Figure
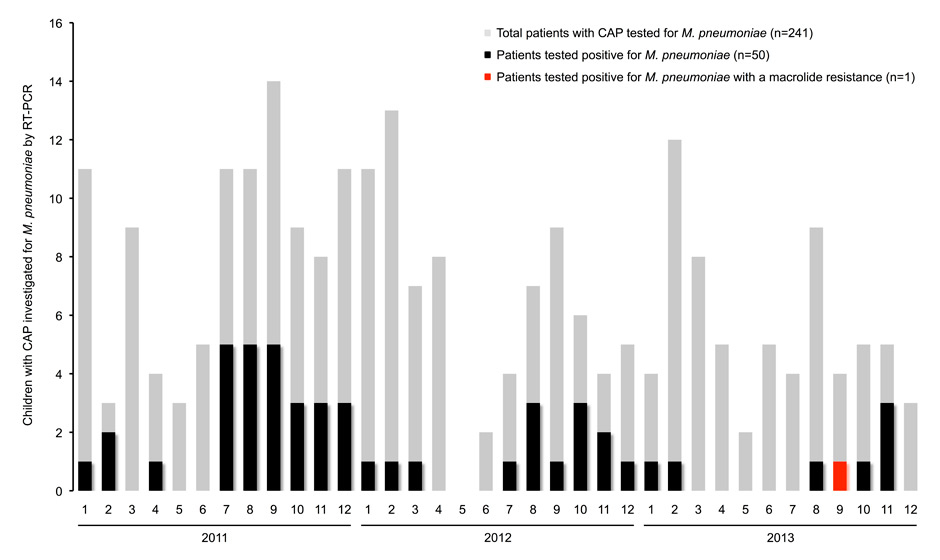
Case distribution of children with community-acquired pneumonia (CAP) investigated for Mycoplasma pneumoniaeby real-time polymerase chain reaction (RT-PCR) in respiratory specimens (n = 241) during the study period between 2011 and 2013.
DOI: https://doi.org/10.4414/smw.2014.14041
Mycoplasma pneumoniae is a leading cause of community-acquired pneumonia (CAP) in children and macrolides are recommended for this entity [1]. Extensive macrolide use led to the rapid, worldwide emergence of macrolide-resistant M. pneumoniae (MRMP) [2] with rates of over 90% in Asia (China, 2012 [3]) and up to 26% in Europe (Italy, 2010 [4]). The first two cases of MRMP in Switzerland were reported in adults in 2012 [5]. We aimed to assess the presence of MRMP in children with CAP.
Mycoplasma pneumoniae strains detected by real-time polymerase chain reaction (RT-PCR) in respiratory specimens (pharyngeal swab, sputum, nasopharyngeal or tracheal secretion, bronchoalveolar lavage, or pleural aspirate) were investigated for macrolide resistance by sequence analysis of the M. pneumoniae 23S rRNA gene. All respiratory specimens were collected from both in-patients and out-patients ≤16 years of age with clinically diagnosed CAP at the University Children's Hospital of Zurich between January 2011 and December 2013. Isolation of M. pneumoniae chromosomal DNA and RT-PCR targeting the P1 adhesion protein gene (P1 gene) was performed on all respiratory specimens as previously described [6, 7]. Amplification of the partial M. pneumoniae 23S rRNA gene (domain V) followed by sequence analysis for the detection of resistance mutations at positions 2063, 2064, and 2617 was conducted on M. pneumoniaeRT-PCR-positive samples according to Matsuoka et al. [8]. Clinical data was gathered from medical records.
During the 3 years, respiratory specimens from 50 out of 241 CAP patients (20.7%; figure) tested positive for M. pneumoniae. The median age of the 50 patients was 9.1 years (range, 1.3–15.9). The clinical diagnosis of CAP was radiologically confirmed in 93% (43/46). Macrolides were administered in 2% of patients before and in 26% after detection of M. pneumoniae.

Figure
Case distribution of children with community-acquired pneumonia (CAP) investigated for Mycoplasma pneumoniaeby real-time polymerase chain reaction (RT-PCR) in respiratory specimens (n = 241) during the study period between 2011 and 2013.
Sequencing of the partial 23S rRNA gene amplicons from these M. pneumoniae RT-PCR-positive respiratory samples (96% pharyngeal swabs) revealed one sample with an A2063G mutation, which confers high-level macrolide resistance [2]. Mutations at position 2064 or 2617 were not found. The resistant strain was detected in a pharyngeal swab specimen of an otherwise healthy 7-year-old boy seen in September 2013 with a mild and self-limiting M. pneumoniae CAP (no hospitalisation nor antimicrobial treatment). The patient had not received prior antimicrobial treatment.
Our survey documents the first existence of MRMP in a child with CAP due to M. pneumoniae in Switzerland. We detected the A2063G mutation that represents the most common cause of high-level macrolide resistance in M. pneumoniae[2]. At least four mutations in the 23S rRNA gene of M. pneumoniae have been reported in vitro, whereby mutations at positions 2063, 2064, and 2617 (corresponding to Escherichia coli 23S rRNA gene positions 2058, 2059, and 2611) were also found in vivo[2]. The point mutation results in modification of the peptidyltransferase loop of domain V of 23S rRNA, which reduces the binding affinity of macrolides to the 23S rRNA component of the large subunit (50S) of the bacterial ribosome.
The frequency of MRMP among our paediatric CAP patients (2.0%, 1/50) is remarkably low compared to that recently detected in children in Japan (87.1%, 176/202) [9] and China (97.0%, 32/33) [3] although study designs and cohorts differ, which limits comparison. The high resistance rate in Asia is linked with broad macrolide use [2]. This may be also true for the alarming resistance rate observed in the neighbouring country Italy (25.6%, 11/43) [4], where selective antibiotic pressure has been demonstrated to lead to de novo macrolide resistance during treatment. However, the emergence of MRMP in our study parallels the lower occurrence of MRMP in other neighbouring countries, e.g., Germany (1.2%, 2/167) [10] and France (9.8%, 5/51) [11]. There was no macrolide treatment history in our patient. This suggests MRMP did not emerge in the patient himself but rather was acquired from the community. Importantly, children have recently been recognised to carry M. pneumoniae in their respiratory tract in up to 21% [12]. This influences current diagnostic procedures, because neither PCR nor single sample serology is a reliable indicator of infection [1, 12], and, in turn, may suggest that MRMP is indeed considerably less frequent in Switzerland compared to in countries with higher prescription numbers of macrolides, as e.g., Asia [3, 9].
Limitations of our survey include the retrospective design (missing M. pneumoniae CAP cases not tested for M. pneumoniae and/or managed by general practitioners only), the patient cohort (both in-patients and out-patients) that was geographically confined to the region of Zurich, Switzerland, and the lack of control samples from asymptomatic children. Moreover, RT-PCR for M. pneumoniae from respiratory specimens other than pharyngeal swabs is less validated.
MRMP can potentially have clinical consequences, i.e., longer duration of symptoms and hospitalisation, more severe CAP, and increased frequency of extrapulmonary manifestations [1]. Thus, further surveys of MRMP in children should be considered also in Switzerland to prevent missing changes in MRMP in the local community. Our results are compatible with the rather judicious prescription of macrolides in Switzerland [13]. To this effect current guidelines confine macrolide treatment for CAP in children at any age as second choice if there is no response to first-line empirical β-lactam antibiotics or in the case of very severe CAP [14].
1 Meyer Sauteur PM, van Rossum AM, Vink C. Mycoplasma pneumoniae in children: carriage, pathogenesis, and antibiotic resistance. Curr Opin Infect Dis. 2014;27(3):220–7.
2 Bebear C, Pereyre S, Peuchant O. Mycoplasma pneumoniae: susceptibility and resistance to antibiotics. Future Microbiol. 2011;6(4):423–31.
3 Zhao F, Liu G, Wu J, Cao B, Tao X, He L, et al. Surveillance of macrolide-resistant Mycoplasma pneumoniae in Beijing, China, from 2008 to 2012. Antimicrob Agents Chemother. 2013;57(3):1521–3.
4 Chironna M, Sallustio A, Esposito S, Perulli M, Chinellato I, Di Bari C, et al. Emergence of macrolide-resistant strains during an outbreak of Mycoplasma pneumoniae infections in children. J Antimicrob Chemother. 2011;66(4):734–7.
5 Tschan A, Weisser M, Frank B, Dumke R, Tamm M, Imhof E, et al. Severe pneumonia due to macrolide-resistant Mycoplasma pneumoniae in Switzerland. Schweiz Med Forum. 2012;12(49):961–3.
6 Nadal D, Bossart W, Zucol F, Steiner F, Berger C, Lips U, et al. Community-acquired pneumonia in children due to Mycoplasma pneumoniae: diagnostic performance of a seminested 16S rDNA-PCR. Diagn Microbiol Infect Dis. 2001;39(1):15–9.
7 Hardegger D, Nadal D, Bossart W, Altwegg M, Dutly F. Rapid detection of Mycoplasma pneumoniae in clinical samples by real-time PCR. J Microbiol Methods. 2000;41(1):45–51.
8 Matsuoka M, Narita M, Okazaki N, Ohya H, Yamazaki T, Ouchi K, et al. Characterization and molecular analysis of macrolide-resistant Mycoplasma pneumoniae clinical isolates obtained in Japan. Antimicrob Agents Chemother. 2004;48(12):4624–30.
9 Okada T, Morozumi M, Tajima T, Hasegawa M, Sakata H, Ohnari S, et al. Rapid effectiveness of minocycline or doxycycline against macrolide-resistant Mycoplasma pneumoniae infection in a 2011 outbreak among Japanese children. Clin Infect Dis. 2012;55(12):1642–9.
10 Dumke R, von Baum H, Luck PC, Jacobs E. Occurrence of macrolide-resistant Mycoplasma pneumoniae strains in Germany. Clin Microbiol Infect. 2010;16(6):613–6.
11 Peuchant O, Menard A, Renaudin H, Morozumi M, Ubukata K, Bebear CM, et al. Increased macrolide resistance of Mycoplasma pneumoniae in France directly detected in clinical specimens by real-time PCR and melting curve analysis. J Antimicrob Chemother. 2009;64(1):52–8.
12 Spuesens EB, Fraaij PL, Visser EG, Hoogenboezem T, Hop WC, van Adrichem LN, et al. Carriage of Mycoplasma pneumoniae in the upper respiratory tract of symptomatic and asymptomatic children: an observational study. PLoS Med. 2013;10(5):e1001444.
13 Achermann R, Suter K, Kronenberg A, Gyger P, Muhlemann K, Zimmerli W, et al. Antibiotic use in adult outpatients in Switzerland in relation to regions, seasonality and point of care tests. Clin Microbiol Infect. 2011;17(6):855–61.
14 Harris M, Clark J, Coote N, Fletcher P, Harnden A, McKean M, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66Suppl 2(ii):1–23.
Funding / potential competing interests: P.M.M.S. is funded by a Swiss National Science Foundation (SNSF) grant (PBZHP3_147290). There are no conflicts of interest.