Figure 1
Illustration of the study design.
DOI: https://doi.org/10.4414/smw.2015.14063
Iron deficiency is the most common cause of anaemia in the western world. With respect to iron metabolism and the mechanisms of iron absorption, the peptide hepcidin is the key regulator [1]. It is mainly produced in the liver and upon binding to ferroportin it causes a reduction of intestinal iron resorption as well as of iron release from the reticuloendothelial system. Despite progress in diagnosis and treatment there is a challenge in establishing the cause of iron deficiency in the absence of obvious reasons such as blood loss or deficient dietary intake. There are no prospectively validated and reproducible oral iron absorption tests (OIAT) that can confidently evaluate iron malabsorption in an iron deficient population.
The preferred first-line treatment of iron deficiency is the per oral (p.o.) administration of iron. In case of an insufficient increase of ferritin and/or haemoglobin despite adequate iron substitution the suspicion of iron malabsorption is often postulated, and a validated OIAT would be helpful in that setting for further evaluation.
Several variants of OIAT in heterogeneous study populations have been published. Iron absorption studies in iron deficient patients and healthy persons have been performed using iron radioisotopes, but they are no longer used in clinical practice due to radiation exposure and logistical difficulties [2, 3]. There are several studies in patients with iron deficiency and/or healthy individuals using several low doses of bivalent iron, ranging from 5 to 20 mg [4–7]. It was postulated that an OIAT with low dose iron might be better at reflecting the physiological iron absorption [7]. Furthermore, a large dose of iron might overwhelm the ability of the normal intestine to reject unneeded iron [4]. Contrarily, there are reports of healthy volunteers receiving 50 mg of bivalent sodium ferrous citrate without a significant or reproducible iron increase, whereas the administration of 100 or 150 mg leads to an increment of plasma iron [8]. A significant plasma iron increase in healthy individuals after oral ingestion of 100 mg ferrous chloride was also shown in a study that used the OIAT to compare iron absorption in healthy volunteers with uremic patients [9]. The most commonly used OIAT in clinical practice in iron deficient patients is the administration of 200 mg bivalent iron in fasting condition, measuring the increase of plasma iron 2 and 4 hours later, although a validation of this test is lacking. As there are no prospectively validated and therefore reproducible reference values of such an OIAT in a homogenous iron deficient study population with proven normal iron absorption, the interpretation of these results to evaluate the cause of iron deficiency with regard to iron malabsorption is not possible. Additionally, there are differing statements about the normal increase after oral iron intake in this iron deficient population, for example a relative increase of 50–200% after 2 or 4 hours or an absolute increase of at least 9 µmol/l, respectively.
The aim of this study therefore was to establish an OIAT and to define reference values of plasma iron in healthy volunteers after oral iron intake. An amount of 200 mg oral iron was applied in order to demonstrate meaningful changes in plasma iron increment. Whether or not this OIAT will be a useful diagnostic tool to evaluate the cause of iron deficiency and especially to exclude iron malabsorption in an iron deficient population has to be prospectively validated in a future study.
Healthy volunteers without iron deficiency were included into this study. Exclusion criteria were relevant co-morbidities (inflammation, pre-existing malabsorption or liver disease) which were analysed by medical history and laboratory tests (full blood count, ferritin, CRP, ASAT). The intake of iron during the last three months before study entry or any other medication during that period was prohibited. We screened 65 apparently healthy adults after written informed consent. After screening, 16 adults had to be excluded. Therefore, 49 adults could be included into the study.

Figure 1
Illustration of the study design.

Figure 2
Absolute plasma iron concentration. Box and whiskers plot representing the median, first and third quartiles and the range of plasma iron concentration after ingestion of bivalent ferrous fumarate. Outliers are shown as circles (females) and squares (males).
Each of the 49 volunteers received, in a first test phase, 200 mg of solid bivalent ferrous fumarate in fasting condition after blood samples were drawn for plasma iron, ferritin, soluble transferrin receptor (sTfR), haemoglobin (Hb), reticulocytes (Ret) and reticulocyte-haemoglobin (Ret-He). Two and four hours later, as well as on days 3 and 5, plasma iron, Hb, Ret and Ret-He were measured, all in fasting condition. Relative plasma iron concentration was calculated in relation to the concentration before iron ingestion, which is defined as 1.0. Haematocrit did not differ relevantly among all measurements. Therefore, no adjustments were necessary. The measurement at days 3 and 5 was performed in 35 persons only as all mentioned parameters were found to be comparable to initial values. After a wash-out phase of two weeks, 23 healthy volunteers proceeded to a second test phase to receive trivalent iron hydroxide polymaltose in fasting condition and 11 healthy volunteers did not receive iron and, thus, served as a negative control. In these latter two groups the identical laboratory analyses were performed in this second test phase. The study design is summarised in figure 1.
All parameters were measured at the central laboratory at the Luzerner Kantonsspital and analysed separately according to gender.
MedCalc v12.3 (MedCalc, Mariakerke, Belgium) was used for data analysis. Results are shown as mean ± standard deviation. Two sided t-test was used for comparison. A p <0.05 was considered significant. Pearson correlation coefficient r was used for correlations.
The local cantonal ethics committee approved the study. Participants were included after written informed consent.
After screening of 65 volunteers, 16 had to be excluded. This was because of a new diagnosis of iron deficiency in 11 patients (10 females, 1 male), because of difficulties with venous puncture in two female patients and because of concomitant intake of medication during the study period in three patients (2 females, 1 male). Finally, 49 healthy persons (24 females, 25 males; age 36 ± 10 years) were studied. Of these, one missed the appointment for blood drawing on day 5, and one was not examined for ferritin, ASAT, CRP and the sTfR on day 1 of the first test phase, but these parameters were examined at day 1 of the second test phase and were all within the normal range. Clinical and initial laboratory findings of the study population are summarised in table 1.
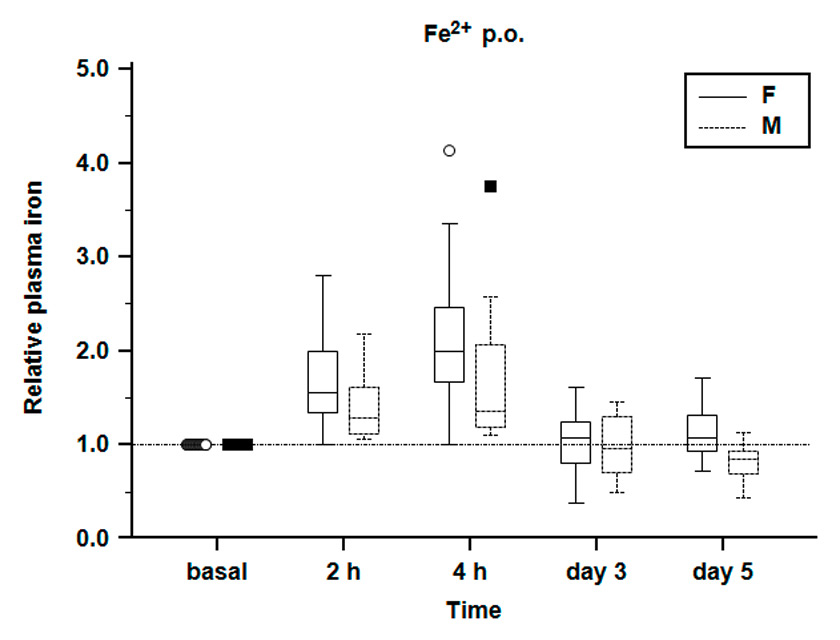
Figure 3
Relative plasma iron concentration. Relative plasma iron concentration during the first test phase. Plasma iron concentration before iron application is defined as reference = 1.0. Females show a greater relative increase of plasma iron. Plasma iron values on day 3 and 5 are comparable to initial values.
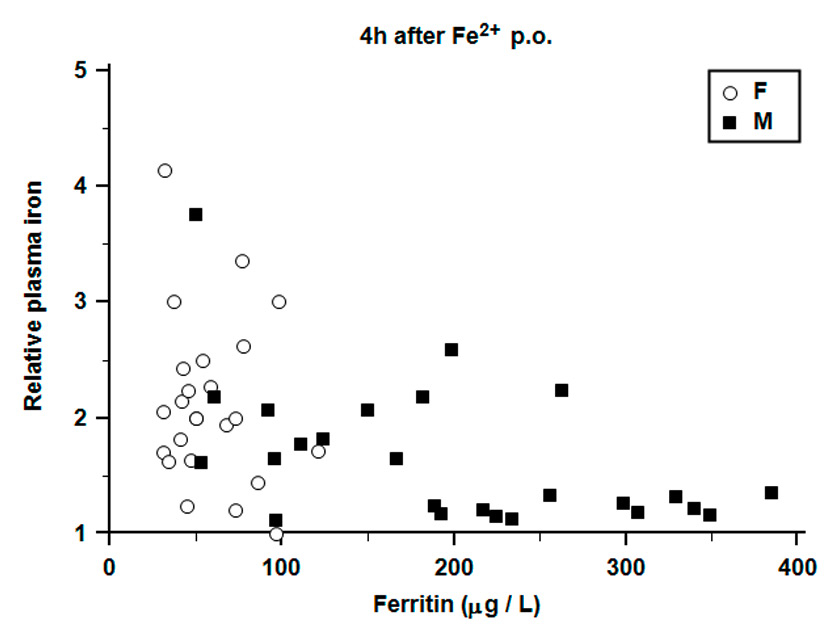
Figure 4
Correlation of initial ferritin and relative plasma iron concentration. Lower initial ferritin is correlated with a greater relative plasma iron increase. In general, females show lower ferritin values compared to males.
Plasma iron increased after taking ferrous fumarate in fasting condition from 17 ± 4 µmol/L to 27 ± 8 µmol/L in females and from 21 ± 7 µmol/l to 27 ± 9 µmol/l in males after two hours, and to 35 ± 12 µmol/l in females and 33 ± 11 µmol/l in males after four hours (both p <0.0001 compared to iron values prior iron ingestion) (fig. 2).
Relative plasma iron concentration increased from 1.0 (defined reference) after two hours to 1.7 ± 0.4 in females and 1.4 ± 0.3 in males, and after four hours to 2.1 ± 0.7 in females and 1.7 ± 0.6 in males (fig. 3). Particularly important is the greater increase of plasma iron in females as opposed to males. All other parameters did not change significantly (table 2).
On days 3 and 5, we found values for all tested parameters comparable to the initial values. Correspondingly, blood sampling was stopped on days 3 and 5 after analysing the values of 35 subjects. There was no significant increase of plasma iron during observation time of four hours after ingestion of trivalent iron in fasting condition or in volunteers not receiving iron (see figs S1 and S2 [supplement]). A negative correlation between ferritin and relative iron increase could be detected in males (r = –0.51, p = 0.008) but not in females (r = –0.17, p = 0.4); females showed lower ferritin values than males (p <0.0001) in general and a wider variation of iron increase (fig. 4).
| Table 1:Clinical and initial laboratory findings of the study population. | |||
| Reference values | Female n = 24 | Male n = 25 | |
| Age (years) | 34 ± 10 | 38 ± 9 | |
| Haemoglobin (g/l) | 115–148 (f) 127–163 (m) | 136 ± 7 | 157 ± 8 |
| Haematocrit | 0.34–0.43 (f) 0.37–0.46 (m) | 0.40 ± 0.02 | 0.45 ± 0.02 |
| Reticulocytes (G/l) | 25–120 (f) 25–100 (m) | 42 ± 10 | 55 ± 14 |
| Ret-He (pg) | 28–35 | 33 ± 2 | 34 ± 1 |
| Ferritin (µg/l) | 13–150 (f) 30–400 (m) | 59 ± 24 | 199 ± 99 |
| sTfR (mg/l) | 1.9–4.4 (f) 2.2–5.0 (m) | 2.4 ± 0.6 | 2.8 ± 0.5 |
| Plasma iron (µmol/l) | 7–26 (f) 11–28 (m) | 17 ± 4 | 21 ± 7 |
| ASAT (U/l) | <35 (f) <50 (m) | 21 ± 6 | 25 ± 5 |
| CRP (mg/l) | <5 | ≤11 | ≤11 |
| Table 2:Laboratory findings before and after ingestion of ferrous fumarate. | |||||||
| Reference values | Female (f) | Male (m) | |||||
| basal | after 2 h | after 4 h | basal | after 2 h | after 4 h | ||
| Hb (g/l) | 115–148 (f) 127–163 (m) | 136 ± 7 | 137 ± 7 | 136 ± 8 | 157 ± 8 | 157 ± 8 | 156 ± 8 |
| Ret (G/l) | 25–120 (f) 25–100 (m) | 42 ± 10 | 42 ± 11 | 40 ± 12 | 55 ± 14 | 53 ± 17 | 52 ± 15 |
| Ret-He (pg) | 28–35 | 33 ± 2 | 33 ± 1 | 33 ± 1 | 34 ± 1 | 33 ± 1 | 33 ± 1 |
| Plasma iron (µmol/l) | 7 – 26 (f) 11–28 (m) | 17 ± 4 | 27 ± 8 | 35 ± 12 | 21 ± 7 | 27 ± 9 | 33 ± 11 |
In the present study reference values of absolute and relative plasma iron concentrations after oral iron ingestion of ferrous fumarate in healthy volunteers could be established. As there are no prospectively validated reference values in an iron deficient population without proven evidence of malabsorption in commonly used OIAT, our intention was to establish these values in healthy non-iron deficient volunteers as the necessary baseline in order to study an iron deficient population in a follow-up study. Therefore we established reference values of an OIAT in healthy volunteers using ferrous fumarate, which is widely used for iron substitution in clinical practice. We are aware that it is not possible to make direct comparisons with other studies which used different iron formulations, doses and which also analysed different study populations.
We measured a maximum increase of plasma iron four hours after oral intake of bivalent iron in fasting condition. On days 3 and 5, all tested parameters were comparable to initial values. Therefore, an OIAT can be performed within four hours. Due to the fact, that all persons had to fast during that time to ensure standardised and reproducible conditions, a sampling point later than 4 hours did not seem feasible for routine clinical application and, therefore, potentially higher values of plasma iron thereafter cannot be completely excluded. It must be assumed that the change in plasma iron values is not only due to iron absorption but due to other factors as well, such as the transport of absorbed iron to iron storing organs, potentially influencing the results [2, 3]. Unfortunately, iron absorption cannot be determined directly without the use of radioisotopes so far. Therefore, one should be aware that the value of plasma iron measured after iron intake is not influenced by iron absorption alone.
A dosage of 200 mg of ferrous fumarate was chosen to be able to detect a meaningful increase of plasma iron within a time scale of four hours, comparable to commonly used OIAT in clinical practice in iron deficient patients. In most previous studies in patients with iron deficiency and / or healthy individuals, dosages of 5–20 mg bivalent iron were chosen. With regard to the significance of plasma iron increase after oral ingestion of low dose bivalent iron, their results differ. Crosby et al. assumed an overburdening of the normal intestine by available iron when using a large dose of iron [4]. In their study, which included exclusively male participants, they demonstrated by the application of a low dose OIAT the possibility of distinguishing normal iron absorption from iron absorption in iron deficiency. However, in subjects with normal iron storage there was only little change in plasma iron that does not allow the definition of a minimum cut off value for healthy persons (positive controls). Joosten et al. examined a low-dose OIAT in elderly hospitalised patients and in healthy controls using a dose of 20 mg ferrous sulphate [5]. The study aimed to investigate if the maximum plasma iron increase after oral iron ingestion of 20 mg ferrous sulphate might serve as parameter to evaluate body iron content variations. They proposed a plasma iron increase of >80 µg/dl to identify iron deficient individuals without analysing data according to gender. In another study individuals using 10 mg of ferrous sulphate in an OIAT, a significant increase of plasma iron within three hours was only shown in 17 of 37 healthy subjects [7].
A study specifically focusing on defining reference values for oral iron absorption in healthy volunteers was undertaken by Jensen et al. [6]. Proposing that the populations of previous studies were not sufficiently large to establish a reference interval for the low-dose OIAT, 122 healthy individuals were included. In this study, a low dose of 10 mg bivalent iron sulphate was chosen with a test procedure comparable to our study, but with a shorter test duration of three hours. They determined two reference intervals for plasma iron increase (presented as Cmax[µmol/l]) after oral iron intake, one for pre-menopausal women (0–34 µmol/l) and one for all other healthy individuals without iron deficiency (0–11 µmol/l). In contrast to our study they used maximum iron values independent of the time of appearance and established reference values for different groups compared to the current study.
The results of the subsequent studies are contrary to the before mentioned thesis of Crosby et al. of an overburdening of the normal intestine by available iron when using a large dose of iron. Ekenved et al. showed in their study in iron deficient patients and healthy persons through offering doses from 25 up to 100 mg of oral bivalent iron that plasma iron increase was proportional to applied iron dose [3].
In a recent study in healthy non-iron deficient individuals performing an OIAT of three hour duration, the optimal dosage of bivalent iron was analysed. After the intake of 50 mg sodium ferrous citrate (SFC) there was no significant increase of plasma iron in some cases whereas both a dosage of 100 mg and of 150 mg SFC were followed by an increase of plasma iron in all individuals [8]. The study by Bastani et al. examined an OIAT among others in healthy volunteers demonstrating a steep increase in plasma iron with a dosage of 260 mg bivalent iron [10]. These values are comparable to the current study. However, in this study the group of healthy volunteers included merely seven individuals, with the majority being males. Therefore, these analyses cannot be considered as representative but support the results of our study.
Summarising the above mentioned studies it is obvious that for the achievement of maximum iron absorption higher doses of iron are favourable compared to lower doses, although the amount of iron is different according to the used formulation. In view of our results we suggest to use 200 mg bivalent ferrous fumarate in fasting condition for an OIAT, although we cannot exclude that lower doses could have a comparable effect on iron absorption.
In our study a proportion of volunteers were tested in the second test phase without having been given iron, serving as a control group. During the test phases we could not observe any significant changes in plasma iron in that group. Thus we can assume that the increase of plasma iron seen in volunteers receiving oral bivalent ferrous fumarate is associated with the iron intake and is not attributed to physiological diurnal variations. We confirmed that oral bivalent iron is the only preparation for an OIAT leading to an increase of plasma iron, whereas patients receiving trivalent iron hydroxide polymaltose in fasting condition did not show a significant increase of plasma iron.
In patients with an unclear aetiology of iron deficiency and in those with an inadequate or absent increase of ferritin and/or haemoglobin despite oral iron substitution, the established reference values from the current study of an OIAT with bivalent ferrous fumarate in fasting condition in healthy volunteers could be helpful in evaluating suspected iron malabsorption. Whether the reference values of this OIAT can safely differentiate between presence and absence of iron malabsorption in patients with iron deficiency needs to be prospectively validated. Patients with known iron malabsorption will be needed to determine whether clinically relevant cut-offs can be established. In this respect our study should be regarded as the first step in establishing a clinically validated OIAT in patients with iron deficiency.
1 Nemeth E, Ganz T. Regulation of Iron Metabolism by Hepcidin. Annu Rev of Nutr. 2006;26:323–42.
2 Henley ED, Christenson WN, Grace WJ, Wolff HG. Absorption of Iron from the Gastrointestinal Tract. A comparative study of the oral iron tolerance test in human beings using stable and radioactive Iron. Am J Clin Nutr. 1956;4:609–18.
3 Ekenved G, Norrby A, Sölvell L. Serum iron increase as a measure of iron absorption studies on the correlation with total absorption. Scand J Haematol Suppl. 1976;28:31–49.
4 Crosby WH, O'Neil-Cutting MA. A small-dose iron tolerance test as an indicator of mild iron deficiency. JAMA. 1984;251:1986–7.
5 Joosten E, Vander Elst B, Billen J. Small-dose oral iron absorption test in anaemic and non-anaemic elderly hospitalized patients. Eur J Haematol. 1997;58: 99–103.
6 Jensen NM, Brandsborg M, Boesen AM, Yde H, Dahlerup JF. Low-dose oral iron absorption test: establishment of a referencal interval. Scand J Clin Lab Invest. 1998;58:511–20.
7 Costa A, Liberato LN, Palestra P, Barosi G. Small-dose iron tolerance test and body iron content in normal subjects. Eur J Haematol. 1991;46:152–7.
8 Kobune M, Miyanishi K, Takada K, Kawano Y, Nagashima H, Kikuchi S, et al. Establishment of a simple test for iron absorption from the gastrointestinal tract. Int J Hematol. 2011;93:715–9.
9 Goch J, Birgegård G, Danielson BG, Wikström B. Iron absorption in patients with chronic uremia on maintenance hemodialysis and in healthy volunteers measured with simple oral iron load test. Nephron. 1996;73:403–6.
10 Bastani B, Islam S, Boroujerdi N. Iron absorption after single pharmacological oral iron loading test in patients on chronic peritoneal dialysis and healthy volunteers. Perit Dial Int. 2000;20:662–6.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.