DOI: https://doi.org/10.4414/smw.2015.14047
Standard duplex ultrasound has been established as an important diagnostic tool in vascular medicine to assess haemodynamics and luminal narrowing within the large- and middle-sized arteries in different vascular beds. During the last decade contrast-enhanced ultrasound (CEUS) has emerged as a valuable imaging modality that complements and enhances standard vascular ultrasound imaging [1]. In contrast to conventional ultrasound, CEUS enables the dynamic assessment and quantification of the microcirculation in clinical and scientific settings. Ultrasound contrast agents are gas-filled microbubbles that are injected intravenously and serve as intravascular tracers [2]. The functionality of the contrast agent is based on the extended distensibility of the microbubbles compared with the surrounding biological tissues resulting in some kind of natural resonant vibration that highly increases the scattering cross-section [3]. Using contrast specific imaging modalities, these backscattering properties of the microbubbles allow to enhance and to quantify the macro- and microvascularisation down to the capillary perfusion level [4].
Based on these properties with the advantage of imaging and also assessing the microcirculation, the use of CEUS for clinical vascular imaging has increased significantly during the last several years [5]. Besides the potential clinical use of ultrasound contrast agents for luminal enhancement in large arteries such as the abdominal aorta, carotid and renal arteries, CEUS has had a great impact on imaging the microvascular perfusion in different organs including the liver, kidney and skeletal muscle, as well as the microvasculature within tumours (angiogenesis) and the vasa vasorum-derived neovascularisation of atherosclerotic plaques and inflamed vessel walls (vasculitis) [6]. Most of these new indications for CEUS in vascular medicine have already been implemented in the latest update of the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) guidelines and recommendations on the clinical practice of CEUS on non-hepatic applications [5].
In the following review article, we will discuss CEUS as a novel approach in vascular medicine to assess the microvasculature using ultrasound contrast agents in different clinical settings (table 1).
| Table 1:Indications for microcirculation assessment by contrast-enhanced ultrasound in vascular medicine. | ||
| Vessel territory / organ | Indications | References |
| Carotid artery | Detection and quantification of neovascularisation (Vasa vasorum) within the atherosclerotic plaque and stenosis for vascular risk stratification | [16, 17, 21, 23–32] |
| Large and middle-size arteries | Detecting inflammation of the vessel wall by imaging the vascularised and hyperaemic inflamed tissue for diagnosis and monitoring of inflammatory vascular diseases (chronic periaortitis, giant cell arteritis, Takayasu disease) | [33–37] |
| Kidney | Detection of renal infarction and cortical necrosis | [38, 39] |
| Transplanted kidney | Detection of devascularised regionsQuantification of parenchymal perfusion in patients with delayed graft function for the detection of acute rejection | [40, 41] |
| Skeletal muscle | Quantification of muscle perfusion and flow reserve in patients with PAD and diabetes mellitus | [42–50] |
Currently, the only contrast agent approved for vascular indications in Switzerland is a second–generation contrast agent, consisting of a low soluble gas (sulphur hexafluorane) in a phospholipid shell (SonoVue®, Bracco, Milan) [5]. It is important to mention that some of the potential useful applications described in this review are currently not all accepted or registered indications and may be considered as off label use. The encapsulated bubbles are slightly smaller than red blood cells and act by their presence in the whole vascular system after injection into a peripheral vein as pure intravascular contrast agents. After a few minutes, the microbubbles are eliminated through the respiratory tract and are therefore not nephrotoxic. Ultrasound contrast agents are very simple to use, well tolerated by the patients and can be safely used in renal impairment unlike computed tomography (CT) and magnetic resonance (MR) contrast agents. In very rare cases severe adverse events including anaphylactoid reactions in less than 1 out of 10,000 patients had been reported [7]. More frequent side effect are temporary complains of headache and discomfort at the injection site (pain, burning, paraesthesia, bruising).
According to the summary of product information provided by the European Medicines Agency, SonoVue® is contraindicated in patients known to have right-to-left shunts, severe pulmonary hypertension (pulmonary artery pressure >90 mm Hg), uncontrolled systemic hypertension and in patients with adult respiratory distress syndrome. Furthermore it should not be used in patients with suggested cardiovascular instability. Beyond that the safety and efficacy of SonoVue® have not been established in pregnant and lactating women nor in children and adolescents.
For vascular applications, a typical whole body human dose ranges between 0.5–2.4 ml of SonoVue® depending on the sensitivity of the equipment and the type of transducer used. Enhancement of the arterial lumen following intravenous bolus injection starts after 10–30 s, peaks after 30–60 s and decreases continuously over 2–5 minutes thereafter. Alternatively, a continuous infusion (VueJect®, Bracco, Milan) of 1 ml per minute to achieve a stable enhancement may be applied.
Depending on the depth of the targeted tissue or vessel a linear higher frequency array transducer (e.g. 3 to 9 MHz) or a low frequency curved array transducer (e.g. 1 to 5 MHz) should be used for vascular indications. For CEUS a low mechanical-index contrast-specific ultrasound mode (harmonic imaging, pulse inversion imaging, amplitude and phase modulation) is mandatory in order to specifically image the non-linear ultrasound echoes from the microbubbles and thereby suppressing the echoes from the surrounding tissue. Eventually, this ultrasound imaging technique in conjunction with contrast agent enables depiction of vessels down to 100 μm in diameter which is well below the 1 mm resolution limit of conventional Doppler techniques. Therefore, CEUS is an excellent tool for assessing the microcirculation of different organs and tissues.
A potential limitation of using CEUS with vascular indications could be a specific artefact due to a contrast-induced nonlinear propagation of the ultrasound, the so called “pseudo-enhancement”, which might lead to misinterpretation of vascularisation, especially in the proximity of the far wall in vascular applications [8].
The quantification of the perfusion of the microcirculation also remains challenging [9]. Besides a simple visual based subjective assessment of tissue enhancement, specific kinetics of contrast enhancement can be used to evaluate the tissue perfusion which is more accurate and reproducible. The time intensity curve (TIC) after bolus injection of the contrast agent represents the average intensity in a region of interest as a function of time. Using dedicated quantification software, specific TIC parameters as time to peak, peak intensity, wash-in- and wash-out-time, and mean transit time can be calculated and reflect the local blood volume and flow. When the contrast agent is administered as a continuous infusion, a disruption-replenishment analysis method can be applied. During a steady-state condition the mechanical index is briefly increased for a few frames which causes bubble disruption within the imaging plane. The quantification of the tissue perfusion within the region of interest can be calculated based on the following replenishment of the microbubbles which reflects the regional flow rate. Currently, different types of quantification software are available to calculate these specific perfusion parameters in order to assess microvascular perfusion [10].
The clinical use of carotid ultrasound to assess the extra-cranial cerebral artery atherosclerosis is well established. In the clinical setting, the main focus is to detect and to estimate the degree of carotid stenosis by measuring the haemodynamics with duplex ultrasound. However, ultrasound imaging also provides additional information on early and late manifestations of atherosclerosis beyond the degree of stenosis. Using high-resolution B-mode carotid ultrasound, the thickening of the intima-media can be measured. Furthermore, the presence and characteristics of focal atherosclerotic plaques can be assessed. Both the carotid intima-media thickness (c-IMT) and carotid plaques are well established indicators of atherosclerosis [11]. Based on large population studies, it is well known that c-IMT predicts cardiovascular events [12]. Nevertheless, the addition of c-IMT alone to traditional risk prediction models in the general population is associated with only limited improvement of cardiovascular risk prediction [13]. The combination of measuring c-IMT and assessing the presence or absence of carotid plaque has been found to significantly improve the prediction of cardiovascular events [14].

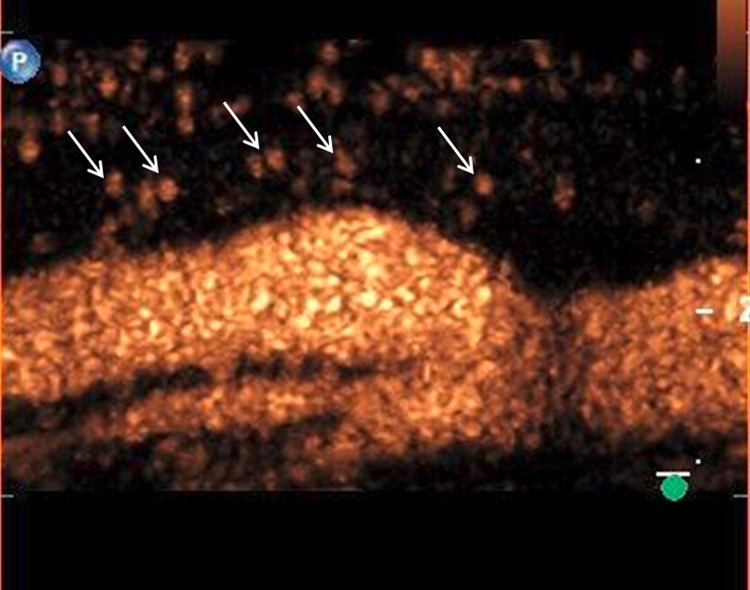
Figure 1
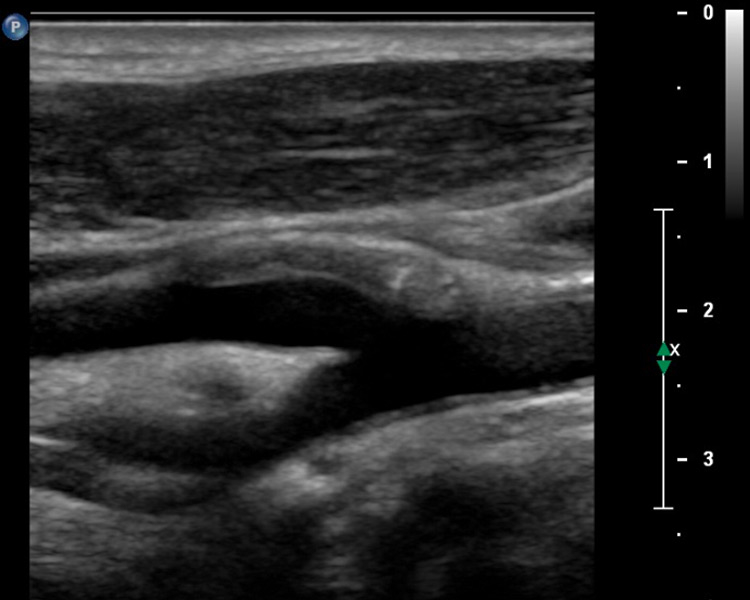
A 58-year-old patient with myocardial infarction 2 months previously with hypoechogenic plaque at the origin of the left internal carotid artery on B-mode ultrasound (A). Extensive intraplaque neovascularisation (arrows) on CEUS imaging (B).
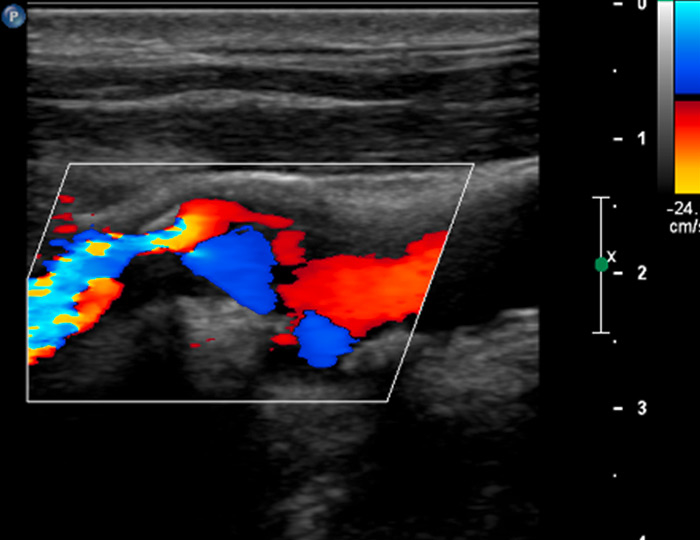
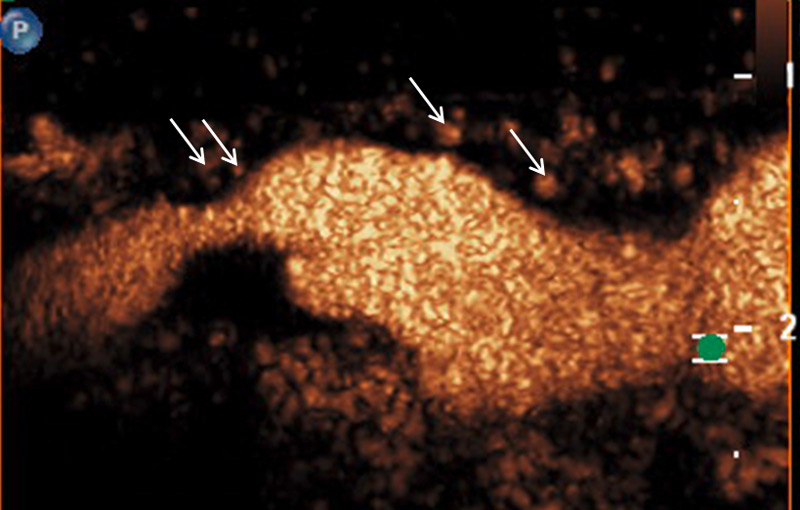
Figure 2
A 61-year-old patient with asymptomatic high-grade stenosis at the origin of the left internal carotid artery on colour Doppler ultrasound (A). Moderate intraplaque neovascularisation within the carotid stenosis at the near wall on CEUS imaging (arrows) and no neovascularisation at the far wall (B).
However, the integration of c-IMT and the presence of plaque into cardiovascular risk assessment strategies are currently still controversial as they do not add enough discriminatory power to standard risk estimation. Therefore, the additional analysis of plaque characteristics assessed by ultrasound could provide deeper insights into individual risk assessment [15].
In CEUS imaging of the carotid artery, the plaques and the intima-media complex appear hypoechoic, whereas the carotid lumen and the adventitial layer appear enhanced. This improves visualisation of vessel wall irregularities including soft plaques and plaque ulcerations [16]. In a recent prospective study in asymptomatic patients with cardiovascular risk factors, CEUS showed additional value for the detection of subclinical atherosclerosis in the carotid arteries [17]. The atherosclerotic plaques that were detected by CEUS but not with standard carotid ultrasound were predominantly hypoechogenic. The authors concluded that CEUS may have an incremental value for the detection of subclinical atherosclerosis, particularly in the carotid arteries.
Furthermore, CEUS may also be helpful not only for plaque detection but also for a more dedicated plaque characterisation by imaging the microcirculation within the plaque [6]. Based on several histopathological studies, it is well known that proliferation of the vasa vasorum in the adventitial layer and the vasa vasorum-derived intraplaque neovascularisation with leaky immature vessels triggered by hypoxia and inflammation play an important role for plaque progression and vulnerability leading to consecutive cardiovascular events [18]. An increased number of abnormal, immature microvessels, which could contribute to plaque instability triggered by vascular leakage, inflammatory cell recruitment, and intraplaque haemorrhage, have been found particularly in symptomatic carotid stenosis. Recently, a prospective study also evaluated whether specific histological features within the atherosclerotic lesion helped to identify patients who are at increased risk to suffer a cardiovascular event within 3 years after carotid endarterectomy [19]. The authors found that patients with increased vessel density and more plaque haemorrhage in the operated atherosclerotic lesion had an increased risk for future cardiovascular events. These results support the concept that carotid intraplaque neovascularisation is an important hallmark of cardiovascular vulnerability and holds major prognostic information. Hence, non-invasive imaging techniques to visualise intraplaque neovascularisation may significantly contribute to the detection of instable and vulnerable plaque prone to rupture and serve for individual risk stratification to prevent cardiovascular events [6, 20].
Therefore, carotid CEUS imaging has emerged as a promising and valuable method to identify and quantify the microcirculation within the atherosclerotic vessel wall by direct visualisation of the vasa vasorum-derived intraplaque neovascularisation. Different studies found a positive correlation between intraplaque neovascularisation on CEUS imaging in vivo and the microvessel density found at histology in animal models as well as in patients scheduled for endarterectomy (CD31 staining on endarterectomy specimens) [21–23]. Most of these studies used a semi-quantitative visual based approach with different scoring systems to grade intraplaque neovascularisation (e.g. no, moderate, extensive enhancement). Other studies used a quantitative analysis method by measuring intraplaque video-intensity [24, 25]. They also found a very good correlation with the histologic density of neovessels. An even more sophisticated quantitative method using a motion tracking algorithm has been demonstrated [26]. These newer software-based quantitative analyses of intraplaque neovascularisation may provide a more accurate assessment of the microcirculation within the atherosclerotic vessel wall, but they still have some methodological limitations. Two very recent studies found not only a positive correlation between intraplaque neovascularisation on CEUS imaging and histology but also a very good accuracy of a semi-quantitative visual based analysis and a quantitative software analysis of intraplaque neovascularisation on CEUS [27, 28]. A visually based approach may therefore be accurate and useful for daily clinical use.
In our own clinical study, we analysed 293 atherosclerotic lesions with standard and CEUS imaging [29]. The degree of intraplaque neovascularisation was graded visually as no, moderate or extensive enhancement. Similar to other investigators we found that predominantly hypoechoic carotid plaques on B-mode ultrasound, known to be associated with an increased risk of cardiovascular events significantly more often, had a higher grade of intraplaque neovascularisation on CEUS (fig. 1). In accordance with the concept that more vulnerable atherosclerotic lesions had a higher degree of neovascularisation, we found also that intraplaque neovascularisation on CEUS correlated well with lesion severity based on the degree of stenosis and plaque thickness (fig. 2).
Several retrospective studies including our own study revealed that intraplaque neovascularisation on CEUS was associated with clinical symptoms [30–32]. Patients with a more pronounced carotid plaque enhancement on CEUS imaging suffered more often from previous cerebrovascular and coronary events, indicating that the risk of an ischemic event assessed from carotid lesion neovascularisation is not limited to the cerebrovascular system, but is also generalisable to other vascular beds, particularly to the coronary vessels.
These promising clinical results led to the recommendation in the latest EFSUMB guidelines on the clinical practice of CEUS on non-hepatic applications, that CEUS examination of the carotid artery should be used for the evaluation of carotid plaque neovascularisation in order to provide a novel, non-invasive tool for plaque risk stratification and for assessing the response to anti-atherosclerotic therapy [5]. The quantification of intraplaque neovascularisation on CEUS may help to identify vulnerable carotid lesions in individual patients that are at increased risk of rupture and thromboembolic event. This eventually improves the prediction of future vascular events and may allow a more accurate treatment selection on a case-by-case basis.
The current management of carotid atherosclerotic lesions focuses only on stroke prediction and treatment recommendations based on the degree of stenosis. Particularly, patients with symptomatic carotid stenosis >70% are at increased risk for recurrent stroke and may benefit from carotid endarterectomy or stenting. The recommendation of carotid endarterectomy in patients with asymptomatic carotid stenosis >70% to prevent cerebrovascular events is more controversial, particularly when considering the development of better medical preventative treatment. Therefore, a better risk stratification method beyond the degree of carotid stenosis in order to discriminate patients with stable atherosclerotic lesions from those with unstable lesions independent of the actual stenosis degree seems mandatory. This could lead to a better selection of patients who benefit from invasive therapy. CEUS imaging particularly has the potential to help the clinician to improve the risk stratification of carotid stenosis. It may identify patients with unstable, vascularised stenosis with moderate luminal narrowing who would already benefit from carotid endarterectomy or endovascular treatment. On the other hand, unnecessary invasive interventions can be avoided in patients with stable, not vascularised high grade carotid stenosis. This approach, however, still has to be demonstrated in prospective studies.
The initial inflammatory phase of different inflammatory or autoimmune vascular diseases of large- and middle-sized arteries including chronic periaortitis, giant cell arteritis and Takayasu disease are characterised by inflammation and proliferation of the vessel vasa vasorum representing the main portal of entry for inflammatory cells into the vessel wall. CEUS imaging may therefore allow the assessment of this inflammation-driven hyperaemia and neovascularisation of the inflamed vessel wall which could represent a marker of disease activity.
In several case reports including one by our group, the use of CEUS imaging in patients with chronic periaortitis including therapy assessment has been published [33, 34]. Chronic periaortitis is usually suggested by ultrasound and appears as a smooth-bordered, hypoechoic or isoechoic mass around the infrarenal aorta and the iliac arteries on B-mode ultrasound. It has been shown, that the additional use of ultrasound contrast agents to visualise the microvasculature within the fibro-inflammatory process can further substantiate the diagnosis and may also be helpful to monitor treatment response documenting a decrease in the vascularisation state of the periaortic tissue after favourable immunosuppressive therapy. The degree of microvascularisation in the periarotic tissue visualised by CEUS for initial diagnosis and follow-up monitoring also correlated with inflammatory activity and glucose metabolism assessed by positron emission tomography / computed tomography in a case report [34]. This supports the use of CEUS imaging in chronic periaortitis, because it seems to be an excellent alternative imaging tool for radiological and nuclear methods without of radiation exposure.
The use of carotid CEUS has also been proposed to assess microvasculature within the inflamed vessel wall in patients with large-vessel vasculitis [35, 36]. In a patient with a long, smooth, homogeneous, and concentric thickening of the carotid arterial wall on B-mode ultrasound, typical for Takayasu arteritis, additional CEUS imaging revealed a large amount of contrast agent microbubbles within the vessel wall thickening. This neovascularisation may represent the initial inflammatory phase of Takayasu arteritis. Further case reports confirmed a marked arterial wall enhancement with direct visualisation of multiple moving microbbubles in the early phase of Takayasu arteritis. Arterial wall enhancement clearly decreased 6 months after beginning an anti-inflammatory therapy. In a recent small prospective case series of patients with Takayasu and giant cell arteritis, carotid CEUS imaging not only improved the visualisation of the lumen border, but also allowed the assessment of carotid wall vascularisation, which could be a useful marker of disease activity [37]. Therefore, CEUS imaging seems to be a valuable tool for the diagnosis and follow-up of inflammatory vessel diseases as a non-invasive and easily reproducible measuring technique for arterial wall inflammation and treatment evaluation.
Conventional Duplex ultrasound is often the first-line imaging technique to assess renal vascular disease. However, the sensitivity of colour Doppler to evaluate microvascular changes within the renal parenchyma is limited. CEUS imaging is overcoming this limitation and allows real-time assessment of the complete renal macro- and microvasculature in order to image the renal blood flow and to quantify renal tissue perfusion [5]. More importantly, the contrast agents used in CEUS are not nephrotoxic and are excreted mainly via the respiratory tract.
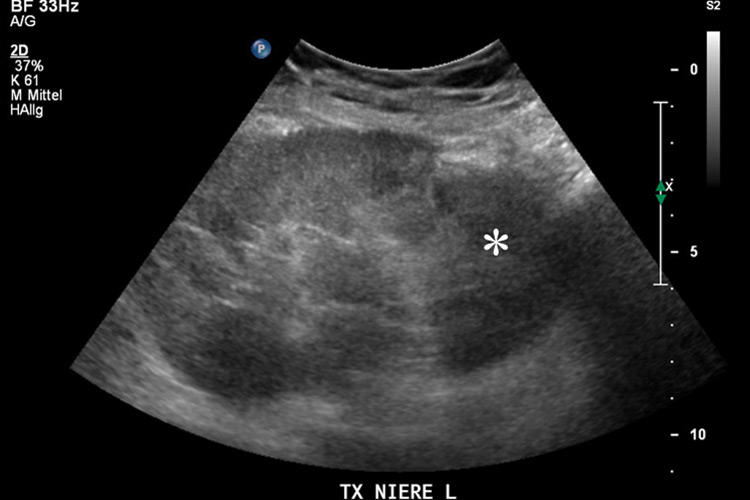
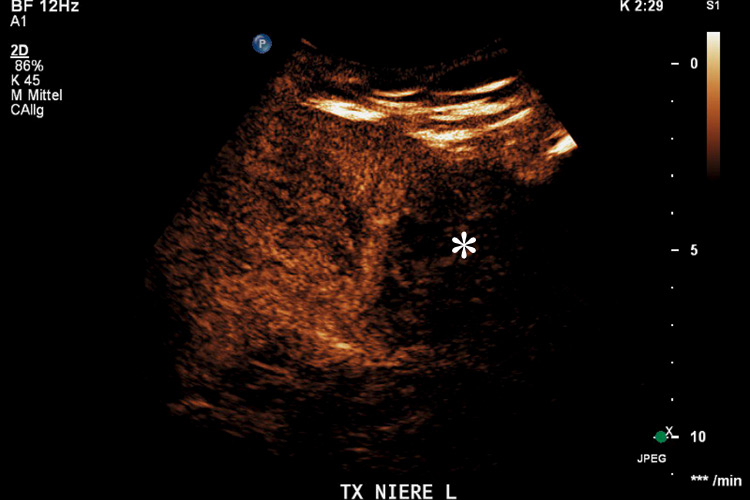
Figure 3
Transplanted kidney with hypoechoic region at the lower pole (*) on B-mode ultrasound (A). Corresponding CEUS imaging with devascularised region at the lower pole (*) in the transplanted kidney (B).
Following bolus contrast agent injection, the arterial pedicle and main branches enhance first. After a few seconds, cortical perfusion is followed by medullary perfusion. This arterial phase lasts between 20–40 s post-injection. During the later phase which lasts for 45–120s, this cortico-medullary differentiation is lost leading to a homogenous enhancement of the kidneys. Subsequently concentration of microbubbles in the circulation decreases and renal enhancement fades.
Renal perfusion can be affected by different vascular diseases including kidney parenchymal infarction, arteriovenous fistula, pseudoaneurysm, renal artery stenosis or renal vein thrombosis. These disorders can generally be diagnosed by conventional Duplex ultrasound. However, compared to colour Doppler ultrasound, CEUS seems to be superior for detecting renal parenchymal ischemia with a very good diagnostic performance, comparable to that of computed tomography [38]. Therefore, CEUS is considered as a recommended imaging tool in patients with suspected renal infarction. Similar to other parenchymatous organs, an infarcted renal area on CEUS imaging appears as a triangular or wedge-shaped area without contrast uptake, while the rest of the renal tissue is normally enhanced. Due to its excellent spatial resolution, CEUS imaging allows differentiation of non-perfused infarcted tissue from cortical necrosis, which appears as a non-enhancing cortical area with preservation of hilar vascularity [39]. Furthermore, CEUS can differentiate renal infarction from parenchymal areas with only reduced perfusion. Even though in both conditions colour Doppler ultrasound shows no flow in the corresponding kidney area, only renal infarction is associated with a complete lack of contrast uptake after application of the ultrasound contrast agent.
Furthermore, the use of CEUS has emerged as an excellent tool for assessing vascular complications of renal transplants by imaging the microcirculation similarly to that of the native kidney [39]. The sensitivity in the detection of transplant infarction is also very high (fig. 3). Due to the ability to image flow in the microcirculation, the infarcted size on CEUS may appear even smaller than on the corresponding colour Doppler ultrasound. Also cortical necrosis in renal transplants can be clearly depicted and is rendered more conspicuous by CEUS which allows a better immediate clinical management.
Quantitative methods can also be applied during CEUS imaging to the transplant kidney in order to assess transplant perfusion and to detect complications in the early transplant period. Particularly, acute tubular necrosis, rejection, and drug toxicity as important causes of early graft dysfunction are difficult to discriminate based on standard ultrasound and laboratory abnormalities alone, so that a biopsy often is needed. Different studies investigated the use of CEUS to detect and discriminate these complications by analysing the dynamic changes of contrast enhancement within the kidney microcirculation [40, 41]. Patients with acute rejection have delayed signal increase in the transplant cortex when compared with patients with normal graft function and those with proven acute tubular necrosis on biopsy. These promising results support the use of CEUS for monitoring graft function, directing further management.
In clinical daily routine the diagnosis of peripheral artery disease (PAD) is usually made by measuring the ankle-brachial index and recording the pulse-volume curve followed by non-invasive imaging modalities to quantify structural changes of the macroperfusion such as standard Doppler ultrasound, magnetic resonance or computed tomography angiography. However, these imaging methods have not only a limited ability to assess diffuse small vessel disease or the fraction of functional collateral perfusion but are also affected by the lack of expressing the impact on nutritive microvascular flow, which is a crucial aspect in the estimation of the end organ damage in PAD patients.
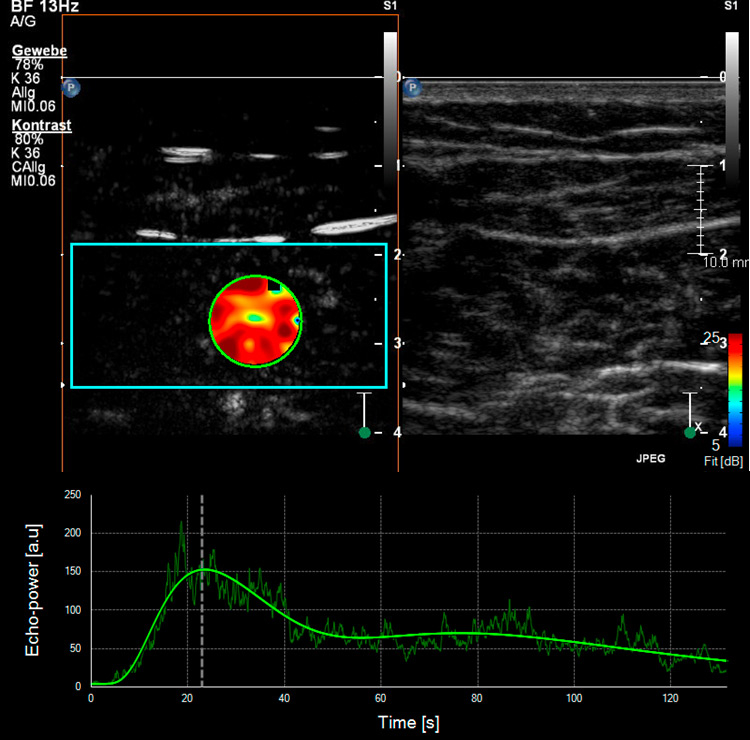
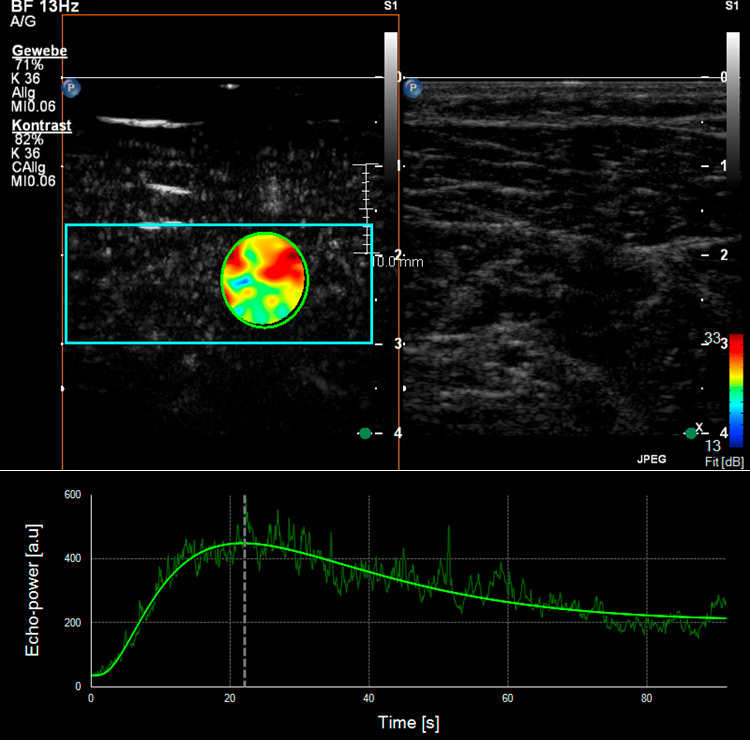
Figure 4
Skeletal muscle microperfusion on CEUS in a healthy volunteer (A) and a patient with peripheral artery disease (B). Corresponding time-intensity-curves of a region of interest (green circle) within the skeletal muscle (soleus muscle) after bolus injection of the ultrasound contrast agent with different shape in the healthy volunteer compared to the patient with peripheral artery disease.
In contrast, CEUS imaging enables the examiner to assess and to quantify the microcirculation of the skeletal muscle in real time by measuring changes in signal intensity over time [42].
Different studies have already used CEUS to analyse skeletal muscle perfusion in patients with PAD and with different cardiovascular risk factors (fig. 4). In one survey, the wash-in curves recorded in calf muscles of patients with symptomatic PAD showed an extended time to reach the maximum intensity as compared to healthy volunteers, and this was even more distinct in patients with reduced collateralisation and progressed disease [43, 44]. The time-to-peak intensity was significantly reduced after effective revascularisation by either percutaneous transluminal angioplasty or bypass surgery [45]. Another research group investigated calf muscle perfusion in symptomatic PAD patients at rest and during exercise (repetitive plantar flexion) by looking at the replenishment kinetics on harmonic power Doppler imaging. Patients with symptomatic PAD showed a significantly lower flow reserve when compared with normal volunteers [46]. Furthermore, the calculated muscle blood flow during exercise on CEUS imaging was impaired in the PAD subject group compared to the controls. By using dynamic CEUS, other investigators illustrated that the maximum signal intensity and the time to decline after temporary arterial occlusion (provoked at mid-thigh via inflatable cuff) were significantly affected in PAD patients [47]. By measuring the time-intensity curve in the muscle and the related arteries or veins selectively, patients with diabetes mellitus revealed a prolonged transit time of the microbubbles from artery to vein which in turn reflects a dysfunctional microcirculation [48, 49]. This was also shown in a similar way using CEUS perfusion imaging on the forearm flexor in patients with complicated advanced stage diabetes [50].
Therefore, CEUS perfusion imaging could play an important role as a non-invasive imaging method to monitor the impact on skeletal microvascularisation in patients with PAD and low grade claudication attending a standardised exercise programme or in patients with critical limb ischaemia receiving invasive or new pro-arteriogenic therapy.
Even in other diseases altering skeletal muscle perfusion, such as myofascial pain syndrome, muscle strain and traumatic muscle lesions or systemic inflammatory disease, CEUS may reliably enable the operator to quantify transitory shift in muscle microvascularity and visualize neoangiogenesis. CEUS has the potential to become a valid tool for monitoring either the degree of muscular involvement, the progress of regenerative processes or even the effect of anti-inflammatory therapy.
CEUS has emerged as a valuable and capable non-invasive imaging tool to assess real-time microcirculation in different vascular territories and organs [1]. Compared to conventional Duplex ultrasound, CEUS as a novel image modality improves the diagnostic performance in the detection and characterisation of various vascular disorders (table 1). In carotid atherosclerotic disease, CEUS imaging provides additional information on plaque vulnerability by illustrating the presence and extent of intraplaque neovascularisation originating from adventitial vasa vasorum. This new imaging modality may be helpful for further risk stratification of atherosclerotic lesions and for detecting patients at risk for vascular events, eventually leading to more specific therapeutic recommendations.
Furthermore, CEUS imaging is also a helpful tool for the diagnosis and monitoring of inflammatory vascular diseases. It increases the diagnostic performance of ultrasound in detecting inflammatory changes of the vessel wall such as hypervascularisation and hyperaemia. Changes in vessel wall enhancement may also reflect the response to anti-inflammatory therapy.
CEUS imaging is also a valuable tool for the assessment of the microcirculation and the tissue perfusion in solid organs including native and transplanted kidneys. It provides more accurate information on perfusion deficits of the parenchyma in patients with kidney infarction, cortical necrosis or graft dysfunction.
Furthermore, CEUS has great potential in the assessment of the microcirculation of the skeletal muscle particularly in patients with PAD or diabetic microangiopathy.
As a consequence, most of these applications for CEUS imaging in vascular medicine have found their way into the latest EFSUMB guidelines and recommendations on the clinical practice of CEUS [15]. However, further clinical studies are needed to establish the scientific basis of using CEUS to assess the microcirculation in various vascular disorders in order to improve diagnostic performance and to guide therapeutic interventions.
Furthermore, by using site-targeted microbubbles which are retained and imaged within regions of disease by ligand-directed binding after intravenous injection, CEUS also has the potential to image non-invasively the molecular or cellular profile of different tissues in the future [51]. These newer techniques can further enhance and expand the diagnostic capabilities of current vascular imaging by assessing specific molecular processes that play a role in the pathophysiology of vascular disease. CEUS molecular imaging has been shown to provide information on disease development and progression in atherosclerosis, tissue ischemia, thrombosis and angiogenesis in various animal models. However, these studies in vascular diseases using site-targeted microbubbles are still on a pre-clinical level and have not yet found the way into the clinical arena.
Moreover, ultrasound contrast agents could also gain great clinical value in the future by the development of novel non-viral, ultrasound directed and site-specific gene and drug delivery systems [52]. A target for therapeutic ultrasound with a potentially great clinical impact in the field of vascular medicine is that of thrombolysis, anti-inflammatory or anti- or angiogenic treatment by delivering small molecules or plasmid DNA. This combination of CEUS for diagnosis and therapy will provide unique opportunities for vascular clinicians to image the microcirculation and directly treat vascular diseases.
1 Staub D, Partovi S, Imfeld S, Uthoff H, Baldi T, Aschwanden M, et al. Novel applications of contrast-enhanced ultrasound imaging in vascular medicine. Vasa. 2013;42(1):17–31.
2 Feinstein S, Coll B, Staub D, Adam D, Schinkel A, ten Cate F, et al. Contrast enhanced ultrasound imaging. J Nucl Cardiol. 2010;17(1):106–15.
3 Faez T, Emmer M, Kooiman K, Versluis M, van der Steen A, de Jong N. 20 years of ultrasound contrast agent modeling. IEEE Trans Ultrason Ferroelectr Freq Control. 2013;60(1):7–20.
4 Clevert DA, D’Anastasi M, Jung EM. Contrast-enhanced ultrasound and microcirculation: efficiency through dynamics – current developments. Clin Hemorheol Microcirc. 2013;53(1–2):171–86.
5 Piscaglia F, Nolsøe C, Dietrich CF, Cosgrove DO, Gilja OH, Bachmann Nielsen M, et al. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med. 2012;33(1):33–59.
6 Staub D, Schinkel A, Coll B, Coli S, van der Steen A, Reed J, et al. Contrast-enhanced ultrasound imaging of the vasa vasorum: from early atherosclerosis to the identification of unstable plaques. JACC Cardiovasc Imaging. 2010;3(7):761–71.
7 Piscaglia F, Bolondi L. The safety of Sonovue in abdominal applications: retrospective analysis of 23188 investigations. Ultrasound Med Biol. 2006;32(9):1369–75.
8 ten Kate GL, Renaud GG, Akkus Z, van den Oord SC, ten Cate FJ, Shamdasani V, et al. Far-wall pseudoenhancement during contrast-enhanced ultrasound of the carotid arteries: clinical description and in vitro reproduction. Ultrasound Med Biol. 2012;38(4):593–600.
9 Dietrich CF, Averkiou MA, Correas JM, Lassau N, Leen E, Piscaglia F. An EFSUMB Introduction into Dynamic Contrast-Enhanced Ultrasound (DCE-US) for Quantification of Tumour Perfusion. Ultraschall Med. 2012;33(4):344–51.
10 Tranquart F, Mercier L, Frinking P, Gaud E, Arditi M. Perfusion quantification in contrast-enhanced ultrasound (CEUS) – ready for research projects and routine clinical use. Ultraschall Med. 2012;33 Suppl 1:S31–8.
11 Sillesen H. Carotid Intima-media Thickness and/or Carotid Plaque: What is Relevant? Eur J Vasc Endovasc Surg. 2014.
12 Den Ruijter HM, Peters SA, Anderson TJ, Britton AR, Dekker JM, Eijkemans MJ, et al. Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis. JAMA. 2012;308(8):796–803.
13 Ziegelbauer K, Schaefer C, Steinmetz H, Sitzer M, Lorenz MW. Clinical usefulness of carotid ultrasound to improve stroke risk assessment: ten-year results from the Carotid Atherosclerosis Progression Study (CAPS). Eur J Prev Cardiol. 2013;20(5):837–43.
14 Nambi V, Chambless L, Folsom AR, He M, Hu Y, Mosley T, et al. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (Atherosclerosis Risk In Communities) study. J Am Coll Cardiol. 2010;55(15):1600–7.
15 Eyding J, Geier B, Staub D. Current strategies and possible perspectives of ultrasonic risk stratification of ischemic stroke in internal carotid artery disease. Ultraschall Med. 2011;32(3):267–73.
16 Partovi S, Loebe M, Aschwanden M, Baldi T, Jäger KA, Feinstein SB, et al. Contrast-enhanced ultrasound for assessing carotid atherosclerotic plaque lesions. AJR Am J Roentgenol. 2012;198(1):W13–9.
17 van den Oord SC, ten Kate GL, Sijbrands EJ, van der Steen AF, Schinkel AF. Effect of carotid plaque screening using contrast-enhanced ultrasound on cardiovascular risk stratification. Am J Cardiol. 2013;111(5):754–9.
18 Sluimer J, Daemen M. Novel concepts in atherogenesis: angiogenesis and hypoxia in atherosclerosis. J Pathol. 2009;218(1):7–29.
19 Hellings WE, Peeters W, Moll FL, Piers SR, van Setten J, Van der Spek PJ, et al. Composition of carotid atherosclerotic plaque is associated with cardiovascular outcome: a prognostic study. Circulation. 2010;121(17):1941–50.
20 Gallino A, Stuber M, Crea F, Falk E, Corti R, Lekakis J, et al. “In vivo” imaging of atherosclerosis. Atherosclerosis. 2012;224(1):25–36.
21 Coli S, Magnoni M, Sangiorgi G, Marrocco-Trischitta M, Melisurgo G, Mauriello A, et al. Contrast-enhanced ultrasound imaging of intraplaque neovascularization in carotid arteries: correlation with histology and plaque echogenicity. J Am Coll Cardiol. 2008;52(3):223–30.
22 Schinkel A, Krueger C, Tellez A, Granada J, Reed J, Hall A, et al. Contrast-enhanced ultrasound for imaging vasa vasorum: comparison with histopathology in a swine model of atherosclerosis. Eur J Echocardiogr. 2010.
23 Shah F, Balan P, Weinberg M, Reddy V, Neems R, Feinstein M, et al. Contrast-enhanced ultrasound imaging of atherosclerotic carotid plaque neovascularization: a new surrogate marker of atherosclerosis? Vasc Med. 2007;12(4):291–7.
24 Hoogi A, Adam D, Hoffman A, Kerner H, Reisner S, Gaitini D. Carotid plaque vulnerability: quantification of neovascularization on contrast-enhanced ultrasound with histopathologic correlation. AJR Am J Roentgenol. 2011;196(2):431–6.
25 Vavuranakis M, Sigala F, Vrachatis DA, Papaioannou TG, Filis K, Kavantzas N, et al. Quantitative analysis of carotid plaque vasa vasorum by CEUS and correlation with histology after endarterectomy. Vasa. 2013;42(3):184–95.
26 van den Oord SC, Akkus Z, Bosch JG, Hoogi A, Ten Kate GL, Renaud G, et al. Quantitative Contrast-Enhanced Ultrasound of Intraplaque Neovascularization in Patients with Carotid Atherosclerosis. Ultraschall Med. 2014.
27 Li C, He W, Guo D, Chen L, Jin X, Wang W, et al. Quantification of carotid plaque neovascularization using contrast-enhanced ultrasound with histopathologic validation. Ultrasound Med Biol. 2014;40(8):1827–33.
28 Müller HF, Viaccoz A, Kuzmanovic I, Bonvin C, Burkhardt K, Bochaton-Piallat ML, et al. Contrast-enhanced ultrasound imaging of carotid plaque neo-vascularization: accuracy of visual analysis. Ultrasound Med Biol. 2014;40(1):18–24.
29 Staub D, Partovi S, Schinkel AF, Coll B, Uthoff H, Aschwanden M, et al. Correlation of carotid artery atherosclerotic lesion echogenicity and severity at standard US with intraplaque neovascularization detected at contrast-enhanced US. Radiology. 2011;258(2):618–26.
30 Staub D, Patel M, Tibrewala A, Ludden D, Johnson M, Espinosa P, et al. Vasa vasorum and plaque neovascularization on contrast-enhanced carotid ultrasound imaging correlates with cardiovascular disease and past cardiovascular events. Stroke. 2010;41(1):41–7.
31 Xiong L, Deng Y, Zhu Y, Liu Y, Bi X. Correlation of carotid plaque neovascularization detected by using contrast-enhanced US with clinical symptoms. Radiology. 2009;251(2):583–9.
32 Faggioli GL, Pini R, Mauro R, Pasquinelli G, Fittipaldi S, Freyrie A, et al. Identification of carotid “vulnerable plaque” by contrast-enhanced ultrasonography: correlation with plaque histology, symptoms and cerebral computed tomography. Eur J Vasc Endovasc Surg. 2011;41(2):238–48.
33 Partovi S, Imfeld S, Aschwanden M, Bilecen D, Jaeger KA, Staub D. The use of contrast-enhanced ultrasound (CEUS) in chronic periaortitis. Ultraschall Med. 2012;Aug 7 [epub ahead of print].
34 Steubl D, Thürmel K, Moog P, Essler M, Heemann U, Stock KF. Comparison of fluorine-18–deoxyglucose positron emission tomography/computed tomography and contrast-enhanced ultrasound in a patient with chronic periaortitis. Vasa. 2013;42(5):370–4.
35 Magnoni M, Dagna L, Coli S, Cianflone D, Sabbadini MG, Maseri A. Assessment of Takayasu arteritis activity by carotid contrast-enhanced ultrasound. Circ Cardiovasc Imaging. 2011;4(2):e1–2.
36 Giordana P, Baqué-Juston MC, Jeandel PY, Mondot L, Hirlemann J, Padovani B, et al. Contrast-enhanced ultrasound of carotid artery wall in Takayasu disease: first evidence of application in diagnosis and monitoring of response to treatment. Circulation. 2011;124(2):245–7.
37 Schinkel AF, van den Oord SC, van der Steen AF, van Laar JA, Sijbrands EJ. Utility of contrast-enhanced ultrasound for the assessment of the carotid artery wall in patients with Takayasu or giant cell arteritis. Eur Heart J Cardiovasc Imaging. 2014;15(5):541–6.
38 Bertolotto M, Martegani A, Aiani L, Zappetti R, Cernic S, Cova MA. Value of contrast-enhanced ultrasonography for detecting renal infarcts proven by contrast enhanced CT. A feasibility study. Eur Radiol. 2008;18(2):376–83.
39 McArthur C, Baxter GM. Current and potential renal applications of contrast-enhanced ultrasound. Clin Radiol. 2012.
40 Fischer T, Filimonow S, Rudolph J, Morgera S, Budde K, Slowinski T, et al. Arrival time parametric imaging: a new ultrasound technique for quantifying perfusion of kidney grafts. Ultraschall Med. 2008;29(4):418–23.
41 Grzelak P, Szymczyk K, Strzelczyk J, Kurnatowska I, Sapieha M, Nowicki M, et al. Perfusion of kidney graft pyramids and cortex in contrast-enhanced ultrasonography in the determination of the cause of delayed graft function. Ann Transplant. 2011;16(1):48–53.
42 Aschwanden M, Partovi S, Jacobi B, Fergus N, Schulte AC, Robbin MR, et al. Assessing the end-organ in peripheral arterial occlusive disease-from contrast-enhanced ultrasound to blood-oxygen-level-dependent MR imaging. Cardiovasc Diagn Ther. 2014;4(2):165–72.
43 Duerschmied D, Olson L, Olschewski M, Rossknecht A, Freund G, Bode C, et al. Contrast ultrasound perfusion imaging of lower extremities in peripheral arterial disease: a novel diagnostic method. Eur Heart J. 2006;27(3):310–5.
44 Duerschmied D, Zhou Q, Rink E, Harder D, Freund G, Olschewski M, et al. Simplified contrast ultrasound accurately reveals muscle perfusion deficits and reflects collateralization in PAD. Atherosclerosis. 2009;202(2):505–12.
45 Duerschmied D, Maletzki P, Freund G, Olschewski M, Bode C, Hehrlein C. Success of arterial revascularization determined by contrast ultrasound muscle perfusion imaging. J Vasc Surg. 2010;52(6):1531–6.
46 Lindner J, Womack L, Barrett E, Weltman J, Price W, Harthun N, et al. Limb stress-rest perfusion imaging with contrast ultrasound for the assessment of peripheral arterial disease severity. JACC Cardiovasc Imaging. 2008;1(3):343–50.
47 Amarteifio E, Wormsbecher S, Krix M, Demirel S, Braun S, Delorme S, et al. Dynamic contrast-enhanced ultrasound and transient arterial occlusion for quantification of arterial perfusion reserve in peripheral arterial disease. Eur J Radiol. 2012.
48 Duerschmied D, Maletzki P, Freund G, Olschewski M, Seufert J, Bode C, et al. Analysis of muscle microcirculation in advanced diabetes mellitus by contrast enhanced ultrasound. Diabetes Res Clin Pract. 2008;81(1):88–92.
49 Amarteifio E, Wormsbecher S, Demirel S, Krix M, Braun S, Rehnitz C, et al. Assessment of skeletal muscle microcirculation in type 2 diabetes mellitus using dynamic contrast-enhanced ultrasound: a pilot study. Diab Vasc Dis Res. 2013;10(5):468–70.
50 Womack L, Peters D, Barrett EJ, Kaul S, Price W, Lindner JR. Abnormal skeletal muscle capillary recruitment during exercise in patients with type 2 diabetes mellitus and microvascular complications. J Am Coll Cardiol. 2009;53(23):2175–83.
51 Lindner JR, Sinusas A. Molecular imaging in cardiovascular disease: Which methods, which diseases? J Nucl Cardiol. 2013;20(6):990–1001.
52 Castle J, Butts M, Healey A, Kent K, Marino M, Feinstein SB. Ultrasound-mediated targeted drug delivery: recent success and remaining challenges. Am J Physiol Heart Circ Physiol. 2013;304(3):H350–7.
Funding / potential competing interests: Prof. Staub is supported by grants from the Swiss National Science Foundation (Grant PZ00P3_142419 and PBZHB-120997), the Swiss Society of Angiology, the University of Basel and by an unrestricted research grant from Bracco Suisse SA, Manno, Switzerland.