Interventional treatment for structural heart disease: who is deciding, and can we afford it?
DOI: https://doi.org/10.4414/smw.2014.14046
Raban
Jeger, Stefan
Toggweiler
Summary
During the last years, the numbers of interventions in structural heart disease such as transcatheter aortic valve implantation (TAVI), percutaneous treatment of mitral regurgitation using the MitraClip, closure of atrial septal defects (ASD) and others have constantly increased. While the 20th century was called the century of surgery, it appears that the present century might be the century of minimally invasive percutaneous therapy. The reduced invasiveness of these procedures and the success in elderly patients make these treatments increasingly attractive for younger and healthier patients. Now that these procedures are moving forward, some questions arise, namely, who is deciding on treatment modality, and can we afford it?
Introduction
Structural heart disease consists of congenital conditions such as complex congenital heart disease, patent foramen ovale (PFO), ASD, ventricular septal defects (VSD), and hypertrophic obstructive cardiomyopathy (HOCM), and conditions which are acquired during life such as dysfunctions of the native aortic and mitral valves and paravalvular leaks following valve replacement. A special entity is the left atrial appendage, which actually is a normal cardiac structure; however, since it may be the source of emboli in atrial fibrillation, it represents a possible target for interventional treatment.
Traditionally, the gold standard in the treatment of structural heart disease has been open heart surgery. However, due to the ease of interventional therapy and the often increased perioperative risk, catheter-based treatment has become a real alternative in many conditions. In parallel, economic aspects have become more and more important.
Costs normally include the costs of the initial treatment and all follow-up therapies as well as the costs of all future events, while effectiveness is measured by quality of life-adjusted life years gained (QUALY) [1]. Cost-effectiveness analysis includes the comparison of two or more therapies with calculation of incremental cost-effectiveness ratios (ICER). ICER are defined as the difference in costs between two therapies divided by the difference in QALYs and are expressed as a certain sum per QUALY which is the value of a therapy in comparison to another. If the ICER are smaller than the so-called willingness-to-pay threshold, the therapy is deemed cost-effective. Most countries do not make explicit statements about their willingness-to-pay ratios. However, common willingness-to-pay ratios are $US 50,000 in the United States and £20,000 to £30,000 in Great Britain. In the case that one therapy is both more effective and cost saving than the other, it is called “dominant”.
In the following, we will review the most frequent interventional treatments in structural heart disease, i.e., TAVI, MitraClip, PFO and ASD closure, and left atrial appendage (LAA) occlusion, with special focus on costs and cost-effectiveness.
Transcatheter aortic valve implantation (TAVI)
TAVI has been proven highly effective in patients with severe aortic valve stenosis who are not candidates for open-heart surgery [2–5]. TAVI is often performed in patients who are operable but at high surgical risk due to a prior open heart surgery, a severely calcified or porcelain aorta, chronic kidney disease, a low ejection fraction, or lung disease [6, 7]. The randomised Placement of AoRTic TraNscathetER Valve Trial (PARTNER) A trial has included such high-risk patients and showed that mortality after TAVI and surgical aortic valve replacement (SAVR) was relatively equal (3.4% vs 6.5% after TAVI and SAVR, respectively, p = 0.07) [6]. However, the combined rate of stroke and transitory ischaemic attacks was significantly higher after TAVI at 30 days (5.5% vs 2.4%, p = 0.04), but there was no difference in strokes at 3 years follow-up (8.2% vs 9.3%, p = 0.76) [8]. In the recently published U.S. CoreValve Study, TAVI was associated with a significantly higher survival rate at 1 year (85.8%) than SAVR (80.9%, p = 0.04), and the rate of strokes at 1 year was 8.8% in the TAVI group and 12.6% in the SAVR group (p = 0.10) [9]. Other complications after TAVI include paravalvular leaks (moderate in about 10% of patients), vascular injury (major vascular complications in about 10% of patients), and need for permanent pacemaker (about 25% of patients with self-expanding valves and about 8%–15% of patients with balloon-expandable valves) [7]. Encouraged by trial results and by personal and institutional experience, many physicians started performing TAVI in selected moderate or low-risk patients. Preliminary studies have shown that improved results may be anticipated from such patients [10]. Such lower risk patients are generally younger and have a longer life expectancy. Therefore, durability of the transcatheter heart valve becomes an issue, and complications such as stroke, paravalvular regurgitation, and need for a permanent pacemaker need to be minimised. Currently, there are many new transcatheter valves on a rapidly evolving market.
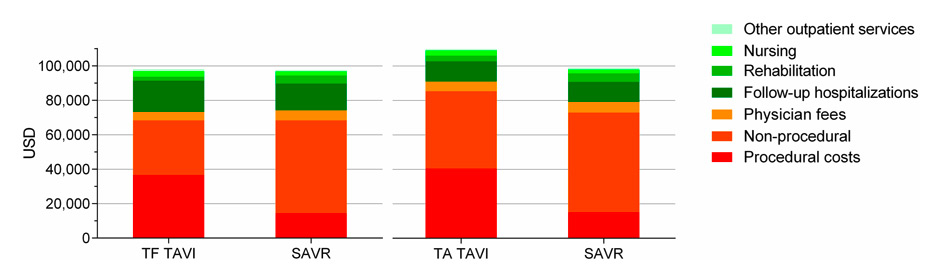
Figure 1
Cumulative 1 year costs of TAVI and SAVR in the PARTNER trial in USD.
Procedural costs were generally higher with TAVI, mainly due to the higher costs of the implant and personnel. However, non-procedural costs were lower with TAVI, mainly due to the shorter hospitalisation period.
TAVI = transcatheter aortic valve implantation; TF = transfemoral; SAVR = surgical aortic valve replacement; TA = transapical
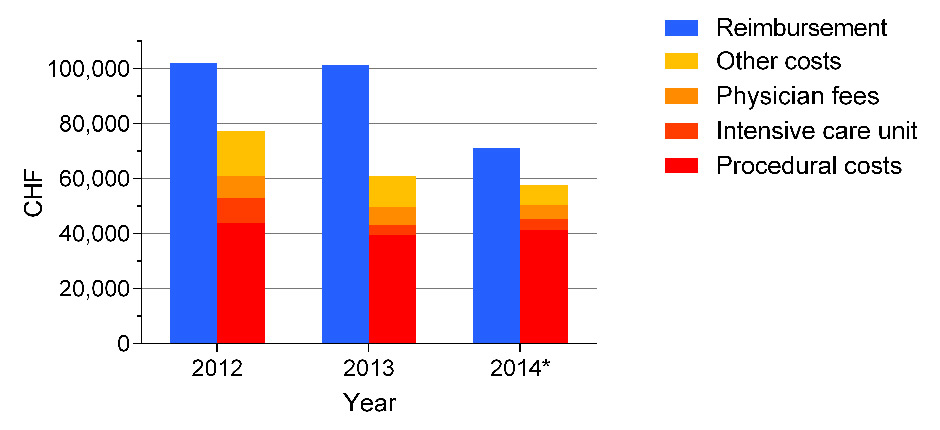
Figure 2
Reimbursement and costs of TAVI in Switzerland 2012–2014.
The case weight for TAVI in Switzerland was 9,707 in 2012, 9,795 in 2013, and decreased to 7,159 in 2014. The case weight will further decrease to 6,216 in 2015. Between 2012 and 2014, costs for intensive care unit, nursing, physiotherapy, and other costs were reduced, but procedural costs including costs for the implant remained relatively unchanged (example of the Cantonal Hospital Lucerne). Median hospital stay decreased from 9 days (2012) to 7 days (2014). *January – June 2014.
There is general agreement that TAVI has a learning curve, and this may be on a personal as well on an institutional basis. On the other hand, SAVR is a mature procedure that has been performed for more than five decades. Indeed, the first SAVR was reported in 1960, 42 years before the first TAVI procedure [11, 12]. Cardiac surgeons have made important advances in operative techniques and postoperative care, and biomedical engineers and cardiac surgeons have improved prosthetic valves [13]. These improvements resulted in a drop in mortality and major complications from 25%–50% to currently 1%–3% [14]. In light of such excellent outcomes with open heart surgery, it is not prudent to reduce invasiveness at the cost of a higher complication rate. In our opinion, TAVI should generally be performed in higher risk patients only. TAVI in moderate risk patients should be restricted to experienced centres with good outcomes in higher risk patients, the benchmark of which being the local SAVR outcomes. Results may not need to be entirely equivalent, but at least, they should be similar.
Currently, the Edwards SAPIEN valve (Edwards Lifesciences, Irvine CA, USA) and the CoreValve (Medtronic, Minneapolis MN, USA) are the only transcatheter valves with Food and Drug Administration (FDA) approval. Following publication of the PARTNER trial, TAVI for inoperable and high-risk patients was reimbursed in the United States at the same rate as open heart surgery [15]. Reimbursement varies between $US 32,000 and $US 94,000 based on the geographic area (average around $US 40,000 – $US 45,000), and also depends on patient comorbidities and complications. However, the implant kit including the SAPIEN valve, the balloon for predilatation, and the sheath costs about $US 32,500. Thus, reimbursement does not cover the costs of TAVI in many hospitals in the United States [16]. In Switzerland, reimbursement in 2014 is around CHF 72,000 for TAVI and CHF 43,000 for SAVR. Implant costs for the Edwards SAPIEN 3 kit are approximately CHF 32,000, whereas the price for a surgical aortic valve bioprosthesis is approximately CHF 3,000.
Several cost-effectiveness analyses have been performed to compare TAVI to medical therapy and to SAVR. Compared to medical therapy, different analyses calculated the ICER from $US 26,302 to $US 61,889 per QALY in inoperable patients included in the PARTNER study [17]. Compared to SAVR, overall costs of TAVI were still higher, mainly due to the higher implant price. This difference was not outweighed by the shorter intensive care unit and hospital stay, and lower expenditure on blood products [17, 18]. However, a cost-effectiveness analysis based on the PARTNER data showed that transfemoral TAVI compared quite favourably to SAVR. Average total 12 month costs were $US 96,743 with transfemoral TAVI compared to $US 97,992 with SAVR. Of note, TAVI patients reported a better quality of life and had a slightly better life expectancy resulting in an ICER <$US 50,000/QALY as long as the difference in the device costs remained <$US 29,390 [19]. Transapical TAVI compared a bit less favourable to SAVR and would be economically attractive only if the difference in valve acquisition costs was less than $US 11,324 (fig. 1) [19]. However, the PARTNER study was conducted with the SAPIEN valve requiring large sheaths for transfemoral (and transapical) access. Furthermore, experience was limited in all centres. Some European centres have published lower costs for TAVI hospitalisations (e.g., € 35,164 in France, € 35,841 in Italy, € 38,739 in Belgium) [20–22]. In these countries, the main drivers for costs in TAVI were costs for the valve, access routes other than transfemoral, implantation of a second valve, implantation of a permanent pacemaker, and vascular complications. According to these studies, costs may be reduced by lower prices for the valve, inclusion of lower risk patients, and with increasing experience through avoidance of complications and shortening of the hospitalisation time.
Percutaneous mitral valve repair (MitraClip)
Transcatheter mitral valve repair with the MitraClip device (Abbott Vascular, Santa Clara CA, USA) is a novel treatment option in surgically high-risk patients with severe structural or degenerative mitral regurgitation. Based on the strictly venous approach and the continuous functional control during intervention, complication rates usually are low and results good.
The system has been tested in the Endovascular Valve Edge-to-Edge Repair Studies (EVEREST) trial programme against mitral valve surgery in high-risk surgical patients [23] or against medical therapy in patients without surgical option [24]. The data shows that, by reducing mitral regurgitation, MitraClip improves heart failure symptoms and quality of life without severe side effects. This finding has been corroborated in real world practice by numerous national and international registries that have documented the efficacy and safety of the technique [25–30]. Until now, >10,000 patients have been treated with the device worldwide.
Data on the cost-effectiveness of MitraClip is sparse. It has been tested against medical therapy in a subgroup analysis of the EVEREST II high-risk study [31] in patients ineligible for conventional surgery [32]. The authors found that MitraClip had incremental QUALY gains of 0.48 and 2.04 over 2 and 10 year periods, respectively, compared to medical therapy. The ICER at 2 and 10 years were £ 52,947 and £14,800 per QUALY gained, respectively. The main cost drivers in the model were implant costs, short-term hospitalisations, and background medication, which was higher due to the longer survival associated with MitraClip treatment. At the thresholds used by the National Institute for Health and Clinical Excellence (NICE), the probability that MitraClip is cost-effective was 37% (£20,000) and 93% (£30,000), respectively. Therefore, MitraClip was considered cost-effective, mainly dependent on the length of the time model used and insensitive to device and procedure costs. However, no comparison of the costs against surgery is available in this high-risk population.
In most European countries, MitraClip is commercially available, but funding may be insufficient. In the United States, the device has been approved by the FDA but there is currently no reimbursement. As in Germany, a MitraClip-specific disease-related group (DRG) budget has been established in Switzerland, which is high enough to cover the costs of the intervention. Reimbursement for Germany for 2014 is € 31,659 (base rate of € 3’157) and for Switzerland CHF 49,533 (base rate of CHF 9,500), with current costs for the device of approximately CHF 30,000.
Closure of PFO and ASD
Both PFO and ASD are defects of the interatrial wall of the heart. PFO is a remnant of the foetal communication between the vena cava inferior and the left atrium, where oxygenated blood is bypassing the foetal lungs [33]. Normally, the two septa fuse, with approximately 20% of humans experiencing persisting patency. Despite the fact that most PFO are innocent, there is an established association between PFO and cryptogenic stroke, migraine, and headache [34, 35]. However, clinical trials in PFO closure have shown non-conclusive results. In patients with cryptogenic stroke, only three randomised trials have been performed so far using the STARFlex septal closure system (NMT Medical Inc., Boston MA, USA) in one and the AMPLATZER PFO occluder device (St. Jude Medical, St. Paul MN, USA) in two [36–38]. While meta-analyses including both devices show at least a strong tendency toward a benefit [39–41], a recent publication showed that PFO closure is able to reduce the incidence of strokes when only AMPLATZER PFO occluder devices were used, probably due to higher rates of thrombosis and atrial fibrillation with the STARFlex device [42]. In contrast to PFO, ASD is one of the most common forms of congenital heart disease presenting in adulthood. There are different forms of ASD, with the ostium secundum type accounting for approximately 75% of all ASD [33]. From the pathophysiological standpoint, ASD may lead to an equalisation of pressures in the left and right atria, left-to-right shunting, volume overload of the right-sided structures, atrial flutter or fibrillation, and finally to pulmonary hypertension which forms an important prognostic factor for the patients [33]. Ostium secundum defects usually can be treated interventionally, while other defects such as ostium primum defects, sinus venosus defects, and coronary sinus defects are subjects of surgical closure.
Currently, most ASD and PFO are closed by interventional techniques, except for large ASD >38 mm or ASD with insufficient septal rims [33]. The intervention is considered low-risk and usually shows good results. While clear indications for ASD closure exist, there is no such thing for PFO closure. ASD should be closed when right atrial or ventricular enlargement is present, or when cryptogenic strokes or orthodeoxia-platypnoea occur [43]. However, PFO closure is still an off-label use in many countries. Accordingly, cost-effectiveness calculations have been performed for ASD closure only. In one of the largest analyses, data from the province-wide Québec Congenital Heart Disease Database were analysed and showed a reduction of five years costs from $CDN 15’304 with surgery to $CDN 11,060 with the interventional approach which was going along with a higher effectiveness [44]. Therefore, interventional ASD closure was deemed a dominant strategy with 80% cost savings and higher effectivity when compared to surgery.
While PFO closure is still an off-label indication in the United States, reimbursement is similar for ASD and PFO closures in most countries throughout Europe. DRG is € 7,406 (base rate € 3,157) in Germany and CHF 14,554 (base rate CHF 9,500) in Switzerland.
LAA occlusion
The LAA is the relict of the embryonic left atrium and represents the main origin of cardiac thrombi. While in sinus rhythm, it is contracting and emptying regularly towards the left atrium, slower flow velocities in atrial fibrillation allow for the formation of thrombi, which carry the risk for embolic stroke. Therefore, in most patients with atrial fibrillation oral anticoagulation, which decreases the risk of stroke by approximately 60% [45], is indicated dependent on their underlying CHA2DS2–VASc score [46]. However, many patients with an indication for oral anticoagulation cannot be treated with vitamin K-antagonists or the novel oral anticoagulants, e.g., the direct thrombin inhibitor or the selective factor Xa inhibitors, because they are either at a higher risk of bleeding dependent on their HAS-BLED score [47] or non-compliant to therapy. In these patients with non-valvular atrial fibrillation but relative or absolute contraindications against oral anticoagulation, interventional closure of the LAA may be an option to reduce the risk of strokes by excluding it from the circulation. LAA occlusion was given a class IIb indication by the European Society of Cardiology [48].
Early clinical studies using the Percutaneous Left Atrial Appendage Transcatheter Occlusion (PLAATO) device have shown that interventional LAA occlusion is feasible and can be performed at an acceptable risk level [49–51]. The randomised Watchman Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation (PROTECT-AF) clinical trial [52] showed the noninferiority of LAA occlusion against warfarin [53]. While the primary efficacy endpoint, i.e., occurrence of ischaemic or haemorrhagic stroke, cardiovascular or unexplained death, or systemic emboli within up to three years, was similar in both the device and the medical therapy groups, rates in the primary safety endpoint were higher in the device group. Complications were pericardial effusion and device embolisations with much lower rates in the later Continued Access Protocol registry that enrolled patients in the same centres using the same protocol as in PROTECT-AF [54] and the Prospective Randomized Evaluation of the Watchman LAA Closure Device in Patients With Atrial Fibrillation Versus Long Term Warfarin Therapy (PREVAIL) [55], suggesting that increasing experience and device modifications to increase safety are important in decreasing complication rates. Other studies such as the ACP trial are under way [56].
Cost-effectiveness of interventional LAA occlusion has been analysed in only one publication. Singh et al. [57] analysed data from the Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) trial [58] that compared dabigatran against warfarin, and from PROTECT-AF [53]. While ischaemic strokes were similar in all treatment groups, there was an overall reduction of bleeding with interventional LAA occlusion. Compared with warfarin, there was an ICER of $CDN 41,565 for interventional LAA occlusion, with cost-efficacy in 43% of simulations using a willingness-to-pay threshold of $CDN 50,000 and in 47% of simulations using a willingness-to-pay threshold of $CDN 100,000. Of note, dabigatran therapy was dominated by interventional LAA occlusion because dabigatran was more expensive per additional unit of effectiveness.
Reimbursement of interventional LAA occlusion does not exist in many European countries and is still an off-label use in the United States. In Germany and Switzerland, there is a DRG code with a reimbursement in 2014 of € 9,669 (base rate € 3,157) and CHF 16,483 (base rate CHF 9,500), respectively.
Who is deciding?
During the last years, the concept of the “Heart Team” has been promoted during teaching sessions and international conference meetings. The idea behind the heart team is that an interdisciplinary team of cardiac surgeons, cardiologists, and also anaesthesiologists or geriatricians decides if a patient with structural heart disease should be treated with surgery, interventional treatment, or medical therapy only. While the heart team approach applies for more complex interventions such as TAVI, MitraClip, and ASD closure, more simple interventions such as PFO or LAA closure remain purely interventional. For patients with severe symptomatic aortic stenosis, one end of the spectrum is currently the USA, where two cardiac surgeons have to agree independently, before TAVI can be offered to a patient. In Europe, decisions are made more liberally. In Germany, which is probably at the other end of the spectrum, more than 50% of patients with severe aortic stenosis are currently treated with TAVI, while other industrialised countries are somewhere between. In Switzerland, the TAVI rate is not as high as in Germany, but TAVI saw a gradual increase from 2008 (127 procedures) to 2013 (650 procedures). At the same time, the number of SAVR remained relatively unchanged at 1,500 per year. In other fields, there are similar developments.
Patients cannot always judge if an operation or an intervention was performed properly, but they always remember how much discomfort they had, and how long their hospital stay and recovery period was. They will ask their general practitioners and cardiologists: Can I go home after one week? When can I return to my daily life? Do I need rehabilitation? There may be an inherent disconnect between what patients want in regards to therapeutics and what surgery can typically deliver [59]. Intuitively, patients are looking for treatment modalities that are minimally invasive and have a short recovery period. In the media, new treatment technologies are often presented in 5–10 minutes, possibly showing a successfully treated patient and how the technology works. This short time simply is not enough to emphasise all the possible pros and cons of a new treatment. In our experience, general practitioners and also non-interventional cardiologists are more conservative with new treatment modalities, probably because they know about the good results of the established treatment.
There is currently no doubt that the Heart Team should make the decision taking into account the preference of the patient and the referring physician. However, in clinical practice, many patients are already primed towards one treatment modality, most often interventional. To allow correct decision making, heart surgeons should see potential candidates in person and talk to them before the meeting takes place. However, this can be very challenging to implement in clinical practice. Last but not least it is important to remember that treatment options for patients with structural heart disease are not only surgery and interventional treatment, but also medical management in some.
Can we afford it?
Innovation in the diagnosis and treatment of disease is a key driver of healthcare expenditure growth, mainly for two reasons [60, 61]. A new procedure may be less invasive or safer – as a consequence, the procedure is performed more frequently and becomes more widely available. A new procedure may also cost more due to more expensive materials and techniques used. TAVI for example is less invasive than SAVR and thus more attractive, resulting in more patients with severe aortic stenosis undergoing treatment. However, the price for a transcatheter valve is higher than for a surgically implanted bioprosthesis, and generates up to 50% of the total hospitalisation costs in many centres. Prices of the TAVI devices have already been lowered during the last 5 years (in Switzerland by about 10%–15%) and will certainly further decline due to increasing numbers of TAVI procedures performed, but also due to the increasing number of competitors. A reduction of valve prices would improve economic feasibility of TAVI [20–22].
Innovation is not the only reason why healthcare costs continue to rise. Other important factors may include demographic trends, lifestyle changes in industrialised countries with its consequences (e.g., obesity, diabetes), increased costs for healthcare administration, or the lack of well-developed competitive healthcare markets [62]. For all these reasons, it can be expected that health care costs will continue to rise [63]. However, whether society is able or willing to pay such costs, is a more political than medical decision.
Innovative ideas and products are essential for further progress in medicine, and sometimes, interventions or operations have to be performed even without having evidence. It is important that these innovations are performed according to the best clinical practice and according to the ethical rules. In our opinion, off-label use of devices is a medical reality but should always be performed as part of a study or registry. Particularly, it is important to avoid “overuse” of new procedures and diagnostic modalities.
Conclusion
Interventional treatment in structural heart disease is a rapidly evolving technique that has overcome surgery in many indications already. The ease of utilisation, high efficacy, low complication rates, short recovery times and lucrative reimbursement will further challenge surgery for the treatment of severe aortic stenosis and mitral regurgitation in the upcoming years. However, assessment of the patient within the Heart Team is essential, and surgery will remain the gold standard for many complex cases where interventional treatment is not possible.
References
1 Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. Oxford University Press; 2005.
2 Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–607.
3 Thomas M. The global experience with percutaneous aortic valve replacement. JACC Cardiovasc Interv. 2010;3:1103–9.
4 Gurvitch R, Wood DA, Tay EL, Leipsic J, Ye J, Lichtenstein SV, et al. Transcatheter aortic valve implantation: Durability of clinical and hemodynamic outcomes beyond 3 years in a large patient cohort. Circulation. 2010;122:1319–27.
5 Ferrari E, von Segesser LK. Transcatheter aortic valve implantation (tavi): State of the art techniques and future perspectives. Swiss Med Wkly. 2010;140:w13127.
6 Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–98.
7 Toggweiler S, Webb JG. Challenges in transcatheter aortic valve implantation. Swiss Med Wkly. 2012;142:w13735.
8 Thourani V. American College of Cardiology Scientifc Session. March 11th, 2013.
9 Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790–8.
10 Wenaweser P, Stortecky S, Schwander S, Heg D, Huber C, Pilgrim T, et al. Clinical outcomes of patients with estimated low or intermediate surgical risk undergoing transcatheter aortic valve implantation. Eur Heart J. 2013;34:1894–905.
11 Harken DE, Soroff HS, Taylor WJ, Lefemine AA, Gupta SK, Lunzer S. Partial and complete prostheses in aortic insufficiency. J Thorac Cardiovasc Surg. 1960;40:744–62.
12 Cribier A, Eltchaninoff H, Bash A, Borenstein N, Tron C, Bauer F, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: First human case description. Circulation. 2002;106:3006–8.
13 Braunwald E. Aortic valve replacement: An update at the turn of the millennium. Eur Heart J. 2000;21:1032–3.
14 Svensson LG. Evolution and results of aortic valve surgery, and a 'disruptive' technology. Cleve Clin J Med. 2008;75:802–4.
15 Barbash IM, Waksman R. Overview of the 2011 food and drug administration circulatory system devices panel of the medical devices advisory committee meeting on the edwards sapien transcatheter heart valve. Circulation. 2012;125:550–5.
16 Svensson LG. Aortic valve replacement: Options, improvements, and costs. Cleve Clin J Med. 2013;80:253–4.
17 Indraratna P, Ang SC, Gada H, Yan TD, Manganas C, Bannon P, Cao C. Systematic review of the cost-effectiveness of transcatheter aortic valve implantation. J Thorac Cardiovasc Surg. 2013
18 Osnabrugge RL, Head SJ, Genders TS, Van Mieghem NM, De Jaegere PP, van der Boon RM, et al. Costs of transcatheter versus surgical aortic valve replacement in intermediate-risk patients. Ann Thorac Surg. 2012;94:1954–60.
19 Reynolds MR, Magnuson EA, Lei Y, Wang K, Vilain K, Li H, et al. Cost-effectiveness of transcatheter aortic valve replacement compared with surgical aortic valve replacement in high-risk patients with severe aortic stenosis: Results of the partner (placement of aortic transcatheter valves) trial (cohort a). J Am Coll Cardiol. 2012;60:2683–92.
20 Bartoli S, Saia F, Marrozzini C, Berti E, Guastaroba P, Fortuna D, et al. The cost of innovation in treating aortic stenosis: Transcatheter aortic valve implantation. G Ital Cardiol (Rome). 2012;13:50–8.
21 Van Gestel R, De Graeve D, Vrints C, Rodrigus I, Bosmans J. Hospitalization costs oftavi in one belgian university hospital. Acta Cardiol. 2013;68:263–70.
22 Chevreul K, Brunn M, Cadier B, Haour G, Eltchaninoff H, Prat A, et al. Cost of transcatheter aortic valve implantation and factors associated with higher hospital stay cost in patients of the france (french aortic national corevalve and edwards) registry. Arch Cardiovasc Dis. 2013;106:209–19.
23 Feldman T, Foster E, Glower DD, Glower DG, Kar S, Rinaldi MJ, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011;364:1395–406.
24 Feldman T, Wasserman HS, Herrmann HC, Gray W, Block PC, Whitlow P, et al. Percutaneous mitral valve repair using the edge-to-edge technique: Six-month results of the everest phase i clinical trial. J Am Coll Cardiol. 2005;46:2134–40.
25 Sürder D, Pedrazzini G, Gaemperli O, Biaggi P, Felix C, Rufibach K, et al. Predictors for efficacy of percutaneous mitral valve repair using the mitraclip system: The results of the mitraswiss registry. Heart. 2013;99:1034–40.
26 Grasso C, Capodanno D, Scandura S, Cannata S, Imme S, Mangiafico S, et al. One- and twelve-month safety and efficacy outcomes of patients undergoing edge-to-edge percutaneous mitral valve repair (from the grasp registry). Am J Cardiol. 2013;111:1482–7.
27 Schillinger W, Hünlich M, Baldus S, Ouarrak T, Boekstegers P, Hink U, et al. Acute outcomes after mitraclip therapy in highly aged patients: Results from the german transcatheter mitral valve interventions (trami) registry. EuroIntervention. 2013;9:84–90.
28 Maisano F, Franzen O, Baldus S, Schäfer U, Hausleiter J, Butter C, et al. Percutaneous mitral valve interventions in the real world: Early and 1–year results from the access-eu, a prospective, multicenter, nonrandomized post-approval study of the mitraclip therapy in europe. J Am Coll Cardiol. 2013;62:1052–61.
29 Armoiry X, Brochet E, Lefevre T, Guerin P, Dumonteil N, Himbert D, et al. Initial french experience of percutaneous mitral valve repair with the mitraclip: A multicentre national registry. Arch Cardiovasc Dis. 2013;106:287–94.
30 Estévez-Loureiro R, Franzen O, Winter R, Sondergaard L, Jacobsen P, Cheung G, et al. Echocardiographic and clinical outcomes of central versus noncentral percutaneous edge-to-edge repair of degenerative mitral regurgitation. J Am Coll Cardiol. 2013;62:2370–7.
31 Whitlow PL, Feldman T, Pedersen WR, Lim DS, Kipperman R, Smalling R, et al. Acute and 12–month results with catheter-based mitral valve leaflet repair: The everest ii (endovascular valve edge-to-edge repair) high risk study. J Am Coll Cardiol. 2012;59:130–9.
32 Mealing S, Feldman T, Eaton J, Singh M, Scott DA. Everest ii high risk study based uk cost-effectiveness analysis of mitraclip® in patients with severe mitral regurgitation ineligible for conventional repair/replacement surgery. J Med Econ. 2013;16:1317–26.
33 Tobis J, Shenoda M. Percutaneous treatment of patent foramen ovale and atrial septal defects. J Am Coll Cardiol. 2012;60:1722–32.
34 Lechat P, Mas JL, Lascault G, Loron P, Theard M, Klimczac M, Drobinski G, Thomas D, Grosgogeat Y. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med. 1988;318:1148–52.
35 Handke M, Harloff A, Olschewski M, Hetzel A, Geibel A. Patent foramen ovale and cryptogenic stroke in older patients. N Engl J Med. 2007;357:2262–8.
36 Furlan AJ, Reisman M, Massaro J, Mauri L, Adams H, Albers GW, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991–9.
37 Carroll JD, Saver JL, Thaler DE, Smalling RW, Berry S, MacDonald LA, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092–100.
38 Meier B, Kalesan B, Mattle HP, Khattab AA, Hildick-Smith D, Dudek D, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368:1083–91.
39 Khan AR, Bin Abdulhak AA, Sheikh MA, Khan S, Erwin PJ, Tleyjeh I, et al. Device closure of patent foramen ovale versus medical therapy in cryptogenic stroke: A systematic review and meta-analysis. JACC Cardiovasc Interv. 2013;6:1316–23.
40 Rengifo-Moreno P, Palacios IF, Junpaparp P, Witzke CF, Morris DL, Romero-Corral A. Patent foramen ovale transcatheter closure vs. Medical therapy on recurrent vascular events: A systematic review and meta-analysis of randomized controlled trials. Eur Heart J. 2013;34:3342–52.
41 Ntaios G, Papavasileiou V, Makaritsis K, Michel P. Pfo closure vs. Medical therapy in cryptogenic stroke or transient ischemic attack: A systematic review and meta-analysis. Int J Cardiol. 2013;169:101–5.
42 Capodanno D, Milazzo G, Vitale L, Di Stefano D, Di Salvo M, Grasso C, et al. Updating the evidence on patent foramen ovale closure versus medical therapy in patients with cryptogenic stroke: A systematic review and comprehensive meta-analysis of 2,303 patients from three randomised trials and 2,231 patients from 11 observational studies. EuroIntervention. 2014;9:1342–9.
43 Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, et al., Cardiology ACo, Disease) AHATFoPGWCtDGotMoAWCH, Echocardiography ASo, Society HR, Disease ISfACH, Interventions SfCAa, Surgeons SoT. Acc/aha 2008 guidelines for the management of adults with congenital heart disease: A report of the american college of cardiology/american heart association task force on practice guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease). Developed in collaboration with the american society of echocardiography, heart rhythm society, international society for adult congenital heart disease, society for cardiovascular angiography and interventions, and society of thoracic surgeons. J Am Coll Cardiol. 2008;52:e143–263.
44 Mylotte D, Quenneville SP, Kotowycz MA, Xie X, Brophy JM, Ionescu-Ittu R, et al. Long-term cost-effectiveness of transcatheter versus surgical closure of secundum atrial septal defect in adults. Int J Cardiol. 2014;172:109–14.
45 Hart RG, Pearce LA, Aguilar MI. Meta-analysis: Antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–67.
46 Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest. 2010;137:263–72.
47 Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (has-bled) to assess 1–year risk of major bleeding in patients with atrial fibrillation: The euro heart survey. Chest. 2010;138:1093–100.
48 Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. 2012 focused update of the esc guidelines for the management of atrial fibrillation: An update of the 2010 esc guidelines for the management of atrial fibrillation. Developed with the special contribution of the european heart rhythm association. Eur Heart J. 2012;33:2719–47.
49 Sievert H, Lesh MD, Trepels T, Omran H, Bartorelli A, Della Bella P, et al. Percutaneous left atrial appendage transcatheter occlusion to prevent stroke in high-risk patients with atrial fibrillation: Early clinical experience. Circulation. 2002;105:1887–9.
50 Ostermayer SH, Reisman M, Kramer PH, Matthews RV, Gray WA, Block PC, et al. Percutaneous left atrial appendage transcatheter occlusion (plaato system) to prevent stroke in high-risk patients with non-rheumatic atrial fibrillation: Results from the international multi-center feasibility trials. J Am Coll Cardiol. 2005;46:9–14.
51 Bayard YL, Omran H, Neuzil P, Thuesen L, Pichler M, Rowland E, et al. Plaato (percutaneous left atrial appendage transcatheter occlusion) for prevention of cardioembolic stroke in non-anticoagulation eligible atrial fibrillation patients: Results from the european plaato study. EuroIntervention. 2010;6:220–6.
52 Sick PB, Schuler G, Hauptmann KE, Grube E, Yakubov S, Turi ZG, et al. Initial worldwide experience with the watchman left atrial appendage system for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2007;49:1490–5.
53 Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: A randomised non-inferiority trial. Lancet. 2009;374:534–42.
54 Reddy VY, Holmes D, Doshi SK, Neuzil P, Kar S. Safety of percutaneous left atrial appendage closure: Results from the watchman left atrial appendage system for embolic protection in patients with af (protect af) clinical trial and the continued access registry. Circulation. 2011;123:417–24.
55 Http:// http://www.Bostonscientific.Com/watchman-eu/clinical-data/prevail-clinical-study.Html? , accessed feb 26 2014.
56 Landmesser U, Holmes DR. Left atrial appendage closure: A percutaneous transcatheter approach for stroke prevention in atrial fibrillation. Eur Heart J. 2012;33:698–704.
57 Singh SM, Micieli A, Wijeysundera HC. Economic evaluation of percutaneous left atrial appendage occlusion, dabigatran, and warfarin for stroke prevention in patients with nonvalvular atrial fibrillation. Circulation. 2013;127:2414–23.
58 Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al., Investigators R-LSCa. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51.
59 Singh HS, Osten M, Horlick E. Future horizons for catheter-based interventions in adult congenital and structural heart disease. Future Cardiol. 2012;8:203–13.
60 Bodenheimer T. High and rising health care costs. Part 2: Technologic innovation. Ann Intern Med. 2005;142:932–7.
61 Conen D. Health economics from the physician’s point of view. Z Arztl Fortbild Qualitatssich. 2007;101:375–80.
62 Bodenheimer T. High and rising health care costs. Part 1: Seeking an explanation. Ann Intern Med. 2005;142:847–54.
63 Zweifel P. How much are we willing to pay for health? Z Arztl Fortbild Qualitatssich. 2007;101:369–73.

