The painful primary hip replacement – review of the literature
DOI: https://doi.org/10.4414/smw.2014.13974
Csaba
Forster-Horvath, Christian
Egloff, Andrej M.
Nowakowski, Victor
Valderrabano
Summary
Total hip replacement is one of the most successful surgical procedures of the 20th century (World Health Organisation).
The success rate is dependent on the chosen endpoint. Evaluation of the outcome in joint replacement surgery has shifted from the revision rate toward patient satisfaction and quality of life. Patient satisfaction is reported to be up to 96% 16 years postoperatively, but the prevalence of groin pain after conventional total hip replacement ranges from 0.4% to 18.3% and activity-limiting thigh pain is still an existing problem linked to the femoral component of uncemented hip replacement in up to 1.9% to 40.9% of cases in some series.
The aim of our article is to review the aetiology, diagnostic procedures and treatment of the painful primary total hip replacement. We discuss the most relevant intrinsic and extrinsic aetiological factors responsible for chronic pain after total hip arthroplasty focusing on comparative studies and randomised controlled trials including diagnostics and management. Detailed analysis of history, clinical examination, imaging and laboratory tests are required prior to any revision for painful total hip arthroplasty. Revision surgery without knowing the underlying pathology should be avoided.
CRP C-reactive protein
ESR erythrocyte sedimentation rate
GTPS greater trochanteric pain syndrome
HO heterotopic ossification
HPF high-power field
LLD leg-length discrepancy
MRI magnetic resonance imaging
NJR National Joint Registry
PCR polymerase chain-reaction
PJI prosthetic joint infection
PMC polymorphonuclear cells
SHAR Swedish Hip Arthroplasty Register
THR total hip replacement
WBC white blood cell
Introduction
Total hip replacement (THR) is one of the most successful surgical procedures in the 20th century. Mainly through technological advances, the 10-year survival rate of THR improved from 86% between 1979 and 1981 to 96% between 2000 and 2002 (Swedish Hip Arthroplasty Register [SHAR] 2011). In some series a 100% survival rate has been achieved [1].
However, the success rate is dependent on the chosen endpoint. Evaluation of the outcome has shifted from the revision rate toward patient satisfaction and quality of life [2]. These data are more controversial and show a broader range than survival rates. Patient satisfaction is reported to be as high as 96% 16 years postoperatively [3] and only 0.948% of primary THRs were revised because of pain without any other reason (SHAR 2011).
However, a nationwide survey in Denmark reported chronic ipsilateral pain in 28.1% of patients and 12.1% had moderate or severe limitations of daily activities [4]. Patient time incidence rates per 1,000 years for pain were found to be 1.37 and for aseptic loosening 1.44 as the cause of revision (10th annual report of the National Joint Registry [NJR] of England and Wales). That means that the revision rate due to pain expressed as the number of revision operations per 1,000 patient years is the second highest after the rate of revision due aseptic loosening.
Looking at pain as cause of revision in terms of incidence per 1,000 patient years by type of bearing and fixation, uncemented metal-on-metal has the highest incidence followed by uncemented ceramic-on-ceramic and uncemented metal-on-polyethylene constructs. Cemented metal-on-polyethylene with a 28 mm head has the lowest pain incidence; however, this construct is mainly applied in the elderly with lower levels of physical activity (9th annual report NJR England 2012).
In the following review we address intrinsic and extrinsic aetiologies. Intrinsic causes originate from the hip joint. All other causes are extrinsic.
Intrinsic causes include aseptic loosening, prosthetic joint infection, instability and impingement, thigh pain (micromotion, modulus mismatch, unnoticed periprosthetic fractures), iliopsoas impingement or hypersensitivity to metal debris.
Local extrinsic causes are those related to the hip region such as trochanteric pain syndrome, heterotopic ossification and insufficiency fractures. Remote extrinsic causes include spinal pathology, postsurgical pain syndrome and causes unrelated to the musculoskeletal system radiating to this area.
In our review we intend to give a comprehensive overview of the recent literature of the last 5 years giving practical information for interested general practitioners and specialists on all the orthopaedic aspects of painful total hip replacement.
Intrinsic causes
Aseptic loosening
Isolated acetabular revision (30%) is twice as common as isolated revision of the stem (15%). Cup and stem revision makes up approximately 45% of all revisions.
Acetabular loosening typically causes pain in the groin, although it also can lead to isolated buttock pain a little more distally than referred pain from the lumbar spine. Femoral stem loosening produces pain in the proximal region of the thigh and occasionally in the groin [5].
No clear answer can be given to the question as to whether cemented or uncemented implants perform best, because the type of bearing and age of the patient plays a significant role as well. However, the 10th annual report of the NJR England 2013 reports slightly higher revision rates for uncemented THRs compared with cemented ones, depending on the type of bearing. The least revision rate has been found with hybrid THRs with ceramic heads and polyethylene liners, which we apply most frequently in our practice. In this construct the acetabular cup is uncemented and the stem is cemented.
To show the complexity of this topic we present results from Scandinavian registers separately for acetabular and femoral components.
Acetabular component
When aseptic loosening is the endpoint, uncemented cups have a lower revision rate according to both the Swedish and the Finnish arthroplasty registers, if the most frequently used brands and patients younger than 55 years are considered [6, 7].
Stem
The most frequently used uncemented stems perform at least as well as or better than cemented ones if aseptic loosening is taken as an endpoint, especially in patients below 55 years of age. Again, this tendency is not supported by the 10th annual report NJR England and Wales.
Pain and the type of bearing and method of fixation
Looking at pain as cause of revision in terms of incidence per 1,000 patient years by bearing type and fixation uncemented metal-on-metal has the highest incidence followed by uncemented ceramic-on-ceramic and uncemented metal-on-polyethylene constructs. Cemented metal-on-polyethylene with 28 mm head has the lowest incidence (10th annual report NJR England 2012).
When the type of bearing and fixation are considered, pain is the third to fourth most frequent cause of revision for ceramic-on-ceramic and metal-on-polyethylene bearings; for metal-on-metal, it is the second after aseptic loosening. Ceramic-on-polyethylene and metal-on-polyethylene bearings have the least incidence rate of revision due to pain per 1,000 patient years (10th annual report, NJR for England and Wales). This is also supported by a randomised controlled trial comparing metal-on-metal, ceramic-on-polyethylene and metal-on-polyethylene bearings at the 7-year follow-up. Rates of aseptic loosening were 3%, 0% and 0%, respectively [8].
Prosthetic joint infection (PJI)
One of the three most frequent causes of postoperative pain after THR is prosthetic joint infection. The incidence of prosthetic infection lies between 0.4% and 1% for THR [9, 10]. Superficial infections are not difficult to diagnose, but low grade infections may often be occult. Distinguishing aseptic from septic loosening is crucial; the preoperative diagnosis relies on anamnestic data, laboratory findings, imaging and aspiration.
One of the most important pieces of anamnestic information is the onset of pain, which occurs significantly earlier in infections than in aseptic loosening (3 vs 9 years, respectively) and is found in 80% to 84% of patients [9, 11]. The index of suspicion has to be high if the patient had prolonged discharge postoperatively and if the patient has been having pain since operation and reports pain at night. In a number of cases modern antithrombotic drugs are responsible for prolonged oozing after THR, increasing the risk of surgical site infection [12].
Elevated synovial fluid white blood cell (WBC) count cut off 1.7×109/l) and elevated differential polymorphonuclear cell (PMC) count in synovial fluid (≥65%) [13]. The C-reactive protein (CRP) level and erythrocyte sedimentation rate (ESR) combined have a very high negative predictive value, reliably excluding aseptic cases [11, 14]. Synovial fluid tumour necrosis factor-α (TNF-α), interleukin-6 and interleukin-1β are also significantly higher in infected cases, but the positive predictive value of these markers is low [11].
According to the 2011 guidelines of the Workgroup of the Musculoskeletal Infection Society (MSIS), the diagnosis of prosthetic joint infection is definitive when four of the following criteria are fulfilled: ESR ≥30 mm/h and CRP ≥10 mg/l, elevated synovial WBC count (≥2,000/μl), elevated differential PMC count in synovial fluid (≥65%), pus in the joint in question, one positive culture of tissue or fluid, and more than five PMCs per high-power field on histological examination [15]. However, the cut off value for the synovial fluid WBC count has been shown to be lower (cut off 1.6×109/l) [13, 16].
Polymerase chain-reaction (PCR) tests are an additional useful tool in diagnosing prosthetic joint infection; however, their value is still controversial. A recent meta-analysis showed 86% and 91% sensitivity and specificity, respectively, that processing tissue samples instead of synovial fluid improved sensitivity, and that quantitative PCR and sonication of prosthesis components improved specificity [17].
A recent study including patients with systemic inflammatory disease and those already receiving antibiotic treatment has shown 100% sensitivity and specificity for the diagnosis of PJI with the application of novel biomarkers. Human α-defensin 1-3, neutrophil elastase 2, bactericidal/permeability-increasing protein, neutrophil gelatinase-associated lipocalin, and lactoferrin, correctly predicted the MSIS classification of all patients [96].
A further novel approach is prerevision synovial biopsy, which proved to be highly valuable for the diagnosis of PJI. The biopsy in combination of the bacteriologic and histologic examinations showed a sensitivity of 82% (95% CI, ± 11%), specificity of 98% (95% CI, ± 4%) [97].
Retention with debridement or change of the prosthesis is indicated, depending on the time of onset of symptoms after the primary or last surgery and the stability of the implant. The decision about one- or two-stage revisions is also governed by the species and resistance profile of the causative agent, and bone loss [9, 18–20].
Instability, impingement
Impingement is the cause of the majority of subluxations and dislocations after THR, and in cases of recurrent dislocation prosthetic impingement could always be detected [21]. Prosthetic impingement and instability not only limit range of movement and activities of daily living, but also increase the risk of cracking and thus third-body wear, especially in hard bearing implants, and results in unexplained pain [22, 23].
Positioning of the components balances minimising instability and maximising range of motion. Technically speaking the surgeon aims at minimal susceptibility to posterior or anterior prosthetic impingement. This can be achieved by the optimal positioning of the components, considering the combined anteversion of the acetabular and femoral component. Simulation studies have shown that anatomical anteversion and medial offset of the femoral component and 45° inclination / 20° antversion of the acetabular component with less than 5 mm protrusion of the anterior or posterior lip of the component produce best results in terms of stability and range of motion.
In clinical studies, significantly reduced rates of dislocation with larger head sizes in cases with intact abductor muscles were found [24]. Instead of absolute head size, significant correlation was found between impingement and head to neck ratio [21]. Notably, larger cup sizes (more than 56 mm) have an adjusted odds ratio of 2.4 (95% CI) for dislocation with the same head diameter [25]. It has to be mentioned that in cases of metal-on-metal bearings, large femoral heads induce groin pain significantly more frequently than small heads [26], possibly because of increased volumetric wear and hypersensitivity to metal debris.
The treatment of recurrent dislocation is surgical and requires restoration of the femoral offset and optimal head to neck ratio above 2. Revision of THR for instability is challenging and has a high redislocation rate, especially if only modular revision is done without correction of malpositioned implants. Redislocation rates in THRs revised for instability with exchange of bone fixed components or modular revisions were 18% and 39%, respectively [27, 28]. Thus, it is advised to refer patients with recurrent dislocation to a specialist arthroplasty surgeon with experience in component revision.
Hypersensitivity and synovitis with metal-on-metal components
The risk of revision with metal-on-metal bearings increases with head size and is 2–3 times higher than for other bearing options. The highest revision rate, predicted for a younger female patient with large head size (46 mm), is 9.1% at 7 years (9th annual report of the NJR of England and Wales).
The main cause for revision for well-fixed metal-on-metal bearing THRs is unexplained pain, well ahead of aseptic loosening [29]. A special feature of this type of bearing is pseudotumour formation within few years after implantation causing pain in patients with normal appearing postoperative X-rays [30]. Aseptic lymphocyte-associated vasculitic lesion (ALVAL) is the histological description of the lesion. It is also referred to as adverse local tissue reaction (ALTR).
Blood cobalt levels were found to be a sensitive and specific marker of abnormal wear in metal on metal bearings. More than 4.5 µg/l is indicative of poor performance [31].
Early magnetic resonance imaging (MRI) is indicated in patients presenting with unexplained pain after metal-on-metal hip arthroplasty to assess maximal synovial thickness and synovial volume in order to identify pseudotumours around the implants.
In these cases revision surgery with change of the components is indicated.
Iliopsoas impingement
Pain is typically exacerbated by getting in and out of bed or a car. Ambulation and weight bearing do not aggravate pain, but straight leg raise and resisted straight leg raise are sensitive tests. Response to diagnostic infiltration strongly suggests the diagnosis. Incidence of iliopsoas impingement after primary THR is about 0.37% at a follow up of 20 months, which is the average time to the presentation of clinical symptoms [32]. Iliopsoas impingement was the established diagnosis in 4.4% of painful THRs [33].
Insufficient anteversion of the cup resulting in prominence above the anterior acetabular rim most frequently interferes with the course of the iliopsoas tendon causing impingement and pain. Lengthening of the operative limb or excessive offset are also predisposing factors [34].
Conservative treatment is successful in 39% of the cases [35] and operative treatment is advocated after a course of physiotherapy and one or two image-guided diagnostic and therapeutic infiltrations with corticosteroids [36]. Dora et al. achieved a success rate of 82% with surgical treatment with either tenotomy or revision of the acetabular component and tendon debridement. An alternative therapy option is the endoscopic release of the iliopsoas tendon [37, 38].
Thigh pain
In spite of significant improvements in implant design and the surface of uncemented stems, activity-limiting thigh pain is still an existing problem linked to the femoral component of uncemented hip replacement. The incidence is 1.9% to 40.4% [39–41].
Possible causes for thigh pain after THR include excessive micromotion, modulus mismatch between bone and prostheses, unnoticed intraoperative fractures and loosening of the stem. Aseptic loosening is associated with excessive micromotion.
Micromotion depends on implant design [42], surface structure and roughness (Ra), as well as surgical technique. Depending on the rate of micromotion, bony or fibrous ingrowth or loosening will result [43–45]. The mechanism of bone apposition or ongrowth onto implant surfaces relies on intramembranous ossification [46]. This process takes approximately 4–12 weeks until the implant gains secondary stability [42]. The prerequisites for this are biocompatible implant material and surface, initial proximity of implant and bone surface, stable fixation and sterile environment [47, 48]. It could be clearly shown that fibrous ingrowth and stem subsidence correlates with thigh pain [39, 49, 50]. In the revision setting thigh pain was seen in 7%, 16% and 75% of bony ingrown, stable fibrous fixated and unstable uncemented extensively porous coated cobalt chrome stems, respectively [51].
The rigidity mismatch between the stem and the femoral diaphysis modulus mismatch is a well-documented cause of thigh pain with uncemented stems [41, 52–54]. However, this is controversial in the literature and others have found no correlation between thigh pain and stem diameter with these type of stems [55].
The dogma of extensively porous coated stems causing thigh pain is also controversial, since proximally coated cobalt-chrome stems were associated with more thigh pain than extensively porous coated stems (19% and 42%, respectively) [40].
Another possible cause of postoperative thigh pain is an occult intraoperative fracture. Up to 1 year postoperatively the periprosthetic fracture rate of uncemented stems is significantly higher, which would imply that these patients are more susceptible to periprosthetic fracture. However, the risk ratio adjusted for age, sex and underlying diagnosis contradicts this hypothesis. It seems to be more likely that these fractures are caused inadvertently at the time of surgery and become apparent later [6].
Local extrinsic causes
Greater trochanteric pain syndrome (GTPS), trochanteric bursitis, gluteal tendon tears
Lateral trochanteric pain was found to be associated with the direct lateral versus the posterior approach in 4.9% and 1.2%, respectively [56]. It seems plausible that it is also associated with excessive offset of the prosthetic stem, but studies could not verify this hypothesis [57].
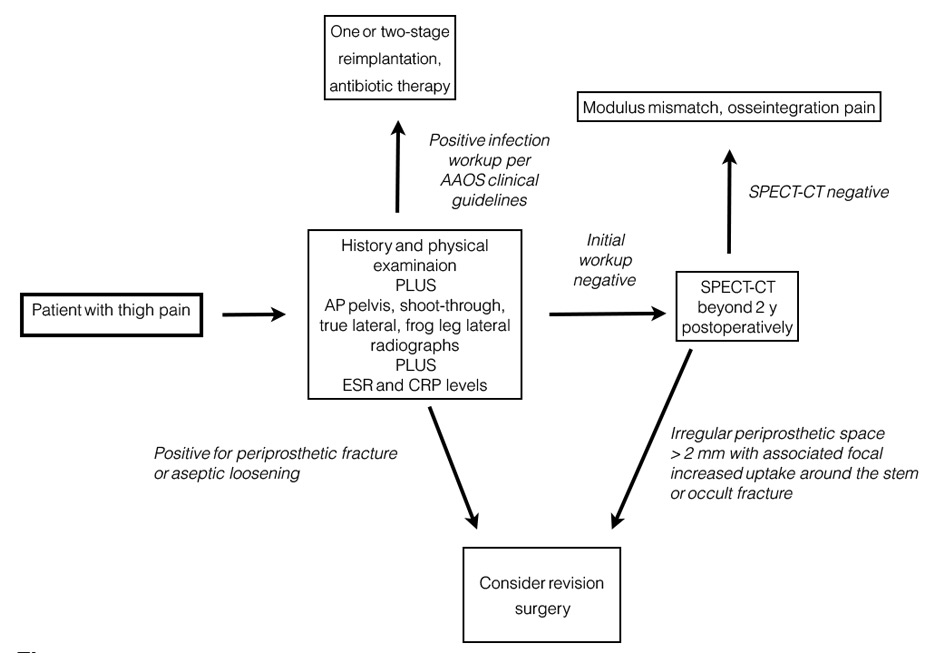
Figure 1
Diagnostic algorithm for thigh pain after total hip replacement (based on the American Academy of Orthopedic Surgeons Clinical Practice Guideline) [ 94].
AP = anteroposterior; CRP = C-reactive protein; CT = computed tomography; ESR = erythrocyte sedimentation rate; SPECT = single photon emission computed tomography
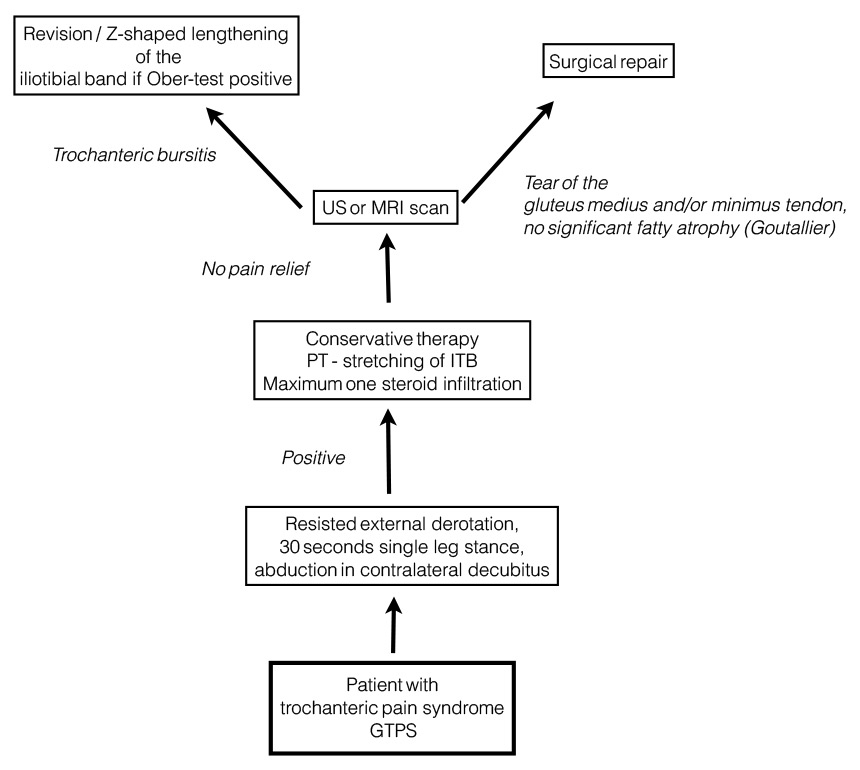
Figure 2
Diagnostic algorithm for greater trochanteric pain after total hip replacement [58, 95].
ITB = iliotibial band; MRI = magnetic resonance imaging; PT = physiotherapy
Clinical diagnosis is established by use of the Ober test or aggravation by active abduction against resistance and direct palpation. Sensitivity and specificity for gluteal tendinopathy and bursitis were 88% and 97.3%, respectively, with resisted external derotation in supine position and were 100% and 97.3%, respectively, with single leg stance for 30 seconds [58]. Weakness of abduction and a positive Trendelenburg's sign is compatible with tendon tears of the gluteus medius [59]. Ultrasound and MRI can differentiate gluteal tendinopathy and partial or full thickness tears from bursitis [60, 98].
Treatment involves stretching exercises of the iliotibial band and ultrasound-guided infltration with corticosteroids [57] and local anaesthetics. Response rate is slightly less than in non-prosthetic trochanteric bursitis (80% vs 90.3%) [61]. In a few recalcitrant cases open or arthroscopic bursectomy and Z-lengthening of the iliotibial band may be indicated [62, 63]. In cases of tendon tear with relevant functional impairment found at the time of primary THR or postoperatively, repair has to be performed before fatty degeneration of the abductors [64, 65].
Notably, leg-length discrepancy (LLD) is an associated factor of greater trochanteric pain [66].
Heterotopic ossification
The incidence of severe (Brooker grade II to IV) heterotopic ossification (HO) limiting the range of motion after major hip surgery has been reported to be about 9% [67]. The ossification in the periarticular musculature develops during the first postoperative year. There is a trend towards male patients with lower body mass index. Age is not an independent factor [68].
The gold standard for prevention is 25 mg indomethacin three times daily for 6 weeks. There is evidence that adding radiotherapy (700 cGy) improves efficacy and thus length of treatment can be reduced to 15 days [69]. Celecoxib, a selective cyclo-oxygenase-2 (COX-2) inhibitor, at a dosage of 200 mg twice daily for 28 days proved to be effective in the prevention of HO after THR [68]. Aspirin 325 mg given twice daily for 6 weeks has been shown to effectively prevent HO and thromboembolism [70]. The additional advantage of this treatment is the reduction of cardiovascular morbidity [71–73].
Leg-length discrepancy
There is no universal definition of LLD after THR, yet there is a consensus that less than 10 mm is acceptable. It appears that lengthening causes more discomfort than shortening [74]. More than 10 mm of lengthening after THR significantly impairs outcome, resulting in a 27% and 18% reduction of mean Oxford Hip Score at 3 and 12 months, respectively. The underlying cause is femoral component malpositioning in 98% of cases [75]. It leads to significant dissatisfaction, limping, lower back pain and nerve palsy [76, 77].
Prevention should be emphasised in terms of preoperative planning and intraoperative referencing to anatomical landmarks, positioning of the patient to allow intraoperative checking of the contralateral leg and restoring femoral offset. Computer navigation is another possible method for minimising LLD [78–80].
Treatment options are to be determined individually, depending on the extent of discrepancy and symptoms of the patient. Operative correction is effective in half of the cases and represents a last option [74].
Insufficiency fractures
Insufficiency fractures after THR are relatively frequent at the superior and inferior pubic ramus or the ischium [81–83]. A rare finding is postoperative fracture of the medial acetabular wall. This occurs as a consequence of osteolysis and thus represents a separate entity, which eventually leads to pelvic discontinuity requiring osteosynthetic stabilisation of the acetabulum [84–86].
Remote extrinsic causes
Spinal pathology
Differential diagnosis and management of spinal pathology and osteoarthritis of the hip can be challenging [87].
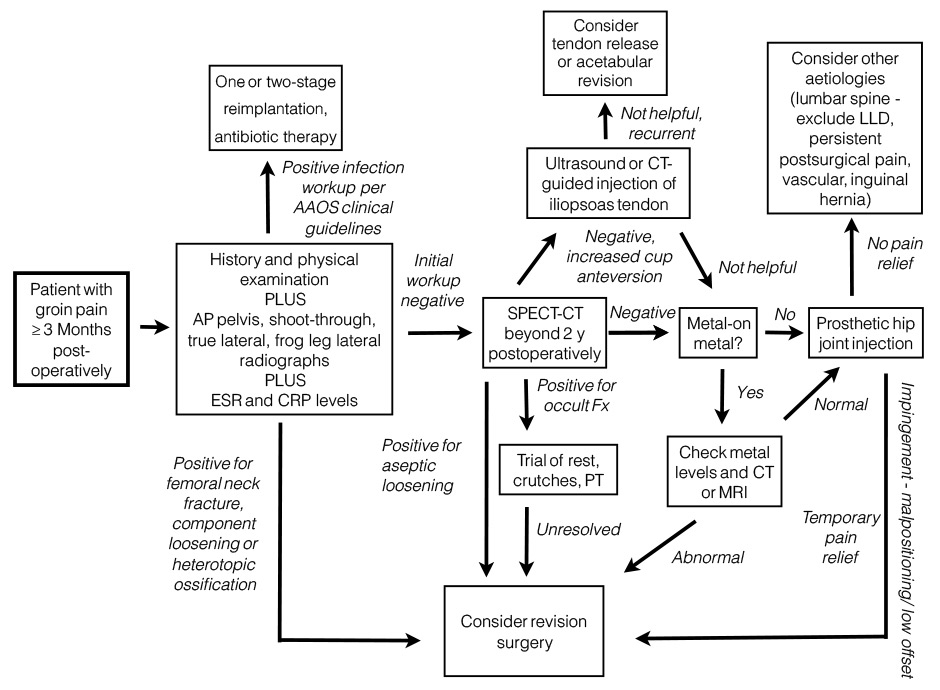
Figure 3
Diagnostic algorithm for groin pain after total hip replacement [93, 94].
AAOS = American Academy of Orthopedic Surgeons; AP = anteroposterior; CRP = C-reactive protein; CT = computed tomography; ESR = erythrocyte sedimentation rate; LLD = leg-length discrepancy; MRI = magnetic resonance imaging; PT = physiotherapy; SPECT = single photon positron emission computed tomography
Pain radiating below knee level from the lower back and resolved by leaning forward is not characteristic for painful THR. In contrast, a trimodal pain pattern with start-off pain, reduction after a few steps and aggravation by prolonged ambulation draws attention to component loosening. Muscular weakness and atrophy, and diminished reflexes and sensibility are signs of spinal stenosis. Lumbar stenosis with disc herniation L4–L5 is a documented cause of groin pain [88]. L5 radicular compression can lead to weakness of the gluteal muscles, mimicking Trendelenburg positivity. Both of these features require a careful diagnostic approach when evaluating a painful THR.
Thus, coexisting osteoarthritis of the hip and spinal stenosis is a challenging situation for the orthopaedic surgeon. Usually the hip should be treated first, in order to give a more predictable relief for the patient. However, patients with lumbar stenosis are at increased risk of peripheral nerve injury and postoperative neurological deficit when THR is performed. Thus, in such cases an asymtomatic lumbar stenosis has to be considered. Decompression of the involved segments does improve symptoms in some cases [89]. On the other hand THR improves low back pain and spinal functional assessment indexes [90].
The above facts are important when counselling and obtaining consent from patients with spinal stenosis awaiting THR.
Persistent postsurgical pain
Pain of any severity and severe to extreme persistent pain was experienced 3 to 4 years postoperatively by 27% and 6% of patients, respectively. Major depression and the number of other painful areas of the body were found to be significant and independent determinants of chronic postoperative pain. Neuropathic type of pain was reported by only 1% of these patients [91]. In contrast, the prevalence of nerve injury-induced neuropathic pain was found to be 6% after THR in another study [92].
It is important to note that there is evidence that the intensity of early postoperative pain does have an effect on the development of chronic pain after THR [4].
|
Table 1: Recommendations for clinical practice. |
|
Topic
|
Recent literature data
|
Recommendation
|
Reference
|
| Aseptic loosening |
Lowest revision rate for hybrid THR with highly crosslinked polyethylene liners and ceramic heads |
Use hard-on-soft bearings with hybrid fixation |
NJR England and Wales 2013 |
| Lowest incidence of pain with cemented metal-on-polyethylene THR and 28 mm heads |
Use metal-on-polyethylene bearing with cemented components in less active patients |
NJR England and Wales 2013 |
| Prosthetic joint infection |
WBC cut off value is less than that of the Workgroup of the Musculoskeletal Infection Society Guideline 2011 |
Use synovial fluid WBC cut off 1.6x109/l instead of 2.0x109/l |
Ref [16] |
| Meta-analysis:
Sensitivity and specificity of PCR are 86% and 91%, respectively, and sonication increases specificity of PCR |
In cases of ongoing antibiotic treatment PCR can be helpful |
Ref [17] |
| Thigh pain |
As a result of unnoticed intraoperative fractures the rate of periprosthetic fractures is higher in uncemented THRs |
Early radiological assessment (X-ray, CT) if patient complains of early thigh pain |
Ref [6] |
| Iliopsoas impingement |
Iatrogenic limb lengthening and increased offset are predisposing factors for iliopsoas impingement |
Avoid LLD and increased offset, precise execution of preoperative plan |
Ref [34] |
| Iatrogenic LLD |
Operative correction is effective in 50% of cases |
Avoid LLD, accurate assessment, planning and good operative technique for determining leg length |
Ref [74] |
| Metal hypersensitivity |
Blood cobalt levels were found to be a sensitive and specific marker of abnormal wear in metal on metal bearings |
Measure blood cobalt ion levels: more than 4.5 μg/l is indicative of poor performance |
Ref [31] |
| Heterotopic ossification |
The gold standard of prevention is 25 mg indomethacin three times daily for 6 weeks. There is evidence that adding radiotherapy (700 cGy) improves efficacy and thus length of treatment can be reduced to 15 days |
|
Ref [69] |
| 200 mg celecoxib, a selective COX-2 inhibitor given twice daily for 28 days proved to be effective in the prevention of HO after THR |
Give celecoxib for 28 days |
Ref [68] |
| Greater trochanteric pain syndrome |
30 seconds single leg stance and resisted external derotation are valuable tools in diagnosing gluteal tendon tears |
Use these tests and in positive cases do ultrasound or MRI |
Ref [58] |
| COX-2 = cyclo-oxygenase-2; CT = computed tomography; HO = heterotopic ossification; LLD = leg-length discrepancy; MRI = magnetic resonance imaging; NJR = National Joint Registry; PCR = polymerase chain-reaction; THR = total hip replacement; WBC = white blood cell; |
Recommendations
Figures 1 to 3 show diagnostic algorithms for groin pain, thigh pain, and greater trochanteric pain after THR. Recommendations for clinical practice are summarised in table 1.
Conclusion
In this review we present the most relevant causes for a painful total hip replacement including the latest scientific results not included in recent review articles in this topic. We support the view that no revision surgery be planned before a meticulous differential diagnostic approach and identification of the underlying cause.
References
1 Berli BJ, Schafer D, Morscher EW. Ten-year survival of the MS-30 matt-surfaced cemented stem. J Bone Joint Surg Br. 2005;87(7):928–33.
2 Ahmad MA, Xypnitos FN, Giannoudis PV. Measuring hip outcomes: common scales and checklists. Injury. 2011;42(3):259–64.
3 Mariconda M, Galasso O, Costa GG, Recano P, Cerbasi S. Quality of life and functionality after total hip arthroplasty: a long-term follow-up study. BMC Musculoskelet Disord. 2011;12:222.
4 Nikolajsen L, Brandsborg B, Lucht U, Jensen TS, Kehlet H. Chronic pain following total hip arthroplasty: a nationwide questionnaire study. Acta Anaesthesiol Scand. 2006;50(4):495–500.
5 Attar SS, M., Clinical evaluation of the failed or painful total hip arthroplasty, in Arthritis and Arthroplasty – The Hip. 2009, Saunders Elsevier: Philadelphia. p. 12–23.
6 Hailer NP, Garellick G, Karrholm J. Uncemented and cemented primary total hip arthroplasty in the Swedish Hip Arthroplasty Register. Acta Orthop. 2010;81(1):34–41.
7 Eskelinen AP, P, Helenius, I, Pulkkinen, P, Remes, V. Total hip arthroplasty for rheumatoid arthritis in younger patients: 2,557 replacements in the Finnish Arthroplasty Register followed for 0–24 years. Acta Orthop. 2006;77(6):853–65.
8 Bjorgul K, Novicoff WN, Andersen ST, Ahlund OR, Bunes A, Wiig M, et al. High rate of revision and a high incidence of radiolucent lines around Metasul metal-on-metal total hip replacements: results from a randomised controlled trial of three bearings after seven years. Bone Joint J. 2013;95–B(7):881–6.
9 Giulieri SG, Graber P, Ochsner PE, Zimmerli W. Management of infection associated with total hip arthroplasty according to a treatment algorithm. Infection. 2004;32(4):222–8.
10 Cui Q, Mihalko WM, Shields JS, Ries M, Saleh KJ. Antibiotic-impregnated cement spacers for the treatment of infection associated with total hip or knee arthroplasty. J Bone Joint Surg Am. 2007;89(4):871–82.
11 Nilsdotter-Augustinsson A, Briheim G, Herder A, Ljunghusen O, Wahlstrom O, Ohman L. Inflammatory response in 85 patients with loosened hip prostheses: a prospective study comparing inflammatory markers in patients with aseptic and septic prosthetic loosening. Acta Orthop. 2007;78(5):629–39.
12 Aquilina AL, Brunton LR, Whitehouse MR, Sullivan N, Blom AW. Direct thrombin inhibitor (DTI) vs. aspirin in primary total hip and knee replacement using wound ooze as the primary outcome measure. A prospective cohort study. Hip Int. 2012;22(1):22–7.
13 Trampuz A, Hanssen AD, Osmon DR, Mandrekar J, Steckelberg JM, Patel R. Synovial fluid leukocyte count and differential for the diagnosis of prosthetic knee infection. Am J Med. 2004;117(8):556–62.
14 Spangehl MJ, Masri BA, O'Connell JX, Duncan CP. Prospective analysis of preoperative and intraoperative investigations for the diagnosis of infection at the sites of two hundred and two revision total hip arthroplasties. J Bone Joint Surg Am. 1999;81(5):672–83.
15 Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011;469(11):2992–4.
16 Dinneen A, Guyo A, Clements J, Bradley N. Synovial fluid white cell and differential count in the diagnosis or exclusion of prosthetic joint infection. Bone Joint J. 2013;95–B(4):554–7.
17 Qu X, Zhai Z, Li H, Li H, Liu X, Zhu Z, et al. PCR-based diagnosis of prosthetic joint infection. J Clin Microbiol. 2013;51(8):2742–6.
18 Klouche S, Leonard P, Zeller V, Lhotellier L, Graff W, Leclerc P, et al. Infected total hip arthroplasty revision: one- or two-stage procedure? Orthop Traumatol Surg Res. 2012;98(2):144–50.
19 De Man FH, Sendi P, Zimmerli W, Maurer TB, Ochsner PE, Ilchmann T. Infectiological, functional, and radiographic outcome after revision for prosthetic hip infection according to a strict algorithm. Acta Orthop. 2011;82(1):27–34.
20 Sendi P, Rohrbach M, Graber P, Frei R, Ochsner PE, Zimmerli W. Staphylococcus aureus small colony variants in prosthetic joint infection. Clin Infect Dis. 2006;43(8):961–7.
21 Marchetti E, Krantz N, Berton C, Bocquet D, Fouilleron N, Migaud H, et al. Component impingement in total hip arthroplasty: frequency and risk factors. A continuous retrieval analysis series of 416 cup. Orthop Traumatol Surg Res. 2011;97(2):127–33.
22 Yamamoto T, Saito M, Ueno M, Hananouchi T, Tokugawa Y, Yonenobu K. Wear analysis of retrieved ceramic-on-ceramic articulations in total hip arthroplasty: Femoral head makes contact with the rim of the socket outside of the bearing surface. J Biomed Mater Res B Appl Biomater. 2005;73(2):301–7.
23 Malik A, Maheshwari A, Dorr LD. Impingement with total hip replacement. J Bone Joint Surg Am. 2007;89(8):1832–42.
24 Kung PL, Ries MD. Effect of femoral head size and abductors on dislocation after revision THA. Clin Orthop Relat Res. 2007;465:170–4.
25 Peter R, Lubbeke A, Stern R, Hoffmeyer P. Cup size and risk of dislocation after primary total hip arthroplasty. J Arthroplasty. 2011;26(8):1305–9.
26 Lavigne M, Laffosse JM, Ganapathi M, Girard J, Vendittoli P. Residual groin pain at a minimum of two years after metal-on-metal THA with a twenty-eight-millimeter femoral head, THA with a large-diameter femoral head, and hip resurfacing. J Bone Joint Surg Am. 2011;93(Suppl 2):93–8.
27 Daly PJ, Morrey BF. Operative correction of an unstable total hip arthroplasty. J Bone Joint Surg Am. 1992;74(9):1334–43.
28 Lachiewicz PF, Soileau E, Ellis J. Modular revision for recurrent dislocation of primary or revision total hip arthroplasty. J Arthroplasty. 2004;19(4):424–9.
29 Nawabi DH, Gold S, Lyman S, Fields K, Padgett DE, Potter HG. MRI predicts ALVAL and tissue damage in metal-on-metal hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):471–81.
30 Hart AJ, Satchithananda K, Liddle AD, Sabah SA, McRobbie D, Henckel J, et al. Pseudotumors in association with well-functioning metal-on-metal hip prostheses: a case-control study using three-dimensional computed tomography and magnetic resonance imaging. J Bone Joint Surg Am. 2012;94(4):317–25.
31 Sidaginamale RP, Joyce TJ, Lord JK, Jefferson R, Blain PG, Nargol AV, et al. Blood metal ion testing is an effectivescreening tool to identify poorly performing metal-on-metal bearingsurfaces. Bone Joint Res. 2013;2(5):84–95.
32 O'Sullivan M, Tai CC, Richards S, Skyrme AD, Walter WL, Walter WK. Iliopsoas tendonitis a complication after total hip arthroplasty. J Arthroplasty. 2007;22(2):166–70.
33 Ala Eddine T, Remy F, Chantelot C, Giraud F, Migaud H, Duquennoy A. Anterior iliopsoas impingement after total hip arthroplasty: diagnosis and conservative treatment in 9 cases. Rev Chir Orthop Reparatrice Appar Mot. 2001;87(8):815–9.
34 Dora C, Houweling M, Koch P, Sierra RJ. Iliopsoas impingement after total hip replacement: the results of non-operative management, tenotomy or acetabular revision. J Bone Joint Surg Br. 2007;89(8):1031–5.
35 Lachiewicz PF, Kauk JR. Anterior iliopsoas impingement and tendinitis after total hip arthroplasty. J Am Acad Orthop Surg. 2009;17(6):337–44.
36 Henderson RA, Lachiewicz PF. Groin pain after replacement of the hip: aetiology, evaluation and treatment. J Bone Joint Surg Br. 2012;94(2):145–51.
37 Gedouin JE, Huten D. Technique and results of endoscopic tenotomy in iliopsoas muscle tendinopathy secondary to total hip replacement: a series of 10 cases. Orthop Traumatol Surg Res. 2012;98(4 Suppl):S19–25.
38 Van Riet A, De Schepper J, Delport HP. Arthroscopic psoas release for iliopsoas impingement after total hip replacement. Acta Orthop Belg. 2011;77(1):41–6.
39 Kinov P, Radl R, Zacherl M, Leithner A, Windhager R. Correlation between thigh pain and radiological findings with a proximally porous-coated stem. Acta Orthop Belg. 2007;73(5):618–24.
40 Barrack RL, Paprosky W, Butler RA, Palafox A, Szuszczewicz E, Myers L, Patients' perception of pain after total hip arthroplasty. J Arthroplasty, 2000;15(5):590–6.
41 Brown TE, Larson B, Shen F, Moskal JT. Thigh pain after cementless total hip arthroplasty: evaluation and management. J Am Acad Orthop Surg. 2002;10(6):385–92.
42 Khanuja HS, Vakil JJ, Goddard MS, Mont MA. Cementless femoral fixation in total hip arthroplasty. J Bone Joint Surg Am. 2011;93(5):500–9.
43 Bauer TW, Schils J. The pathology of total joint arthroplasty.II. Mechanisms of implant failure. Skeletal Radiol. 1999;28(9):483–97.
44 Engh CA, D. O'Connor, Jasty M, McGovern TF, Bobyn JD, Harris WH. Quantification of implant micromotion, strain shielding, and bone resorption with porous-coated anatomic medullary locking femoral prostheses. Clin Orthop Relat Res. 1992(285):13–29.
45 Jasty M, Bragdon C, Burke D, O'Connor D, Lowenstein J, Harris WH. In vivo skeletal responses to porous-surfaced implants subjected to small induced motions. J Bone Joint Surg Am. 1997;79(5):707–14.
46 Brånemark PI. Osseointegration and its experimental background. J Prosthet Dent. 1983;50(3):399–410.
47 Sumner DR, Turner TM, Urban RM. Animal models relevant to cementless joint replacement. J Musculoskelet Neuronal Interact. 2001;1(4):333–45.
48 Bauer TW, Schils J. The pathology of total joint arthroplasty. I. Mechanisms of implant fixation. Skeletal Radiol. 1999;28(8):423–32.
49 Davies MS, Parker BC, Ward DA, Hua J, Walker PS. Migration of the uncemented CLS femoral component. Orthopedics. 1999;22(2):225–8.
50 Kim YH, Kim VE. Early migration of uncemented porous coated anatomic femoral component related to aseptic loosening. Clin Orthop Relat Res. 1993(295):146–55.
51 Moreland JR, Moreno MA. Cementless femoral revision arthroplasty of the hip: minimum 5 years followup. Clin Orthop Relat Res. 2001(393):194–201.
52 Skinner HB, Curlin FJ. Decreased pain with lower flexural rigidity of uncemented femoral prostheses. Orthopedics. 1990;13(11):1223–8.
53 Vresilovic EJ, Hozack WJ, Rothman RH. Incidence of thigh pain after uncemented total hip arthroplasty as a function of femoral stem size. J Arthroplasty. 1996;11(3):304–11.
54 Maloney WJ, Harris WH. Comparison of a hybrid with an uncemented total hip replacement. A retrospective matched-pair study. J Bone Joint Surg Am. 1990;72(9):1349–52.
55 Engh CA, Jr., Mohan V, Nagowski JP, Sychterz Terefenko CJ, Engh CA, Sr., Influence of stem size on clinical outcome of primary total hip arthroplasty with cementless extensively porous-coated femoral components. J Arthroplasty. 2009;24(4):554–9.
56 Iorio R, Healy WL, Warren PD, Appleby D. Lateral trochanteric pain following primary total hip arthroplasty. J Arthroplasty. 2006;21(2):233–6.
57 Farmer KW, Jones LC, Brownson KE, Khanuja HS, Hungerford MW. Trochanteric bursitis after total hip arthroplasty: incidence and evaluation of response to treatment. J Arthroplasty. 2010;25(2):208–12.
58 Lequesne M, Mathieu P, Vuillemin-Bodaghi V, Bard H, Djian P. Gluteal tendinopathy in refractory greater trochanter pain syndrome: diagnostic value of two clinical tests. Arthritis Rheum. 2008;59(2):241–6.
59 Bird PA, Oakley SP, Shnier R, Kirkham BW. Prospective evaluation of magnetic resonance imaging and physical examination findings in patients with greater trochanteric pain syndrome. Arthritis Rheum. 2001;44(9):2138–45.
60 Klauser AS, Martinoli C, Tagliafico A, Bellmann-Weiler R, Feuchtner GM, Wick M, et al. Greater trochanteric pain syndrome. Semin Musculoskelet Radiol. 2013;17(1):43–8.
61 Schapira D, Nahir M, Scharf Y. Trochanteric bursitis: a common clinical problem. Arch Phys Med Rehabil. 1986;67(11):815–7.
62 Chirputkar K, Weir P, Gray A. Z-lengthening of the iliotibial band to treat recalcitrant cases of trochanteric bursitis. Hip Int. 2007;17(1):31–5.
63 Craig RA, Jones DP, Oakley AP, Dunbar JD. Iliotibial band Z-lengthening for refractory trochanteric bursitis (greater trochanteric pain syndrome). ANZ J Surg. 2007;77(11):996–8.
64 Miozzari HH, Dora C, Clark JM, Notzli HP. Late repair of abductor avulsion after the transgluteal approach for hip arthroplasty. J Arthroplasty. 2010;25(3):450–457 e1.
65 Odak S, Ivory J. Management of abductor mechanism deficiency following total hip replacement. Bone Joint J. 2013;95–B(3):343–7.
66 Sayed-Noor AS, Sjoden GO. Greater trochanteric pain after total hip arthroplasty: the incidence, clinical outcome and associated factors. Hip Int. 2006;16(3):202–6.
67 Neal B, Gray H, MacMahon S, Dunn L. Incidence of heterotopic bone formation after major hip surgery. ANZ J Surg. 2002;72(11):808–21.
68 Lavernia CJ, Contreras JS, Villa JM, Rossi MD. Celecoxib and Heterotopic Bone Formation After Total Hip Arthroplasty. J Arthroplasty. 2013.
69 Le Duff MJ, Takamura KB, Amstutz HC. Incidence of heterotopic ossification and effects of various prophylactic methods after hip resurfacing. Bull NYU Hosp Jt Dis. 2011;69(Suppl 1):S36–41.
70 Beksac B, Gonzalez Della Valle A, Anderson J, Sharrock NE, Sculco TP, Salvato EA. Symptomatic thromboembolism after one-stage bilateral THA with a multimodal prophylaxis protocol. Clin Orthop Relat Res. 2007;463:114–9.
71 Bek D, Beksac B, Della Valle AG, Sculco TP, Salvati EA. Aspirin decreases the prevalence and severity of heterotopic ossification after 1–stage bilateral total hip arthroplasty for osteoarthrosis. J Arthroplasty. 2009;24(2):226–32.
72 Pavlou G, Kyrkos M, Tsialogiannis E, Korres N, Tsiridis E. Pharmacological treatment of heterotopic ossification following hip surgery: an update. Expert Opin Pharmacother. 2012;13(5):619–22.
73 Parry M, Wylde V, Blom AW. Ninety-day mortality after elective total hip replacement: 1549 patients using aspirin as a thromboprophylactic agent. J Bone Joint Surg Br. 2008;90(3):306–7.
74 McWilliams AB, Grainger AJ, O'Connor PJ, Redmond AC, Stewart TD, Stone MH. A review of symptomatic leg length inequality following total hip arthroplasty. Hip Int. 2013;23(1):6–14.
75 Konyves A, Bannister GC. The importance of leg length discrepancy after total hip arthroplasty. J Bone Joint Surg Br. 2005;87(2):155–7.
76 Roder C, Vogel R, Burri L, Dietrich D, Staub LP. Total hip arthroplasty: leg length inequality impairs functional outcomes and patient satisfaction. BMC Musculoskelet Disord. 2012;13:95.
77 Clark CR, HuddlestonHD, Schoch EP, 3rd, and Thomas BJ. Leg-length discrepancy after total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(1):38–45.
78 Manzotti A, Cerveri P, De Momi E, Pullen C, Confalonieri N. Does computer-assisted surgery benefit leg length restoration in total hip replacement? Navigation versus conventional freehand. Int Orthop. 2011;35(1):19–24.
79 Cuckler JM. Limb length and stability in total hip replacement. Orthopedics. 2005;28(9):951–3.
80 Desai A, Barkatali B, Dramis A, Board TN. A simple intraoperative technique to avoid limb length discrepancy in total hip arthroplasty. Surgeon. 2010;8(2):119–21.
81 Oh I, Hardacre JA. Fatigue fracture of the inferior pubic ramus following total hip replacement for congenital hip dislocation. Clin Orthop Relat Res. 1980(147):154–6.
82 Marmor L. Stress fracture of the pubic ramus stimulating a loose total hip replacement. Clin Orthop Relat Res. 1976(121):103–4.
83 Launder WJ, Hungerford DS. Stress fracture of the pubis after total hip arthroplasty. Clin Orthop Relat Res. 1981(159):183–5.
84 Kanaji A, Ando K, Nakagawa M, Fukaya E, Date H, Yamada H. Insufficiency fracture in the medial wall of the acetabulum after total hip arthroplasty. J Arthroplasty. 2007;22(5):763–7.
85 Callaghan JJ, Kim YS, Pederson DR, Brown TD. Periprosthetic fractures of the acetabulum. Orthop Clin North Am. 1999;30(2):221–34.
86 Della Valle CJ, Momberger NG, Paprosky WG. Periprosthetic fractures of the acetabulum associated with a total hip arthroplasty. Instr Course Lect. 2003;52:281–90.
87 Fogel GR, Esses SI. Hip spine syndrome: management of coexisting radiculopathy and arthritis of the lower extremity. Spine J. 2003;3(3):238–41.
88 Yukawa Y, Kato F, Kajino G, Nakamura S, Nitta H. Groin pain associated with lower lumbar disc herniation. Spine. (Phila Pa 1976), 1997;22(15):1736–9; discussion 1740.
89 Pritchett JW. Lumbar decompression to treat foot drop after hip arthroplasty. Clin Orthop Relat Res. 1994(303):173–7.
90 Peleg Ben-Galim TB-G, Nahshon Rand, Shmuel Dekel, Yizhar Floman, Hip-Spine Syndrome: The Effect of Total Hip Replacement Surgery Upon Low Back Pain in Patients With Severe Osteoarthritis of the Hip. The Spine Journal. 2006;6(5 Supplement):31S–32S.
91 Wylde V, Hewlett S, Learmonth ID, Dieppe P. Persistent pain after joint replacement: prevalence, sensory qualities, and postoperative determinants. Pain. 2011;152(3):566–72.
92 Haroutiunian S, Nikolajsen L, Finnerup NB, Jensen TS. The neuropathic component in persistent postsurgical pain: a systematic literature review. Pain. 2013;154(1):95–102.
93 Parvizi J, Della Valle CJ. AAOS Clinical Practice Guideline: diagnosis and treatment of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg. 2010;18(12):771–2.
94 Tam HH, Bhaludin B, Rahman F, Weller A, Ejindu V, Parthipun A. SPECT-CT in total hip arthroplasty. Clin Radiol. 2014;69(1):82–95.
95 Lequesne M, Djian P, Vuillemin V, Mathieu P. Prospective study of refractory greater trchanter pain syndrome. MRI findings of gluteal tendon tears seen at surgery. Clinical and MRI results of tendon repair. Joint Bone Spine. 2008;75(4):458–64.
96 Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing Periprosthetic Joint Infection: Has the Era of the Biomarker Arrived? Clin Orthop Relat Res. 2014 Mar 4.
97 Fink B, Gebhard A, Fuerst M, Berger I, Schäfer P. High diagnostic value of synovial biopsy in periprosthetic joint infection of the hip. Clin Orthop Relat Res. 2013;471(3):956-64.
98 Westacott DJ, Minns JI, Foguet P. The diagnostic accuracy of magnetic resonance imaging and ultrasonography in gluteal tendon tears - a systematic review. Hip Int. 2011;21(6):637–45.


