Intensive care without walls – introduction of a Medical Emergency Team system in a Swiss tertiary care centre
DOI: https://doi.org/10.4414/smw.2014.14027
Reto
Etter, Jukka
Takala, Tobias Michael
Merz
Summary
QUESTIONS UNDER STUDY: To improve the response of deteriorating patients during their hospital stay, the University Hospital Bern has introduced a Medical Emergency Team (MET). Aim of this retrospective cohort study is to review the preceding factors, patient characteristics, process parameters and their correlation to patient outcomes of MET calls since the introduction of the team.
METHODS: Data on patient characteristics, parameters related to MET activation and intervention and patient outcomes were evaluated. A Vital Sign Score (VSS), which is defined as the sum of the occurrence of each vital sign abnormalities, was calculated for all physiological parameters pre MET event, during event and correlation with hospital outcomes.
RESULTS: A total of 1,628 MET calls in 1,317 patients occurred; 262 (19.9%) of patients with MET calls during their hospital stay died. The VSS pre MET event (odds ratio [OR] 1.78, 95% confidence interval [CI] 1.50–2.13; AUROC 0.63; all p <0.0001) and during the MET call (OR 1.60, 95% CI 1.41–1.83; AUROC 0.62; all p <0.0001) were significantly correlated to patient outcomes. A significant increase in MET calls from 5.2 to 16.5 per 1000 hospital admissions (p <0.0001) and a decrease in cardiac arrest calls in the MET perimeter from 1.6 in 2008 to 0.8 per 1000 admissions was observed during the study period (p = 0.014).
CONCLUSIONS: The VSS is a significant predictor of mortality in patients assessed by the MET. Increasing MET utilisation coincided with a decrease in cardiac arrest calls in the MET perimeter.
Abbreviations
AE Adverse event
AUROC Area under the receiver operator characteristic curve
CI Confidence interval
GCS Glasgow Coma Scale
ICU Intensive care unit
IMC Intermediate care unit
IQR Interquartile range
MET Medical emergency team
OR Odds ratio
RRS Rapid response system
VSS Vital sign score
Introduction
Adverse events (AE) leading to the deterioration of a patient’s condition, unplanned intensive care unit (ICU) admission, or unexpected deaths do occur on hospital wards [1–3]. These events are often preceded by abnormalities in vital sign parameters [4–6]. We can assume that the underlying physiological processes leading to AE are potentially treatable in a majority of patients and that any intervention will be more efficient if initiated as early as possible. Less than optimal care in this setting is due to a failure to recognise signs of organ dysfunction [7], delayed notification to the appropriate medical decision makers [8] and subsequent failure to address a patient’s deteriorating condition rapidly and adequately [9]. To improve the timely recognition of and response to the deterioration of patients during their hospital stay, the introduction of a rapid response system (RRS) has been recommended by health care quality improvement organisations worldwide [10–12].
RRS consist of four different integrated components [13, 14]. The afferent limb of the system is comprised of a systematic process that ensures early identification and assessment of the deteriorating patient and a mechanism for activating the response team. Most RRS use predefined and objective triggering criteria (calling criteria), which are based on neurological, respiratory and circulatory vital signs that can be assessed easily and rapidly by the first health care provider evaluating a patient at risk. The response team is commonly referred to as the medical emergency team (MET) and represents the efferent limb of the RRS. The European Resuscitation Council summarises the requirements of this team as follows: “…a designated outreach service or resuscitation team capable of responding in a timely fashion to acute clinical crises identified by the track and trigger system or other indicators. This service must be available 24 hours per day. The team must include staff with the appropriate acute or critical care skills [11].” The evaluative and process improvement limb of the RRS consists of a systematic, regular evaluation of MET events on hospital wards. Quality improvement initiatives for suboptimal care processes that are identified to be causative factors for MET calls are then implemented. Finally, these components of the RRS are overseen by organisational and administrative structures which also ensure the coordination of on-going training of the team members and the continuous education of hospital staff regarding the rapid-response process.
As the first tertiary care provider in Switzerland, the University Hospital Bern (Inselspital) has introduced a RRS based on a quality improvement initiative of the Department of Intensive Care Medicine. The aim of this retrospective cohort study is to review patient characteristics and process parameters of MET calls since the introduction of the team in October 2009 and to establish the prognostic significance of antecedents of MET calls on patient outcomes.
Methods
Patients and study design
This retrospective cohort study includes all patients who were assessed by the MET between the introduction of the team on 19 October 2009 and 31 December 2013.
Setting
The study was performed at the Department of Intensive Care Medicine of the Bern University Hospital. The hospital is a 960-bed tertiary care medical centre, in Bern, Switzerland. The Department of Intensive Care Medicine (ICU) is the sole provider of intensive care for adults at the hospital. It currently operates a total of 51 intensive care and intermediate care beds and is managed as a closed unit, handling all types of medical, surgical and trauma patients. The ICU operates with 24 hour intensivist coverage in three shifts; a minimum of two board certified intensive care staff specialists are present at any given time.
Medical Emergency Team
The MET is staffed by the ICU and consists of a staff specialist in intensive care and an accredited intensive care nurse both of whom are available 24/7 everywhere on the hospital campus with the exception of the emergency department and operating theatres. The MET members are rostered for standard clinical shifts in their respective positions in the ICU and operate as MET only on an as-needed basis. The MET equipment comprises a backpack with advanced airway management materials, a cardiac and respiratory monitor, a defibrillator and emergency drugs and consumables. Any health care professional involved in the treatment of patients throughout the hospital can alert the MET using a standard set of calling criteria based upon physiological changes which have been validated as antecedents to a life threatening deterioration and/or cardiopulmonary arrest (table 1) [6, 15]. The MET calling criteria include, besides objective physiological parameters, a subjective “concern” criterion. This represents a fall-back position for hospital staff if their concerns about the clinical status of a patient do not fit the physiological MET criteria but the patient’s condition is still considered critical, for example in the event of the onset of chest pain or the occurrence of focal neurological deficits. The MET is alerted via the hospital paging system; the call is answered by the MET physician. The MET can be called for hospital inpatients, outpatients, visitors or staff who meet any of the criteria. Depending on the immediate urgency the MET can be on scene within a maximum of 10 minutes. Whenever possible, we require a doctor from the patient’s primary care team to be present at a MET incident to get access to vital information on the patient and to discuss treatment options, as well as to ensure the implementation of the MET management plan. It is the responsibility of the calling health care provider to contact the treating physician after they have called the MET to their ward. The organisational and administrative structures of the MET system are integrated in the Department of Intensive Care Medicine and are overseen by a senior staff specialist and two staff consultants who are responsible for regular evaluation and quality improvement of the MET system as well as the continuous education of hospital staff. All hospital departments are contacted on a regular basis and information material and educational sessions on MET use are offered. Tuition is provided by an intensive care specialist on an annual or biannual basis. The hospital also operates a “traditional” cardiac arrest team which is led by the Department of Anaesthesiology. This team has a shorter response time than the MET, and attends to all respiratory and circulatory arrests. The MET is fully equipped for advanced cardiac life support in the event of an arrest occurring during a MET call; however, usually the cardiac arrest team is called for additional assistance.
Study data
According to current guidelines [16] data on MET events is routinely collected during the MET call and recorded in a separate data base for quality improvement initiatives and audits. Data consists of patient characteristics, available data on physiological parameters recorded in the 24 hours pre-event and during event, parameters related to MET activation and intervention and patient outcomes. For auditing and clinical purposes, we use a simple severity scoring system (Vital Sign Score [VSS]) based on the MET calling criteria for heart rate, systolic blood pressure, respiratory rate, oxygen saturation and GCS; additionally peripheral perfusion is assessed (capillary refill time of > three seconds is considered abnormal). To calculate the VSS, the occurrence of each of the six potential vital sign abnormalities is considered as one VSS point, the VSS is defined as the total sum of all VSS points at one point in time [17]. For study purposes, the hospital outcomes of patients with MET calls were extracted from the hospital patient administration system. Additionally, information on the occurrence of cardiac arrest team calls within the MET perimeter was extracted from the cardiac arrest event data base which is operated by the Department of Anaesthesiology.
Statistical analysis
The data are presented as mean ± standard deviation or median and interquartile ranges (IQR) as appropriate. Comparisons of data in the strata of years during the study period were performed using Kruskal-Wallis test followed by Dunn's Multiple Comparison Test. Comparison of outcome groups defined on the basis of hospital survival/non-survival was performed using the nonparametric Mann-Whitney test or the Chi-square test, as appropriate. For survival analyses, the data of the last MET call per hospital admission were included in the analysis for patients with several MET calls. The predictive value of vital sign abnormalities pre-MET event and during event in relation to hospital mortality was assessed by univariate and multivariate logistic regression. Additionally, receiver operating characteristic (ROC) curves were constructed and the area under the curve (AUC) was calculated to assess the capability of VSS pre MET and during MET event to discriminate survivors from non-survivors. In all analyses a p-value of 0.05 or less was considered statistically significant. Statistical analyses were performed using the software package SPSS version 20.0 (SPSS, Inc., Chicago, IL, USA).
Ethical approval and patient consent
The institutional review board (IRB) of the Canton of Bern waived the need for IRB and patient consent due to the retrospective and observational nature of the analysis of data which were collected in conjunction with routine clinical management. Nevertheless, all patients admitted to the Bern University Hospital are routinely informed of their right to specify whether data related to their stay can be used in observational studies; data of patients who declined were not included in the study.
|
Table 1: MET calling criteria used by the University Hospital Bern (Inselspital). |
| Airway
– Threatened airway |
– Necessity for intratracheal suctioning, insertion of oro- or nasopharyngeal tubes, intubation, bronchoscopy |
| Breathing
– Respiratory rate
– Oxygen saturation: |
– Respiratory rate <6/minute or >36/minute
– SaO2 <90% despite supplementary oxygen delivered by any means (nasal cannula, bag mask, tracheostoma) |
| Circulation
– Blood pressure
– Heart rate
– Peripheral perfusion* |
– Systolic blood pressure <90 mm Hg
– <40/minute or >140/minute
– Capillary refill time of >3 seconds |
| Neurology
– Glasgow Coma Scale (GCS)
– Seizures |
– GCS <13 or decrease by ≥2 points
– Repeated or prolonged (>5 minutes) seizures |
| Concern |
– Staff member is worried about the patient for any other reason |
| * Peripheral perfusion; not part of the MET calling criteria, used for calculation of Vital Sign Score (VSS) only. |
|
Table 2:Patient characteristics. |
| |
Survivors
(N = 1055)
|
Non-survivors
(N = 262)
|
Significance p
|
| Age |
65.20 (IQR 53.6–75) |
68.50 (IQR 58.6–77.1) |
0.0008 |
| Gender (male/female) |
622/433 |
138/124 |
0.07 |
|
Time of MET call (day shift vs late/night shift)
|
318 (30%) vs 737 (70%) |
100 (38%) vs 162 (62%) |
0.014 |
|
Reason for MET call
|
N
|
(%)
|
N
|
(%)
|
0.010 |
| – Concern |
311 |
29.5 |
58 |
22.1 |
|
| – Heart rate |
80 |
7.6 |
15 |
5.7 |
|
| – Low GCS |
86 |
8.2 |
33 |
12.6 |
|
| – Low oxygen saturation |
237 |
22.5 |
69 |
26.3 |
|
| – Respiratory rate |
100 |
9.5 |
34 |
13.0 |
|
| – Seizures |
18 |
1.7 |
|
|
|
| – Systolic BP <90 mm Hg |
188 |
17.8 |
43 |
16.4 |
|
| – Threatened airway |
35 |
3.3 |
10 |
3.8 |
|
|
MET diagnosis
|
N
|
(%)
|
N
|
(%)
|
0.165 |
| – Cardiovascular |
223 |
21.1 |
44 |
16.8 |
|
| – GI |
50 |
4.7 |
9 |
3.4 |
|
| – Infection |
195 |
18.5 |
58 |
22.1 |
|
| – Metabolic/drug related |
69 |
6.5 |
15 |
5.7 |
|
| – Neurologic |
174 |
16.5 |
38 |
14.5 |
|
| – Other |
102 |
9.7 |
24 |
9.2 |
|
| – Respiratory |
192 |
18.2 |
52 |
19.8 |
|
| – Shock |
50 |
4.7 |
22 |
8.4 |
|
| • Haemorrhagic
• Cardiac
• Septic |
33
15
2 |
3.1
1.4
0.2 |
14
7
1 |
5.3
2.7
0.4 |
|
Results
MET calls
A total of 1,628 MET calls in 1,317 patients (69% male) occurred during the study period. 262 (19.9%) of 1,317 patients with MET calls during their hospital stay died. Patient characteristics stratified by hospital survivors and non-survivors are presented in table 2.
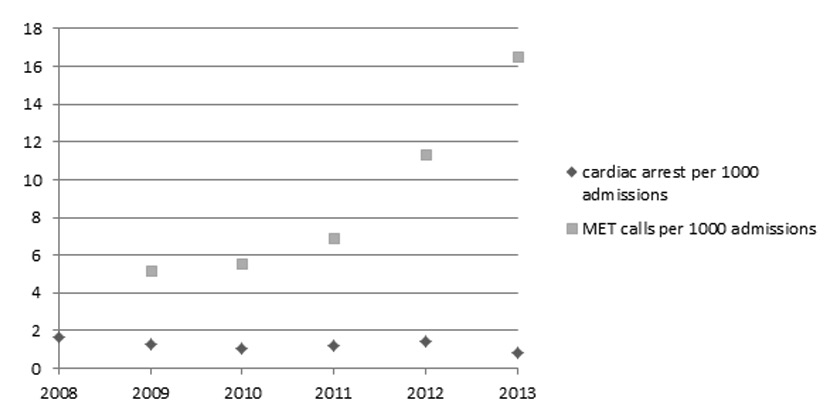
Figure 1
Incidence of MET calls and cardiac arrest calls per 1000 admissions during the study period.
During the study period an increase in MET calls from 20 ± 9 calls per month in 2009 to 54 ±11 calls per month in 2013 occurred (p <0.0001), corresponding to an increase in MET calls from 5.2 to 16.5 per 1000 hospital admissions. In the same time period we observed a decrease in cardiac arrest calls in the MET perimeter from 1.6 in 2008 (pre-MET) to 0.8 per 1000 admissions in 2013 (p = 0.014).
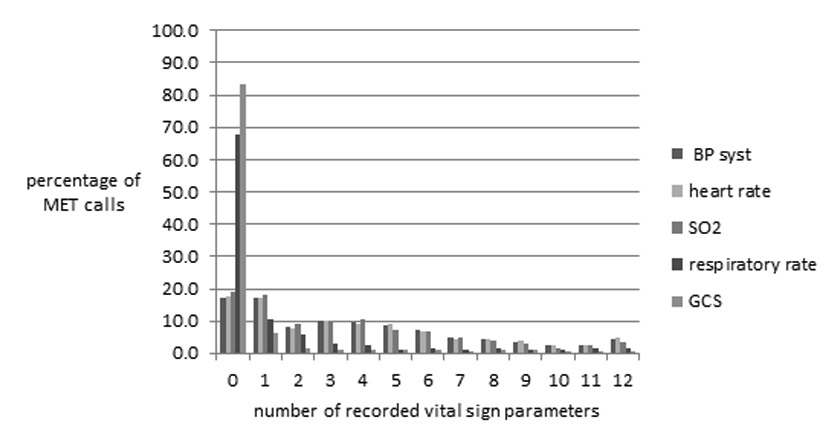
Figure 2
Number of vital sign parameters recorded during the 24 hours before MET calls.
In a substantial number of MET calls vital sign measurements had been performed only infrequently and incompletely in the antecedent 24 hours before the MET event. In 83% of MET calls no assessments of GCS and in 68% no assessment of respiratory rate was recorded.
We did register a significant increase in MET calls from 20 ± 9 calls per month in 2009 to 54 ±11 calls per month in 2013 (p <0.0001). This corresponds to an increase in MET calls from 5.2 to 16.5 per 1000 hospital admissions. In the same time period we observed a decrease in cardiac arrest calls in the MET perimeter from 1.6 in 2008 to 0.8 per 1000 admissions in 2013 (p = 0.014) (fig. 1). 524 (32%) MET calls occurred during the day shift (08.00–16.00 o’clock), 651 (40%) during the late shift (16.00–00.00 o’clock) and 453 (28%) during the night (00.00 to 08.00 o’clock). The median time delay from the first occurrence of vital sign abnormality to MET call was 8.7 hours (interquartile range [IQR] 1.7 to 18.4 hours). This time delay did not change during the study period (p = 0.445). The median time delay from alarm to the arrival of the MET on scene was 5 minutes (IQR 5 to 10 minutes) and the median time on scene was 25 minutes (IQR 15 to 30 minutes). In 801 (49%) MET calls the patient was stabilised on the original ward, a transfer to the ICU or an intermediate care unit was necessary in 647 (40%) and 180 (11%) cases respectively. In non-survivors of hospital stay, the median time period from arrival of the MET on scene to occurrence of death was 53 hours (IQR 21 to 125 hours). During the study period 12 patients died while the MET was on scene. In 6 of these events the cardiac arrest team was alerted, while in 3 patients, a DNR order was in place before the MET call. In the remaining 3 patients resuscitation efforts were terminated by the MET. In 151 (9%) MET calls the patient had a “do not resuscitate” (DNR) order in place and in 41 (3%) calls the patient’s DNR status was changed to or defined as DNR for the first time on the initiative of the MET. Univariate logistic regression analysis revealed no correlation between the delay in time from the first occurrence of vital sign instability to MET alarm or the delay in time from MET alarm to the arrival of the team on scene to hospital mortality. All single vital sign parameters recorded during the MET call had a significant correlation to hospital mortality in the univariate analysis (table 3). The VSS as assessed by the MET was a significant predictor of outcome (AUROC 0.63; p <0.0001).
Multivariate logistic regression revealed the parameters “respiratory rate” and “GCS” to be independent predictors of survival (table 4).
Antecedents to MET calls
The number of recorded vital signs as antecedents to MET calls varied considerably. The percentage of patients who had three or less measurements of vital sign parameters before the MET event was 53% for systolic blood pressure and heart rate, 56% for oxygen saturation, 87% for respiratory rate and 93% for GCS, respectively. In 14% of all MET calls, no vital sign parameters had been recorded in the twenty-four hours pre MET event in the patient’s charts. Figure 2 shows the frequency of recorded different vital sign parameters expressed as percentage of MET calls. In a univariate logistic regression analysis of single vital signs and VSS pre MET, the parameters heart rate and maximal VSS showed a significant correlation to hospital mortality (table 5). The maximal VSS in the time period of 24 hours before the MET event was a significant predictor of outcome (AUROC 0.62; p <0.0001).
|
Table 3: Results of univariate logistic regression analysis of operational time delays and single vital sign parameters as recorded by the MET in relation to hospital mortality. |
| |
|
|
95% CI of OR
|
|
Significance (p)
|
OR
|
Lower value
|
Upper value
|
|
Time delay from MET alarm to MET arrival on scene
|
0.828 |
1.001 |
0.992 |
1.010 |
|
Time delay from occurrence of First vital sign instability and MET alarm
|
0.643 |
1.004 |
0.986 |
1.024 |
|
VSS recorded by MET
|
<0.0001 |
1.606 |
1.411 |
1.827 |
| – Peripheral perfusion |
<0.0001 |
1.947 |
1.366 |
2.775 |
| – BP systolic |
<0.0001 |
0.992 |
0.988 |
0.996 |
| – Heart rate |
0.003 |
1.008 |
1.003 |
1.013 |
| – Respiratory rate |
<0.0001 |
1.035 |
1.020 |
1.051 |
| – GCS |
<0.0001 |
0.882 |
0.842 |
0.923 |
| – Oxygen saturation |
<0.0001 |
0.968 |
0.953 |
0.984 |
| – Threatened airway |
<0.0001 |
2.118 |
1.388 |
3.232 |
|
Table 4: Results of multivariate logistic regression analysis of single vital sign parameters recorded by the MET in relation to hospital mortality. |
| |
OR
|
95% CI of OR
|
|
|
Lower value
|
Upper value
|
Significance (p)
|
|
Peripheral perfusion
|
1.492 |
0.981 |
2.269 |
0.062 |
|
BP systolic
|
0.994 |
0.982 |
1.005 |
0.282 |
|
Heart rate
|
1.002 |
0.995 |
1.010 |
0.565 |
|
Respiratory rate
|
1.043 |
1.019 |
1.068 |
<0.0001 |
|
GCS
|
0.886 |
0.820 |
0.958 |
0.002 |
|
Oxygen saturation
|
0.978 |
0.953 |
1.003 |
0.082 |
|
Threatened airway
|
1.280 |
0.670 |
2.447 |
0.455 |
|
Table 5: Results of univariate logistic regression of single vital sign parameters occurring in the 24h before MET call in correlation to hospital outcome. |
| |
OR
|
95% CI of OR
|
Significance p
|
|
Lower value
|
Upper value
|
|
Peripheral perfusion
|
0.803 |
0.231 |
2.795 |
0.730 |
|
BP systolic
|
0.995 |
0.989 |
1.000 |
0.068 |
|
Heart rate
|
1.008 |
1.003 |
1.013 |
0.001 |
|
Respiratory rate
|
1.000 |
0.999 |
1.001 |
0.720 |
|
GCS
|
0.935 |
0.830 |
1.053 |
0.268 |
|
Oxygen saturation
|
0.994 |
0.981 |
1.007 |
0.335 |
|
Maximal VSS
|
1.781 |
1.489 |
2.131 |
<0.0001 |
Discussion
Based on the data from this retrospective cohort study on antecedents, patient characteristics, process parameters and patient outcomes of MET calls, a number of conclusions can be made.
Our experience indicates that at-risk patients can be identified by the established single vital sign criteria and that the reported VSS using a combination of these parameters represents a simple but significant predictor of mortality in patients assessed by the MET. At the time of the MET intervention, all assessed single vital sign parameters and the calculated VSS showed a significant correlation to hospital outcome in the univariate analysis, whereas a number of single parameter lost prognostic significance in the multivariate analysis. This result is consistent with previous studies on the prognostic significance of vital signs in ward patients and in other cohorts such as emergency department or ICU admissions [6, 17, 18]. The analysis of physiological parameters recorded in the lead up to the MET intervention revealed VSS but not the single vital sign abnormalities to be of prognostic significance. The potential benefits of using a triggering system based on physiological criteria can only be realised if these parameters are routinely measured. Our data suggests that vital sign parameters were often assessed infrequently and incompletely in a proportion of patients prior to MET events. Additionally, we registered a substantial time delay between the first recording of occurrence of vital sign instability and the subsequent MET call. These factors could lead to a low sensitivity of the triggering system, as reflected by our data, and may result in a number of patients who require intervention to be missed [19]. The cohort of patients evaluated by the MET did have a relatively high hospital mortality of nearly 20%. We do not have data to establish the mortality of ward patients in whom abnormal vital signs did develop but for whom the MET was not alerted and data from other studies is scarce. Buist et al reported a comparable 26% in-hospital mortality rate in a cohort of ward patients with abnormal vital sign parameters in whom the frequency of abnormal clinical observations was prospectively collected [6].
The MET system offers a simple and rapid way to assess and treat at-risk patients with reasonable resource use. The reaction time of the MET was within the predefined time limit of ten minutes and the time on scene was below 30 minutes in the majority of calls. The majority of calls occurred after hours, thus requiring the MET to be fully staffed at any time of the day. The point must be made, however, that the resource use of a RRS is not solely determined by the time expenditure of the efferent limb of the system. The establishment and maintenance of the necessary organisational and administrative structures, the training of the team members and the on-going education of hospital staff has to be taken into account as well. The total resource use by the RRS must be weighed against the fact that nearly half of the patients in our cohort were stabilised on the original ward, thus not requiring any ICU or IMC resources for their further care.
To date, the effectiveness of RRS to improve patient outcomes remains a controversial matter. Studies have been limited, were not always adequately powered and have reported mixed results [20]. In the only multi-centre, cluster randomised study on the implementation of a RRS, investigators randomly assigned 12 hospitals to MET implementation and 11 to continued standard care including the on-going use of established cardiac arrest teams [21]. The study failed to show a positive effect of the RRS on the occurrence of cardiac arrests, ICU admissions, or unexpected deaths when compared with the control hospitals; although primary outcomes significantly improved in both arms of the study. However, in a post hoc analysis, the authors reported that nearly half of the cardiac arrest calls in the control hospital were not associated with a cardiac arrest but with a less severe or “early” deterioration of the patient, thus resulting in a “MET-like” intervention. Additionally, in hospitals with MET implementation, a substantial proportion of patients with documented MET criteria were not subsequently assessed by the MET [22]. Studies assessing the effects of complex interventions like the introduction of a RRS in the open and evolving hospital environment are notoriously challenging to conduct. It is conceivable that the scarcity of evidence supporting a positive effect of RRS also reflects the difficulties in the design and execution of such trials. Nevertheless, the assumption that the introduction of a RSS can improve relevant patient outcomes seems inherently logical and is supported by two recent meta-analyses [23, 24].
Since the introduction of the Inselspital MET in 2009, we observed a steady increase in the monthly number of MET calls coinciding with a decrease in cardiac arrest calls on hospital wards. A progressive increase in MET utilisation and concurring decrease in cardiac arrests in the first years after introduction of RRS has been reported by other authors [25, 26]. Jones et al introduced the concept of “MET dose” by establishing that hospitals with higher MET utilisation rates per 1000 admissions are more likely to report improved patient outcomes after introducing a RRS [27]. Based on our data, we cannot conclude that the introduction of the MET system in our hospital has contributed to the decrease in cardiac arrests. It may well represent an association rather than evidence of causation. Mechanisms by which the MET may have had a positive effect on outcome include – aside from stabilisation of deteriorating patients who would have proceeded to cardiac arrest without MET intervention – improved education of ward staff, more specific DNR designations and therefore improved decision making in the context of end-of-life care.
Whereas RRSs have been introduced in many hospitals of different sizes and care levels internationally, this has not taken place on a large scale in Switzerland. On a tertiary care level, only the University Hospital of Basel operates a Rapid Response Team staffed by ICU and anaesthesia personnel for in-hospital resuscitation [28]. This team is similar to the efferent limb of our RRS. However, it mainly attends to immediately life-threatening conditions, including cardiac arrests, which are specifically not the focus of MET interventions at our institution. An important principle underlying RRSs is that early intervention can improve patient outcomes. Thus the system’s afferent limb – designed to identify clinical deterioration in patients and trigger a response – is a key factor to ensure the RSS effectiveness. We believe that this component of a RRS could be introduced in other Swiss hospitals even within the constraints of limited resources. The MET itself requires 24 hour in-hospital presence of health care professionals who are competent to assess and treat critically ill patients. Our ICU’s policy to operate with around the clock coverage by trained intensivists enabled us to implement the MET without structural changes to operational ICU staffing. In hospitals without 24 hour intensivist coverage, alternative models such as nurse-led METs or staffing with experienced specialist registrars could be considered [29, 30].
The main limitations of our study are related to the single-centre design and the need to retrospectively extract data from patient records. Our hospital serves as a primary care centre for a large urban area as well as a tertiary care centre for specialised evaluation and treatment of a population of approximately 1.5 million. With regard to structure and organisation, our institution is comparable to other university hospitals in Switzerland, thus our results might have implications for planning of RRS in other hospitals. Inter-observer variation in the accuracy of measuring vital sign parameters pre and during MET events or changes of such practice during the study period was not assessed. Determination of inter-observer variation of all the involved health care professionals would not have been possible for logistical reasons. All collected data consist of established vital sign assessments which were already implemented in the clinical routine. Most data originated from automatic monitoring systems. Therefore, we do not expect a significant bias from high inter-observer variation. Assessing the impact of a RRS on resource use and hospital mortality of all hospital admissions or in subgroups such as ICU patients before and after the introduction of the MET was not part of the study at hand. During the study period more than 140,000 and 22,000 patients were admitted to the hospital and to the ICU respectively, compared to 1628 MET calls in 1,317 patients. Given the very small proportion of patients with a MET intervention, it seems very unlikely that a difference in resource use or hospital mortalities could be established using a before – after study design.
Conclusion: Patients at risk for an unfavourable outcome can be identified using established criteria used in the context of a RRS. These patients can be assessed rapidly and with reasonable resource use by MET members who are familiar with the treatment of critically ill patients. The introduction of a RRS is associated with a number of logistical, organisational and medical challenges and the support of senior medical and nursing personnel is crucial. The key points for improvement, based on our experience, are to promote regular assessments of ward patients for signs of deterioration and to encourage early activation of the MET. The barriers to the successful use of the MET can only be overcome by continuous information and education of all health care professionals involved in the care of at-risk patients.
References
1 Baker GR, Norton PG, Flintoft V, Blais R, Brown A, Cox J, et al. The Canadian Adverse Events Study: the incidence of adverse events among hospital patients in Canada. CMAJ: Canadian Medical Association journal = journal de l’Association medicale canadienne. 2004;170(11):1678–86.
2 Brennan TA, Leape LL, Laird NM, Hebert L, Localio AR, Lawthers AG, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370–6.
3 Zegers M, de Bruijne MC, Wagner C, Hoonhout LH, Waaijman R, Smits M, et al. Adverse events and potentially preventable deaths in Dutch hospitals: results of a retrospective patient record review study. Quality & safety in health care. 2009;18(4):297–302.
4 Kause J, Smith G, Prytherch D, Parr M, Flabouris A, Hillman K. A comparison of antecedents to cardiac arrests, deaths and emergency intensive care admissions in Australia and New Zealand, and the United Kingdom– the ACADEMIA study. Resuscitation. 2004;62(3):275–82.
5 Storm-Versloot MN, Verweij L, Lucas C, Ludikhuize J, Goslings JC, Legemate DA, et al. Clinical relevance of routinely measured vital signs in hospitalized patients: a systematic review. Journal of nursing scholarship: an official publication of Sigma Theta Tau International Honor Society of Nursing / Sigma Theta Tau. 2014;46(1):39–49.
6 Buist M, Bernard S, Nguyen TV, Moore G, Anderson J. Association between clinically abnormal observations and subsequent in-hospital mortality: a prospective study. Resuscitation. 2004;62(2):137–41.
7 Hillman KM, Bristow PJ, Chey T, Daffurn K, Jacques T, Norman SL, et al. Duration of life-threatening antecedents prior to intensive care admission. Intensive care medicine. 2002;28(11):1629–34.
8 Wilson RM, Harrison BT, Gibberd RW, Hamilton JD. An analysis of the causes of adverse events from the Quality in Australian Health Care Study. Med J Aust. 1999;170(9):411–5.
9 Hodgetts TJ, Kenward G, Vlackonikolis I, Payne S, Castle N, Crouch R, et al. Incidence, location and reasons for avoidable in-hospital cardiac arrest in a district general hospital. Resuscitation. 2002;54(2):115–23.
10 Institute for Healthcare Improvement. 5 Million Lives Campaign. [http://www.ihi.org/engage/initiatives/completed/5MillionLivesCampaign/Pages/default.aspx]
11 Nolan JP, Soar J, Zideman DA, Biarent D, Bossaert LL, Deakin C, et al. European Resuscitation Council Guidelines for Resuscitation 2010 Section 1. Executive summary. Resuscitation. 2010;81(10):1219–76.
12 Acutely Ill Patients in Hospital: Recognition of and Response to Acute Illness in Adults in Hospital. London: National Institute for Health and Clinical Excellence; 2007.
13 Manthous CA: Rapid-response teams. N Engl J Med. 2011;365(14):1356; author reply 1356–7.
14 Devita MA, Bellomo R, Hillman K, Kellum J, Rotondi A, Teres D, et al. Findings of the first consensus conference on medical emergency teams. Crit Care Med. 2006;34(9):2463–78.
15 Cretikos M, Parr M, Hillman K, Bishop G, Brown D, Daffurn K, et al. Guidelines for the uniform reporting of data for Medical Emergency Teams. Resuscitation. 2006;68(1):11–25.
16 Peberdy MA, Cretikos M, Abella BS, DeVita M, Goldhill D, Kloeck W, et al. Recommended guidelines for monitoring, reporting, and conducting research on medical emergency team, outreach, and rapid response systems: an Utstein-style scientific statement: a scientific statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian Resuscitation Council, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, and the New Zealand Resuscitation Council); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiopulmonary, Perioperative, and Critical Care; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research. Circulation. 2007;116(21):2481–500.
17 Merz TM, Etter R, Mende L, Barthelmes D, Wiegand J, Martinolli L, et al. Risk assessment in the first fifteen minutes: a prospective cohort study of a simple physiological scoring system in the emergency department. Crit Care. 2011;15(1):R25.
18 Etter R, Ludwig R, Lersch F, Takala J, Merz TM. Early prognostic value of the medical emergency team calling criteria in patients admitted to intensive care from the emergency department. Crit Care Med. 2008;36(3):775–81.
19 Gao H, McDonnell A, Harrison DA, Moore T, Adam S, Daly K, et al. Systematic review and evaluation of physiological track and trigger warning systems for identifying at-risk patients on the ward. Intensive care medicine. 2007;33(4):667–79.
20 Price RJ, Cuthbertson BH, Cairns CJ. The findings of the International Conference on Medical Emergency Teams are biased and misleading. Crit Care Med. 2007;35(3):992–3.
21 Hillman K, Chen J, Cretikos M, Bellomo R, Brown D, Doig G, et al. Introduction of the medical emergency team (MET) system: a cluster-randomised controlled trial. Lancet. 2005;365(9477):2091–97.
22 Chen J, Bellomo R, Flabouris A, Hillman K, Finfer S. The relationship between early emergency team calls and serious adverse events. Crit Care Med. 2009;37(1):148–53.
23 Chan PS, Jain R, Nallmothu BK, Berg RA, Sasson C. Rapid Response Teams: A Systematic Review and Meta-analysis. Arch Intern Med. 2010;170(1):18–26.
24 Winters BD, Weaver SJ, Pfoh ER, Yang T, Pham JC, Dy SM. Rapid-response systems as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 Pt 2):417–25.
25 Foraida MI, DeVita MA, Braithwaite RS, Stuart SA, Brooks MM, Simmons RL. Improving the utilization of medical crisis teams (Condition C) at an urban tertiary care hospital. J Crit Care. 2003;18(2):87–94.
26 Baxter AD, Cardinal P, Hooper J, Patel R. Medical emergency teams at The Ottawa Hospital: the first two years. Canadian journal of anaesthesia = Journal canadien d’anesthesie. 2008;55(4):223–31.
27 Jones D, Bellomo R, DeVita MA. Effectiveness of the Medical Emergency Team: the importance of dose. Crit Care. 2009;13(5):313.
28 Harm F, Ummenhofer W, Luethy M, Zuercher M. In-hospital cardiac arrest after leaving a monitored bed-do we transfer patients too early? Resuscitation. 2012;83:e7.
29 Morris DS, Schweickert W, Holena D, Handzel R, Sims C, Pascual JL, et al. Differences in outcomes between ICU attending and senior resident physician led medical emergency team responses. Resuscitation. 2012;83(12):1434–7.
30 Mitchell A, Schatz M, Francis H. Designing a critical care nurse-led rapid response team using only available resources: 6 years later. Crit Care Nurse. 2014;34(3):41–55; quiz 56.

