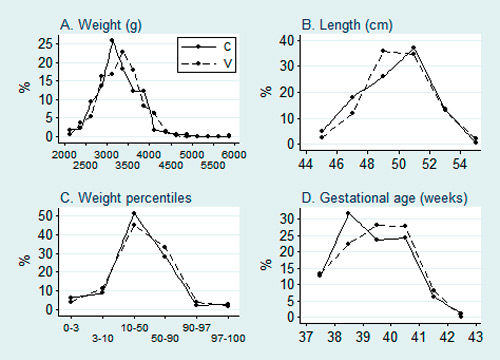
Figure 1
Distribution of birthweight, birthlength, percentiles of birthweight and exact gestational age at birth of the two distinct groups.
V = Vitrolife®; C = Cook®.
DOI: https://doi.org/10.4414/smw.2014.14038
Since the introduction of in vitro fertilisation (IVF) – intracytoplasmatic sperm injection (ICSI), several studies have shown that birthweight of infants conceived after fresh IVF-ICSI cycles is lower than birthweight of neonates after spontaneous conception, even in singleton pregnancies [1, 2]. The reasons for these differences still remain uncertain. Many different aspects are taken into consideration, such as intrinsic effects of the patients (infertility, age, smoking etc.), hormonal stimulation of the ovaries or the effects of the treatment itself, i.e., in-vitro culture which may cause imprinting disorders and epigenetic changes.
In 2010, Dumoulin et al. postulated that specific culture media could be a predisposing factor of inferior birthweight [3]. They compared in a non-randomised, retrospective cohort study the effect of two different commercially available culture media on the outcome of singleton pregnancies after fresh IVF-ICSI cycles and observed that neonates, cultured as embryos in the Cook® embryo culture media, had lower birthweight compared to those of the Vitrolife® culture media. In a second retrospective cohort study (same time frame as the first study and including partly the same cohort), they analysed the effect of these two culture media on frozen cycles and twin pregnancies and did not find any significant difference in birthweight, although a trend in the same direction was observed [4]. When analysing the same patient group in a third retrospective cohort study, they found that differences in intrauterine growth could be detected as early as the second trimester [5]. A very recently published Norwegian study, comparing birthweight after embryo culture in three different media with birthweight after spontaneous conception, showed significant differences in birthweight and also in placental weight in relation to birthweight [6]. However, other research groups, investigating the same and other commercialised culture media, could not find any differences in birthweight related to the use of varying embryo culture media [7–10]. Unfortunately, the exact composition of the culture media is a manufacturing secret of the different companies producing embryo culture media. Only the type of ingredients can be found out, not the precise composition. As epigenetic modifications can occur already during the first days of the embryonic life, it would be thinkable that some ingredients (or concentrations of ingredients), present in one culture medium, but not in another, could modify birthweight by influencing epigenetic processes and could result accordingly in higher respectively lower birthweight. Research on this topic is therefore very important.
Hence, there are many unanswered questions on this theme and the debate is ongoing. As birthweight is an important predictor for long-term health implications, it is primordial to ascertain if there is an association between specific culture media and adverse fetal outcomes with potentially long lasting consequences in adulthood.
In the current study, we investigated birthweight of singleton term infants after culture in embryo culture media from the same distributors (Vitrolife and Cook) as Dumoulin et al. and Carrasco et al. [3, 10]. The primary objective was to evaluate and compare birthweight and length of singleton term newborn infants, conceived after IVF-ICSI (fresh and frozen cycles) and cultured in two distinct, widely used commercial culture media systems (Vitrolife® and Cook®). We tested the hypothesis that both culture media are equivalent with respect to birthweight and length.
All patients who underwent IVF-ICSI fresh or frozen treatment cycles at the University Hospital of Lausanne (CHUV) from 1 January 2000 to 31 December 2004 and had singleton live births at term were considered for the study. Additional inclusion criteria were age ≤43 years, pre-treatment hormonal values in the normal range and normal gynaecological ultrasound and cervical smear. Exclusion criteria for an IVF/ICSI-treatment were acute or chronic infectious diseases (patient and/or her partner), severe psychiatric illness, or known carrier of a severe genetic disease.
To sum up, in an IVF-ICSI fresh cycle, stimulation of several follicles in the ovaries is undertaken until maturation of at least three follicles is seen by ultrasound, with corresponding oestradiol levels. On the day of oocyte retrieval, the sperm is prepared in the IVF-laboratory and mature oocytes are fertilised either by IVF or ICSI. The next day, supernumerary fertilised oocytes at the pronuclei stage are cryopreserved; one or two are cultured until embryo transfer (two or three days after oocyte pick-up). Quantitative pregnancy test is undertaken 14 days after embryo transfer.
A total of 525 patients fulfilled the inclusion criteria; 352 had embryos cultured in Vitrolife® media and 173 in Cook® media. Only healthy neonates were included. Infants with major congenital malformations or genetic defects were excluded. All included cycles were cycles with own gametes and without gestational carrier (according to the Swiss law). ICSI was performed in the case of male infertility or if less than five oocytes could be obtained.
Patients were addressed to one of the two laboratory sites, one using culture media from Vitrolife® (Vitrolife AB, Goteborg, Sweden) and the other from Cook® (Cook Medical, Brisbane, Australia), in order not to be dependent on only one distributor. Separation of the laboratory in two sites was undertaken for space and organisational reasons. The only difference between the two laboratory sites was the embryo culture medium. Otherwise, the setting (laboratory and medical staff, laboratory equipment and procedures, stimulation protocols etc.) was the same. At the site using Vitrolife® culture media, more IVF-ICSI cycles were performed, explaining the difference in the number of newborns between the two groups. Attribution to one or the other site was randomly performed and followed practical and not medical reasons (availability of consultation space, accessibility, insurance reasons etc.). However, it was not performed following a clear randomisation protocol.
Measurements of weight and length of newborn infants were obtained through the local IVF-ICSI registry (collection of the data by medical reports). Anthropometric measurements were performed in both centres by trained nurses and midwives in accordance to the methods described by Cheik Ismail et al. [11]. The attitude was not different in the measurements between the two groups. The birthweight of each newborn infant was adjusted for gestational age/gender and attribution to growth percentiles and growth categories was performed. Factors influencing neonatal birthweight, such as parental weight and height measurements, smoking habits and complications during pregnancy were collected by contacting the patients. Due to the fact, that the latter information was collected by a questionnaire sent to the parents and return rate was not a 100%, all birthweight adjustments could not be undertaken for the whole study population. Thus, two analyses were performed separately: one within the whole study population and the other in the sample with the complete data set based on the additional information of the filled in questionnaires.
The study was approved by the institutional ethical committee.
The “long” stimulation protocol was used for controlled ovarian hyperstimulation. Downregulation was performed with the agonist Triptorelin, 0.1 mg per day SC; for ovarian stimulation recombinant FSH was administered SC. To assess follicular maturation, ultrasound and serum oestradiol measurements were carried out. Selection criteria for ovulation induction included at least three follicles exceeding 17 mm in diameter and an oestradiol concentration per mature follicle exceeding 1000 pmol/l. Ovulation induction was performed with 5000 to 10000 IU hCG and 35 to 36 hours later, the follicular fluid with the oocytes was retrieved from all follicles by needle aspiration. Oocyte pick-up (OPU) was performed with transvaginal ultrasound guidance and under routine general anaesthesia.
The transfer of the embryos was performed two days after the OPU; only in the case of week-ends, the transfer was performed three days after the OPU. As embryo cryopreservation in Switzerland is forbidden by law, elective single embryo transfer could not be performed.
Vaginal progesterone was used to support the luteal phase.
In the case of a cryocycle, oestradiol in form of tablets or patches was used (artificial cycles), in order to promote a sufficient endometrial thickness (>7 mm).
Two weeks after the transfer, a quantitative pregnancy test was performed. In the event of pregnancy, support of the luteal phase was continued until nine weeks of gestation. The first obstetric ultrasound was performed four weeks after embryo transfer.
Cumulus-oocyte complexes were incubated in fertilisation medium K-SIFM (Sydney IVF Fertilization Medium, Cook®, Brisbane, Australia) or IVF (IVF Science Scandinavia, part of Vitrolife®group, Goteborg, Sweden) immediately after OPU. On day 1 (16–18 hours post insemination with conventional IVF or ICSI), oocytes were checked for the presence of two pronuclei and two polar bodies. One to three of them were then transferred to a new four-well dish [12, 13] with either culture medium K-SICM (Sydney IVF Cleavage Medium, Cook® Brisbane, Australia), corresponding in our study to the Cook®-group or IVF (same medium as for insemination, Vitrolife®Goteborg, Sweden) corresponding to Vitrolife study group, until embryonic transfer on day 2 (day 3 in the case of week-ends). The zygotes were cultured in a CO2 incubator with 5% O2 (CYTOPERM 2, HERAUS, Thermo Fisher Scientific Inc.). The other oocytes were cryopreserved at pronuclei stage according to conventional slow freezing protocol adopted from Lassalle et al. [14]. The freezing and thawing solutions were made by mixing cryoprotectants with Human Tubal Fluid (HTF) medium (University Hospital, Lausanne, Switzerland) containing Synthetic Serum Substitute (SSS) (Irvine Scientific, Santa Ana, USA). Briefly, zygotes were incubated for 15 minutes at room temperature in a freezing solution containing 1.5 M PrOH (1,2-propanediol) (SIGMA-ALDRICH, St. Louis, USA) and 0.1 M sucrose (SIGMA-ALDRICH, St. Louis, USA), then loaded in plastic straws (CryoBioSystem, St. Ouens, France) and placed in an automated freezer (Minicool 40PC, Carbagas, Switzerland). They were further thawed with cryoprotectants (1.0 M PrOH and 0.2 M sucrose); removal in three steps. Embryo culture and embryo transfer were performed as in fresh cycles, on day 2 (or day 3). Culture conditions were 37 °C, 6% O2 and 6% CO2.
The effect of culture medium on birthweight was estimated by means of linear least square regression adjusted for the confounding factors. Our primary objective was to test whether both culture media are equivalent in terms of mean birthweight. In an equivalence test, one tries to reject the null hypothesis saying a difference exists which is larger than a pre-defined maximum irrelevant difference. We considered a mean birthweight difference up to 150 g as being clinically insignificant because it represents less than 5% of mean birthweight of term neonates and <10% of the normal variation between term born at 37 and 42 weeks of gestation (for newborn females: percentile [P]10%: 2,500 g, P90%: 4,200 g; for newborn males: P10%: 2,550 g, P90%: 4350 g) [15]. Thus, in the present study, for a two-sided test with alpha = 0.05, the null hypothesis will be rejected, and both culture media are to be considered as equivalent, if the 95% confidence interval of the regression’s coefficient will lie completely within the interval (–150 g, 150 g). The regression coefficient estimates the mean birthweight difference between culture media. A power calculation was performed prior to data collection. Statistical power was calculated to be 79% to detect equivalence between 352 Vitrolife and 173 Cook embryos, with a maximum tolerated difference of 150 g, standard deviation of 550 g in each group [3], and alpha of 5%.
The effect of culture medium on birthweight was adjusted in a multiple linear regression by the sex of the infants, type of IVF-cycle (fresh versus frozen), gestational age, height and weight of mother and father, mother’s smoking status (smoker versus non-smokers), maternal age, multiparity (multiparous versus primiparous) and pregnancy complications (yes versus no).
Birthlength was compared between culture media using the same methods as for birthweight.
Further characteristics of parents and infants were compared between culture media by means of t-tests (quantitative, normally distributed variables), chi-square tests (categorical variables with cell frequencies >4) or Fisher’s exact tests for categorical variables with cell frequencies <5). The statistical package Stata version 12 (StataCorp LP, College Station, USA) was used for the analyses.
Data from 525 patients who delivered singleton infants at term were analysed. Among them, 352 had embryos cultured in Vitrolife® media and 173 in Cook® media. The characteristics of the male and female newborns are shown in table 1. Weight, length, gestational age and birthweight percentiles did not differ between the groups for both genders. The distribution of these parameters is presented in figure 1. Again, no significant differences were observed, although a trend to higher percentage of births between 38 and 39 weeks of gestation is to be noted in the Cook® group.

Figure 1
Distribution of birthweight, birthlength, percentiles of birthweight and exact gestational age at birth of the two distinct groups.
V = Vitrolife®; C = Cook®.
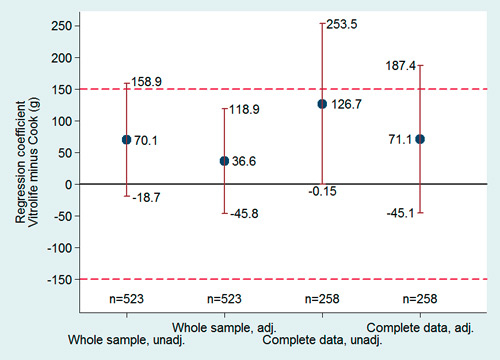
Figure 2
Birthweight. Regression coefficients of the adjusted and unadjusted models with 95% confidence intervals. The whole sample was adjusted for infant sex, gestational age and type of IVF-cycle. The sample with complete data was adjusted for sex of the infants, type of IVF-cycle, gestational age, height and weight of parents, mother’s smoking status maternal age, multiparity and pregnancy complications. The coefficients represent the mean birth weight difference between culture media. The dashed lines represent the equivalence limits.
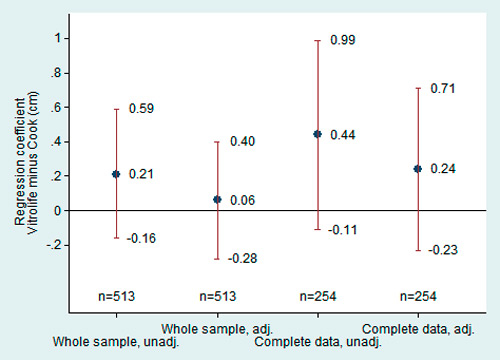
Figure 3
Birthlength. Regression coefficients of the adjusted and unadjusted models with 95% confidence intervals. The coefficients represent the mean birthlength difference between culture media. See legend of figure 2 for the details about the adjustments.
After adjustment for infant sex, gestational age and type of IVF-cycle, analysis of the whole study population suggests the two culture media being equivalent regarding their effect on birthweight, since the confidence interval lies completely within the equivalence boundary (fig. 2). The same regression model could not provide enough evidence of an effect of cycle type (fresh versus frozen) on neonatal birthweight (frozen cycle averaging 2.2 g less than fresh cycles, 95% CI from –75.3 to –79.8 g).
No evidence for a difference in birthlength between culture media was found. After adjustment for infant sex, gestational age and type of IVF-cycle, Vitrolife® children were in the average 0.06 cm longer than Cook® children (fig. 3). As for birthweight, our data do not provide sufficient evidence that cycle type results in differential birthlength (mean difference in the adjusted model: 0.07 cm in favour of the fresh cycle: 95% CI from –0.25 to 0.39 cm).
In half of the patients (175/352 [49.7%] in the Vitrolife® group; 83/173 [47.9%] in the Cook® group), additional information was obtained by a secondarily filled in questionnaire. The characteristics of these patients with complete data set are shown in table 2. Maternal and paternal as well as IVF-ICSI-treatment characteristics did not differ between the groups. Significant differences could not be found concerning neonatal characteristics, either (table 2). The mean raw birthweight for the term infants was 3,355.7 g (±476 g) in the Vitrolife® group and 3,229.1 g (±499 g) in the Cook® group, corresponding to a mean weight difference of 127 g, with 95%CI ranging from ‒0.15 to 253 g (fig. 2). After adjustment for the confounding variables, the mean difference between the two culture media groups was 71 g in favour of Vitrolife®, with 95%CI ranging from –45 to 187 g (fig. 2). The interaction between sex and medium was also analysed and no significant difference was found (interaction 95%CI: –273.3 g to 197.6 g). The variance in the data, however, did not allow to prove the equivalence of the two culture media. The same regression model did not provide sufficient evidence that cycle type (fresh versus frozen) had an effect on birthweight (mean difference 24.5 g in favour of fresh cycle, 95%CI: –83.9 to 131.9 g).
In conclusion, birthweight of term newborns was not significantly different in the two groups, although a trend towards higher weight was found in the Vitrolife® group. Cycle type (fresh or frozen) had no impact on birthweight.
No conclusive evidence for a difference in birthlength between culture media was found (fig. 3). Before adjustment for the confounding factors, Vitrolife® children were in the average 0.44 cm longer than Cook® children, with 95%CI ranging from –0.11 to 0.99. After adjustment, the difference remained in favour of Vitrolife®, but with smaller amplitude (mean difference: 0.24 cm; 95%CI from –0.23 to 0.71 cm). Again, our data provide no evidence for a difference in birthlength between cycle types (mean difference 0.07 cm in favour of fresh cycle, with 95%CI from –0.36 to 0.50).
Even though not influencing mean birthweight of a study population, culture media could have an impact by increasing percentage of small and/or large for gestational age infants, meaning more newborn infants below P10 or above P90. We therefore analysed birthweight distribution (fig. 1A) in relation to birthweight according to birthweight percentile categories (fig. 1B) and gestational age (fig. 1C). There were no differences between the two groups, particularly, in the percentage of small for gestational age infants (fig. 1B), according to the two clinical definitions of small for gestational age infants (birthweight below P3 and below P10).
| Table 1: Neonatal outcome of live born term singletons (whole sample). | |||
| Vitrolife® | Cook® | P-value | |
| Number of singletons | 352 | 173 | |
| Fresh cycles (%) | 191 (54.3) | 93 (53.8) | 0.91a |
| Frozen cycles (%) | 161 (45.7) | 80 (46.2) | 0.91a |
| Gestational age (weeks) | 39.4 (1.1) | 39.2 (1.2) | 0.12b |
| Average weight newborn (g) | 3,315 (476) | 3,250 (479) | 0.15b |
| Average weight female newborn (g) | 3,284 (429) | 3,215 (476) | 0.23b |
| Average weight male newborn (g) | 3,349 (520) | 3,290 (482) | 0.39b |
| Average height newborn (cm) | 49.5 (2.0) | 49.3 (2.1) | 0.37b |
| Average height female newborn (cm) | 49.3 (1.7) | 48.8 (2.0) | 0.05b |
| Average height male newborn (cm) | 49.7 (2.2) | 49.9 (2.1) | 0.55b |
| Low birth weight (<2500 g) | 16 (4.5) | 7 (4.0) | 0.79a |
| High birth weight (>4500 g) | 2 (0.6) | 2 (1.2) | 0.60c |
| Small for gestational age (<10th percentile) | 55 (15.7) | 26 (15.1) | 0.86a |
| Very small for gestational age (<3th percentile) | 15 (4.3) | 11 (6.4) | 0.30a |
| Large for gestational age (90–97th percentile) | 15 ( 4.3) | 4 ( 2.3) | 0.28a |
| Very large for gestational age (>97th percentile) | 7 (2.0) | 5 (2.9) | 0.53a |
| Data are mean (± SD) or n (%). a Chi-squared test; b Two-tailed t-test for independent samples; c Fisher's exact test. | |||
| Table 2:Characteristics of the patients with complete data. | |||
| Vitrolife® | Cook® | P-value | |
| Number of patients (%) | 175 (49.7) | 83 (48.0) | 0.71a |
| Maternal characteristics | |||
| Age of the patients (years) | 33.7 (3.8) | 34.5 (4.5) | 0.13b |
| Height (cm) | 166.9 (6.14) | 167.6 (6.27) | 0.41b |
| Weight (kg) | 62.9 (10.4) | 61.8 (10.2) | 0.43b |
| BMI (kg/m2) | 22.6 (3.47) | 22.0 (3.01) | 0.16b |
| Smoking | 30 (17.1) | 11 (13.2) | 0.42a |
| Multiparity | 14 (8.0) | 12 (14.5) | 0.11a |
| Complications during pregnancy (%) | |||
| Arterial hypertension | 4 (2.3) | 3 (3.6) | 0.68c |
| Gestational diabetes | 1 (0.6) | 1 (1.2) | 0.54c |
| Paternal characteristics | |||
| Height (cm) | 178.1 (6.44) | 179.4 (6.35) | 0.13b |
| Weight (kg) | 79.2 (11.2) | 79.0 (10.4) | 0.91b |
| BMI (kg/m2) | 25.0 (2.91) | 24.5 (2.55) | 0.27b |
| IVF-ICSI treatment characteristics | |||
| Fresh cycles (%) | 93 (53.1) | 44 (53.0) | 0.98a |
| Frozen cycles | 82 (46.9) | 39 (47.0) | 0.98 |
| Newborn characteristics | |||
| Gestational age (weeks) | 39.5 (1.06) | 39.3 (1.15) | 0.21b |
| Weight (g) | 3,355.7 (475.64) | 3,229.1 (498.97) | 0.05b |
| Height (g) | 49.6 (2.01) | 49.2 (2.13) | 0.10b |
| Data are mean (SD) or n (%). a Chi-squared test; b Two-tailed t-test for independent samples; c Two-tailed Fisher's exact test. | |||
The present study explores the embryo culture media from the same distributors as Dumoulin et al. and Carrasco et al. [3, 10]. These two groups presented very different study results, Dumoulin et al. showing a significantly increased birthweight (+ 245 g) after culture in Vitrolife® media compared to Cook® media, while Carrasco et al. could not find any differences, either in the retrospective or in the prospective arm of their study.
Our results suggest that there is no statistically significant difference of birthweight after embryo culture in Vitrolife® or Cook® media. In the overall study population adjusted for infant’s sex and gestational age, data showed equivalence of the two culture media regarding birthweight. Considering only those patients in which a complete data set was available, permitting adjustment also for maternal risk factors and paternal growth parameters, equivalence of the two culture media could not be shown anymore, as this patient group was underpowered for detecting equivalence between culture media. Therefore, the adjusted whole-sample analysis suggesting equivalence has to be taken with care because bias due to insufficient adjustment for confounders cannot be excluded. Indeed, the adjusted complete-data analysis (fig. 2) suggests a mean difference of 71 g between the two groups, representing only a trend to higher birthweight in the Vitrolife® group. In accordance with the study by Lin et al., we neither found a significant difference in length [9]. Unfortunately, the latter item was not evaluated in the Dumoulin study.
Besides their potential effect on mean birthweight differences, culture media could impact fetal growth by favouring high risk growth alterations, meaning a higher percentage of small or large for gestational age infants. However, our data did not show either a significant difference in the number of low for gestational age infants (P3 or P10) between the two groups, or in the number of large for gestational age infants (P90 or P97) (fig. 1B).
A further important finding of this study is that there is no difference in birthweight between the two culture media, if fresh and frozen cycles are regarded separately. It is worth noting that neonatal birthweight after fresh IVF cycles was not significantly different when compared to neonatal birthweight after frozen cycles, in contrast to the literature [16]. A hypothesis to explain this latter contrasting finding could be that in Switzerland, due to legal restrictions, freezing is performed at the stage of a fertilised oocyte with two pronuclei and two polar bodies, and not at the embryonic stage.
The published studies concerning the influence of culture media on birthweight are quite difficult to compare because of different study designs and research questions. For example, in their recently published trial, Carrasco et al. included a study arm with prospective randomisation of 98 term and preterm singleton live births after culture in Vitrolife® or Cook® media following fresh IVF-ICSI cycles [10]. However, Dumoulin et al. included in their first retrospective study singleton pregnancies already at >20 weeks gestation after fresh IVF-ICSI cycles, 110 after culture with Vitrolife® medium and 78 with Cook® and so comparing a wider range of gestational age neonates [3]. Furthermore, their second retrospective study comprised singleton pregnancies of >20 weeks gestation after frozen cycles (22 after culture with Vitrolife® medium and 45 with Cook®) as well as 20, respectively 24 twin pregnancies following fresh IVF-ICSI cycles [4]. On the other hand, Eaton et al. explored, in their retrospective study, fresh IVF-ICSI cycles with day 3 transfer after culture with three other culture media (G1.3®, Global® and G1.5®), and analysing singletons with gestational age >34 weeks [8]. In the same year, Vergouw et al. published a retrospective study including singletons >22 weeks gestation after fresh IVF-ICSI cycles with day 3 transfer and frozen cycles with day 5 transfer, after culture with again two disparate culture media, namely HTF® and Sage Quinn’s advantage protein® [7]. A retrospective Chinese study compared birthweight of singletons and twins >20 weeks gestation after IVF-ICSI fresh cycles and culture with, yet again, distinct culture media (G5™ ®, Global® and Quinn’s advantage protein®) and day 3 transfer [9]. A retrospective observational study, investigating again 3 other culture media (Medicult ISM1®, n = 402; Vitrolife G-1 PLUS®, n = 449; and Medicult Universal®, n = 1,584), showed mean birthweights of 3,352 g, 3441 g and 3,448 g, respectively, the corresponding mean placental weights were 693 g, 704 g and 684 g, respectively. Due to the high numbers included, these minimal differences in birthweight between the groups (i.e., 7 g to maximal 96 g) were statistically significant [6]. Lastly, the most recently published study from the Dumoulin group, analysing again a cohort of 265 singletons, found that growth velocity and head circumference were similar between the two groups, but growth differences persisted (weight measurements were not shown, only standard deviation scores). A reason for caution and limitation of this study is that they did not correct for factors influencing post-natal growth (i.e., childhood diseases, nutrition, etc.) [17].
The question if day 2 versus day 3 transfer could influence birthweight is completely unclear until today. The studies published on birthweight differences are very heterogeneous, some including newborns after 2 days of culture [10], others including newborns after 2 and 3 days of culture [3–5], again others including newborns after 3 days of culture [8, 9], and once again others including newborns after 3 days and 5 days of culture [7]. In summary, most studies did not find any birthweight difference between the different culture media, even though embryos were in one study group cultured until 5 days. However, the group of Dumoulin showed birthweight differences after only 2 and 3 days of culture (however, no difference noted between 2 versus 3 days). More data are available in literature concerning differences in viability and outcome of day 2 versus day 3 embryos. With culture duration, the risk of arrested embryos rises, which is in general due to aneuploidy. Several randomised controlled trials have been conducted in order to find out if it would be preferable to transfer the embryos on day 2 or day 3 and a Cochrane analysis has been done [18]. In this systematic review, the authors found no evidence to suggest a significant improvement in live birth when delaying embryo transfer from day 2 to day 3.
Whether a difference is statistically significant or not is among other things a question of numbers. However, more important than the statistical significance may be the clinical relevance. Today, it is difficult to conclude that the significant respectively not significant differences in birthweight are clinically relevant. However, considering the Barker hypothesis, it could be very possible that a few days in a certain environment as an embryo could have an impact in later life and an adverse environmental influence on the embryo by in-vitro culture as a cause of cardiovascular and cardiometabolic dysfunction has already been hypothesised [19, 20]. Yet, it should be emphasised that IVF-ICSI is an extremely complex process and many different factors, besides the utilisation of different commercialised culture media, the known parental clinical confounding factors and underlying parental genetic factors, could have repercussions on epigenetic processes of the infants and might potentially influence their outcome: different laboratory techniques, culture length (2 days versus 5 days), culture conditions (percent of oxygen concentration), etc. The complexity of this issue highlights how difficult it is to have comparable study results.
One of the strengths of the present study is the homogenous study population: we included only full term births after a singleton pregnancy. Another one is its sample size, being amongst the largest to analyse Vitrolife® and Cook® culture media, despite the fact that only full term births were included. Even though, as a drawback of this study, it is not a properly prospective randomised study, allocation to the one or the other culture medium has been done randomly, depending on the site of the laboratory to which the patient was addressed. Both laboratories have been located in the same city and within a distance of one kilometre, excluding a geographical bias. As the same laboratory staff worked on both sites, potential biases caused by different laboratory techniques, equipments and protocols can also be excluded in this study.
In conclusion, the present study results show neither a significant difference in birthweight and length of singleton term newborn infants after IVF-ICSI treatment comparing the two embryo culture media Vitrolife® and Cook®, nor a significant difference in the number of low for gestational age infants (P3 or P10) respectively large for gestational age infants (P90 or P97) between the two groups.
Further research is required, in order to elucidate the impact of embryo culture media and other aspects of the culture system that may be associated with adverse outcomes on the health of children conceived by IVF-ICSI.
1 Henningsen AK, Pinborg A, Lidegaard Ø, Vestergaard C, Forman JL, Andersen AN. Perinatal outcome of singleton siblings born after assisted reproductive technology and spontaneous conception: Danish national sibling-cohort study. Fertil Steril. 2011;95:959–63.
2 Pinborg A, Loft A, Nyboe Andersen A. Neonatal outcome in a Danish national cohort of 8602 children born after in vitro fertilization or intracytoplasmic sperm injection: the role of twin pregnancy. Acta Obstet Gynecol Scand. 2004;83:1071–8.
3 Dumoulin JC, Land JA, Van Montfoort AP, Nelissen EC, Coonen E, Derhaag JG, et al. Effect of in vitro culture of human embryos on birthweight of newborns. Hum Reprod. 2010;25:605–12.
4 Nelissen EC, Van Montfoort AP, Coonen E, Derhaag JG, Geraedts JP, Smits LJ. Further evidence that culture media affect perinatal outcome: findings after transfer of fresh and cryopreserved embryos. Hum Reprod. 2012;27:1966–76.
5 Nelissen EC, Van Montfoort AP, Smits LJ, Menheere PP, Evers JL, Coonen E, et al. IVF culture medium affects human intrauterine growth as early as the second trimester of pregnancy. Hum Reprod. 2013;28:2067–74.
6 Eskild A, Monkerud L, Tanbo T. Birthweight and placental weight; do changes in culture media used for IVF matter? Comparisons with spontaneous pregnancies in the corresponding time periods. Hum Reprod. 2013;28:3207–14.
7 Vergouw CG, Kostelijk EH, Doejaaren E, Hompes PG, Lambalk CB, Schats R. The influence of the type of embryo culture medium on neonatal birthweight after single embryo transfer in IVF. Hum Reprod. 2012;27:2619–26.
8 Eaton JL, Lieberman ES, Stearns C, Chinchilla M, Racowsky C. Embryo culture media and neonatal birthweight following IVF. Hum Reprod. 2012;27:375–9.
9 Lin S, Li M, Lian Y, Chen L, Liu P. No effect of embryo culture media on birthweight and length of newborns. Hum Reprod. 2013;28:1762–7.
10 Carrasco B, Boada M, Rodriguez I, Coroleu B, Barri PN, Veiga A. Does culture medium influence offspring birth weight? Fertil Steril. 2013;100:1283–8.
11 Cheikh Ismail L, Knight HE, Bhutta Z, Chumlea WC; International Fetal and Newborn Growth Consortium for the 21st Century. Anthropometric protocols for the construction of new international fetal and newborn growth standards: the INTERGROWTH-21st Project. BJOG 2013;120(Suppl 2):42–7.
12 Vajta G, Korösi T, Du Y, Nakata K, Leda S, Kuwayama M, Nagy ZP. The Well-of-the-Well system: an efficient approach to improve embryo development. Reprod Biomed Online. 2008;17:73–81
13 Ebner T, Shebl O, Moser M, Mayer RB, Arzt W, Tews G. Group culture of human zygotes is superior to individual culture in term of blastulation, implantation and life birth. Reprod Biomed online. 2010;21:762–8.
14 Lassalle B, Testart J, Renard JP. Human embryo features that influence the success of cryopreservation with the use of 1,2 propanediol. Fertil Steril. 1985;44:645–51.
15 Voigt M, Schneider KT, Jahrig K. Analysis of a 1992 birth sample in Germany. 1: New percentile values of the body weight of newborn infants. Geburtshilfe Frauenheilkd. 1996;56:550–8.
16 Wennerholm UB, Henningsen AK, Romundstad LB, Bergh C, Pinborg A, Skjaerven R, et al. Perinatal outcomes of children born after frozen-thawed embryo transfer: a Nordic cohort study from the CoNARTaS group. Hum Reprod. 2013;28:2545–53.
17 Kleijkers SH, van Montfoort AP, Smits LJ, Viechtbauer W, Roseboom TJ, Nelissen EC, et al. IVF culture medium affects post-natal weight in humans during the first 2 years of life. Hum Reprod. 2014;29:661–9.
18 Oatway C, Gunby J, Daya S. Day three versus day two embryo transfer following in vitro fertilization or intracytoplasmic sperm injection. Cochrane Database Syst Rev 2004;(2):CD004378. Review
19 Scherrer U, Rimoldi SF, Rexhaj E, Stuber T, Duplain H, Garcin S, et al. Systemic and pulmonary vascular dysfunction in children conceived by assisted reproductive technologies. Circulation. 2012;125:1890–6.
20 Yeung EH, Druschel C. Cardiometabolic health of children conceived by assisted reproductive technologies. Fertil Steril. 2013;99:318–26.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article were reported.