen TI, Asvold BO, Forsmo S, Schei B, Bjoro T, et al. Does thyroid function influence fracture risk? Prospective data from the HUNT2 study, Norway. European journal of endocrinology / European Federation of Endocrine Societies. 2013;169(6):845–52. Epub 2013/09/14. doi: 10.1530/EJE-13–0546. PubMed PMID: 24031093.
38 Monzani F, Del Guerra P, Caraccio N, Pruneti CA, Pucci E, Luisi M, et al. Subclinical hypothyroidism: neurobehavioral features and beneficial effect of L-thyroxine treatment. Clin Investig. 1993;71(5):367–71.
39 Roberts LM, Pattison H, Roalfe A, Franklyn J, Wilson S, Hobbs FD, et al. Is subclinical thyroid dysfunction in the elderly associated with depression or cognitive dysfunction? Ann Intern Med. 2006;145(8):573–81. Epub 2006/10/18. PubMed PMID: 17043339.
40 Pae CU, Mandelli L, Han C, Ham BJ, Masand PS, Patkar AA, et al. Thyroid hormones affect recovery from depression during antidepressant treatment. Psychiatry Clin Neurosci. 2009;63(3):305–13.
41 Ceresini G, Lauretani F, Maggio M, Ceda GP, Morganti S, Usberti E, et al. Thyroid function abnormalities and cognitive impairment in elderly people: results of the Invecchiare in Chianti study. J Am Geriatr Soc. 2009;57(1):89–93. Epub 2008/12/05. doi: 10.1111/j.1532–5415.2008.02080.x. PubMed PMID: 19054181; PubMed Central PMCID: PMC2631617.
42 Parsaik AK, Singh B, Roberts RO, Pankratz S, Edwards KK, Geda YE, et al. Hypothyroidism and risk of mild cognitive impairment in elderly persons: a population-based study. JAMA Neurol. 2014;71(2):201–7.
43 Parle J, Roberts L, Wilson S, Pattison H, Roalfe A, Haque MS, et al. A randomized controlled trial of the effect of thyroxine replacement on cognitive function in community-living elderly subjects with subclinical hypothyroidism: the Birmingham Elderly Thyroid study. J Clin Endocrinol Metab. 2010;95(8):3623–32. Epub 2010/05/27. doi: 10.1210/jc.2009–2571. PubMed PMID: 20501682.
44 Canpolat AG, Kav T, Sivri B, Yildiz BO. Effects of L-thyroxine on gastric motility and ghrelin in subclinical hypothyroidism: a prospective study. J Clin Endocrinol Metab. 2013;98(11):2013–1488.
45 Chung GE, Kim D, Kim W, Yim JY, Park MJ, Kim YJ, et al. Non-alcoholic fatty liver disease across the spectrum of hypothyroidism. J Hepatol. 2012;57(1):150–6.
46 Bremner AP, Feddema P, Joske DJ, Leedman PJ, O’Leary PC, Olynyk JK, et al. Significant association between thyroid hormones and erythrocyte indices in euthyroid subjects. Clin Endocrinol. 2012;76(2):304–11.
47 Cinemre H, Bilir C, Gokosmanoglu F, Bahcebasi T. Hematologic effects of levothyroxine in iron-deficient subclinical hypothyroid patients: a randomized, double-blind, controlled study. J Clin Endocrinol Metab. 2009;94(1):151–6.
48 Christ-Crain M, Meier C, Huber P, Zulewski H, Staub JJ, Muller B. Effect of restoration of euthyroidism on peripheral blood cells and erythropoietin in women with subclinical hypothyroidism. Hormones (Athens). 2003;2(4):237–42.
49 Casey BM, Dashe JS, Wells CE, McIntire DD, Byrd W, Leveno KJ, et al. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol. 2005;105(2):239–45.
50 Benhadi N, Wiersinga WM, Reitsma JB, Vrijkotte TG, Bonsel GJ. Higher maternal TSH levels in pregnancy are associated with increased risk for miscarriage, fetal or neonatal death. European journal of endocrinology / European Federation of Endocrine Societies. 2009;160(6):985–91.
51 Wilson KL, Casey BM, McIntire DD, Halvorson LM, Cunningham FG. Subclinical thyroid disease and the incidence of hypertension in pregnancy. Obstet Gynecol. 2012;119(2 Pt 1):315–20.
52 Lazarus JH, Bestwick JP, Channon S, Paradice R, Maina A, Rees R, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366(6):493–501.
53 Lazarus J, Brown RS, Daumerie C, Hubalewska-Dydejczyk A, Negro R, Vaidya B. 2014 European thyroid association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur Thyroid J. 2014;3(2):76–94.
54 Kong WM, Sheikh MH, Lumb PJ, Naoumova RP, Freedman DB, Crook M, et al. A 6–month randomized trial of thyroxine treatment in women with mild subclinical hypothyroidism. Am J Med. 2002;112(5):348–54. Epub 2002/03/21. PubMed PMID: 11904108.
55 Müller B. “Subklinische” Hypo- und Hyperthyreose. Schweizerische Gesellschaft für Endokrinologie und Diabetologie. http://www.sgedssed.ch/fileadmin/files/dokumente/EDM_Key_Slides_2010–2011/SGED_Subklinische_Hypo_Hyperthyresose.pdf http://effectivehealthcare.ahrq.gov/ehc/products/129/750/Hypo-Hyper-Thyroid_CER24_20111114.pdf 2010–2011.
56 Pearce SH, Brabant G, Duntas LH, Monzani F, Peeters RP, et al. 2013 ETA Guideline: Management of subclinical hypothyroidism. Eur Thyroid J. 2013;2(4):215–28.
57 Flynn RW, Bonellie SR, Jung RT, MacDonald TM, Morris AD, Leese GP. Serum thyroid-stimulating hormone concentration and morbidity from cardiovascular disease and fractures in patients on long-term thyroxine therapy. J Clin Endocrinol Metab. 2010;95(1):186–93.
58 Rodondi N, Bauer DC. Subclinical hypothyroidism and cardiovascular risk: how to end the controversy. J Clin Endocrinol Metab. 2013;98(6):2267–9. Epub 2013/05/02. doi: 10.1210/jc.2013–1875. PubMed PMID: 23633199; PubMed Central PMCID: PMC3667255.
59 Blum MR, Collet TH, Krebs D, Stettler C, Christ E, Virgini V, et al. Subklinische Hypothyreose. Schweiz Med Forum. 2013;13(39):772–5.
