Enterococci, Clostridium difficile and ESBL-producing bacteria: epidemiology, clinical impact and prevention in ICU patients
DOI: https://doi.org/10.4414/smw.2014.14009
Jan A.
Sidler, Manuel
Battegay, Sarah
Tschudin-Sutter, Andreas F.
Widmer, Maja
Weisser
Summary
Most hospital-acquired infections arise from colonising bacteria. Intensive care patients and immunocompromised individuals are at highest risk for microbial invasion and subsequent infection due to multiple invasive procedures in addition to frequent application of chemotherapeutics and presence of poor microperfusion leading to mucosal disruption. In this narrative review, we summarise the literature on bacterial colonisation in intensive care patients, in particular the epidemiology, the clinical impact and respective infection control strategies of three pathogens, i.e., Enterococcusspp., extended-spectrum ß-lactamase producing gram-negative bacteria and Clostridium difficile, which have evolved from commensals to a public health concern today.
Abbreviations
ARE ampicillin-resistant enterococci
CI confidence interval
ESBL extended-spectrum ß-lactamase
ESBL-GNB extended-spectrum ß-lactamase producing gram-negative bacteria
ICU intensive care unit
MRSA methicillin-resistant Staphylococcus aureus
OR odds ratio
VRE vancomycin-resistant enterococci
Introduction
Infections are the leading cause of death in intensive care units (ICUs) worldwide and mortality in infected ICU patients is more than twice as high compared to non-infected patients [1, 2]. Despite significant advances in intensive care therapy and infection prevention, incidence of nosocomial infections in ICU patients has remained high [1, 3]. The bacteria causing most hospital-acquired infections are staphylococci including methicillin-resistant S. aureus (MRSA), enterococci including vancomycin-resistant enterococci (VRE), Candidaspp., Clostridium difficile and different often multidrug-resistant gram-negative bacteria [1].
In healthy individuals, an ecological community of commensals, symbiotes and pathogens – the microbiome – is in equilibrium with the host. If anatomical barriers or host defenses are disrupted, invasion of colonising bacteria and subsequent infection can arise [4].
In ICU patients, multiple invasive procedures (e.g., central venous catheters) and the presence of poor microperfusion lead to integrity loss of skin and mucosae with risk of invasive infection [5]. Furthermore, ICU patients are per se immunocompromised due to the severity of the disease [6].
Selected by pressure of antibiotic treatments, colonising multidrug-resistant bacteria can outgrow commensals from the microbiome [7] and become invasive. In view of the global rise of infections with multidrug-resistant bacteria and a concomitant lean development pipeline for antimicrobial agents, the “Infectious Diseases Society of America” stated that we should consequently maximise infection control strategies [8].
In this narrative review, we summarise the literature on bacterial colonisation in ICU patients, in particular the epidemiology, the clinical impact and respective infection control strategies focusing on three intestinal bacteria, i.e. Enterococcusspp., extended-spectrum ß-lactamase producing gram-negative bacteria (ESBL-GNB) and Clostridium difficile.
Methods
We searched PubMed/MEDLINE in November 2013, without restrictions, using the following search strategy with Boolean operators: “(“enterococcus” OR “enterococci” OR “VRE” OR “ARE” OR “ESBL” OR “Clostridium difficile” OR “C. difficile”) AND (“intensive care unit” OR “ICU”)”. In addition, we searched the references of cited articles in this review for other appropriate studies.
Only articles focusing on adult populations (≥18 years) written in English, German, French or Italian language were included. Several other search terms were applied to identify appropriate studies regarding specific questions considered in this narrative review (e.g., to describe the global epidemiology of enterococci).
Enterococci
Background
E. faecalis and E. faecium – the species most frequently encountered in clinical isolates [9] – have evolved from intestinal commensals to the third highest ranking cause of nosocomial infections in the United States [10]. Enterococci are characterised by a remarkable genomic flexibility [11] with the ability to incorporate foreign mobile genetic elements carrying e.g., resistance genes to multiple antibiotics in addition to chromosomal resistance genes [12, 13]. The increasing rate of antimicrobial resistance in enterococci, e.g. to ampicillin or vancomycin, are of major clinical importance [14, 15]. Since the first description of VRE in a clinical isolate in Europe in 1988 [16], VRE are increasing in prevalence worldwide, capable of spreading vancomycin resistance genes (mainly vanAand vanB) via transposons to vancomycin-susceptible enterococci and rarely to other bacteria (e.g., MRSA) [17, 18]. VanA is widely prevalent in the United States and Korea, whereas vanB has been introduced as main genotype in VRE epidemics in Australia and Singapore [19]. Chromosomal vancomycin resistance genes are less transmissible (e.g., vanC) and are related to the use of the animal growth promoter avoparcin in Europe until 1997 and rarely cause infection [20, 21].
Whereas in the Unites States VRE nowadays dominate the epidemiology of nosocomial enterococcal infections, the situation in Europe is more diverse: Germany, Greece, England, Ireland and Portugal have high VRE rates of >10%, whereas in most European countries an increase in ampicillin-resistant enterococci (ARE) is observed since the year 2000 [22, 23]. According to the European Center for Disease Prevention and Control (ECDC) report 2012, overall ampicillin-susceptibility rates of E. faecalis isolates were >75% in all European countries (mostly >95%) compared to <50% in E. faecium (range 0.8–33.3%) [22].
Clinical impact of infection and colonisation with enterococci in ICU patients
According the Unites States “National Healthcare Safety Network” 2006–7, enterococci accounted for 12.1% of hospital-acquired infections in ICU patients (16.0% of central-line-associated bloodstream infections and 14.9% of catheter-associated urinary tract infections) [10]. In a global ICU point-prevalence study from 2007, VRE rates among enterococcal clinical isolates differed widely in the geographic regions of the world: Western Europe 31.1%, Eastern Europe 31.4%, Central/South America 46.9%, North America 47.8%, Oceania 52.6%, and Asia 37.0% [1].
In hospitalised patients, densities of ampicillin-resistant E. faecium colonisation in stool increases 10–fold compared to E. faecalis, leading to overgrowth [24] and possibly facilitated invasive infection. Table 1 gives an overview of the published VRE and ARE colonisation and infection rates in ICU patients. On admission, VRE colonisation rates range from 0.6% [25] to 42.6% [26]. VRE acquisition rates during hospitalisation range from 1.2% [25] to 41.4% [27]. Major risk factors for VRE colonisation/infection are length of hospital stay [28–35], high VRE colonisation pressure (high rate of colonised patients on the same ward) [28, 36, 37], and antimicrobial therapy with broad-spectrum antibiotics [25, 27, 30, 32, 33, 35–44]. To note, not only antimicrobial therapy with vancomycin [27, 30, 32, 37, 38, 40, 41, 44, 45] but also metronidazole [30, 33, 38], quinolones [30, 36, 38, 44], cephalosporins [27, 30, 33, 36, 37, 39], carbapenems [25, 36, 40, 44], and other broad-spectrum antibiotic classes were associated with VRE in ICU patients.
In contrast to VRE, only few studies focused on ARE colonisation in ICU patients [46–50]. In two prospective studies from the Unites States, ARE were found in 5.0% and 5.4% of patients admitted to a general ward or ICU, and acquisition during hospitalisation in 18.9% [49, 50]. In a Dutch ICU, ARE were present on admission screening in 28% of patients; new ARE clones were acquired during hospitalisation in 83% [46]. Documented risk factors for ARE colonisation are previous hospitalisation [49], prior antimicrobial therapy [49, 50], enteral tube feeding [49], urinary bladder catheter [50], and total nursing care [50].
The relative contribution of both cross-transmission and selection under antibiotic pressure to the burden of VRE/ARE colonisation is largely unknown. Typing methods such as multilocus sequence typing have allowed identification of nosocomial E. faecium clones, such as clonal-complex 17 [51], which contain genes conferring virulence and resistance to multiple antibiotics. Several studies have demonstrated the importance of cross-transmission and environmental contamination of VRE [27, 28, 30, 36, 37, 52–54] and ARE [50, 55] estimating cross-transmission as the cause of VRE colonisation in up to 85% [27].
Once colonised with VRE/ARE, ICU patients or haematological patients have the highest risk to develop an invasive infection [56]. In VRE-colonised ICU patients, infection rates of up to 45% [57] and bacteremia rates of up to 16% [32] have been reported [58]. In contrast, VRE infection prevalence among non-colonised ICU patients is negligible with <2% [58]. The prevalence of ARE infections in colonised ICU patients or other high-risk populations has not been described so far, but infected patients were colonised with the same ARE type in 100% of patients in a European study [46].
Colonised ICU patients developing an enterococcal infection are sicker, i.e. have more co-morbidities, higher disease severity scores on admission and have increased mortality rates compared to non-infected patients [1]. At highest risk are neutropenic patients with a VRE infection rate up to 27% once colonised [59] and a mortality rate up to 60% [60–62]. The question, whether this is due to virulence of VRE or an effect of the underlying condition is still a matter of debate [63–67]. Besides host factors, bacterial virulence and treatment might affect outcome. A meta-analysis including a total of 1,614 enterococcal bloodstream infections indicated that patients with VRE were more likely to die than those with vancomycin-susceptible enterococci (odds ratio (OR) 2.52; 95% confidence interval (CI) 1.9–3.4) [67].
|
Table 1:Studies on vancomycin- and ampicillin-resistant enterococci: rates and risk factors for colonisation/infection in adult patients (intensive care unit setting only). |
|
Study design and reference
|
Study year
|
Country
|
Total of colonised patients
|
Rate of co-lonisation on admissiona, %
|
Rate of HA colonisationa, %
|
Rate of infections, %
|
Risk factors for VRE/ARE colonisation and/or infection
|
|
Vancomycin-resistant enterococci
|
| ROS [38] |
2013 |
Saudi Arabia |
30 |
NA |
NA |
NA |
Multiorgan failure, chronic renal failure, VAN, MN, P/T, QN, gastrointestinal contrast procedure |
| POS [39] |
2012 |
Taiwan |
97 |
5.8 |
5.4 |
10.6b
|
Septic shock, cardiovascular disease, endocrine disorder, 1st /2nd-gen. cephalosporin, antifungal agent |
| ROS [40] |
2012 |
Brazil |
78 |
15.0 |
9.9 |
NA |
Diabetes mellitus, nephropathy, any antimicrobial therapy, VAN, CAR |
| POS [41] |
2012 |
Korea |
290 |
17.6 |
12.3 |
15.2c
|
Polymicrobial infection, haemodialysis catheter, intra-abdominal procedure, long duration of VAN therapy |
| ROS [45] |
2012 |
Korea |
153 |
3.4 |
NA |
NA |
Polymicrobial infection, haemodialysis catheter, intra-abdominal procedure, long duration of VAN therapy |
| ROS [36] |
2011 |
United States |
885 |
8.0 |
2.9 |
NA |
Chronic renal failure, wounds, rash, surgery, surgical drain, intubation, central line catheter, low albumin, high VRE colonisation pressure, macrolide, QN, AG, 3rd-gen. CEPH, CAR |
| POS [42] |
2011 |
United States |
19 |
2.5 |
NA |
NA |
Any antimicrobial therapy, rehospitalisation, intravenous drug user, haemodialysis, immunocompromised status |
| ROS [28] |
2009 |
Korea |
52 |
6.1d
|
NA |
NA |
Female gender, GCS <8, co-morbidity, invasive catheters, long duration of mechanical ventilation, long hospital stay, presence of nearby VRE positive patient |
| POS [43] |
2009 |
Korea |
34 |
4.4 |
NA |
NA |
Infectious disease, rehospitalisation, any antimicrobial therapy |
| POS [208] |
2008 |
United States |
168 |
8.9 |
4.1 |
NA |
Environmental VRE contamination, prior room occupant with VRE colonisation |
| POS [209] |
2006 |
Italy |
56 |
2.6 |
8.7 |
3.6 |
NA |
| POS [210] |
2006 |
United States |
309 |
9.7 |
7.5 |
NA |
NA |
| POS [44] |
2005 |
United States |
312 |
12.8 |
NA |
NA |
Any antimicrobial therapy, liver disease, renal disease, VAN, IMI, QN |
| POS [29] |
2005 |
Brazil |
48 |
NA |
32.6 |
NA |
Long hospital stay, long duration of any antimicrobial therapy, rehospitalisation, nosocomial infection |
| POS [84] |
2004 |
United States |
136 |
10.0 |
NA |
NA |
NA |
| POS [211] |
2004 |
Taiwan |
816 |
8.0 |
10.8 |
1.1 |
NA |
| POS [25] |
2003 |
Australia |
66 |
0.6 |
1.2 |
|
Rehospitalisation, CAR, ticarcillin-clavulanate |
| ROS [30] |
2003 |
United States |
63 |
18.9 |
22.6 |
3.2b
|
Long hospital stay, acute respiratory failure, sepsis, multiorgan failure, central venous catheter, VAN, CEPH, MN, QN, location in high-risk room |
| POS [31] |
2003 |
United States |
201 |
24.5 |
21.0 |
NA |
COPD, high APACHE score, sucralfate, C. difficile diarrhea, vasopressor, tracheostomy, long duration of mechanical ventilation, long hospital stay, chronic dialysis, rehospitalisation |
| POS [26] |
2002 |
United States |
26 |
42.6e
|
22.2e
|
NA |
Rehospitalisation, enteral tube feeding |
| POS [57] |
2001 |
Unites States |
23 |
9.4f
|
11.3f
|
45.0 |
NA |
| POS [212] |
2001 |
Argentina |
1 |
0.7 |
NA |
NA |
NA |
| POS [213] |
1999 |
United States |
10 |
6.3 |
9.8 |
|
NA |
| POS [32] |
1999 |
Israel |
14 |
9.8 |
14.5 |
7.1 |
Young age, any antimicrobial therapy, long duration of any antimicrobial therapy, VAN, rehospitalisation, long hospital stay |
| POS [214] |
1999 |
Australia |
1 |
0.7 |
NA |
NA |
NA |
| POS [33] |
1999 |
United States |
46 |
12.1 |
14.1 |
NA |
Gastrointestinal disease, prior solid organ transplantation, long hospital stay, rehospitalisation, high APACHE score, 2nd/3rd-gen. CEPH, MN |
| POS [34] |
1999 |
United States |
13 |
15.7c
|
NA |
NA |
Immunosuppression, neutropenia, long hospital stay |
| POS [35] |
1998 |
Netherlands |
98 |
14.3 |
18.3 |
NA |
Long hospital stay, any antimicrobial therapy |
| POS [37] |
1998 |
United States |
45 |
NA |
29.4 |
NA |
High VRE colonisation pressure, VAN, 3rd-gen. CEPH, enteral tube feeding, sucralfate, high APACHE score |
| POS [27] |
1996 |
United States |
31 |
13.0g
|
41.4g
|
NA |
Old age, 3rd-gen. CEPH, VAN |
|
Ampicillin-resistant enterococci
|
| POS [46] |
2012 |
Netherlands |
21 |
27.6d
|
NA |
NA |
NA |
| POS [49] |
1996 |
United States |
19h
|
5.4 |
NA |
NA |
Rehospitalisation, treatment with >3 antibiotics, 3rd-gen. CEPH, enteral tube feeding |
| POS [50] |
1992 |
United States |
23h
|
5.0 |
18.9 |
NA |
Any antimicrobial therapy, CEPH, urinary bladder catheter, need for total nursing care |
| Partly adapted from Ziakas et al. [58]. Interventional trials were excluded.
HA = hospital-acquired; VRE = vancomycin-resistant enterococci; NA = not assessed; ROS = retrospective observational study; POS = prospective observational study; VAN = vancomycin; AG = aminoglycoside; QN = quinolone; MN = metronidazole; CAR = carbapenem; P/T = piperacillin/tazobactam; CEPH = cephalosporin; IMI = imipenem; APACHE = acute physiology and chronic health evaluation; COPD = chronic obstructive pulmonary disease; GCS = Glasgow Coma Score; ICU = intensive care unit.
a Rectal/perirectal colonisation if not stated otherwise. HA colonisation was defined as negative culture within the first 48h of ICU admission and subsequent positive culture; b among patients with HA VRE colonisation; c among patients with VRE colonisation on admission; d total VRE colonization rate (on admission or HA); e based on rectal, faecal, and/or urine cultures; f based on rectal, faecal, respiratory, and/or urine cultures; g based on rectal, integumental (groin and arm), oropharyngeal, tracheal, and/or gastric cultures; h ICU and/or medical ward. |
Extended-spectrum ß-Lactamase (ESBL) producing gram-negative bacteria
Background
One of the most important resistance mechanisms of gram-negative bacteria are ß-lactamases conferring resistance to ß-lactam antibiotics by hydrolisation of their ß-lactam-ring [68]. Of the many different ß-lactamases, ESBLs comprise the largest group of enzymes [68], causing resistance to newer ß-lactam antibiotics, including the third-generation cephalosporins and monobactams, but not the cephamycins and carbapenems [69, 70].
ESBLs were initially recognised in clinical bacterial isolates in the 1980’s and are a rapidly increasing public health threat today [68, 71]. The different gene classes encoding ESBLs are located on plasmids or chromosomes. Plasmids encoding ESBLs easily spread among Enterobacteriaceae, mainly Escherichia coli and Klebsiella pneumoniae but also among non-fermentative gram-negative bacteria, such as Pseudomonas aeruginosa and Acinetobacter baumannii[71]. A variety of distinct ESBL genotypes predominate in certain regions of the world (e.g., CTX-M-15 is becoming dominant in most European countries) [72–74].
Based on a recent surveillance trial, the rate of ESBL-producers among clinical K. pneumoniae isolates, was highest in Latin America (44.0%), followed by Asia/Pacific Rim (22.4%), Europe (13.3%), and North America (7.5%) [75]. The same geographical ranking order of ESBL producers was observed among E. coliisolates, although lower for all four regions (13.5%, 12.0%, 7.6%, and 2.2%, respectively) [75]. In Europe, 2012, resistance to third-generation cephalosporins ranged from 0% (Romania) to 74.8% (Bulgaria) and from 4.4% (Sweden) to 38.1% (Bulgaria) for K. pneumoniae and E. coli, respectively [22].
In addition to ESBLs, the large plasmids commonly harbor genes encoding for resistance to other antibiotic classes [76] such as aminoglycosides (5.6–83.5%), tetracyclines (44.4–61.1%), trimethoprim-sulfamethoxazole (5.6–25.4%), and ciprofloxacin (22.2–44.2%) [77].
In a recent Swiss hospital-wide surveillance study of patients with any clinical ESBL-GNB isolate and no current ESBL-GNB specific antimicrobial therapy, urine samples were positive in 110 of 133 patients (82.7%), rectal swabs in 69.2%, skin swabs of the groin in 35.3%, and throat swabs in 12.8% [78].
Clinical impact of infection and colonisation with ESBL-GNB in ICU patients
Global surveillance data from a 1–day point prevalence study on 1,265 ICUs in 2007 showed an overall ESBL rate of 3.0% among clinical isolates of gram-negative bacteria (North America 0.4%, Western Europe 3.0%, Asia 4.5%) [1].
The published ESBL-GNB colonisation rates of ICU patients (table 2) range from 2.2% [79] to 49.0% [80] with important geographical differences. The highest ESBL-GNB colonisation rates on ICU admission have been found in Korean [81] (42.5%), Indian [80] (49.0%), and Spanish [82] (38.3%) ICUs, whereas especially ICUs from the Unites States [79, 83, 84] and Belgium [85] exhibited low colonisation rates (2.2–6.2%).
The three main risk factors for colonisation/infection with ESBL-GNB in ICU patients are length of hospital stay [80, 86], high ESBL-GNB colonisation pressure [86, 87], and broad-spectrum antibiotics [79, 80, 82, 86–88] (table 2).
ESBL-GNB infection rates in colonised ICU patients range from 4.9% [87] to 68.8% [85]. The largest of these studies was a prospective 3.5–year single-centre study from the Unites States. Out of 5,209 ICU patients, 2.2% were rectally colonised with ESBL-producing E. coli or Klebsiella spp. on admission, and in 24.8% the same ESBL-GNB was found in a clinical sample thereafter [79]. In contrast, among the 5,092 patients not colonised with ESBL-GNB, only 0.6% had a subsequent positive clinical culture [79]. One of the few prospective studies on outcome of rectal ESBL-GNB colonisation was performed in 513 hospitalised haematological and oncological patients in Germany. Colonised patients had a risk ratio of 4.5 to develop a subsequent ESBL-GNB bloodstream infection (95% CI 2.9–7.0) [89]. A 10–year prospective French study including 710 liver transplant patients showed an even higher infection rate in patients with pre-transplant fecal ESBL-GNB colonisation (44.8%) compared to non-carriers (3.8%; p <0.0001), proven by identical PCR typing in 76.9%. Another study including 4 high-risk units (2 ICUs, 1 solid organ transplant unit, and 1 haematology/oncology unit) in the Unites States found an ESBL-GNB bloodstream infection rate of 8.5% (35/413) in colonised patients [90]. On the other hand, one study in patients with acute leukaemia or haematopoietic stem cell transplantation could not confirm an association between colonisation and infection with ESBL-producing E. coli or an increased in-hospital mortality (bloodstream infections rate with ESBL-producing E. coli in 1.5% of colonised vs 1% of non-colonised patients; p = 0.7) [91].
Several, mostly retrospective studies showed significantly longer length of hospital stay and higher mortality rates in patients with bloodstream infections due to ESBL-producing versus non-producing GNB [90, 92–98]. The increased mortality in bloodstream infections with ESBL-GNB is mainly caused by inadequate initial therapy and is likely not a consequence of higher bacterial virulence [99].
|
Table 2:Studies on extended-spectrum ß-lactamase producing gram-negative bacteria: rates and risk factors for colonisation/infection in adult patients (intensive care unit setting only). |
|
Study design and reference
|
Study year
|
Country
|
Total of colonised patients
|
Rate of co-lonisation on admissiona, %
|
Rate of HA colonisationa, %
|
Rate of infection, %
|
Risk factors for ESBL-GNB colonisation and/or infection
|
| ROS [86] |
2013 |
United States |
267 |
NA |
3.5b
|
NA |
Rehospitalisation, long hospital stay, high ESBL-GNB colonisation pressure, malignancy, cerebrovascular disease, renal disease, P/T, CFM, antifungal agents, anti-pseudomonas ß-lactams, anti-MRSA therapy |
| POS [81] |
2013 |
Korea |
40 |
42.5c
|
NA |
NA |
NA |
| POS [87] |
2012 |
France |
110 |
15.4 |
13.2 |
4.9 |
Male gender, old age, severe sepsis/septic shock, high ESBL-GNB colonisation pressure, long broad-spectrum antibiotic therapy, penicillin/beta-lactamase inhibitor, QN, 3rd-gen. CEPH, long hospital stay, surgery within past year, hospital admission in another country, rehospitalisation, neurological disease, transfer from another ICU, urinary tract disease |
| POS [88] |
2012 |
France |
63 |
4.2 |
4.2 |
NA |
ß-lactams and CAR |
| POS [80] |
2010 |
India |
47 |
49.0c,d
|
NA |
NA |
Long mechanical ventilation, long hospital stay, comorbidities, use of ≥3 antibiotic groupse |
| POS [79] |
2007 |
United States |
117 |
2.2 |
NA |
24.8 |
Old age, high infectious disease-specific chronic disease score, VAN, P/T, CFM, IMI |
| POS [83] |
2007 |
United States |
97 |
4.1 |
1.3 |
NA |
Horizontal transmissionf
|
| POS [85] |
2006 |
Belgium |
32 |
6.2 |
8.6 |
68.8 |
NA |
| POS [84] |
2004 |
United States |
32 |
2.3g
|
NA |
31.2 |
NA |
| POS [82] |
1997 |
Spain |
72 |
38.3c
|
NA |
NA |
High clinical severity score at admission, arterial or urinary catheterisation, total parenteral nutrition, mechanical ventilation, antimicrobial therapy |
| Interventional trials were excluded.
HA = hospital-acquired; ESBL = extended-spectrum ß-lactamase; GNB = gram-negative bacteria; POS = prospective observational study; ROS = retrospective observational study; VAN = vancomyin; QN = quinolone; MN = metronidazole; CAR = carbapenem; P/T = piperacillin/tazobactam; CEPH = cephalosporin; CFM = cefepime; IMI = imipenem; ICU = intensive care unit; MRSA = methicillin-resistant Staphylococcus aureus; NA = not assessed.
a Rectal/perirectal colonisation if not stated otherwise. HA colonisation was defined as negative culture within the first 48h of ICU admission and subsequent positive culture; b HA colonisation and/or infection; c total VRE colonisation rate (on admission or HA); d based on cultures from the nares, oropharynx, and/or rectum; e other pathogens than ESBL-GNB included in analysis; f based on an ecological correlation; g colonisation within the first 72 hours after admission. |
Clostridium difficile
Background
C. difficileis a gram-positive, anaerobic and spore-forming rod causing mainly antibiotic-associated diarrhea. Symptoms range from uncomplicated diarrhea to severe pseudomembranous colitis and toxic megacolon [100]. C. difficile is a public health concern worldwide representing the leading cause of hospital-associated infectious diarrhea [101]. Increases in incidence, morbidity, and recurrence rate have been reported in the United States, Canada, and Europe [102]. In contrast, little is known on the epidemiology of C. difficile infection in Asian countries [103].
The increased virulence of C. difficile has been attributed to the spread of fluoroquinolone-resistant ribotype 027 (RT027, BI/NAP01), which produces, in addition to toxins A and B, a binary toxin of unspecified significance [102, 104–106]. C. difficile RT027 is the cause of multiple healthcare-associated outbreaks in the United States, Canada, and Europe [107–109]. Furthermore, community-acquired C. difficile infection is increasing, with another hypervirulent ribotype (078), as the main culprit. Compared to North America and Europe, ribotype 017 and 018 have been shown to be the most prevalent types in Asian hospitals [103].
C. difficile is thought to be mainly transmitted via hands of healthcare workers and by the contaminated environment [110]. Hand hygiene with alcoholic solutions is not associated with a higher risk of transmission despite the fact that alcohol does not have any antimicrobial effect against C. difficile [111]. Healthcare workers could be asymptomatic intestinal C. difficile carriers acting as a reservoir for cross-transmission in the hospital. However, in a non-outbreak setting, intestinal colonisation of healthcare workers occurs at similar frequency as among healthy adults [112–115]. The importance of nosocomial transmission of C. difficile has been questioned by a recent study from Oxfordshire, United Kingdom [116]. Using whole-genome sequencing, 45% of patients with C. difficile had genetically distinct strains compared to patients previously diagnosed with C. difficile. Noteworthy, even within a single patient, diverse subtypes were detected indicating different transmission events. These observations suggest that genetically diverse sources play a major role in C. difficile transmission [116].
Clinical impact of infection and colonisation with Clostridium difficile in ICU patients
C. difficile is found as a part of the normal intestinal flora in 1.0% [117] to 12.9% [114] of healthy individuals. Most studies analysing C. difficile colonisation in hospitalised patients have been performed on geriatric wards [118–121]. One study performed in ICU patients documented a colonisation rate of 34.6% [122].
Whether C. difficile colonisation is a risk for infection [123] or has a protective effect [124] is not clear yet. The published prevalence of C. difficile infection and corresponding mortality rate among ICU patients range from 0.5% [125] to 7.3% [126] and from 19.7% [127] to 36.7% [128], respectively (table 3). For ICU patients, C. difficile infection has not been associated with an increase in mortality [126, 128, 129]. The recurrence rates of C. difficile infection in ICU patients are highly variable as follow-up periods differ in most studies. Following treatment for C. difficile, recurrence rates in ICU patients can be as high as 12.7% [129].
Several risk factors for C. difficile infection and related mortality in ICU patients have been described (table 3). Antibiotic treatment is strongly associated with C. difficile infection (OR 6.67; 95% CI 1.76–25.31) [130] probably due to the fact that antibiotics interfere with intestinal colonisation resistance leading to overgrowth of C. difficile and toxin production, eventually causing infection [131]. Most classes of antibiotics have been associated with C. difficile infection in the hospital and community setting [132] with highest risks described for quinolones, cephalosporins, and clindamycin [133]. Other risk factors are older age, use of proton pump inhibitor and the presence of hypervirulent strains [130].
|
Table 3:Studies on Clostridium difficile: infection rate, recurrence rate, mortality, and risk factors for infection/death in adult patients (intensive care unit setting only). |
|
Study design and reference
|
Study year
|
Country
|
CDI rate, %
|
CDI recurrence rate, %
|
Mortality in CDI, %
|
Risk factors for CDI or CDI-associated mortality
|
| ROS [129] |
2013 |
United States |
6.6 |
12.7 |
25.1a
|
Old age, long hospital stay, medical patients, high APACHE II score, end-stage renal-disease, end-stage liver disease, hospital ward-to-ICU transfer, vasopressors, vancomycin enema |
| ROS [125] |
2011 |
United Kingdom |
0.5 |
0.0 |
25.9b
|
NA |
| ROS [126] |
2011 |
Unites States |
7.3 |
NA |
25.3a
|
Male sex, long hospital stay, high APACHE III score, prior room occupant with CDI |
| ROS [215] |
2008 |
United Kingdom |
1.5–4.8c
|
NA |
33.9a
|
Old age, male sex, high APACHE II score |
| ROS [128] |
2007 |
United States |
NA |
NA |
36.7d
|
Old age, medical patients, high APACHE II score, malignancy, low serum albumin, septic shock, ward-to-ICU-transfer, colonic thickening on CT |
| ROS [216] |
2007 |
United States |
NA |
NA |
27.6a
|
Old age, renal failure, hepatic failure, high SOFA score, organ failure, septic shock, respiratory failure |
| ROS [127] |
2007 |
United States |
4.1 |
2.6 |
19.7e
|
Long hospital stay, enteral tube feeding, mechanical ventilation, Pseudomonas aeruginosa bacteremia, VRE colonisation or infection, gastric acid suppressive therapy, C. difficile colonisation pressure, antimicrobial therapy |
| CDI = Clostridium difficile infection; ROS = retrospective observational study; APACHE = acute physiology and chronic health evaluation; ICU = intensive care unit; NA = not assessed; CT = computed tomography; SOFA=sequential organ failure assessment; VRE = vancomycin-resistant enterococci.
a in-hospital mortality rate; b 30–day in-hospital mortality rate; c 3 consecutive surveillance periods; d crude 30–day mortality rate; e mortality rate during ICU stay. |
Infection control strategies
Background
Generally, infection control on ICUs includes a bundle of prevention strategies. Wenzel and Edmond suggested to stratify such bundles in vertical and horizontal strategies (fig. 1), although considerable overlap between the two approaches exists [134, 135]. Vertical strategies include all pathogen-specific modules to reduce colonisation and/or infection (e.g., selective decolonisation in MRSA carriers). In contrast, horizontal interventions focus on minimising the spread of all pathogens between patients by using universal approaches (e.g., hand hygiene, chlorhexidine body washing) [134, 136].
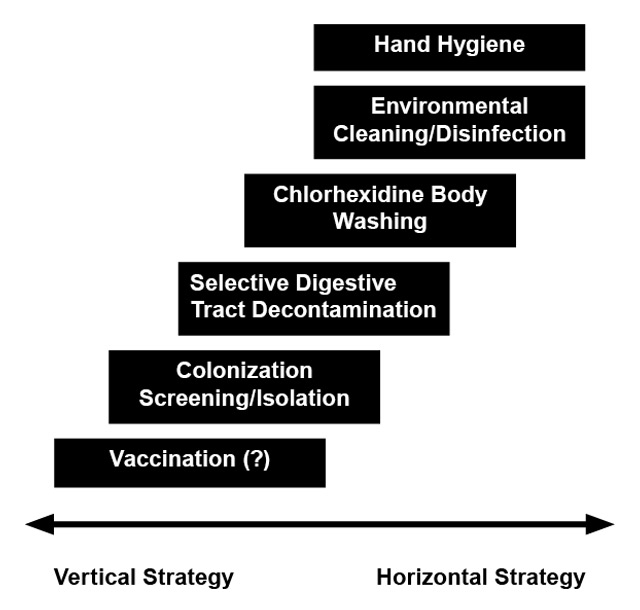
Figure 1
Infection control strategies in intensive care units: A continuum.
Infection control in intensive care units can involve vertical and horizontal strategies [134, 135]. While vertical interventions aim to reduce colonisation and infection with a certain pathogen, horizontal interventions try to minimise the spread of pathogens in general by using a universal approach [134, 135]. For prevention of bacterial infection in ICU patients, vaccines may be of interest in the future.
Hand hygiene as an essential part of infection control is highly effective in reducing all sorts of hospital-acquired infections [137] and therefore is recommended for the prevention of infections caused by VRE, ESBL-GNB and C. difficile[138–141].
A limitation of infection control studies is the fact that results from a study performed in a specific epidemiological context may not apply to other epidemiological settings. Furthermore, steadily evolving and changing antimicrobial resistance patterns make it difficult to draw long-term conclusions.
Colonisation screening and contact precautions (vertical strategy)
Screening for nosocomial pathogens in asymptomatic carriers aims to early identify colonised patients and to timely apply appropriate isolation precautions to prevent spread in the hospital. Interventions in patients with a positive screening result are e.g., contact isolation and decolonisation [142]. A challenge is selection of patients at risk – ranging from targeted screening of high-risk patients (e.g., haematopoietic stem cell recipients, ICU patients) to universal screening performed on every admitted patient [143].
The effect of screening on ICU admission on acquisition and infection rates is mainly documented for VRE/MRSA [144–151]. Few studies focused on ESBL-GNB or C. difficile [152–155]: Due to the increasing prevalence of VRE in the Unites States, the Centers for Disease Control and Prevention (CDC) [156], 1995, and the Society for Healthcare Epidemiology of America (SHEA) [139], 2003, recommended VRE screening on hospital admission with subsequent isolation of colonised patients [52, 157–162]. A recent randomised trial did not find an additional benefit of universal screening for MRSA/VRE on ICU admission and strict contact precautions compared to pre-existing practice (standard hand hygiene and use of gloves for contact with patient’s mucous membranes, wounds, and body fluids) [144]. The limitation of this trial was poor compliance with hand hygiene and wearing of gloves and gowns in intervention ICUs, as well as application of barrier precautions in only 35.0–50.7% of all ICU patient-days due to late reporting [163]. The findings of this study were confirmed by another recent cluster-randomised cross-over trial including 20 medical and surgical ICUs in the Unites States [145]. Universal care with gloves and gowns did not reduce the acquisition of MRSA or VRE compared to contact isolation of colonised patients (difference in acquisition density for MRSA or VRE –1.71 acquisitions per 1,000 person-days; 95% CI -6.15–2.73; p = 0.57) [145]. A European study on 13 ICUs analysed the effect of different vertical and horizontal infection control strategies on acquisition density of VRE, MRSA, and ESBL-GNB [146]. After a 6 month baseline surveillance period (phase 1) starting in May 2008, hand hygiene improvement programmes and chlorhexidine body washing were implemented at all ICUs (phase 2), followed by a cluster-randomised trial (phase 3, until April 2011) analysing the additional effect of admission colonisation screening for VRE, MRSA, and ESBL-GNB with subsequent contact isolation of carriers on acquisition incidence. Interventions in phase 2 significantly reduced MRSA acquisition, but had no impact on VRE and ESBL-GNB. Additional screening with subsequent contact isolation of carriers (phase 3) – whether performed by rapid testing (PCR) or conventional testing with chromogenic media – did not further reduce the acquisition incidence of antimicrobial-resistant bacteria (MRSA, VRE, and ESBL-GNB). However, it has to be taken into account that these results are only generalisable to settings with sustained high level of compliance to hand hygiene and chlorhexidine body washing. In a study from our centre we could demonstrate that with the use of strict contact precautions (i.e., hand hygiene, gloves, gowns, single room) for every VRE-colonised patient (no active surveillance), the incidence of VRE at our university hospital decreased to zero, after multiple cases in the mid 90’s [21].
Altogether, these studies show that screening with contact isolation of carriers might not be equally effective for different bacteria. The failure to reduce VRE and ESBL-GNB compared to MRSA may be partly explained by differences in colonisation characteristics [146]. In contrast to MRSA, VRE and ESBL-GNB mainly colonise the intestinal tract, which is not affected by chlorhexidine body washing [146]. Furthermore, the colonisation of the environment probably plays a much more important role in nosocomial enterococcal transmission than previously thought [30]. In addition, around 5% of healthcare workers are colonised with MRSA [164] and may also spread the pathogen adding to the difficulties to identify key factors for transmission.
Selective digestive tract decontamination (mainly vertical strategy)
Over the last years, the effect of selective digestive tract decontamination regimens on colonisation and infection rates of ICU patients has been studied using different non-absorbable antibiotics reducing intestinal carriage of mainly gram-negative bacteria (e.g., ESBL-GNB) but also S. aureus and yeasts, sparing the anaerobic flora [165].
In a large cluster-randomised trial from the Netherlands, selective digestive tract decontamination and selective oropharyngeal decontamination both significantly reduced incidence of ICU-acquired bacteremia and overall mortality [166]. A review article of 65 randomised-controlled trials and 11 meta-analyses showed a reduction in lower airway infections of 72%, bloodstream infection of 37% and overall mortality of 29% with the use of selective digestive tract decontamination regimens [165].
Only a few studies analysed the effect of selective digestive tract decontamination on VRE [167, 168], C. difficile[169, 170], and ESBL-GNB [171–176] colonisation or infection rate in critical ill patients. A recent randomised placebo-controlled trial showed a reduced rectal ESBL-GNB colonisation rate during an oral decontamination regimen with colistin sulfate and neomycin sulfate for 10 days, but no effect 3–5 weeks after treatment [171]. The temporary effect could be of interest for certain high-risk populations such as oncological or surgical patients during vulnerable treatment phases [171].
The use of selective digestive tract decontamination has raised the concern of selection pressure and increase in antimicrobial resistance [177]. In a recent meta-analysis, however, equal prevalence of colonisation and infection with MRSA (OR 1.46; 95% CI 0.90–2.37), VRE (OR 0.63; 95% CI 0.39–1.02), aminoglycoside- (OR 0.73; 95% CI 0.51–1.05) and polymyxin-resistant GNB (OR 0.58; 95% CI 0.46–0.72) was noted in ICU patients with or without selective digestive tract decontamination [177]. Ecological data from 38 Dutch ICUs showed reduction in resistance rates with the use of selective oropharyngeal and digestive tract decontamination for all antimicrobial agents included in the analysis (ciprofloxacin, ceftazidime, cefotaxime/ceftriaxone, tobramycin, colistin) [178]. The lack of documented resistance could be explained by reduced antibiotic treatment rates for hospital-acquired infections. An important limitation is the short follow-up of studies, possibly not allowing detection of long-term changes in resistance [166, 177–182].
Vaccination (vertical strategy)
Developing vaccines against nosocomial pathogens such as S. aureus and enterococci has been complicated, as the mechanisms leading to protective immunity are only partly understood [183]. A temporary effect has been shown for a S. aureus conjugate vaccine in dialysis patients [184]. Other vaccines are currently being investigated; the most recent S. aureusvaccine (V710) failed to prevent surgical site infections after cardiothoracic surgery [185]. To date, enterococcal vaccines have been solely evaluated in animal studies [186–188] and its clinical use needs to be determined. The importance of humoral immune response to C. difficile toxins A and B [189] lead to the development of vaccines as a promising strategy against C. difficile infection. Different vaccines, containing toxoid A and/or B, have been proven safe, immunogenic, and possibly effective in the prevention of C. difficile infection and recurrence [190–194].
Chlorhexidine body washing (horizontal strategy)
The effect of chlorhexidine body washing on bloodstream infection rates and on cross-transmission of multidrug-resistant bacteria has been demonstrated in a cluster-randomised trial in 9 ICUs in the United States showing a significant reduction in hospital-acquired bloodstream infections of 28% with daily chlorhexidine body washing [195]. Of note, the reduction was significant only for coagulase-negative staphylococci and not MRSA or VRE. In contrast, a recent meta-analysis showed significantly lowered MRSA/VRE colonisation and infection densities in patients treated with daily chlorhexidine body washing compared to patients without (incidence rate ratio 0.51; 95% CI 0.36–0.73 and 0.57; 95% CI 0.33–0.97; for VRE colonisation and VRE infection, respectively) [196]. So far, only a few studies have addressed the effect of chlorhexidine body washing on ESBL-GNB [146, 149] and C. difficile[197, 198] acquisition, not allowing the drawing of definite conclusions for these pathogens.
Antimicrobial stewardship
Antimicrobial stewardship programs encompass interventions promoting a responsible use of antimicrobial agents in order to improve patient outcome, enhance patient safety, reduce antimicrobial resistance and cut health-care costs [199]. A recently published Cochrane systematic review showed that hospital-wide antimicrobial stewardship is safe, reduces antimicrobial resistance and hospital-acquired infection incidence [200]. In a current meta-analysis, implementation of antimicrobial stewardship programmes on ICUs, reduced antibiotic use up to 55.4% and direct antibiotic costs by 4.6–72.3 US$ per patient-day [201]. More importantly, antimicrobial stewardship was associated with reductions in antimicrobial resistance and adverse events, without compromise of short-term clinical outcome [201].
The value of antibiotic cycling or mixing on prevention of multidrug-resistance is unknown [202]. A recent Spanish interventional study in ICU patients with ventilator-associated pneumonia indicated that mixing might prevent the emergence of antimicrobial resistance [203]. Nevertheless, currently, no definitive conclusions can be drawn on the value of antibiotic cycling/mixing [202–207].
Gaps in knowledge
The degree and full extent of health consequences following changes in the human microbiome have only recently been studied and are still little understood. Few studies could show a change in nosocomial infection rate after interventions targeting colonisation with VRE, ESBL-GNB and C. difficile. The rise of multidrug-resistant bacteria in the colonising flora of hospitalised patients but also healthy persons is one of the major challenges of future medicine. Internationally standardised, evidence-based and mandatory policies to control the emergence of multidrug-resistance are urgently needed and the ideal “bundle” of infection control strategies in ICU patients has yet to be defined.
Conclusion
The shift from a normal intestinal microbiome to a ‘selected’ gut flora dominated by antibiotic-resistant enterococci, ESBL-GNB and C. difficile in critically ill patients is a major risk factor for subsequent infection. The global rise of antimicrobial resistance, the increasing spread of bacteria and antimicrobial-resistance genes in the community and healthcare setting endanger patients at highest risk for nosocomial difficult-to-treat infections, especially in the ICU or on transplant units. Known infection control measures such as hand hygiene and antimicrobial stewardship urgently need to be implemented all over the world. New infection control measures need to be studied in order to halter further spread of resistant bacteria. The concurrent paucity of new antibiotics being developed stresses the importance of preventive measures even more. Especially horizontal infection control strategies could gain in importance as new multidrug-resistant pathogens constantly emerge.
Acknowledgement:We thank the Stiftung Forschung Infektionskrankheiten (SFI) for supporting JAS for this review.
References
1 Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323–9.
2 Fridkin SK, Welbel SF, Weinstein RA. Magnitude and prevention of nosocomial infections in the intensive care unit. Infect Dis Clin North Am. 1997;11(2):479–96.
3 Mertens K, Morales I, Catry B. Infections acquired in intensive care units: results of national surveillance in Belgium, 1997–2010. J Hosp Infect. 2013;84(2):120–5.
4 Taur Y, Pamer EG. The intestinal microbiota and susceptibility to infection in immunocompromised patients. Curr Opin Infect Dis. 2013;26(4):332–7.
5 Valles J, Ferrer R. Bloodstream infection in the ICU. Infect Dis Clin North Am. 2009;23(3):557–69.
6 Conway Morris A, Anderson N, Brittan M, Wilkinson TS, McAuley DF, Antonelli J, et al. Combined dysfunctions of immune cells predict nosocomial infection in critically ill patients. Br J Anaesth. 2013;111(5):778–87.
7 Doyle JS, Buising KL, Thursky KA, Worth LJ, Richards MJ. Epidemiology of infections acquired in intensive care units. Semin Respir Crit Care Med. 2011;32(2):115–38.
8 Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(1):1–12.
9 Murray BE. The life and times of the Enterococcus. Clin Microbiol Rev. 1990;3(1):46–65.
10 Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol. 2008;29(11):996–1011.
11 van Schaik W, Top J, Riley DR, Boekhorst J, Vrijenhoek JE, Schapendonk CM, et al. Pyrosequencing-based comparative genome analysis of the nosocomial pathogen Enterococcus faecium and identification of a large transferable pathogenicity island. BMC Genomics. 2010;11:239.
12 Paulsen IT, Banerjei L, Myers GS, Nelson KE, Seshadri R, Read TD, et al. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science. 2003;299(5615):2071–4.
13 Lebreton F, van Schaik W, McGuire AM, Godfrey P, Griggs A, Mazumdar V, et al. Emergence of epidemic multidrug-resistant Enterococcus faecium from animal and commensal strains. MBio. 2013;4(4).
14 Kristich CJ, Rice LB, Arias CA. Enterococcal Infection-Treatment and Antibiotic Resistance. In: Gilmore MS, Clewell DB, Ike Y, Shankar N. Enterococci: From Commensals to Leading Causes of Drug Resistant Infection. Boston, 2014.
15 Patterson JE, Sweeney AH, Simms M, Carley N, Mangi R, Sabetta J, et al. An analysis of 110 serious enterococcal infections. Epidemiology, antibiotic susceptibility, and outcome. Medicine. 1995;74(4):191–200.
16 Leclercq R, Derlot E, Duval J, Courvalin P. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N Engl J Med. 1988;319(3):157–61.
17 Zirakzadeh A, Patel R. Vancomycin-resistant enterococci: colonization, infection, detection, and treatment. Mayo Clin Proc. 2006;81(4):529–36.
18 Perichon B, Courvalin P. VanA-type vancomycin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2009;53(11):4580–7.
19 Molton JS, Tambyah PA, Ang BS, Ling ML, Fisher DA. The global spread of healthcare-associated multidrug-resistant bacteria: a perspective from Asia. Clin Infect Dis. 2013;56(9):1310–8.
20 van den Bogaard AE, Bruinsma N, Stobberingh EE. The effect of banning avoparcin on VRE carriage in The Netherlands. J Antimicrob Chemother. 2000;46(1):146–8.
21 Tschudin Sutter S, Frei R, Dangel M, Gratwohl A, Bonten M, Widmer AF. Not all patients with vancomycin-resistant enterococci need to be isolated. Clin Infect Dis. 2010;51(6):678–83.
22 Ecdc.europa.eu [homepage on the internet]. European Centre for Disease Prevention and Control [updated 2013 October 01; cited 2013 October 18]. Available from: http://www.ecdc.europa.eu .
23 Weisser M, Capaul S, Dangel M, Elzi L, Kuenzli E, Frei R, et al. Additive effect of Enterococcus faecium on Enterococcal bloodstream infections: a 14–year study in a Swiss Tertiary Hospital. Infect Control Hosp Epidemiol. 2013;34(10):1109–12.
24 Ruiz-Garbajosa P, de Regt M, Bonten M, Baquero F, Coque TM, Canton R, et al. High-density fecal Enterococcus faecium colonization in hospitalized patients is associated with the presence of the polyclonal subcluster CC17. Eur J Clin Microbiol Infect Dis. 2012;31(4):519–22.
25 Padiglione AA, Wolfe R, Grabsch EA, Olden D, Pearson S, Franklin C, et al. Risk factors for new detection of vancomycin-resistant enterococci in acute-care hospitals that employ strict infection control procedures. Antimicrob Agents Chemother. 2003;47(8):2492–8.
26 Gardiner D, Murphey S, Ossman E, Jungkind D. Prevalence and acquisition of vancomycin-resistant enterococci in a medical intensive care unit. Infect Control Hosp Epidemiol. 2002;23(8):466–8.
27 Bonten MJ, Hayden MK, Nathan C, van Voorhis J, Matushek M, Slaughter S, et al. Epidemiology of colonisation of patients and environment with vancomycin-resistant enterococci. Lancet. 1996;348(9042):1615–9.
28 Se YB, Chun HJ, Yi HJ, Kim DW, Ko Y, Oh SJ. Incidence and risk factors of infection caused by vancomycin-resistant enterococcus colonization in neurosurgical intensive care unit patients. J Korean Neurosurg Soc. 2009;46(2):123–9.
29 Furtado GH, Martins ST, Coutinho AP, Wey SB, Medeiros EA. Prevalence and factors associated with rectal vancomycin-resistant enterococci colonization in two intensive care units in Sao Paulo, Brazil. Braz J Infect Dis. 2005;9(1):64–9.
30 Martinez JA, Ruthazer R, Hansjosten K, Barefoot L, Snydman DR. Role of environmental contamination as a risk factor for acquisition of vancomycin-resistant enterococci in patients treated in a medical intensive care unit. Arch Intern Med. 2003;163(16):1905–12.
31 Warren DK, Kollef MH, Seiler SM, Fridkin SK, Fraser VJ. The epidemiology of vancomycin-resistant Enterococcus colonization in a medical intensive care unit. Infect Control Hosp Epidemiol. 2003;24(4):257–63.
32 Dan M, Poch F, Leibson L, Smetana S, Priel I. Rectal colonization with vancomycin-resistant enterococci among high-risk patients in an Israeli hospital. J Hosp Infect. 1999;43(3):231–8.
33 Ostrowsky BE, Venkataraman L, D'Agata EM, Gold HS, DeGirolami PC, Samore MH. Vancomycin-resistant enterococci in intensive care units: high frequency of stool carriage during a non-outbreak period. Arch Intern Med. 1999;159(13):1467–72.
34 Bhorade SM, Christenson J, Pohlman AS, Arnow PM, Hall JB. The incidence of and clinical variables associated with vancomycin-resistant enterococcal colonization in mechanically ventilated patients. Chest. 1999;115(4):1085–91.
35 Bonten MJ, Slaughter S, Hayden MK, Nathan C, van Voorhis J, Weinstein RA. External sources of vancomycin-resistant enterococci for intensive care units. Crit Care Med. 1998;26(12):2001–4.
36 Huang SS, Datta R, Rifas-Shiman S, Kleinman K, Placzek H, Lankiewicz JD, et al. Colonization with antibiotic-susceptible strains protects against methicillin-resistant Staphylococcus aureus but not vancomycin-resistant enterococci acquisition: a nested case-control study. Crit Care. 2011;15(5):R210.
37 Bonten MJ, Slaughter S, Ambergen AW, Hayden MK, van Voorhis J, Nathan C, et al. The role of “colonization pressure” in the spread of vancomycin-resistant enterococci: an important infection control variable. Arch Intern Med. 1998;158(10):1127–32.
38 Shorman M, Al-Tawfiq JA. Risk factors associated with vancomycin-resistant enterococcus in intensive care unit settings in saudi arabia. Interdiscip Perspect Infect Dis. 2013;2013:369674.
39 Pan SC, Wang JT, Chen YC, Chang YY, Chen ML, Chang SC. Incidence of and risk factors for infection or colonization of vancomycin-resistant enterococci in patients in the intensive care unit. PLoS One. 2012;7(10):e47297.
40 Batistao DW, Gontijo-Filho PP, Conceicao N, Oliveira AG, Ribas RM. Risk factors for vancomycin-resistant enterococci colonisation in critically ill patients. Mem Inst Oswaldo Cruz. 2012;107(1):57–63.
41 Kim YJ, Kim SI, Kim YR, Lee JY, Park YJ, Kang MW. Risk factors for vancomycin-resistant enterococci infection and mortality in colonized patients on intensive care unit admission. Am J Infect Control. 2012;40(10):1018–9.
42 Minhas P, Perl TM, Carroll KC, Shepard JW, Shangraw KA, Fellerman D, et al. Risk factors for positive admission surveillance cultures for methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci in a neurocritical care unit. Crit Care Med. 2011;39(10):2322–9.
43 Song JY, Cheong HJ, Jo YM, Choi WS, Noh JY, Heo JY, et al. Vancomycin-resistant Enterococcus colonization before admission to the intensive care unit: a clinico-epidemiologic analysis. Am J Infect Control. 2009;37(9):734–40.
44 Furuno JP, Perencevich EN, Johnson JA, Wright MO, McGregor JC, Morris JG, Jr., et al. Methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococci co-colonization. Emerg Infect Dis. 2005;11(10):1539–44.
45 Yoon YK, Kim HJ, Lee WJ, Lee SE, Yang KS, Park DW, et al. Clinical prediction rule for identifying patients with vancomycin-resistant enterococci (VRE) at the time of admission to the intensive care unit in a low VRE prevalence setting. J Antimicrob Chemother. 2012;67(12):2963–9.
46 Weisser M, Oostdijk EA, Willems RJ, Bonten MJ, Frei R, Elzi L, et al. Dynamics of ampicillin-resistant Enterococcus faecium clones colonizing hospitalized patients: data from a prospective observational study. BMC Infect Dis. 2012;12:68.
47 Hanberger H, Hoffmann M, Lindgren S, Nilsson LE. High incidence of antibiotic resistance among bacteria in 4 intensive care units at a university hospital in Sweden. Scand J Infect Dis. 1997;29(6):607–14.
48 Hanberger H, Nilsson LE. [Increased antibiotic resistance of intestinal bacteria]. Lakartidningen. 1996;93(3):148–51,54.
49 Weinstein JW, Roe M, Towns M, Sanders L, Thorpe JJ, Corey GR, et al. Resistant enterococci: a prospective study of prevalence, incidence, and factors associated with colonization in a university hospital. Infect Control Hosp Epidemiol. 1996;17(1):36–41.
50 Chirurgi VA, Oster SE, Goldberg AA, McCabe RE. Nosocomial acquisition of beta-lactamase – negative, ampicillin-resistant enterococcus. Arch Intern Med. 1992;152(7):1457–61.
51 Willems RJ, Top J, van Santen M, Robinson DA, Coque TM, Baquero F, et al. Global spread of vancomycin-resistant Enterococcus faecium from distinct nosocomial genetic complex. Emerg Infect Dis. 2005;11(6):821–8.
52 Boyce JM, Mermel LA, Zervos MJ, Rice LB, Potter-Bynoe G, Giorgio C, et al. Controlling vancomycin-resistant enterococci. Infect Control Hosp Epidemiol. 1995;16(11):634–7.
53 Morris JG, Jr., Shay DK, Hebden JN, McCarter RJ, Jr., Perdue BE, Jarvis W, et al. Enterococci resistant to multiple antimicrobial agents, including vancomycin. Establishment of endemicity in a university medical center. Ann Intern Med. 1995;123(4):250–9.
54 Drees M, Snydman DR, Schmid CH, Barefoot L, Hansjosten K, Vue PM, et al. Prior environmental contamination increases the risk of acquisition of vancomycin-resistant enterococci. Clin Infect Dis. 2008;46(5):678–85.
55 de Regt MJ, van der Wagen LE, Top J, Blok HE, Hopmans TE, Dekker AW, et al. High acquisition and environmental contamination rates of CC17 ampicillin-resistant Enterococcus faecium in a Dutch hospital. J Antimicrob Chemother. 2008;62(6):1401–6.
56 Patel R. Clinical impact of vancomycin-resistant enterococci. J Antimicrob Chemother. 2003;51(Suppl 3):iii13–21.
57 Hendrix CW, Hammond JM, Swoboda SM, Merz WG, Harrington SM, Perl TM, et al. Surveillance strategies and impact of vancomycin-resistant enterococcal colonization and infection in critically ill patients. Ann Surg. 2001;233(2):259–65.
58 Ziakas PD, Thapa R, Rice LB, Mylonakis E. Trends and Significance of VRE Colonization in the ICU: A Meta-Analysis of Published Studies. PLoS One. 2013;8(9):e75658.
59 Zirakzadeh A, Gastineau DA, Mandrekar JN, Burke JP, Johnston PB, Patel R. Vancomycin-resistant enterococcal colonization appears associated with increased mortality among allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2008;41(4):385–92.
60 Kraft S, Mackler E, Schlickman P, Welch K, DePestel DD. Outcomes of therapy: vancomycin-resistant enterococcal bacteremia in hematology and bone marrow transplant patients. Support Care Cancer. 2011;19(12):1969–74.
61 Yoo JH, Lee DG, Choi SM, Choi JH, Shin WS, Kim M, et al. Vancomycin-resistant enterococcal bacteremia in a hematology unit: molecular epidemiology and analysis of clinical course. J Korean Med Sci. 2005;20(2):169–76.
62 DiazGranados CA, Jernigan JA. Impact of vancomycin resistance on mortality among patients with neutropenia and enterococcal bloodstream infection. J Infect Dis. 2005;191(4):588–95.
63 Avery R, Kalaycio M, Pohlman B, Sobecks R, Kuczkowski E, Andresen S, et al. Early vancomycin-resistant enterococcus (VRE) bacteremia after allogeneic bone marrow transplantation is associated with a rapidly deteriorating clinical course. Bone Marrow Transplant. 2005;35(5):497–9.
64 Dubberke ER, Hollands JM, Georgantopoulos P, Augustin K, DiPersio JF, Mundy LM, et al. Vancomycin-resistant enterococcal bloodstream infections on a hematopoietic stem cell transplant unit: are the sick getting sicker? Bone Marrow Transplant. 2006;38(12):813–9.
65 Weinstock DM, Conlon M, Iovino C, Aubrey T, Gudiol C, Riedel E, et al. Colonization, bloodstream infection, and mortality caused by vancomycin-resistant enterococcus early after allogeneic hematopoietic stem cell transplant. Biol Blood Marrow Transplant. 2007;13(5):615–21.
66 Cheah AL, Spelman T, Liew D, Peel T, Howden BP, Spelman D, et al. Enterococcal bacteraemia: factors influencing mortality, length of stay and costs of hospitalization. Clin Microbiol Infect. 2013;19(4):E181–9.
67 DiazGranados CA, Zimmer SM, Klein M, Jernigan JA. Comparison of mortality associated with vancomycin-resistant and vancomycin-susceptible enterococcal bloodstream infections: a meta-analysis. Clin Infect Dis. 2005;41(3):327–33.
68 Gazin M, Paasch F, Goossens H, Malhotra-Kumar S, Mosar WP, Teams SWS. Current trends in culture-based and molecular detection of extended-spectrum-beta-lactamase-harboring and carbapenem-resistant Enterobacteriaceae. J Clin Microbiol. 2012;50(4):1140–6.
69 Bradford PA. Extended-spectrum beta-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev. 2001;14(4):933–51.
70 Pitout JD, Laupland KB. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008;8(3):159–66.
71 Falagas ME, Karageorgopoulos DE. Extended-spectrum beta-lactamase-producing organisms. J Hosp Infect. 2009;73(4):345–54.
72 Canton R, Coque TM. The CTX-M beta-lactamase pandemic. Curr Opin Microbiol. 2006;9(5):466–75.
73 Hawkey PM, Jones AM. The changing epidemiology of resistance. J Antimicrob Chemother. 2009;64(Suppl 1):i3–10.
74 Livermore DM, Canton R, Gniadkowski M, Nordmann P, Rossolini GM, Arlet G, et al. CTX-M: changing the face of ESBLs in Europe. J Antimicrob Chemother. 2007;59(2):165–74.
75 Reinert RR, Low DE, Rossi F, Zhang X, Wattal C, Dowzicky MJ. Antimicrobial susceptibility among organisms from the Asia/Pacific Rim, Europe and Latin and North America collected as part of TEST and the in vitro activity of tigecycline. J Antimicrob Chemother. 2007;60(5):1018–29.
76 Paterson DL. Resistance in gram-negative bacteria: enterobacteriaceae. Am J Med. 2006;119(6 Suppl 1):S20–8;discussion S62–70.
77 Winokur PL, Canton R, Casellas JM, Legakis N. Variations in the prevalence of strains expressing an extended-spectrum beta-lactamase phenotype and characterization of isolates from Europe, the Americas, and the Western Pacific region. Clin Infect Dis. 2001;32(Suppl 2):S94–103.
78 Tschudin-Sutter S, Frei R, Dangel M, Stranden A, Widmer AF. Sites of colonization with extended-spectrum beta-lactamases (ESBL)-producing enterobacteriaceae: the rationale for screening. Infect Control Hosp Epidemiol. 2012;33(11):1170–1.
79 Harris AD, McGregor JC, Johnson JA, Strauss SM, Moore AC, Standiford HC, et al. Risk factors for colonization with extended-spectrum beta-lactamase-producing bacteria and intensive care unit admission. Emerg Infect Dis. 2007;13(8):1144–9.
80 Azim A, Dwivedi M, Rao PB, Baronia AK, Singh RK, Prasad KN, et al. Epidemiology of bacterial colonization at intensive care unit admission with emphasis on extended-spectrum beta-lactamase- and metallo-beta-lactamase-producing Gram-negative bacteria – an Indian experience. J Med Microbiol. 2010;59(8):955–60.
81 Ko YJ, Moon HW, Hur M, Park CM, Cho SE, Yun YM. Fecal carriage of extended-spectrum beta-lactamase-producing Enterobacteriaceae in Korean community and hospital settings. Infection. 2013;41(1):9–13.
82 Pena C, Pujol M, Ricart A, Ardanuy C, Ayats J, Linares J, et al. Risk factors for faecal carriage of Klebsiella pneumoniae producing extended spectrum beta-lactamase (ESBL-KP) in the intensive care unit. J Hosp Infect. 1997;35(1):9–16.
83 Harris AD, Kotetishvili M, Shurland S, Johnson JA, Morris JG, Nemoy LL, et al. How important is patient-to-patient transmission in extended-spectrum beta-lactamase Escherichia coli acquisition. Am J Infect Control. 2007;35(2):97–101.
84 Harris AD, Nemoy L, Johnson JA, Martin-Carnahan A, Smith DL, Standiford H, et al. Co-carriage rates of vancomycin-resistant Enterococcus and extended-spectrum beta-lactamase-producing bacteria among a cohort of intensive care unit patients: implications for an active surveillance program. Infect Control Hosp Epidemiol. 2004;25(2):105–8.
85 Christiaens G, Ciccarella Y, Damas P, Hayette MP, Melin P, Nys M, et al. Prospective survey of digestive tract colonization with enterobacteriaceae that produce extended-spectrum beta-lactamases in intensive care units. J Hosp Infect. 2006;62(3):386–8.
86 Ajao AO, Johnson JK, Harris AD, Zhan M, McGregor JC, Thom KA, et al. Risk of acquiring extended-spectrum beta-lactamase-producing Klebsiella species and Escherichia coli from prior room occupants in the intensive care unit. Infect Control Hosp Epidemiol. 2013;34(5):453–8.
87 Razazi K, Derde LP, Verachten M, Legrand P, Lesprit P, Brun-Buisson C. Clinical impact and risk factors for colonization with extended-spectrum beta-lactamase-producing bacteria in the intensive care unit. Intensive Care Med. 2012;38(11):1769–78.
88 Thiebaut AC, Arlet G, Andremont A, Papy E, Sollet JP, Bernede-Bauduin C, et al. Variability of intestinal colonization with third-generation cephalosporin-resistant Enterobacteriaceae and antibiotic use in intensive care units. J Antimicrob Chemother. 2012;67(6):1525–36.
89 Liss BJ, Vehreschild JJ, Cornely OA, Hallek M, Fatkenheuer G, Wisplinghoff H, et al. Intestinal colonisation and blood stream infections due to vancomycin-resistant enterococci (VRE) and extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBLE) in patients with haematological and oncological malignancies. Infection. 2012;40(6):613–9.
90 Reddy P, Malczynski M, Obias A, Reiner S, Jin N, Huang J, et al. Screening for extended-spectrum beta-lactamase-producing Enterobacteriaceae among high-risk patients and rates of subsequent bacteremia. Clin Infect Dis. 2007;45(7):846–52.
91 Arnan M, Gudiol C, Calatayud L, Linares J, Dominguez MA, Batlle M, et al. Risk factors for, and clinical relevance of, faecal extended-spectrum beta-lactamase producing Escherichia coli (ESBL-EC) carriage in neutropenic patients with haematological malignancies. Eur J Clin Microbiol Infect Dis. 2011;30(3):355–60.
92 Endimiani A, Luzzaro F, Brigante G, Perilli M, Lombardi G, Amicosante G, et al. Proteus mirabilis bloodstream infections: risk factors and treatment outcome related to the expression of extended-spectrum beta-lactamases. Antimicrob Agents Chemother. 2005;49(7):2598–605.
93 Henshke-Bar-Meir R, Yinnon AM, Rudensky B, Attias D, Schlesinger Y, Raveh D. Assessment of the clinical significance of production of extended-spectrum beta-lactamases (ESBL) by Enterobacteriaceae. Infection. 2006;34(2):66–74.
94 Ho PL, Chan WM, Tsang KW, Wong SS, Young K. Bacteremia caused by Escherichia coli producing extended-spectrum beta-lactamase: a case-control study of risk factors and outcomes. Scand J Infect Dis. 2002;34(8):567–73.
95 Schwaber MJ, Navon-Venezia S, Kaye KS, Ben-Ami R, Schwartz D, Carmeli Y. Clinical and economic impact of bacteremia with extended- spectrum-beta-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother. 2006;50(4):1257–62.
96 Tumbarello M, Spanu T, Sanguinetti M, Citton R, Montuori E, Leone F, et al. Bloodstream infections caused by extended-spectrum-beta-lactamase-producing Klebsiella pneumoniae: risk factors, molecular epidemiology, and clinical outcome. Antimicrob Agents Chemother. 2006;50(2):498–504.
97 Lautenbach E, Patel JB, Bilker WB, Edelstein PH, Fishman NO. Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for infection and impact of resistance on outcomes. Clin Infect Dis. 2001;32(8):1162–71.
98 Ben-Ami R, Schwaber MJ, Navon-Venezia S, Schwartz D, Giladi M, Chmelnitsky I, et al. Influx of extended-spectrum beta-lactamase-producing enterobacteriaceae into the hospital. Clin Infect Dis. 2006;42(7):925–34.
99 Rottier WC, Ammerlaan HS, Bonten MJ. Effects of confounders and intermediates on the association of bacteraemia caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae and patient outcome: a meta-analysis. J Antimicrob Chemother. 2012;67(6):1311–20.
100 Bouza E, Munoz P, Alonso R. Clinical manifestations, treatment and control of infections caused by Clostridium difficile. Clin Microbiol Infect. 2005;11(Suppl 4):57–64.
101 Bobo LD, Dubberke ER, Kollef M. Clostridium difficile in the ICU: the struggle continues. Chest. 2011;140(6):1643–53.
102 Kuijper EJ, Coignard B, Tull P. Emergence of Clostridium difficile-associated disease in North America and Europe. Clin Microbiol Infect. 2006;12(Suppl 6):2–18.
103 Collins DA, Hawkey PM, Riley TV. Epidemiology of Clostridium difficile infection in Asia. Antimicrob Resist Infect Control. 2013;2(1):21.
104 Warny M, Pepin J, Fang A, Killgore G, Thompson A, Brazier J, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366(9491):1079–84.
105 McDonald LC, Killgore GE, Thompson A, Owens RC, Jr., Kazakova SV, Sambol SP, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353(23):2433–41.
106 Loo VG, Poirier L, Miller MA, Oughton M, Libman MD, Michaud S, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353(23):2442–9.
107 Smith A. Outbreak of Clostridium difficile infection in an English hospital linked to hypertoxin-producing strains in Canada and the US. Euro Surveill. 2005;10(6):E050630 2.
108 Kuijper EJ, van den Berg RJ, Debast S, Visser CE, Veenendaal D, Troelstra A, et al. Clostridium difficile ribotype 027, toxinotype III, the Netherlands. Emerg Infect Dis. 2006;12(5):827–30.
109 Kuijper EJ, Barbut F, Brazier JS, Kleinkauf N, Eckmanns T, Lambert ML, et al. Update of Clostridium difficile infection due to PCR ribotype 027 in Europe, 2008. Euro Surveill. 2008;13(31).
110 Gerding DN, Muto CA, Owens RC, Jr. Measures to control and prevent Clostridium difficile infection. Clin Infect Dis. 2008;46(Suppl 1):S43–9.
111 Boyce JM, Ligi C, Kohan C, Dumigan D, Havill NL. Lack of association between the increased incidence of Clostridium difficile-associated disease and the increasing use of alcohol-based hand rubs. Infect Control Hosp Epidemiol. 2006;27(5):479–83.
112 Friedman ND, Pollard J, Stupart D, Knight DR, Khajehnoori M, Davey EK, et al. Prevalence of Clostridium difficile colonization among healthcare workers. BMC Infect Dis. 2013;13(1):459.
113 Viscidi R, Willey S, Bartlett JG. Isolation rates and toxigenic potential of Clostridium difficile isolates from various patient populations. Gastroenterology. 1981;81(1):5–9.
114 Ozaki E, Kato H, Kita H, Karasawa T, Maegawa T, Koino Y, et al. Clostridium difficile colonization in healthy adults: transient colonization and correlation with enterococcal colonization. J Med Microbiol. 2004;53(Pt 2):167–72.
115 Kato H, Kita H, Karasawa T, Maegawa T, Koino Y, Takakuwa H, et al. Colonisation and transmission of Clostridium difficile in healthy individuals examined by PCR ribotyping and pulsed-field gel electrophoresis. J Med Microbiol. 2001;50(8):720–7.
116 Eyre DW, Cule ML, Wilson DJ, Griffiths D, Vaughan A, O’Connor L, et al. Diverse sources of C. difficile infection identified on whole-genome sequencing. N Engl J Med. 2013;369(13):1195–205.
117 Clayton EM, Rea MC, Shanahan F, Quigley EM, Kiely B, Hill C, et al. The vexed relationship between Clostridium difficile and inflammatory bowel disease: an assessment of carriage in an outpatient setting among patients in remission. Am J Gastroenterol. 2009;104(5):1162–9.
118 McCoubrey J, Starr J, Martin H, Poxton IR. Clostridium difficile in a geriatric unit: a prospective epidemiological study employing a novel S-layer typing method. J Med Microbiol. 2003;52(Pt 7):573–8.
119 Kyne L, Warny M, Qamar A, Kelly CP. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med. 2000;342(6):390–7.
120 Rudensky B, Rosner S, Sonnenblick M, van Dijk Y, Shapira E, Isaacsohn M. The prevalence and nosocomial acquisition of Clostridium difficile in elderly hospitalized patients. Postgrad Med J. 1993;69(807):45–7.
121 Campbell RR, Beere D, Wilcock GK, Brown EM. Clostridium difficile in acute and long-stay elderly patients. Age Ageing. 1988;17(5):333–6.
122 Cheng SH, Lu JJ, Young TG, Perng CL, Chi WM. Clostridium difficile – associated diseases: comparison of symptomatic infection versus carriage on the basis of risk factors, toxin production, and genotyping results. Clin Infect Dis. 1997;25(1):157–8.
123 Hung YP, Tsai PJ, Hung KH, Liu HC, Lee CI, Lin HJ, et al. Impact of toxigenic Clostridium difficile colonization and infection among hospitalized adults at a district hospital in southern Taiwan. PLoS One. 2012;7(8):e42415.
124 Shim JK, Johnson S, Samore MH, Bliss DZ, Gerding DN. Primary symptomless colonisation by Clostridium difficile and decreased risk of subsequent diarrhoea. Lancet. 1998;351(9103):633–6.
125 Musa SA, Moran C, Thomson SJ, Cowan ML, McAnulty G, Grounds M, et al. Clostridium difficile-associated disease acquired in the cardiothoracic intensive care unit. J Cardiothorac Vasc Anesth. 2011;25(2):263–7.
126 Shaughnessy MK, Micielli RL, DePestel DD, Arndt J, Strachan CL, Welch KB, et al. Evaluation of hospital room assignment and acquisition of Clostridium difficile infection. Infect Control Hosp Epidemiol. 2011;32(3):201–6.
127 Lawrence SJ, Puzniak LA, Shadel BN, Gillespie KN, Kollef MH, Mundy LM. Clostridium difficile in the intensive care unit: epidemiology, costs, and colonization pressure. Infect Control Hosp Epidemiol. 2007;28(2):123–30.
128 Kenneally C, Rosini JM, Skrupky LP, Doherty JA, Hollands JM, Martinez E, et al. Analysis of 30–day mortality for clostridium difficile-associated disease in the ICU setting. Chest. 2007;132(2):418–24.
129 Micek ST, Schramm G, Morrow L, Frazee E, Personett H, Doherty JA, et al. Clostridium difficile infection: a multicenter study of epidemiology and outcomes in mechanically ventilated patients. Crit Care Med. 2013;41(8):1968–75.
130 Loo VG, Bourgault AM, Poirier L, Lamothe F, Michaud S, Turgeon N, et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011;365(18):1693–703.
131 Britton RA, Young VB. Role of the Intestinal Microbiota in Resistance to Colonization by Clostridium difficile. Gastroenterology. 2014;146(6):1547–53.
132 Stevens V, Dumyati G, Fine LS, Fisher SG, van Wijngaarden E. Cumulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clin Infect Dis. 2011;53(1):42–8.
133 Howell MD, Novack V, Grgurich P, Soulliard D, Novack L, Pencina M, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170(9):784–90.
134 Wenzel RP. Minimizing surgical-site infections. N Engl J Med. 2010;362(1):75–7.
135 Wenzel RP, Edmond MB. Infection control: the case for horizontal rather than vertical interventional programs. Int J Infect Dis. 2010;14(Suppl 4):S3–5.
136 Edmond MB, Wenzel RP. Screening inpatients for MRSA–case closed. N Engl J Med. 2013;368(24):2314–5.
137 Tschudin-Sutter S, Pargger H, Widmer AF. Hand hygiene in the intensive care unit. Crit Care Med. 2010;38(8 Suppl):S299–305.
138 Vonberg RP, Kuijper EJ, Wilcox MH, Barbut F, Tull P, Gastmeier P, et al. Infection control measures to limit the spread of Clostridium difficile. Clin Microbiol Infect. 2008;14(Suppl 5):2–20.
139 Muto CA, Jernigan JA, Ostrowsky BE, Richet HM, Jarvis WR, Boyce JM, et al. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and enterococcus. Infect Control Hosp Epidemiol. 2003;24(5):362–86.
140 Siegel JD, Rhinehart E, Jackson M, Chiarello L, Healthcare Infection Control Practices Advisory C. Management of multidrug-resistant organisms in health care settings, 2006. Am J Infect Control. 2007;35(10 Suppl 2):S165–93.
141 Tacconelli E, Cataldo MA, Dancer SJ, De Angelis G, Falcone M, Frank U, et al. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin Microbiol Infect. 2014;20(Suppl 1):1–55.
142 Calfee DP. Methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci, and other Gram-positives in healthcare. Curr Opin Infect Dis. 2012;25(4):385–94.
143 Struelens MJ, Hawkey PM, French GL, Witte W, Tacconelli E. Laboratory tools and strategies for methicillin-resistant Staphylococcus aureus screening, surveillance and typing: state of the art and unmet needs. Clin Microbiol Infect. 2009;15(2):112–9.
144 Huskins WC, Huckabee CM, O’Grady NP, Murray P, Kopetskie H, Zimmer L, et al. Intervention to reduce transmission of resistant bacteria in intensive care. N Engl J Med. 2011;364(15):1407–18.
145 Harris AD, Pineles L, Belton B, Johnson JK, Shardell M, Loeb M, et al. Universal glove and gown use and acquisition of antibiotic-resistant bacteria in the ICU: a randomized trial. JAMA. 2013;310(15):1571–80.
146 Derde LP, Cooper BS, Goossens H, Malhotra-Kumar S, Willems RJ, Gniadkowski M, et al. Interventions to reduce colonisation and transmission of antimicrobial-resistant bacteria in intensive care units: an interrupted time series study and cluster randomised trial. Lancet Infect Dis. 2014;14(1):31–9.
147 Kjonegaard R, Fields W, Peddecord KM. Universal rapid screening for methicillin-resistant Staphylococcus aureus in the intensive care units in a large community hospital. Am J Infect Control. 2013;41(1):45–50.
148 Camus C, Bellissant E, Legras A, Renault A, Gacouin A, Lavoue S, et al. Randomized comparison of 2 protocols to prevent acquisition of methicillin-resistant Staphylococcus aureus: results of a 2–center study involving 500 patients. Infect Control Hosp Epidemiol. 2011;32(11):1064–72.
149 Gould IM, MacKenzie FM, MacLennan G, Pacitti D, Watson EJ, Noble DW. Topical antimicrobials in combination with admission screening and barrier precautions to control endemic methicillin-resistant Staphylococcus aureus in an Intensive Care Unit. Int J Antimicrob Agents. 2007;29(5):536–43.
150 Clancy M, Graepler A, Wilson M, Douglas I, Johnson J, Price CS. Active screening in high-risk units is an effective and cost-avoidant method to reduce the rate of methicillin-resistant Staphylococcus aureus infection in the hospital. Infect Control Hosp Epidemiol. 2006;27(10):1009–17.
151 Lucet JC, Paoletti X, Lolom I, Paugam-Burtz C, Trouillet JL, Timsit JF, et al. Successful long-term program for controlling methicillin-resistant Staphylococcus aureus in intensive care units. Intensive Care Med. 2005;31(8):1051–7.
152 Goddard S, Muller MP. The efficacy of infection control interventions in reducing the incidence of extended-spectrum beta-lactamase-producing Enterobacteriaceae in the nonoutbreak setting: A systematic review. Am J Infect Control. 2011;39(7):599–601.
153 Prospero E, Barbadoro P, Esposto E, Manso E, Martini E, Savini S, et al. Extended-spectrum beta-lactamases Klebsiella pneumoniae: multimodal infection control program in intensive care units. J Prev Med Hyg. 2010;51(3):110–5.
154 Tschudin-Sutter S, Frei R, Dangel M, Stranden A, Widmer AF. Rate of transmission of extended-spectrum beta-lactamase-producing enterobacteriaceae without contact isolation. Clin Infect Dis. 2012;55(11):1505–11.
155 Laurent C, Rodriguez-Villalobos H, Rost F, Strale H, Vincent JL, Deplano A, et al. Intensive care unit outbreak of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae controlled by cohorting patients and reinforcing infection control measures. Infect Control Hosp Epidemiol. 2008;29(6):517–24.
156 Hospital Infection Control Practices Advisory C. Recommendations for preventing the spread of vancomycin resistance. Infect Control Hosp Epidemiol. 1995;16(2):105–13.
157 Boyce JM, Opal SM, Chow JW, Zervos MJ, Potter-Bynoe G, Sherman CB, et al. Outbreak of multidrug-resistant Enterococcus faecium with transferable vanB class vancomycin resistance. J Clin Microbiol. 1994;32(5):1148–53.
158 Byers KE, Anglim AM, Anneski CJ, Germanson TP, Gold HS, Durbin LJ, et al. A hospital epidemic of vancomycin-resistant Enterococcus: risk factors and control. Infect Control Hosp Epidemiol. 2001;22(3):140–7.
159 Karanfil LV, Murphy M, Josephson A, Gaynes R, Mandel L, Hill BC, et al. A cluster of vancomycin-resistant Enterococcus faecium in an intensive care unit. Infect Control Hosp Epidemiol. 1992;13(4):195–200.
160 Dembry LM, Uzokwe K, Zervos MJ. Control of endemic glycopeptide-resistant enterococci. Infect Control Hosp Epidemiol. 1996;17(5):286–92.
161 Jochimsen EM, Fish L, Manning K, Young S, Singer DA, Baker R, et al. Control of vancomycin-resistant enterococci at a community hospital: efficacy of patient and staff cohorting. Infect Control Hosp Epidemiol. 1999;20(2):106–9.
162 Armstrong-Evans M, Litt M, McArthur MA, Willey B, Cann D, Liska S, et al. Control of transmission of vancomycin-resistant Enterococcus faecium in a long-term-care facility. Infect Control Hosp Epidemiol. 1999;20(5):312–7.
163 Widmer AF. Transmission of resistant bacteria in intensive care. N Engl J Med. 2011;365(8):763;author reply 4–5.
164 Albrich WC, Harbarth S. Health-care workers: source, vector, or victim of MRSA? Lancet Infect Dis. 2008;8(5):289–301.
165 Silvestri L, de la Cal MA, van Saene HK. Selective decontamination of the digestive tract: the mechanism of action is control of gut overgrowth. Intensive Care Med. 2012;38(11):1738–50.
166 de Smet AM, Kluytmans JA, Cooper BS, Mascini EM, Benus RF, van der Werf TS, et al. Decontamination of the digestive tract and oropharynx in ICU patients. N Engl J Med. 2009;360(1):20–31.
167 Arnow PM, Carandang GC, Zabner R, Irwin ME. Randomized controlled trial of selective bowel decontamination for prevention of infections following liver transplantation. Clin Infect Dis. 1996;22(6):997–1003.
168 Hellinger WC, Yao JD, Alvarez S, Blair JE, Cawley JJ, Paya CV, et al. A randomized, prospective, double-blinded evaluation of selective bowel decontamination in liver transplantation. Transplantation. 2002;73(12):1904–9.
169 Delmee M, Vandercam B, Avesani V, Michaux JL. Epidemiology and prevention of Clostridium difficile infections in a leukemia unit. Eur J Clin Microbiol. 1987;6(6):623–7.
170 Johnson S, Homann SR, Bettin KM, Quick JN, Clabots CR, Peterson LR, et al. Treatment of asymptomatic Clostridium difficile carriers (fecal excretors) with vancomycin or metronidazole. A randomized, placebo-controlled trial. Ann Intern Med. 1992;117(4):297–302.
171 Huttner B, Haustein T, Uckay I, Renzi G, Stewardson A, Schaerrer D, et al. Decolonization of intestinal carriage of extended-spectrum beta-lactamase-producing Enterobacteriaceae with oral colistin and neomycin: a randomized, double-blind, placebo-controlled trial. J Antimicrob Chemother. 2013;68(10):2375–82.
172 Brun-Buisson C, Legrand P, Rauss A, Richard C, Montravers F, Besbes M, et al. Intestinal decontamination for control of nosocomial multiresistant gram-negative bacilli. Study of an outbreak in an intensive care unit. Ann Intern Med. 1989;110(11):873–81.
173 Taylor ME, Oppenheim BA. Selective decontamination of the gastrointestinal tract as an infection control measure. J Hosp Infect. 1991;17(4):271–8.
174 Paterson DL, Singh N, Rihs JD, Squier C, Rihs BL, Muder RR. Control of an outbreak of infection due to extended-spectrum beta-lactamase – producing Escherichia coli in a liver transplantation unit. Clin Infect Dis. 2001;33(1):126–8.
175 Troche G, Joly LM, Guibert M, Zazzo JF. Detection and treatment of antibiotic-resistant bacterial carriage in a surgical intensive care unit: a 6–year prospective survey. Infect Control Hosp Epidemiol. 2005;26(2):161–5.
176 Buehlmann M, Bruderer T, Frei R, Widmer AF. Effectiveness of a new decolonisation regimen for eradication of extended-spectrum beta-lactamase-producing Enterobacteriaceae. J Hosp Infect. 2011;77(2):113–7.
177 Daneman N, Sarwar S, Fowler RA, Cuthbertson BH; SuDDICU Canadian Study Group. Effect of selective decontamination on antimicrobial resistance in intensive care units: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13(4):328–41.
178 Houben AJ, Oostdijk EA, van der Voort PH, Monen JC, Bonten MJ, van der Bij AK, et al. Selective decontamination of the oropharynx and the digestive tract, and antimicrobial resistance: a 4 year ecological study in 38 intensive care units in the Netherlands. J Antimicrob Chemother. 2014;69(3):797–804.
179 Oostdijk EA, de Smet AM, Kesecioglu J, Bonten MJ; Dutch SOD-SDD Trialists Group. The role of intestinal colonization with gram-negative bacteria as a source for intensive care unit-acquired bacteremia. Crit Care Med. 2011;39(5):961–6.
180 Silvestri L, van Saene HK, Casarin A, Berlot G, Gullo A. Impact of selective decontamination of the digestive tract on carriage and infection due to Gram-negative and Gram-positive bacteria: a systematic review of randomised controlled trials. Anaesth Intensive Care. 2008;36(3):324–38.
181 Silvestri L, van Saene HK, Milanese M, Gregori D, Gullo A. Selective decontamination of the digestive tract reduces bacterial bloodstream infection and mortality in critically ill patients. Systematic review of randomized, controlled trials. J Hosp Infect. 2007;65(3):187–203.
182 Oudhuis GJ, Bergmans DC, Dormans T, Zwaveling JH, Kessels A, Prins MH, et al. Probiotics versus antibiotic decontamination of the digestive tract: infection and mortality. Intensive Care Med. 2011;37(1):110–7.
183 Proctor RA. Is there a future for a Staphylococcus aureus vaccine? Vaccine. 2012;30(19):2921–7.
184 Shinefield H, Black S, Fattom A, Horwith G, Rasgon S, Ordonez J, et al. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med. 2002;346(7):491–6.
185 Fowler VG, Allen KB, Moreira ED, Moustafa M, Isgro F, Boucher HW, et al. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA. 2013;309(13):1368–78.
186 Burnie J, Carter T, Rigg G, Hodgetts S, Donohoe M, Matthews R. Identification of ABC transporters in vancomycin-resistant Enterococcus faecium as potential targets for antibody therapy. FEMS Immunol Med Microbiol. 2002;33(3):179–89.
187 Huebner J, Quaas A, Krueger WA, Goldmann DA, Pier GB. Prophylactic and therapeutic efficacy of antibodies to a capsular polysaccharide shared among vancomycin-sensitive and -resistant enterococci. Infect Immun. 2000;68(8):4631–6.
188 McCormick JK, Hirt H, Waters CM, Tripp TJ, Dunny GM, Schlievert PM. Antibodies to a surface-exposed, N-terminal domain of aggregation substance are not protective in the rabbit model of Enterococcus faecalis infective endocarditis. Infect Immun. 2001;69(5):3305–14.
189 Wullt M, Noren T, Ljungh A, Akerlund T. IgG antibody response to toxins A and B in patients with Clostridium difficile infection. Clin Vaccine Immunol. 2012;19(9):1552–4.
190 Greenberg RN, Marbury TC, Foglia G, Warny M. Phase I dose finding studies of an adjuvanted Clostridium difficile toxoid vaccine. Vaccine. 2012;30(13):2245–9.
191 Leav BA, Blair B, Leney M, Knauber M, Reilly C, Lowy I, et al. Serum anti-toxin B antibody correlates with protection from recurrent Clostridium difficile infection (CDI). Vaccine. 2010;28(4):965–9.
192 Taylor CP, Tummala S, Molrine D, Davidson L, Farrell RJ, Lembo A, et al. Open-label, dose escalation phase I study in healthy volunteers to evaluate the safety and pharmacokinetics of a human monoclonal antibody to Clostridium difficile toxin A. Vaccine. 2008;26(27–28):3404–9.
193 Sougioultzis S, Kyne L, Drudy D, Keates S, Maroo S, Pothoulakis C, et al. Clostridium difficile toxoid vaccine in recurrent C. difficile-associated diarrhea. Gastroenterology. 2005;128(3):764–70.
194 Kotloff KL, Wasserman SS, Losonsky GA, Thomas W, Jr., Nichols R, Edelman R, et al. Safety and immunogenicity of increasing doses of a Clostridium difficile toxoid vaccine administered to healthy adults. Infect Immun. 2001;69(2):988–95.
195 Climo MW, Yokoe DS, Warren DK, Perl TM, Bolon M, Herwaldt LA, et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med. 2013;368(6):533–42.
196 Chen W, Li S, Li L, Wu X, Zhang W. Effects of daily bathing with chlorhexidine and acquired infection of methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus: a meta-analysis. J Thorac Dis. 2013;5(4):518–24.
197 Rupp ME, Cavalieri RJ, Lyden E, Kucera J, Martin M, Fitzgerald T, et al. Effect of hospital-wide chlorhexidine patient bathing on healthcare-associated infections. Infect Control Hosp Epidemiol. 2012;33(11):1094–100.
198 Popovich KJ, Hota B, Hayes R, Weinstein RA, Hayden MK. Daily skin cleansing with chlorhexidine did not reduce the rate of central-line associated bloodstream infection in a surgical intensive care unit. Intensive Care Med. 2010;36(5):854–8.
199 McGowan JE. Antimicrobial stewardship – the state of the art in 2011: focus on outcome and methods. Infect Control Hosp Epidemiol. 2012;33(4):331–7.
200 Davey P, Brown E, Charani E, Fenelon L, Gould IM, Holmes A, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2013;4:CD003543.
201 Kaki R, Elligsen M, Walker S, Simor A, Palmay L, Daneman N. Impact of antimicrobial stewardship in critical care: a systematic review. J Antimicrob Chemother. 2011;66(6):1223–30.
202 Bal AM, Kumar A, Gould IM. Antibiotic heterogeneity: from concept to practice. Ann N Y Acad Sci. 2010;1213:81–91.
203 Sandiumenge A, Lisboa T, Gomez F, Hernandez P, Canadell L, Rello J. Effect of antibiotic diversity on ventilator-associated pneumonia caused by ESKAPE Organisms. Chest. 2011;140(3):643–51.
204 Chong Y, Shimoda S, Yakushiji H, Ito Y, Miyamoto T, Kamimura T, et al. Antibiotic rotation for febrile neutropenic patients with hematological malignancies: clinical significance of antibiotic heterogeneity. PLoS One. 2013;8(1):e54190.
205 Nijssen S, Fluit A, van de Vijver D, Top J, Willems R, Bonten MJ. Effects of reducing beta-lactam antibiotic pressure on intestinal colonization of antibiotic-resistant gram-negative bacteria. Intensive Care Med. 2010;36(3):512–9.
206 Martinez JA, Nicolas JM, Marco F, Horcajada JP, Garcia-Segarra G, Trilla A, et al. Comparison of antimicrobial cycling and mixing strategies in two medical intensive care units. Crit Care Med. 2006;34(2):329–36.
207 van Loon HJ, Vriens MR, Fluit AC, Troelstra A, van der Werken C, Verhoef J, et al. Antibiotic rotation and development of gram-negative antibiotic resistance. Am J Respir Crit Care Med. 2005;171(5):480–7.
208 Drees M, Snydman DR, Schmid CH, Barefoot L, Hansjosten K, Vue PM, et al. Antibiotic exposure and room contamination among patients colonized with vancomycin-resistant enterococci. Infect Control Hosp Epidemiol. 2008;29(8):709–15.
209 Peta M, Carretto E, Barbarini D, Zamperoni A, Carnevale L, Perversi L, et al. Outbreak of vancomycin-resistant Enterococcus spp. in an Italian general intensive care unit. Clin Microbiol Infect. 2006;12(2):163–9.
210 Shadel BN, Puzniak LA, Gillespie KN, Lawrence SJ, Kollef M, Mundy LM. Surveillance for vancomycin-resistant enterococci: type, rates, costs, and implications. Infect Control Hosp Epidemiol. 2006;27(10):1068–75.
211 Yeh KM, Siu LK, Chang JC, Chang FY. Vancomycin-resistant enterococcus (VRE) carriage and infection in intensive care units. Microb Drug Resist. 2004;10(2):177–83.
212 Marin ME, Podesta O, Llambias P, Galdon F, Scilingo VM, Stamboulian D, et al. Detection of carriage of vancomycin-resistant enterococci in an intensive care unit in Buenos Aires. Infect Control Hosp Epidemiol. 2001;22(6):332–3.
213 Zuckerman RA, Steele L, Venezia RA, Tobin EH. Undetected vancomycin-resistant Enterococcus in surgical intensive care unit patients. Infect Control Hosp Epidemiol. 1999;20(10):685–6.
214 Grayson ML, Grabsch EA, Johnson PD, Olden D, Aberline M, Li HY, et al. Outcome of a screening program for vancomycin-resistant enterococci in a hospital in Victoria. Med J Aust. 1999;171(3):133–6.
215 Ang CW, Heyes G, Morrison P, Carr B. The acquisition and outcome of ICU-acquired Clostridium difficile infection in a single centre in the UK. J Infect. 2008;57(6):435–40.
216 Marra AR, Edmond MB, Wenzel RP, Bearman GM. Hospital-acquired Clostridium difficile-associated disease in the intensive care unit setting: epidemiology, clinical course and outcome. BMC Infect Dis. 2007;7:42.
