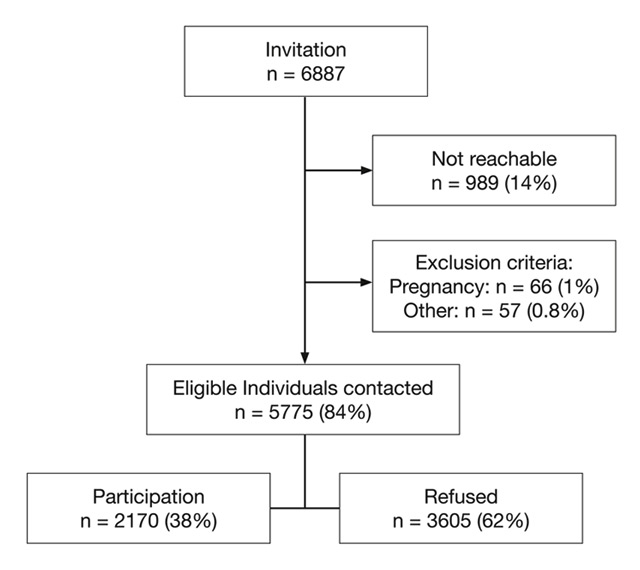
Figure 1
Recruitment of the GAPP cohort study. Flow chart of the recruitment of the GAPP study.
DOI: https://doi.org/10.4414/smw.2014.14019
Cigarette smoking is a leading cause of avoidable morbidity and mortality worldwide [1]. In a recent study, tobacco smoking alone accounted for 6.3% of all global disability-adjusted life-years [1]. Accordingly, both male and female smokers lose at least 10 years of lifespan compared to never smokers [2–5]. A better understanding on how smoking relates to death and other adverse events is therefore of major public health importance.
Several studies have shown a strong relationship between cigarette smoking and type 2 Diabetes mellitus (T2D), independent of other diabetes related risk factors [6–13]. Similar to smoking, T2D is also one of the most important risk factors for global disease burden [1]. Thus, a pro-diabetic effect could be a potential mediator for some of the adverse effects of smoking, but the causality of this relationship has not been well established [8], and its underlying mechanisms are poorly understood. Differences in physical activity and body composition, especially fat distribution, could be involved [13, 14]. A recent study also suggested that genetic polymorphisms in the nicotinic acetylcholine receptor genes may contribute to the occurrence of insulin resistance and T2D, raising the intriguing possibility that nicotine dependence may be mechanistically involved in the relationship between smoking and T2D as well [15].
Furthermore, little information is available on the time course of the relationship between smoking and T2D. Demonstrating an association between smoking and pre-diabetes, i.e., elevations in blood glucose levels that do not yet fulfill the criteria of T2D among young individuals would support this to be an early effect, and would underscore the importance of primary smoking prevention and early smoking cessation. However, few studies have been performed in this context [8]. Finally, controversial data have been published on the effect of smoking cession on the occurrence of T2D [10–12].
Therefore, the aim of this analysis was to assess the relationship between smoking, cumulative smoking exposure and nicotine dependence with pre-diabetes in a large, well-characterised sample of young and healthy adults from the general population.
The genetic and phenotypic determinants of blood pressure and other cardiovascular risk factors (GAPP) study is a population-based cohort study from the Principality of Liechtenstein. Details about recruitment and study methodology have been published previously [16]. In brief, between 2010 and 2013, all inhabitants of the Principality of Liechtenstein aged 25–41 years, namely 6887 individuals, were invited to participate in this study. 5775 individuals could be contacted by phone and were eligible. A study flow chart is provided in (fig. 1). Main exclusion criteria were known cardiovascular disease, renal failure, known sleep apnoea syndrome, current intake of antidiabetic drugs, any other major illness, and a Body Mass Index (BMI) >35 kg/m2. Up to December 2013, 2170 participants have been included in GAPP (participation rate of 38%). 13 participants (0.6%) with missing HbA1c levels, 3 with missing smoking status (0.1%) and 12 participants (0.3%) with HbA1c levels >6.4% were excluded from the current analysis, leaving 2142 participants. The study protocol was approved by the local ethics committee. Informed written consent is obtained from each participant.

Figure 1
Recruitment of the GAPP cohort study. Flow chart of the recruitment of the GAPP study.
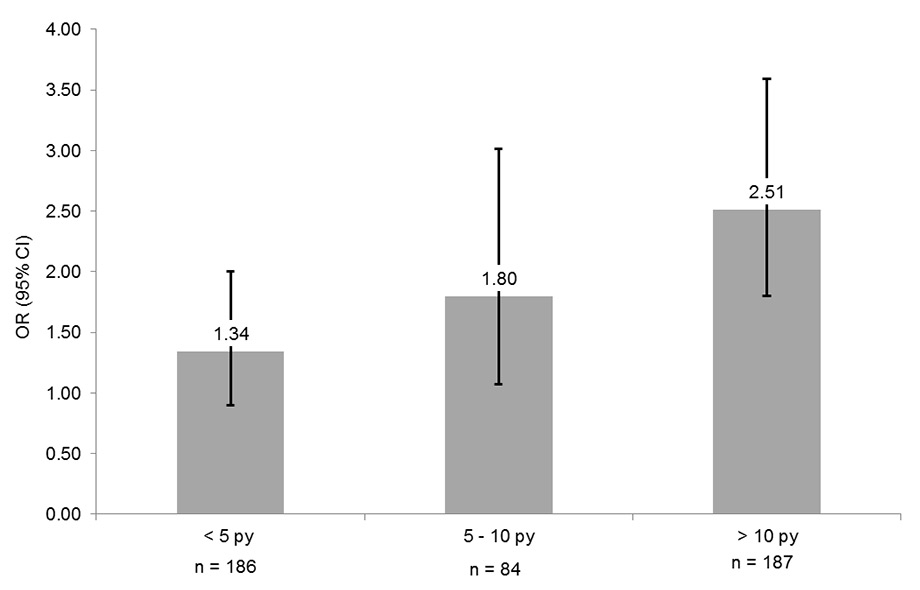
Figure 2
Relationship of pre-diabetes with cumulative smoking exposure. Never smokers represent the reference group. Boxes indicate odds ratios, whiskers are 95% confidence intervals. Data are adjusted for age, sex, Body Mass Index, hypertension, alcohol consumption, education, low density lipoprotein and high density lipoprotein cholesterol, physical activity, fruit/vegetable consumption and body composition.
Details about smoking history were obtained by questionnaire. Current smoking was defined as answering yes to the question “Do you currently smoke”. Participants were classified as past smokers if they reported active smoking in the past but not currently. Past smokers also indicated the year of smoking cessation. The remaining participants who did not report smoking currently or in the past were classified as never smokers. Pack-years of smoking were calculated by multiplying the number of years smoked by the average number of cigarette packs smoked per day. We used the validated Fagerström questionnaire to quantify nicotine dependence among current smokers [17]. The Fagerström questionnaire includes the following six questions about smoking behaviour: (1) “When do you smoke your first cigarette after getting up?”, (2) “Is it difficult for you to not smoke in places where smoking is prohibited?”, (3) “Which cigarette would you be least willing to give-up?”, (4) “How many cigarettes do you generally smoke per day?”, (5) “Do you generally smoke more in the morning than during the rest of the day?”, (6) “Is it the case that you smoke even though you are so ill that you have to spend most of the day in bed?”. Based on these questions, a score between 0 and 10 is obtained, with higher scores indicating stronger dependence to nicotine. Secondhand smoke exposure of all individuals was quantified using three questions about exposition to secondhand smoke at home, in restaurants or bars and at the workplace. If one of the three questions was answered with ”yes”, the duration of exposure was also obtained.
Pre-diabetes was defined as glycated haemoglobin (HbA1c) between 5.7% and 6.4%, as recommended by the current guidelines of the American Diabetes Association [18]. HbA1c was measured with a standardised assay from fasting venous blood samples using high performance liquid chromatography (Bio-Rad D-10, Bio-Rad Laboratories AG, Switzerland).
Information about personal, lifestyle, medical and nutritional factors was obtained by questionnaire. Height, weight and office blood pressure were measured in a standardised manner using validated devices, as described previously [16]. BMI was calculated as body weight in kilograms divided by height in meters squared. Hypertension was defined as mean systolic blood pressure of 140, mean diastolic blood pressure 90 and/or intake of blood pressure lowering drugs. Physical activity was assessed with the validated individual physical activity questionnaire (IPAQ) [19]. Regular physical activity for the current analysis was defined as >180 min of vigorous activity per week. Regular consumption of fruits and/or vegetables was defined as 5 servings per day. Body composition (percent of fat mass, muscle mass and body water) was measured using bioelectrical impedance analysis. Lipid levels were measured from fasting venous blood samples using standard methodology (Roche Cobas 6000, F. Hoffmann – La Roche, Switzerland).
Baseline characteristics were compared according to the presence or absence of pre-diabetes. The normality of the distribution for continuous variables was checked using skewness, kurtosis and visual inspection of the histogram. Normally distributed variables were compared using t-tests, otherwise we used Wilcoxon rank sum tests. Categorical variables were compared by Chi-square tests.
Multivariable logistic regression models were constructed to compare odds ratios (OR) and 95% confidence intervals (CI) for pre-diabetes among current, past and never smokers, and to adjust for potential confounders. Age and sex adjusted models were further adjusted for BMI, hypertension, alcohol consumption, low density lipoprotein cholesterol, high density lipoprotein cholesterol, education, physical activity, dietary factors and body composition variables. To evaluate the effect of long term tobacco exposure and to assess a potential dose-response relationship, a similar series of regression models was constructed using the number of pack-years as predictor of interest according to three pre-defined categories: <5, 5–10 and >10 pack-years.
To assess the relationship between nicotine dependence and prediabetes, we divided current smokers into three pre-defined groups based on the Fagerström test score (2, 3–5 and >5) [20, 21]. As the number of pack-years smoked is part of this questionnaire, we repeated the analyses using a modified scale that does not include the question about cumulative smoking burden (maximum score 7; ≤1,2-3, ≥4), in order to evaluate whether the obtained findings were independent of the cumulative tobacco exposure. We then evaluated whether the relationship between smoking and pre-diabetes may be potentially reversible by comparing the odds of pre-diabetes according to whether past smokers had stopped smoking <2 years, 2–4 years or >4 years before baseline examination [11]. Again, multivariable models were constructed as detailed above.
For all the above mentioned logistic regression analyses participants who never smoked were the reference group.
The effect of secondhand smoke on pre-diabetes was assessed by comparing the odds of prediabetes among those with and without any exposure to secondhand smoke and by comparing the prevalence according to approximate tertiles of secondhand smoke exposure in similar multivariable logistic regression models.
Categorical variables were entered in the multivariable models using binary indicator variables. Tests for linear trend were performed using category-specific median values. All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc, Cary, North Carolina). A p-value <0.05 was pre-specified to indicate statistical significance.
| Table 1: Baseline characteristics according to the presence or absence of pre-diabetes. | |||
| N = 2142 | Normoglycaemia N = 1643 (76.7) | Pre-diabetes N = 499 (23.3) | p1 |
| Male sex (%) | 735 (44.7) | 272 (54.5) | 0.0001 |
| Age, years | 36 (31; 40) | 38 (33; 41) | <0.0001 |
| Height, cm | 172 ± 9 | 173 ± 9 | 0.10 |
| Weight, kg | 72.2 ± 14.6 | 75.9 ± 14.8 | <0.0001 |
| Body Mass Index, kg/m2 | 24.3 ± 3.7 | 25.4 ± 4.0 | <0.0001 |
| Smoking (%) | <0.0001 | ||
| Current | 324 (19.7) | 147 (29.5) | |
| Past | 398 (24.2) | 105 (21.0) | |
| Never | 921 (56.1) | 247 (49.5) | |
| Pack-years2 | 5.5 (3.5; 13.5) | 11.3 (4.5; 17.25) | <0.0001 |
| Highest education level achieved (%) | 0.27 | ||
| High school degree | 128 (7.9) | 47 (9.5) | |
| Hollege degree | 923 (57.0) | 291 (59.0) | |
| University degree | 568 (35.1) | 155 (31.4) | |
| Regular consumption of fruits/vegetables (%) | 306 (18.6) | 106 (21.2) | 0.19 |
| Daily alcohol consumption (g/24 hours) | 0.64 (0.00; 1.71) | 0.64 (0.00; 2.01) | 0.47 |
| Physical activity (%) | 788 (48.0) | 282 (56.5) | 0.0008 |
| Fat mass (%) | 25 ± 6 | 25 ± 7 | 0.44 |
| Muscle mass (%) | 35 ± 4 | 36 ± 4 | 0.08 |
| Body water (%) | 54 ± 5 | 54 ± 6 | 0.82 |
| Systolic BP, mm Hg | 120 ± 13 | 122 ± 13 | <0.0001 |
| Diastolic BP, mm Hg | 78 ± 9 | 79 ± 9.0 | 0.007 |
| Hypertension (%) | 211 (12.8) | 81 (16.2) | 0.05 |
| LDL Cholesterol, mmol/l | 2.89 ± 0.81 | 3.22 ± 0.94 | <0.0001 |
| HDL Cholesterol, mmol/l | 1.55 ± 0.41 | 1.47 ± 0.42 | 0.0001 |
| Data are presented as mean ± SD, median (interquartile range) or number (percentage). N = 30 missing information on education. 1 P values were based on t-tests, Wilcoxon rank sum tests or Chi-square tests, as appropriate 2 Among current smokers | |||
| Table 2: Multivariable logistic regression analysis of the relationship between smoking and pre-diabetes. | |||
| N = 2142 | Never smokers N = 1168 | Past smokers N = 503 | Current smoker N = 471 |
| Unadjusted model | Ref. | 0.98 (0.76; 1.27) | 1.69 (1.33; 2.15) |
| Age and sex adjusted model | Ref. | 0.96 (0.74; 1.24) | 1.80 (1.41; 2.31) |
| Fully adjusted model1 | Ref. | 0.96 (0.73; 1.26) | 1.82 (1.39; 2.38) |
| Data are odds ratios (95% confidence intervals) 1 Adjusted for sex, age, Body Mass Index, hypertension, alcohol consumption, low density lipoprotein cholesterol, high density lipoprotein cholesterol, education, physical activity, fruit/vegetable consumption and body composition; 2107 participants included | |||
Median age of the 2142 participants was 37 years and 47% of the participants were male. The proportion of current, past and never smokers in the overall sample was 22%, 23% and 55%, respectively. Baseline characteristics stratified by the presence or absence of pre-diabetes are shown in table 1. Individuals with pre-diabetes (n = 499, 23.3%) were significantly older, more often male and they had a significantly higher BMI compared with those without pre-diabetes. Individuals with pre-diabetes also had a higher prevalence of current smokers, higher blood pressure levels and worse lipid profiles compared with normoglycemic individuals. Furthermore, smokers with pre-diabetes had a significantly higher lifetime tobacco exposure (5.5 versus 11.3 pack-years, p <0.0001).
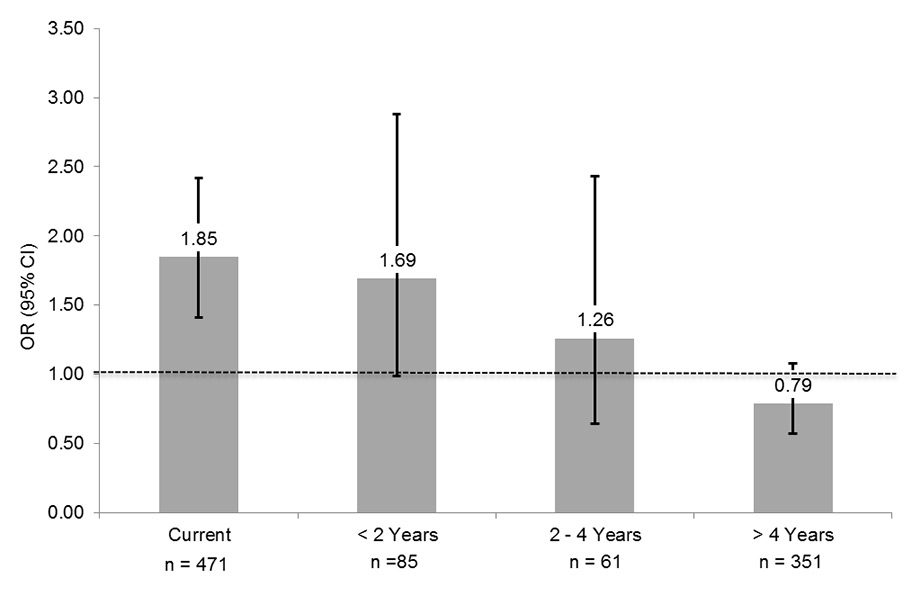
Figure 3
Relationship of pre-diabetes with time since smoking cessation.
Never smokers represent the reference group. Boxes indicate odds ratios, whiskers are 95% confidence intervals. Data are adjusted for age, sex, Body Mass Index, hypertension, alcohol consumption, education, low density lipoprotein and high density lipoprotein cholesterol, physical activity, fruit/vegetable consumption and body composition.
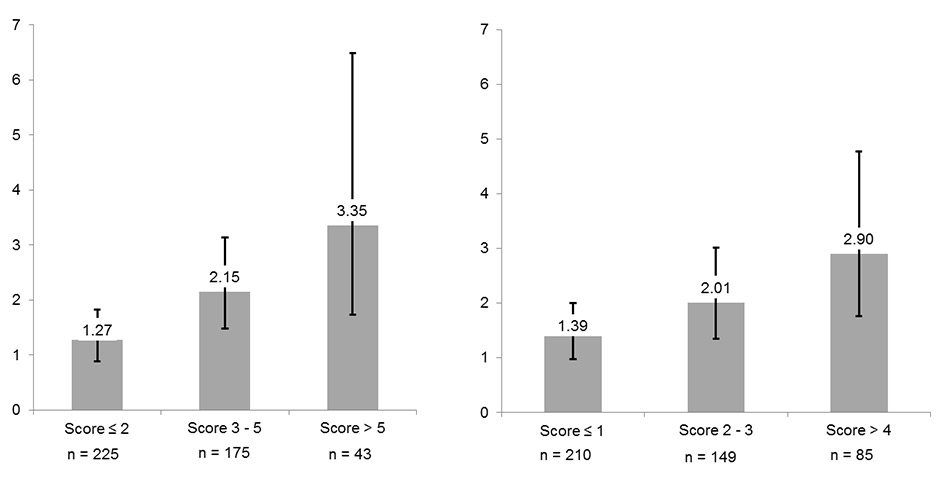
Figure 4
Relationship between nicotine dependence and pre-diabetes Data are with (left side) and without (right side) including information on cumulative smoking exposure into the score. Never smokers represent the reference group. Boxes indicate odds ratios, whiskers are 95% confidence intervals adjusted for age, sex, Body Mass Index, hypertension, alcohol consumption, education, low density lipoprotein and high density lipoprotein cholesterol, physical activity, fruit/vegetable consumption and body composition.
The prevalence of pre-diabetes was 31.2%, 20.9% and 21.2% among current, past and never smokers, respectively (p <0.0001). These results were confirmed in multivariable logistic regression analyses, as shown in table 2. In the unadjusted model, the OR of pre-diabetes for current smoking was 1.69 (95% CI 1.33; 2.15, p <0.0001) compared with never smokers. Multivariable adjustment, based on data of 2107 participants (due to missing values) only slightly changed this relationship, such that in the fully adjusted model, the OR for current smokers became 1.82 (95% CI 1.39; 2.38, p <0.0001). Past smokers did not have an increased odds of pre-diabetes compared with never smokers (fully adjusted OR 0.96 (95% CI 0.73; 1.26, p = 0.75).
Dose response relationships using pack-years of smoking are shown in table 3 and (fig. 2). A cumulative smoking exposure of <5, 5–10 and >10 pack-years was associated with a crude OR (95% CI) of 1.05 (0.73; 1.53), 1.67 (1.03; 2.71) and 2.60 (1.89; 3.61), respectively (p for linear trend <0.0001). Again, this relationship was only minimally affected by multivariable adjustment, such that the fully adjusted ORs (95% CI) were 1.34 (0.90; 2.00), 1.80 (1.07; 3.01) and 2.51 (1.80; 3.59), respectively (p for linear trend <0.0001).
Among past smokers, there was an inverse linear relationship between time since smoking cessation and prevalence of pre-diabetes (p for linear trend <0.0001), as shown in table 4 and (fig. 3). While none of the individual relative risk estimates was statistically significant, these data nevertheless suggest that the prevalence of pre-diabetes is similar to that of never smokers after at most four years of smoking cessation.
Results of the association between Fagerström scores and pre-diabetes among current smokers are shown in table 5 and (fig. 4). Compared with never smokers, current smokers with a Fagerström score ≤2, 3–5 and >5 had an unadjusted OR (CI 95%) for pre-diabetes of 1.18 (0.84; 1.64), 1.90 (1.35; 2.67) and 3.47 (1.88; 6.41), respectively. Adding potential confounders had a minimal influence on this relationship, the fully adjusted OR (CI 95%) for these three groups being 1.27 (0.89; 1.82), 2.15 (1.48; 3.13) and 3.35 (1.73; 6.48), respectively (p for linear trend <0.0001). These results were very similar when the question about cumulative smoking exposure was excluded from the Fagerström questionnaire, as shown in table 5 and figure 4.
The fully adjusted OR for exposure to any secondhand smoke was 1.00 (95% CI 0.93; 1.08) compared with never smokers. Among individuals indicating an exposure to secondhand smoke of <1, 1–2 and >2 hours per day, the multivariable adjusted ORs (95% CI) were 0.97 (0.68; 1.39), 0.92 (0.48; 1.78) and 1.00 (0.60; 1.68), respectively.
| Table 3: Multivariable logistic regression analysis between pre-diabetes and pack-years of smoking. | ||||||
| Current smokers N = 4711 | ||||||
| N = 2142 | Never smokers N = 1168 | Past smokers N = 503 | <5 pack-years N = 186 | 5–10 pack-years N = 84 | >10 pack-years N = 187 | p linear trend |
| Unadjusted model | Ref. | 0.98 (0.76; 1.27) | 1.05 (0.73; 1.53) | 1.67 (1.03; 2.71) | 2.60 (1.89; 3.61) | <0.0001 |
| Age and sex adjusted model | Ref. | 0.96 (0.74; 1.24) | 1.31 (0.89; 1.93) | 1.78 (1.09; 2.91) | 2.40 (1.72; 3.33) | <0.0001 |
| Fully adjusted model2 | Ref. | 0.96 (0.74; 1.26) | 1.34 (0.90; 2.00) | 1.80 (1.07; 3.01) | 2.51 (1.80; 3.59) | <0.0001 |
| Data are odds ratios (95% confidence intervals). 1 N = 14 with missing information on pack-years 2 Adjusted for past smoking, sex, age, Body Mass Index, hypertension, alcohol consumption, low density lipoprotein cholesterol, high density lipoprotein cholesterol, education, physical activity, fruit/vegetable consumption and body composition; 2093 participants included. | ||||||
| Table 4: Multivariable logistic regression analysis between pre-diabetes and time since smoking cessation. | ||||||
| Past smokers N = 5031 | ||||||
| N = 2142 | Never smokers N = 1168 | Current smokers N = 471 | <2 years since quitting N = 85 | 2–4 years since quitting N = 61 | >4 years since quitting N = 351 | p for linear trend |
| Unadjusted model | Ref. | 1.69 (1.33; 2.15) | 1.55 (0.95; 2.53) | 1.01 (0.54; 1.89) | 0.85 (0.63; 1.51) | <0.0001 |
| Age and sex adjusted model | Ref. | 1.82 (1.42; 2.33) | 1.71 (1.04; 2.84) | 1.25 (0.66; 2.38) | 0.77 (0.57; 1.05) | <0.0001 |
| Fully adjusted model2 | Ref. | 1.85 (1.41; 2.42) | 1.69 (0.99; 2.88) | 1.26 (0.64; 2.43) | 0.79 (0.57; 1.08) | <0.0001 |
| Data are odds ratios (95% confidence intervals) 1 N = 6 with missing information on time since smoking cessation. 2 Adjusted for current smoking, sex, age, Body Mass Index, hypertension, alcohol consumption, low density lipoprotein cholesterol, high density lipoprotein cholesterol, education, physical activity, fruit/vegetable consumption and body composition; 2102 participants included. | ||||||
| Table 5:Multivariable logistic regression analysis between pre-diabetes and Fagerström scores. | ||||||
| Current smokers N = 4711 | ||||||
| N = 2142 | Never smokers n = 1168 | Past smokers n = 503 | Fagerström score ≤2 N = 225 | Fagerström score 3–5 N = 175 | Fagerström score >5 N = 43 | p for linear trend |
| Unadjusted model | Ref. | 0.96 (0.74; 1.24) | 1.18 (0.84; 1.64) | 1.90 (1.35; 2.67) | 3.47 (1.88; 6.41) | <0.0001 |
| Age and sex adjusted model | Ref. | 0.93 (0.72; 1.21) | 1.32 (0.94; 1.86) | 1.99 (1.40; 2.82) | 3.22 (1.72; 6.01) | <0.0001 |
| Fully adjusted model2 | Ref. | 0.93 (0.71; 1.22) | 1.27 (0.89; 1.82) | 2.15 (1.48; 3.13) | 3.35 (1.73; 6.48) | <0.0001 |
| Never smokers N = 1168 | Past Smokers N = 503 | Fagerström score ≤1 N = 210 | Fagerström score 2–3 n = 149 | Fagerström score >4 N = 85 | p for linear trend | |
| Unadjusted model | Ref. | 0.98 (0.76; 1.27) | 1.29 (0.92; 1.81) | 1.77 (1.22; 2.57) | 2.61 (1.66; 4.11) | <0.0001 |
| Age and sex adjusted model | Ref. | 0.96 (0.74; 1.24) | 1.44 (1.01; 2.04) | 1.85 (1.27; 2.71) | 2.67 (1.68; 4.25) | <0.0001 |
| Fully adjusted model2 | Ref. | 0.96 (0.73; 1.26) | 1.39 (0.97; 2.00) | 2.01 (1.35; 3.01) | 2.90 (1.76; 4.77) | <0.0001 |
| Data are odds ratios (95% confidence intervals). 1 N = 28 with missing information for normal Fagerström score calculation and N = 27 with missing information for adapted Fagerström score calculation. 2 Adjusted for past smoking, sex, age, Body Mass Index, hypertension, alcohol consumption, low density lipoprotein cholesterol, high density lipoprotein cholesterol, education, physical activity, fruit/vegetable consumption and body composition; 2107 participants included. | ||||||
In this large population based sample of young and healthy individuals, we found that current smoking was strongly associated with pre-diabetes. Compared with never smokers, participants currently smoking cigarettes had an OR for pre-diabetes of 1.82 (95% CI 1.39; 2.38) even after adjustment for multiple confounders and potential mediators. We also found a linear risk gradient across categories of cumulative smoking exposure. Even a cumulative exposure to as few as 5–10 pack-years was associated with a highly significant OR (95% CI) for pre-diabetes of 1.80 (1.07; 3.01). These data therefore are in line with previous studies showing an association between smoking and T2D [6–10].
In addition, this is one of the first investigations in younger individuals with a shorter exposure to environmental risk factors and a lower cumulative smoking exposure. For example, in a prior population based study in an older population the cumulative smoking exposure was 2–3 times higher than in the current analysis [10]. Our data therefore suggest that glucose disturbances among smokers is a relatively early phenomenon, as has been suggested previously [6]. Accordingly, a prior experimental study showed that cigarette smoking directly decreased insulin action and increased insulin resistance [22]. In line with this potential direct effect, none of the various covariates introduced in the multivariable models changed the strength of the relationship between smoking and pre-diabetes. Because prior studies hypothesised on a possible role of body composition and low socioeconomic status in this association, the lack of effect of these variables is particularly noteworthy [8, 13, 14]. In contrast to prior studies on this issue [23, 24], we did not see a relationship between education level and prevalent prediabetes, as shown in table 1. A potential explanation for this lack of association could be the high overall socio-economic status in the Principality of Liechtenstein, and different results may be observed in populations with a larger socio-economic spread.
A second novel observation of our study was that higher scores of the Fagerström questionnaire, a well validated tool of nicotine dependence [17], were strongly associated with pre-diabetes in this study, even after exclusion of cumulative smoking exposure from the score. These data suggest that the nicotinergic system and nicotine dependence may play a role in smoking related hyperglycaemia, which is in line with a small experimental study showing that the long-term use of nicotine containing chewing gums was associated with insulin resistance and hyperinsulinemia [25]. Furthermore, the majority of inhaled nicotine is catabolised into cotinine by an enzymatic activity, which is mediated by CYP2A6. Liu et al. have shown that heavy smokers with a slow or poor metabolizer genotype were more susceptible to develop T2D compared to heavy smokers with a fast metabolizer genotype [26]. Finally, a recent study suggested that genetic polymorphisms within the nicotinic acetylcholine receptor genes may confer an increased risk of T2D, again suggesting that the nicotinergic system may be implicated in the pathogenesis of T2D [15]. In this context it is noteworthy that functional nicotinic receptors have been demonstrated on pancreatic beta cells [27].
Third, we found an inverse linear relationship between prevalence of pre-diabetes and time since smoking cessation among past smokers, suggesting that the adverse effects of smoking on glucose metabolism may be reversible. Although the individual risk estimates were not statistically significant, our data suggest that the excess risk of pre-diabetes is no longer visible among those who stopped smoking four or more years earlier, supporting the beneficial effect of smoking cessation. These data are consistent with several earlier reports [11, 12]. However, in at least one prior study the risk of T2D increased in the first three years after smoking cessation and declined much slower thereafter compared with the current study [10]. It was hypothesised that this higher risk might be due to an increase in body weight after smoking cessation and this effect may be stronger in older individuals with a higher cumulative exposure to tobacco smoke. These differential findings may also suggest that nicotine induced changes in glucose homeostasis become much more difficult to reverse over time [10, 12]. If this hypothesis is confirmed in future studies, it would be another strong motivation to advocate early smoking cessation and primary smoking prevention.
Strengths of this study include its population based design, and the availability of a large sample of well-characterised young and healthy adults with a relatively short exposure history to environmental confounders. Potential limitations that should be considered in the interpretation of this study are the following: First, we enrolled mainly white adults in our study and the generalisability to other population groups is uncertain. Second, this is a cross-sectional analysis, precluding inference of causality to the observed associations. Third, although our comprehensive dataset is very complete, there are some missing values for several covariates, such that the number of individuals slightly varies for individual analyses.
In this large sample of young and healthy individuals, current smoking was strongly related to pre-diabetes. Accumulating as few as 5–10 pack-years of smoking carried a nearly 2-fold increased odds of having pre-diabetes, even after multivariable adjustment. These data suggest that hyperglycaemia is an early event among smokers which occurs independent of other potential confounders, and may be reversible upon smoking cessation. These data therefore reinforce the importance of both prevention of smoking initiation and early smoking cessation. Finally, our data show an intriguing relationship between nicotine dependence and prediabetes, suggesting that alterations in the nicotinergic system could be responsible for the hyperglycaemic changes observed among smokers. Because of the cross-sectional method, assumptions about the causality of the mentioned relationships are not possible. To prove causality of the results and to get a better understanding of the underlying mechanisms of those relationships further investigations are needed.
1 Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380(9859):2224–60.
2 Pirie K, Peto R, Reeves GK, Green J, Beral V. Million Women Study C. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet. 2013;381(9861):133–41.
3 Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328(7455):1519.
4 Thun M, Carter B, Feskanich D, Freedman N, Prentice R, Lopez AD, et al. 50–Year Trends in Smoking-Related Mortality in the United States. N Engl J Med. 2013;368(4):351–64.
5 Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun M, Anderson RN, et al. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368(4):341–50.
6 Houston TK PS, Pletcher MJ, Liu K, Iribarren C, Kiefe CI. Active and passive smoking and development of glucose intolerance among young adults in a prospective cohort: CARDIA study. BMJ. 2006;6(332):1064–69.
7 Wannamethee SG SA, Perry IJ. Smoking as a modifiable risk factor for type 2 diabetes in middle-aged men. British Regional Heart Study. Diabetes care. 2001;24:1590–95.
8 Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2007;298(22):2654–64.
9 Manson JE, Ajani UA, Liu S, Nathan DM, Hennekens CH. A prospective study of cigarette smoking and the incidence of diabetes mellitus among US male physicians. Ann Intern Med. 2000;109(7):538–42.
10 Yeh HC, Duncan BB, Schmidt MI, Wang NY, Brancati FL. Smoking, smoking cessation, and risk for type 2 diabetes mellitus: a cohort study. Ann Intern Med. 2010;152(1):10–7.
11 Hur NW, Kim HC, Nam CM, Jee SH, Lee HC, Suh I. Smoking cessation and risk of type 2 diabetes mellitus: Korea Medical Insurance Corporation Study. Eur J Cardiovasc Prev Rehabil. 2007;14(2):244–9.
12 Will JC, Galuska DA, Ford ES, Mokdad A, Calle EE. Cigarette smoking and diabetes mellitus: evidence of a positive association from a large prospective cohort study. Int J Epidemiol. 2001;30(3):540–6.
13 Patja K, Jousilahti P, Hu G, Valle T, Qiao Q, Tuomilehto J. Effects of smoking, obesity and physical activity on the risk of type 2 diabetes in middle-aged Finnish men and women. J Intern Med. 2005;258(4):356–62.
14 Chiolero A, Faeh D, Paccaud F, Cornuz J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr. 2008;87(4):801–9.
15 Yang J, Zhu Y, Cole SA, Haack K, Zhang Y, Beebe LA, et al. A gene-family analysis of 61 genetic variants in the nicotinic acetylcholine receptor genes for insulin resistance and type 2 diabetes in American Indians. Diabetes. 2012;61(7):1888–94.
16 Conen D, Schon T, Aeschbacher S, Pare G, Frehner W, Risch M, et al. Genetic and phenotypic determinants of blood pressure and other cardiovascular risk factors (GAPP). Swiss Med Wkly. 2013;143:w13728.
17 Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–27.
18 American Diabetes Association. Standards of medical care in diabetes-2012. Diabetes care. 2012;35Suppl1:S11–63.
19 Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12–country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95.
20 Storr CL, Reboussin BA, Anthony JC. The Fagerstrom test for nicotine dependence: a comparison of standard scoring and latent class analysis approaches. Drug Alcohol Depend. 2005;80(2):241–50.
21 Fagerstrom KO, Heatherton TF, Kozlowski LT. Nicotine addiction and its assessment. Ear Nose Throat J. 1990;69(11):763–5.
22 Bergman BC, Perreault L, Hunerdosse D, Kerege A, Playdon M, Samek AM, et al. Novel and Reversible Mechanisms of Smoking-Induced Insulin Resistance in Humans. Diabetes. 2012.
23 Lee TC, Glynn RJ, Pena JM, Paynter NP, Conen D, Ridker PM, et al. Socioeconomic status and incident type 2 diabetes mellitus: data from the Women's Health Study. PLoS One. 2011;6(12):14.
24 Conen D, Glynn RJ, Ridker PM, Buring JE, Albert MA. Socioeconomic status, blood pressure progression, and incident hypertension in a prospective cohort of female health professionals. Eur Heart J. 2009;30(11):1378–84.
25 Eliasson B, Taskinen MR, Smith U. Long-term use of nicotine gum is associated with hyperinsulinemia and insulin resistance. Circulation. 1996;94(5):878–81.
26 Liu T, Chen WQ, David SP, Tyndale RF, Wang H, Chen YM, et al. Interaction between heavy smoking and CYP2A6 genotypes on type 2 diabetes and its possible pathways. Eur J Endocrinol. 2011;165(6):961–7.
27 Yoshikawa H, Hellstrom-Lindahl E, Grill V. Evidence for functional nicotinic receptors on pancreatic beta cells. Metabolism. 2005;54(2):247–54.
Funding / potential competing interests: The GAPP study was supported by the Liechtenstein Government, the Swiss Heart Foundation, the Swiss Society of Hypertension, the University of Basel, the University Hospital Basel, the Hanela Foundation, Schiller AG and Novartis. David Conen was supported by a grant of the Swiss National Science Foundation (PP00P3_133681)