Figure 1
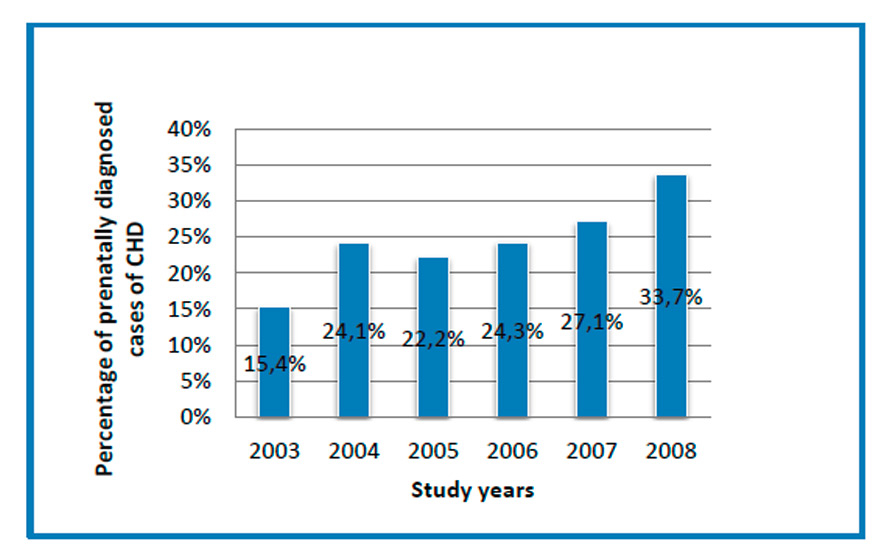
The percentage of prenatally diagnosed cases of CHD by year, demonstrating a significant increase between 2003 and 2008 (p = 0.021).
DOI: https://doi.org/10.4414/smw.2014.14068
Although there has been increasing interest in the prenatal diagnosis of congenital heart disease (CHD) over the last decade, there is little epidemiological evidence regarding the effectiveness and impact of regionally organised screening programmes in this field.
Nevertheless, with the introduction of foetal ultrasonography in the early 1980s and the routine incorporation of cardiac scanning, it is now possible to diagnose a wide range of CHDs during foetal life with a high degree of diagnostic accuracy, particularly when the examination is performed at a specialised centre [1].
Several studies have reported an average antenatal CHD detection rate of 23–28% in the general population [2–4]. Population-based data should, however, be considered separately from tertiary centres' reports, which cite much higher detection rates [5].
Allan et al. reported a decrease in the prevalence of hypoplastic left heart syndrome (HLHS) in infancy due to the impact of prenatal diagnosis [6, 7]. These data were confirmed by Bull in 1999 [2].
Carvalho et al. showed that the addition of outflow tract views to the four-chamber view and operator training resulted in an antenatal detection rate of 75% for major CHD [8].
Meanwhile, the potential impact of the widespread use of foetal echocardiography, increased prenatal diagnosis, and termination of pregnancy (TOP) in cases of foetal anomalies on reducing the prevalence of CHD at birth has not yet been assessed. However, all of these factors are likely to result in a significant change in the paediatric population with congenital cardiac disease, with a major impact on healthcare costs. Thus, the aim of this study was to analyse the evolution of prenatal diagnosis of CHD in our local population and its repercussions for the prevalence rate of CHD at birth, using the reliable database of the Eurocat Registry of Vaud-Switzerland [9, 10].
| Table 1:Groups 1–5 were based on the type and severity of CHD. The category definition and associated pathologies in each group are provided. | ||
| Group | Definition | Pathologies included |
| 1 | Severe heart disease for which only palliative surgery is available (Fontan-like circulation) | HLHS, single-ventricle heart |
| 2 | Heart disease with immediate severe neonatal morbidity and mortality requiring early surgery | Transposition of the great arteries |
| 3 | Heart disease requiring immediate post-natal care and/or deferred surgical or interventional correction | Conotruncal abnormalities (tetralogy of Fallot, pulmonary atresia, truncus arteriosus, double-outlet right ventricle), malalignment VSD, AVSD |
| 4 | Heart disease requiring post-natal follow-up | Aortic stenosis, isolated discrete coarctation, pulmonary stenosis, Ebstein's anomaly, large perimembranous VSDs (requiring an operation), cardiomyopathy, CHD associated with other anomalies |
| 5 | Minor CHD with no impact on outcome | Small VSDs, ASD II |
| CHD = congenital heart disease; HLHS = hypoplastic left heart syndrome; AVSD = atrioventricular septal defect; VSD = ventricular septal defect; ASD = atrial septal defect. | ||
We performed a retrospective analysis to evaluate the evolution of prenatal diagnosis of CHD and its repercussions for the prevalence of CHD at birth in the Canton of Vaud, in south-western Switzerland. The study period was from 1.5.2003 to 31.12.2008. The Canton of Vaud has a population of 684,922 and a birth rate of 112/10,000 (2008). The Eurocat Registry of Vaud-Switzerland provided data on all cases of CHD (live births, stillbirths, and TOP) during this period. EUROCAT is a European network of population-based registries for the epidemiological surveillance of congenital anomalies, incorporating more than 1.5 million births per year in Europe (with a total of 43 registries in 23 countries, covering 29% of European births) [9]. Eurocat Vaud is one of those registries, covering the Canton of Vaud [10]. The method of data collection and the Eurocat membership criteria are well described elsewhere [9–10]. The Eurocat Registry of Vaud uses multiple sources of information for case ascertainment to cover all outcomes of congenital anomalies, including live birth, foetal death from 20 weeks' gestation and TOP. Sources include files from foetal medicine units, cytogenetic and medical genetic records, neonatology, maternity, paediatric, cardio-paediatric and surgical units, paediatricians in private practise, and anatomopathological reports.
The data extracted from the registry were pregnancy outcomes (live births, stillbirths, and TOP), all diagnosed malformations and syndromes, and the time of diagnosis (prenatally, at birth or later).
Cases of patent ductus arteriosus (PDA) and patent foramen ovale (PFO) and cases considered as variations of normal were excluded.
The population data were provided by the Swiss federal statistics database and the Service Cantonal de Recherche et d'Information Statistiques (SCRIS). Prenatal ultrasound screening for congenital malformations in Switzerland is routinely performed in the second trimester, either by a general obstetrician-gynaecologist or by specialists in foetal-maternal medicine, with an estimated percentage of 90% of pregnant women undergoing a scan in the Canton of Vaud. Prenatally suspected cardiac malformations are most often referred for confirmation to the foetal-maternal unit of the University Hospital of Vaud in Lausanne, a secondary and tertiary referral centre for patients living in the Canton of Vaud. In the present study, a team consisting of a paediatric cardiologist and specialists in foetal-maternal medicine performed the cardiac ultrasound investigations. Every case of CHD was explained and discussed with the parents by a multidisciplinary team after the examination. Counselling included an explanation of the normal foetal heart, the particular pathology diagnosed, the short-term outcome of possible interventions, and the long-term clinical condition and quality of life of CHD patients. Co-morbidities, if present, were taken into account in these discussions. Parents were assured of support, regardless of their final decision.
The obstetricians' teaching programme and postgraduate training consisted of repeated courses in foetal cardiology with hands-on training. The in-house training and teaching of obstetric ultrasonographers was intensified during the study period to include different cardiac views, with outflow tract views added to the four-chamber view. Moreover, since 2003, the joint presence of obstetricians and paediatric cardiologists during echocardiographic sessions has permitted direct feedback from and teaching by the specialised foetal cardiologists. High-resolution ultrasound equipment with cineloop technology was used (Voluson 730 Expert, GE Healthcare, Glattbrugg, CH, from 2003 to March 2007 and Voluson E8 Expert, GE Healthcare, Glattbrugg, CH, since March 2007).
Referral indications for echocardiography were both maternal and foetal. Maternal referral indications included a family history of CHD, maternal pathology (e.g., maternal diabetes), lupus erythematosus, Sjögren's disease, and exposure to toxins. Foetal referral indications included abnormal nuchal translucency, suspected cardiac anomaly, foetal arrhythmia, and foetal hydrops. All data from the prenatal cardiac evaluations were recorded in a local database.
Cardiac anomalies diagnosed at primary or secondary centres and ending in TOP without requiring a referral and cases observed at tertiary centres outside the canton were included in the prenatally diagnosed group.
All CHD cases extracted from the local Eurocat database were analysed and compared with the results of the clinical database, when available. The cases were then assigned to one of five different groups based on CHD severity, as defined in table 1. Foetuses with more than one cardiac malformation were listed only once and included in the group corresponding to the most severe malformation. The outcomes were categorised into the following three groups: live birth, TOP and intrauterine death. Cases were classified according to the presence or absence of an associated chromosomal anomaly. Non-chromosomal cases were classified as isolated in the absence of an additional major malformation; otherwise, they were classified as multiple. We compared the overall percentages of prenatally diagnosed defects during the study period, stratified by severity category. We also calculated and compared the overall prevalence rates and live birth prevalence rates for all CHD groups and non-chromosomal CHD groups and for each group separately.
Statistical analysis was performed using Stata 11 (Stata Corporation, College Station, TX, USA). The data are expressed as frequencies, percentages, or means. The prenatal detection rates, overall prevalence rates, and prevalence rates at birth were compared using Fisher's exact test for categorical variables. Data with p-values <0.05 were considered significant.
| Table 2:Numbers of births, CHD cases, prenatally diagnosed CHD cases, and TOP decisions; total prevalence rates; and prevalence rates at birth for CHD (any CHD, CHD after exclusion, non-chromosomal CHD, and group 1 CHD), as well as a comparison of total CHD and CHD prevalence rates at birth in the Canton of Vaud, Switzerland (1.5.2003 to 31.12.2008). | |||||||
| Number of births during the study period (1.5.2003 to 31.12.2008) | CHD, n | Prenatally diagnosed CHD, n | TOP, n | Total prevalence of CHD per 10,000 (LB, SB, TOP) | Prevalence of CHD at birth per 10,000 (LB, SB) | p-value | |
| Any CHD (Eurocat data) | 41573 | 572 | 137.6 | 119.6 | 0.023 | ||
| CHD (after exclusion*) | 535 | 135 | 74 | 128.6 | 110.9 | 0.0199 | |
| Non-chromosomal CHD | 471 | 81 | 30 | 113.3 | 106.1 | 0.3178 | |
| Group 1 CHD | 32 | 28 | 24 | 7.7 | 1.9 | <0.0001 | |
| CHD = congenital heart disease; LB = live births; SB, stillbirths; TOP = termination of pregnancy. * Number of CHD cases after exclusion of patent foramen ovale, patent ductus arteriosus, and cases considered as variations of the norm. | |||||||
During the study period, there were 41,573 births in the Canton of Vaud (Switzerland). The Eurocat Registry of Vaud-Switzerland reported 1,599 cases of congenital anomalies, including 572 cases of CHD, resulting in an overall CHD prevalence of 137.6/10,000. In total, 37 cases were excluded from our study (PFO, PDA, and anomalies considered as variations of the norm), resulting in a total of 535 CHD cases included in our study.

Figure 1
The percentage of prenatally diagnosed cases of CHD by year, demonstrating a significant increase between 2003 and 2008 (p = 0.021).
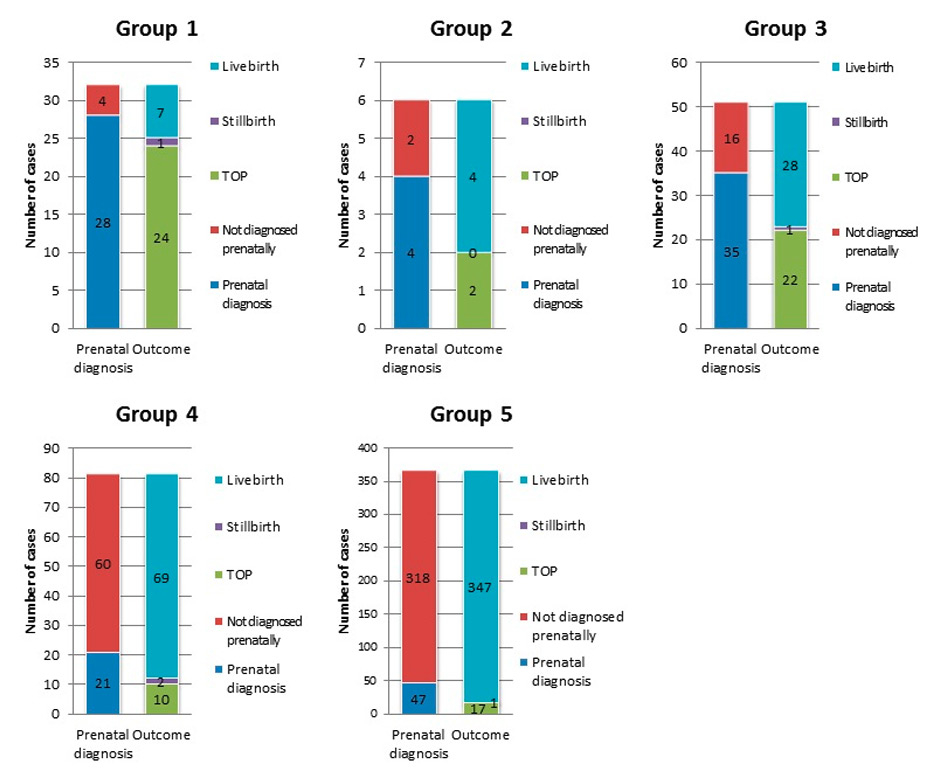
Figure 2
The total number of cases of CHD in groups 1–5, along with the number of prenatally diagnosed cases and their outcomes (live birth, stillbirth, and TOP).
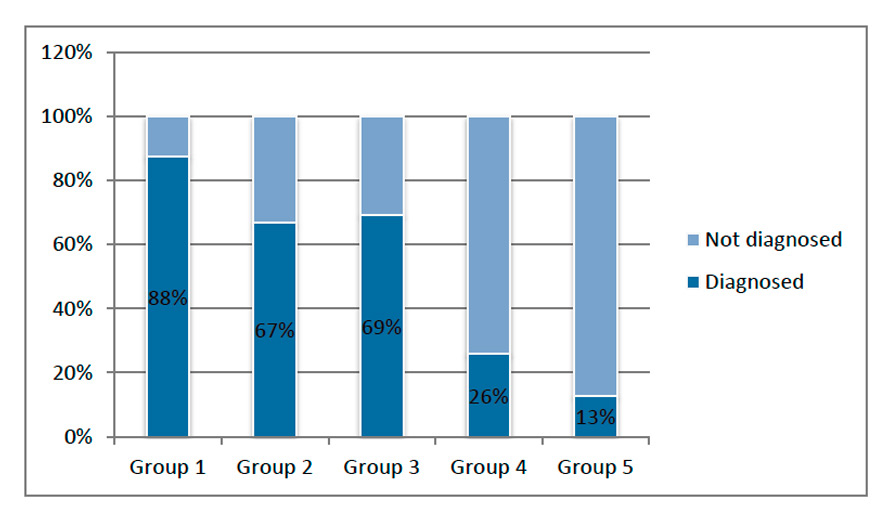
Figure 3
The percentage of prenatally diagnosed CHD cases in groups 1–5.
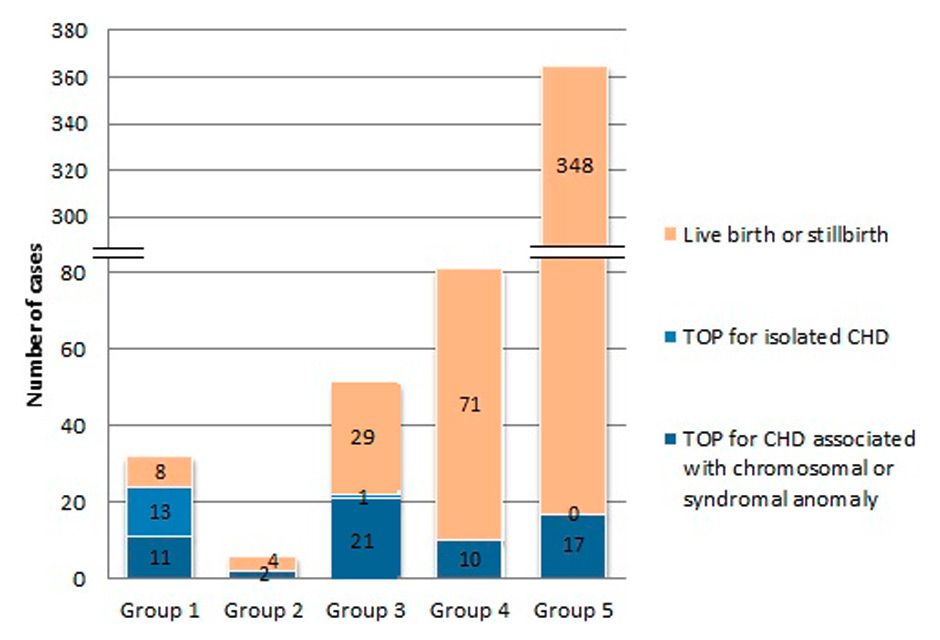
Figure 4
The outcomes of pregnancies in groups 1–5 (live birth or stillbirth, TOP for isolated CHD, or TOP for CHD associated with a chromosomal or syndromal anomaly).
During the study period, 135 cases of CHD were diagnosed prenatally, corresponding to a mean detection rate of 25.2%. The detection rate increased significantly between the years 2003 and 2008 (from 15.4 to 33.7%, p = 0.021) (fig. 1).
Figure 2 shows the total number of cases of CHD in each severity group, along with the number of prenatally diagnosed cases and their outcomes in each group. Group 1 accounted for 6% of all CHD, group 2 for 1.1%, group 3 for 9.5%, group 4 for 15.1%, and group 5 for 68.2%.
The CHD cases included in the groups 1–4 were considered significant in our study and accounted for 31.8% of all CHD (170/535). The overall prenatal detection rate for CHD in the first four groups (groups 1–4) was 51.8% (88/170). Figure 3 shows the rates of prenatal diagnosis in the different groups, with the highest detection rate (87.5%) found in the group with the most severe CHD (group 1). The detection rates in the groups 2 and 3 were 66% (group 2) and 68.6% (group 3), respectively, with a lower rate in group 4 (25.9%). The fifth group consisted of 365 cases of minor CHD with no impact on the outcome, and prenatal diagnosis was the least frequent in this group (12.9%).
Approximately half of the prenatally diagnosed cases were examined in our echocardiographic unit, and the other cases were observed at primary or secondary centres or tertiary centres outside the canton. Most of the cases that were not referred to tertiary centres led to TOP due to associated chromosomal, syndromal, or other major congenital anomalies, and post-mortem examination results were available in 68% of the TOP cases.
Group 1 consisted of 32 cases, 87.5% of which were detected antenatally, leading to TOP in 85.7% of the cases. A total of 46% cases were associated with a chromosomal or other anomaly.
In groups 2–5, the proportion of TOP varied among the different groups: 33.3% in group 2, 43.1% in group 3, 12% in group 4 and 4.6% in group 5. All TOP procedures were performed due to associated chromosomal, syndromal, or other major congenital anomalies, with the exception of one case of truncus arteriosus (fig. 4).
In group 3, 66% of CHD cases were associated with chromosomal, syndromal, or other major congenital anomalies (34/51). The rate of prenatal diagnosis in this group was 68.6%, and the rate was even higher in the case of associated anomalies (75.6%). In particular, atrioventricular septal defect (AVSD) was associated in 90.9% of cases with other anomalies (mostly chromosomal), and the rates of prenatal diagnosis and TOP in this subgroup were 86% and 68%, respectively.
In total, 88% of CHD cases were not associated with a chromosomal anomaly, yielding an overall non-chromosomal CHD prevalence rate of 113.3/10,000 and a prevalence rate at birth of 106.1/10,000 (table 2). Most were live births, and 78.5% were isolated CHD cases.
The overall prevalence rate of CHD during the study period was 137.6/10,000, and the rate was 128.6/10,000 after excluding cases of PFO and PDA (table 2). In the specific groups, the overall CHD prevalence rates were 7.7/10,000 in group 1, 1.4/10,000 in group 2, 12.3/10,000 in group 3, 19.5/10,000 in group 4, and 87.8/10,000 in group 5. Additionally, the prevalence rates at birth were 110.9/10,000 for all CHD cases, 106.1/10,000 for non-chromosomal CHD cases, and 1.9/10,000 for group 1 CHD cases. In group 1, the rate of CHD at birth was significantly lower than the overall prevalence rate (table 2).
This was a retrospective study on the effect of a prenatal screening programme on the prevalence of congenital cardiac malformations at birth in a small but well-defined population. This study differs from previous studies because of its highly accurate epidemiological data. The Eurocat Registry of Vaud provided data on all cases with a diagnosis of cardiac congenital malformation (stillbirth, live birth, and TOP, including CHD diagnosed up to 1 year of life), and our tertiary care centre provided precise foetal and neonatal data for our cases. The total prevalence rate of CHD during the study period was 137.6/10,000, which is considerably higher than the previously reported prevalence. Van der Linde et al. showed a gradual increase of <1% in the incidence of congenital heart defects over time [11]. However, prevalence rates similar to ours were reported by Dolk et al. in 2011 [5]. This increased prevalence may be due to the over-reporting of intrauterine-diagnosed defects that correct themselves at term, such as a prenatally diagnosed muscular ventricular septal defect (VSD), which tends to close before birth [12]. Another explanation could be a higher rate of high-risk pregnancies, such as maternal diabetes associated with septal hypertrophy during pregnancy [13].
We observed a significant increase in prenatally diagnosed CHD between 2003 and 2008, likely due to our organised and focused training efforts, including outreach teaching courses. These efforts led to increased awareness and better knowledge of the diagnostic tools available in our obstetric community [2, 8, 14–16].
Improved imaging technology may also have contributed to our success. Although our study shows prenatal diagnosis rates similar to those in previous studies [2, 3, 17, 18], the present study shows a clearer relationship between the severity of CHD and the decision to terminate the pregnancy. For example, in 1999, Bull et al. reported a prenatal detection rate of 66–69% for hypoplastic left heart and univentricular heart [2], and our study revealed a prenatal detection rate of 88% for group 1 CHD cases. However, the proportion of parents deciding to terminate the pregnancy remained consistent over time. This result could mean that despite the improvement of the diagnostic and therapeutic armamentarium, parents' perceptions of the future quality of life of their offspring have remained unchanged.
Counselling parents after prenatal diagnosis of CHD is therefore an important task for our multidisciplinary team, and the prognosis will be based on not only the diagnosis itself but also its accuracy; the gestational age at diagnosis; any association with extracardiac malformation; and the known results of treatment options, including local surgical results. If parents decide to terminate the pregnancy, we suggest a period of reflection of 2–3 days, which could be implemented to ensure a prudent decision (the minimum time accepted would be 24 hours in special cases). In Switzerland, TOP is practised until the age of viability (24 weeks of gestation). The parental choice regarding TOP in cases of severe heart disease varies among different groups of parents, depending on socio-religious background, educational status, and geographical location, with large differences especially identified between the US and Europe [19]. In Switzerland, the rate of termination matches the rates reported in France [20] and the UK [2] but is higher than those in the Czech Republic [17] and The Netherlands [19].
The division of CHD cases into groups was based on the severity of CHD and an immediate need for intervention. Transposition of the great arteries (TGA) was evaluated separately, considering the high risk of immediate postnatal morbidity and mortality [2].
In the most severe group (group 1), in which only palliative intervention could be offered, TOP was prevalent, occurring in 86% of cases. This choice can be explained by the prospect of a limited quality of life and frequently associated complications, including the need for multiple operations, the risk of neuro-developmental delay, protein-losing enteropathy, arrhythmias, cirrhosis, and thromboembolic events, and the need for cardiac transplantation. These complications have all been observed and reported in long-term follow-up studies [21–33].
The in-hospital delivery option for babies with prenatally diagnosed CHD allows immediate neonatal treatment and prevention of hypoxia and cerebral damage [34–37]. Although the limited size of our study did not allow definitive conclusions regarding in-hospital delivery, previous publications were conclusive [38].
In groups 2–5, the number of terminations was limited and occurred nearly exclusively in cases of foetuses with associated chromosomal, syndromal, or other major congenital anomalies (fig. 3) [39].
Neonates in group 5, with minor CHD, were less frequently prenatally diagnosed, but this had no impact on their outcome.
The Eurocat Registry provided data on all CHD anomalies registered and on whether the anomalies were detected prenatally, permitting us to calculate the percentage of prenatally diagnosed cases of CHD in the population of the Canton of Vaud. However, these data did not allow any conclusion regarding the specificity of prenatal screening, as the real number of all false-positive cases was not assessed for this population.
The impact of the prenatal diagnosis of CHDs in general paediatric cardiology practise is important. In countries where prenatal sonographic evaluation is standard practise, a measurable reduction in the prevalence of live births with CHD has already been demonstrated [2, 40]. In particular, Germanakis et al. calculated an assumed overall reduction of 15% in the prevalence of the most severe malformations [40]. Our study showed a significant reduction (14%) in the overall CHD prevalence and a difference of 6.4% when excluding cases with chromosomal anomalies. The impact of prenatal diagnosis on the prevalence rate at birth was the highest in group 1. Although this study did not investigate the direct changes in the number of surgical interventions, we can assume that if this result is accurate, prenatal diagnosis must have a significant impact on the care of patients with CHD. Additionally, it is likely that all of these factors can lead to a change in the paediatric population with congenital cardiac disease.
Acknowledgements:We thank the sonographers, midwives, and doctors in the Fetal Medicine Department of CHUV, without whose contributions this work would not have been completed. Special thanks also go to Karine Lepigeon for her support and contributions to the statistical analyses.
1 Allan LD, Sharland GK, Milburn A, Lockhart SM, Groves AM, Anderson RH, et al. Prospective diagnosis of 1,006 consecutive cases of congenital heart disease in the fetus. J Am Coll Cardiol. 1994;23:1452–8.
2 Bull C. Current and potential impact of fetal diagnosis on prevalence and spectrum of serious congenital heart disease at term in the UK. British Paediatric Cardiac Association. Lancet. 1999;354:1242–7.
3 Garne E, Stoll C, Clementi M. Evaluation of prenatal diagnosis of congenital heart diseases by ultrasound: experience from 20 European registries. Ultrasound Obstet Gynecol. 2001;17:386–91.
4 Grandjean H, Larroque D, Levi S. The performance of routine ultrasonographic screening of pregnancies in the Eurofetus Study. Am J Obstet Gynecol. 1999;181:446–54.
5 Dolk H, Loane M, Garne E. Congenital heart defects in Europe: prevalence and perinatal mortality, 2000 to 2005. Circulation. 2011;123:841–9.
6 Allan LD, Apfel HD, Printz BF. Outcome after prenatal diagnosis of the hypoplastic left heart syndrome. Heart. 1998;79:371–3.
7 Allan LD, Cook A, Sullivan I, Sharland GK. Hypoplastic left heart syndrome: effects of fetal echocardiography on birth prevalence. Lancet. 1991;337:959–61.
8 Carvalho JS, Mavrides E, Shinebourne EA, Campbell S, Thilaganathan B. Improving the effectiveness of routine prenatal screening for major congenital heart defects. Heart. 2002;88:387–91.
9 EUROCAT. EUROCAT Guide 1.3. Instructions for the Registration and Surveillance of Congenital Anomalies. Available from: www.eurocat-network.eu/ABOUTUS/DataCollection/GuidelinesforRegistration/Guide1_3InstructionManual (28 August 2013)
10 EUROCAT. Member Registries. Available from: www.eurocat-network.eu/aboutus/memberregistries (15 August 2013)
11 van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58:2241–7.
12 Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–900.
13 Ullmo S, Vial Y, Di Bernardo S, Roth-Kleiner M, Mivelaz Y, Sekarski N, et al. Pathologic ventricular hypertrophy in the offspring of diabetic mothers: a retrospective study. Eur Heart J. 2007;28:1319–25.
14 Hunter S, Heads A, Wyllie J, Robson S. Prenatal diagnosis of congenital heart disease in the northern region of England: benefits of a training programme for obstetric ultrasonographers. Heart. 2000;84:294–8.
15 McBrien A, Sands A, Craig B, Dornan J, Casey F. Impact of a regional training program in fetal echocardiography for sonographers on the antenatal detection of major congenital heart disease. Ultrasound Obstet Gynecol. 2010;36:279–84.
16 Ogge G, Gaglioti P, Maccanti S, Faggiano F, Todros T. Prenatal screening for congenital heart disease with four-chamber and outflow-tract views: a multicenter study. Ultrasound Obstet Gynecol. 2006;28:779–84.
17 Marek J, Tomek V, Skovranek J, Povysilova V, Samanek M. Prenatal ultrasound screening of congenital heart disease in an unselected national population: a 21–year experience. Heart. 2010;97:124–30.
18 McBrien A, Sands A, Craig B, Dornan J, Casey F. Major congenital heart disease: antenatal detection, patient characteristics and outcomes. J Matern Fetal Neonatal Med. 2009;22:101–5.
19 Verheijen PM, Lisowski LA, Plantinga RF, Hitchcock JF, Bennink GB, Stoutenbeek P, et al. Prenatal diagnosis of the fetus with hypoplastic left heart syndrome management and outcome. Herz. 2003;28:250–6.
20 Khoshnood B, De Vigan C, Vodovar V, Goujard J, Lhomme A, Bonnet D, et al. Trends in prenatal diagnosis, pregnancy termination, and perinatal mortality of newborns with congenital heart disease in France, 1983–2000: a population-based evaluation. Pediatrics. 2005;115:95–101.
21 Coon PD, Rychik J, Novello RT, Ro PS, Gaynor JW, Spray TL. Thrombus formation after the Fontan operation. Ann Thorac Surg. 2001;71:1990–4.
22 Dadlani GH, Braley K, Perez-Colon E, Stapleton G, Crawford M, Turpin D, et al. Long-term management of patients with hypoplastic left heart syndrome: the diagnostic approach at All Children's Hospital. Cardiol Young. 2011;21(Suppl 2):80–7.
23 Diller GP, Giardini A, Dimopoulos K, Gargiulo G, Muller J, Derrick G, et al. Predictors of morbidity and mortality in contemporary Fontan patients: results from a multicenter study including cardiopulmonary exercise testing in 321 patients. Eur Heart J. 2010;31:3073–83.
24 Feinstein JA, Benson DW, Dubin AM, Cohen MS, Maxey DM, Mahle WT, et al. Hypoplastic left heart syndrome: current considerations and expectations. J Am Coll Cardiol. 2012;59:S1–42.
25 Goldberg CS, Mussatto K, Licht D, Wernovsky G. Neurodevelopment and quality of life for children with hypoplastic left heart syndrome: current knowns and unknowns. Cardiol Young. 2011;21(Suppl 2):88–92.
26 Idorn L, Olsen M, Jensen AS, Juul K, Reimers JI, Sorensen K, et al. Univentricular hearts in Denmark 1977 to 2009: Incidence and survival. Int J Cardiol. 2013;167:1311–6.
27 Mondesert B, Marcotte F, Mongeon FP, Dore A, Mercier LA, Ibrahim R, et al. Fontan circulation: success or failure? Can J Cardiol. 2013;29:811–20.
28 Motoki N, Ohuchi H, Miyazaki A, Yamada O. Clinical profiles of adult patients with single ventricular physiology. Circ J. 2009;73:1711–6.
29 Puosi R, Korkman M, Sarajuuri A, Jokinen E, Mildh L, Mattila I, et al. Neurocognitive development and behavioral outcome of 2–year-old children with univentricular heart. J Int Neuropsychol Soc. 2011;17:1094–103.
30 Sarajuuri A, Jokinen E, Mildh L, Tujulin AM, Mattila I, Valanne L, et al. Neurodevelopmental burden at age 5 years in patients with univentricular heart. Pediatrics. 2012;130:e1636–46.
31 Sugimoto A, Ota N, Ibuki K, Miyakoshi C, Murata M, Tosaka Y, et al. Risk factors for adverse neurocognitive outcomes in school-aged patients after the Fontan operation. Eur J Cardiothorac Surg. 2013;44:454–61.
32 van den Bosch AE, Roos-Hesselink JW, Van Domburg R, Bogers AJ, Simoons ML, Meijboom FJ. Long-term outcome and quality of life in adult patients after the Fontan operation. Am J Cardiol. 2004;93:1141–5.
33 Walker HA, Gatzoulis MA. Prophylactic anticoagulation following the Fontan operation. Heart. 2005;91:854–6.
34 Hutter PA, Kreb DL, Mantel SF, Hitchcock JF, Meijboom EJ, Bennink GB. Twenty-five years' experience with the arterial switch operation. J Thorac Cardiovasc Surg. 2002;124:790–797.
35 Verheijen PM, Lisowski LA, Wassink S, Visser GH, Meijboom EJ. Preoperative acidosis and infant development following surgery for congenital heart disease. Herz. 2010;35:358–63.
36 Verheijen PM, Lisowski LA, Stoutenbeek P, Hitchcock JF, Brenner JI, Copel JA, et al. Prenatal diagnosis of congenital heart disease affects preoperative acidosis in the newborn patient. J Thorac Cardiovasc Surg. 2001;121:798–803.
37 Verheijen PM, Lisowski LA, Stoutenbeek P, Hitchcock JF, Bennink GB, Meijboom EJ. Lactacidosis in the neonate is minimized by prenatal detection of congenital heart disease. Ultrasound Obstet Gynecol. 2002;19:552–5.
38 Bonnet D, Coltri A, Butera G, Fermont L, Le Bidois J, Kachaner J, et al. Detection of transposition of the great arteries in fetuses reduces neonatal morbidity and mortality. Circulation. 1999;99:916–8.
39 Mogra R, Zidere V, Allan LD. Prenatally detectable congenital heart defects in fetuses with Down syndrome. Ultrasound Obstet Gynecol. 2011;38:320–4.
40 Germanakis I, Sifakis S. The impact of fetal echocardiography on the prevalence of liveborn congenital heart disease. Pediatr Cardiol. 2006;27:465–72.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article were reported.