The current role of imaging in head and neck cancer: a clinician’s perspective
DOI: https://doi.org/10.4414/smw.2014.14015
Michael F
Schlumpf, Stephan
Haerle
Summary
Imaging head and neck cancer is crucial for treatment decisions and follow-up of patients. The choice of the appropriate imaging modality for staging and re-staging head and neck cancer can be troublesome. This review highlights the important questions of imaging from a clinician's perspective. The recommendations focus on mucosal squamous cell carcinoma of the head and neck since this is the most common one.
Introduction
With an incidence of 500,000 new cases a year, malignant tumours of the head and neck rank fifth of all carcinomas [1]. Malignant epithelial tumours of the head and neck are histologically divided in verrucous carcinomas, spindle cell carcinomas, adenocarcinoma, adenosquamous carcinoma, lymphoepithelial carcinoma, giant cell carcinoma, malignant salivary gland-type tumours and squamous cell carcinomas. Head and neck squamous cell cancer (HNSCC), the most common one with over 90%. Over 300,000 deaths worldwide and around 450 deaths in Switzerland occur every year due to this type of cancer [1, 2]. There is a geographic variety due to different risk factors [3]. 80% of HNSCC in the eastern world are located in the oral cavity and (naso-) pharynx, whereas the carcinoma of the larynx takes over a third of all HNSCC in the western world [4]. The mean age of primary HNSCC diagnosis is about 60 years, yet the incidence of younger patients is increasing [5]. In most countries men more often contract HNSCC than women [3]. Besides the risk factors alcohol and tobacco, infection with human papilloma virus (HPV) is an increasing independent risk factor for developing oropharyngeal squamous cell carcinomas (OPSCC) [6, 7]. The role of HPV in the pathogenesis of HNSCC was first described in 1983 [8]. Many studies suggest that patients with an HPV affected OPSCC have a better prognosis and argue that a customised therapeutic approach is warranted for those patients. Traditional therapy regimens of OPSCC, 9–11 high doses of radiation or chemotherapy, may be an overtreatment for HPV-positive OPSCC, which is part of several clinical trials [12]. In general, the survival of HNSCC patients is mostly dependent on the stage of disease and personal medical health. Over 60% of the patients are at an advanced stage (Stage III and IV) at the time of diagnosis [13]. Therefore, adequate staging strategies including different imaging modalities are crucial to determine the stage of the disease and the directly related treatment strategy.
Pre-operative staging
Assessing the primary tumour precisely is crucial for treatment planning and interdisciplinary therapy strategies. The surgeon’s questions for assessing the primary are threefold: Firstly, the exact delineation and extension of the tumour, secondly, the potential infiltration of adjacent structures (e.g., vessels, cranial nerves), and thirdly, the differentiation between tumour infiltration and inflammatory reaction of surrounding tissue. Furthermore, there might be the scenario when the primary tumour is missing, so called ‘cancer of unknown primary’ (CUP). Many reports could show for staging purposes of the primary local extension and its related questions are best seen on contrast-enhanced (Ce) computed tomography (CT) or magnetic resonance imaging (MRI) [14]. Soft tissue definition, perineural spread, skull base invasion, intracranial extension, vascularisation and potential bone involvement is best evaluated with MRI [15, 16]. CeCT scans provide a great resolution, are fast and show exact details in bony structures. In clinical examination detection of deeper local invasion or infiltration in structures nearby of cancers is limited. Furthermore, ceCT is particularly useful because criteria for T4 classification like bone or cartilage invasion can be shown [17]. The addition of contrast-agent is crucial since cystic or necrotic lesions may be missed with a native CT scan [18]. In case of CUP, recent studies were able to show the benefit of using metabolic imaging. In up to 30% of cases with cervical lymph node involvement without a corresponding primary when clinical exam and conventional imaging (CT, MRI) are not able to reveal the primary tumour, [18F] fluoro-2–deoxy-D-glucose (18F-FDG) positron emission tomography (PET) in combination with CT (PET/CT) is able to detect as such [19, 20]. Rudmik et al. 21 showed a change in treatment due to the detection of the primary by the use of metabolic imaging in 30%. This is significantly related to the patient’s treatment plan and costs.
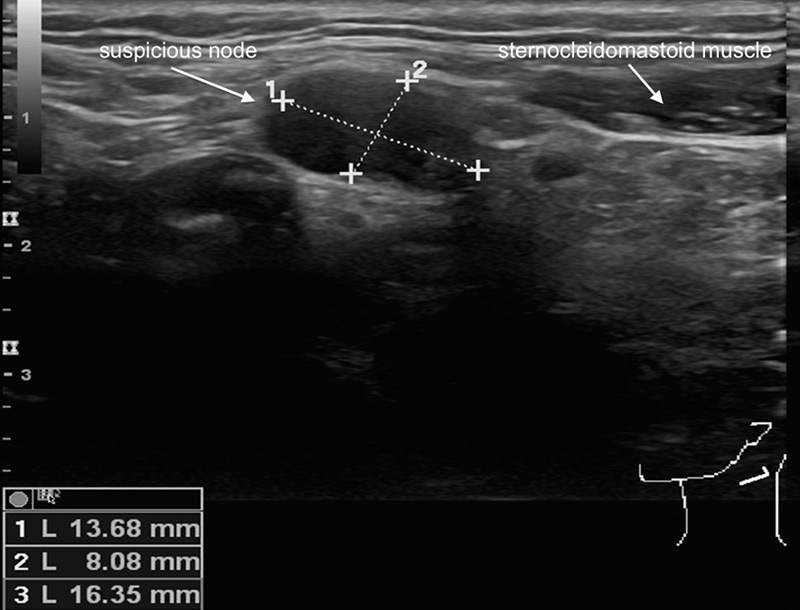
Figure 1
Sonographic appearance of a suspicious lymph node on palpation in level II left from a patient with a small squamous cell carcinoma of the right lateral tongue. The absence of an echogenic hilus, the size of more than 1.5cm and the shape of the lymph node renders it sonographically suspicious. The cytology result after ultrasound-guided fine needle aspiration confirms the presence of a contralateral positive node.
The classic approach to detect a CUP in the otolaryngologists eyes is to perform a panendoscopy (including direct pharyngoscopy, laryngoscopy, oesophagoscopy, and tracheo-bronchoscopy), taking blind biopsies form the base of tongue (BOT) and nasopharynx, performing uni-or bilateral tonsillectomy and obtain any kind of head and neck imaging. Having a 18F-FDG-PET/CT scan available in the operating room indicating the surgeon where to search and biopsy for the potential primary reduces the rate of missing primaries, the morbidity caused by blind biopsies, and therefore the patient’s burden significantly. Consequently, the gold standard in case of CUP shall be to obtain a 18F-FDG-PET/CT scan prior to panendoscopy with the warranted biopsies.
Regional lymphatic involvement for patients suffering from HNSCC is the strongest prognostic factor in these patients [22]. Patients with no nodal involvement count for a 5–year survival of 63% to 86%, whereas 5–year survival rates of 20–36% for patients with lymph node metastases are noted [23]. Clinical examination and appropriate imaging leads to optimal clinical staging and classification of the neck, which has important impact on the treatment decision regarding surgical and non-surgical treatment options. The staging options for patients with a clinical negative neck are manifold and there is a lack of prospective comparative studies in homogeneous patient cohorts with uniform inclusion criteria for the evaluation of the most accurate imaging modality- Ultrasound-guided fine needle aspiration cytology (USgFNAC) seems to correlate best with the exact histologic staging (fig. 1) [24]. There is a correlated sensitivity regarding this technique with the investigator’s skills [25]. However, there seems to be an increasing number of head and neck Surgeons performing neck ultrasounds by themselves. Most centres throughout the world still perform a CT scan if ultrasound is not available in an office-based set up. Since the primary tumour cannot be assessed using ultrasound there is no doubt performing a CT scan for staging reasons is arguable. However, as stated above, maybe the primary will better be assessed by an MRI. Constrast-enhanced CT and MRI are the methods of choice to evaluate the primary tumour, but their accuracy for nodal metastases is discussed in recent reports [26]. Therefore the authors advocate performing an ultrasound of the neck, and, if necessary, an USgFNAC, for staging the clinical N0 neck. In surgical cases patients with a clinical N0–neck should be offered minimally invasive sentinel node biopsy (SNB) [27] or risk-level-based elective neck dissections (ENDs) [28] for most accurate staging purposes. There is a different algorithm if patients present with clinically positive neck involvement. More than a single node is involved (N2b or N2c) or an involved lymph node of more than 3cm in maximum diameter (N2a or N3) is related with a higher risk of distant metastases, and therefore, 18F-FDG-PET/CT is indicated to exclude such [29]. Nevertheless, because of its low costs and its additional value for the assessment of the neck, a neck ultrasound will be added at our institution. For surgical candidates, final staging will be completed after histological assessment of the tissue specimen obtained from the neck dissection specimen [30]. On the other hand, in patients who receive a primary (chemo) radiation, the staging of the neck is ‘solely’ based on imaging. In these cases, the authors advocate performing metabolic imaging for two reasons: First, the metabolic information retrieved from a 18F-FDG-PET/CT can be further used for dose-painting in the planning phase, and second, treatment response after therapy can be assessed by using the metabolic part of the multimodality imaging [31, 32].
In general, advanced tumours (T3/T4 and/or N2/3), laryngopharyngeal tumours, and low level involved lymph node metastases (level III/IV) harbour a high risk of distant disease, and for all patients with such disease, a 18F-FDG-PET/CT scan should be added to exclude distant metastasis [29]. Further, due to alcohol and nicotine abuse which is often encountered in patients with HNSCC they have an increased risk of developing synchronous and metachronous SCC in other regions of the upper aerodigestive tract [33]. Therefore, again, metabolic imaging should be added to exclude second primaries in advanced staged tumours. For small tumours in patients without risk factors panendoscopy performed by the head and neck surgeon is sufficient. In cases of advanced tumours and a negative 18F-FDG-PET/CT regarding second primaries, panendoscopy can be reduced to endoscopic assessment of the primary tumour only. In these cases it is important to perform metabolic imaging prior to planned panendoscopy [29].
Post-treatment imaging and re-staging
HNSCC is treated in many different ways. Small primaries with a clinical N0–neck or small volume regional disease is either treated surgically or with primary irradiation depending on tumour location and/or relevant patient’s comorbidities. On the other hand advanced tumours are mostly treated in a combined approach meaning surgery plus (chemo-) irradiation or vice versa. In either treatment setting imaging plays an important role in the clinician’s view. Post-treatment imaging is mostly used to monitor treatment response and to detect persistent or recurrent disease. Patients who received potential curative treatment for HNSCC are at risk for recurrence between <10% and up to 48% [34, 35]. Most recurrent disease, second primary tumours or metastases occur within 2–3 years after initial treatment [36–38]. Early tumour recurrence may be difficult to confine from tissue changes induced by therapy.
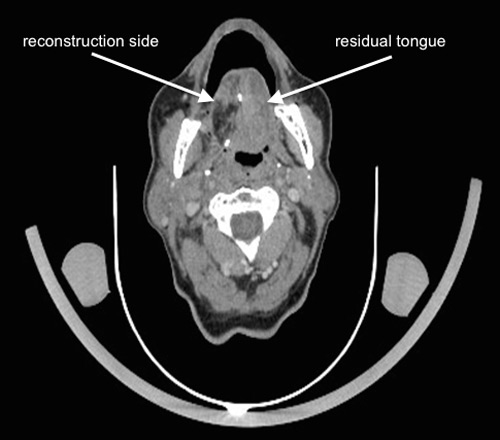
Figure 2
Follow-up contrast enhanced computed tomography part from a 18F-FDG-PET/CT scan 6 months following right partial glossectomy, elective neck dissection and reconstruction of the tongue with a radial forearm free flap for a pT2 pN1 squamous cell carcinoma. The primary site cannot be evaluated exactly regarding local recurrence. There are no evident suspicious lymph nodes to be detected.
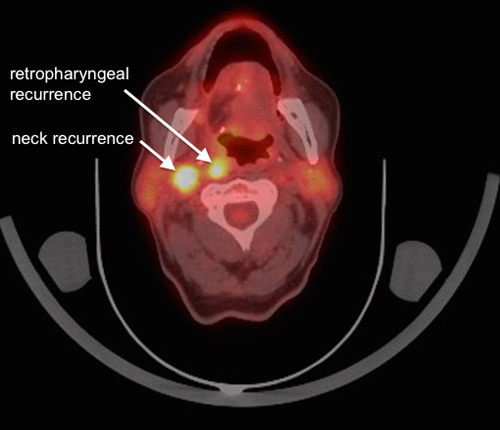
Figure 3
The same follow-up 18F-FDG-PET/CT scan showing the fused 18F-FDG-PET part with the CT part. There is no evidence for local recurrence at the reconstruction site however there are two highly suspicious lymph nodes in level IIb ipsilateral and retropharyngeal.
For initially early staged disease without any neck involvement (e.g., laryngeal SCC cT1 cN0) post-treatment imaging may not be warranted routinely. For small disease with lymphatic involvement conventional imaging (CT or MRI) of the primary will be the first choice for post-treatment imaging. As stated previously, the neck is best addressed by USgFNAC as a baseline and follow-up imaging [39]. Various studies not only show a superior efficacy of US and USgFNAC in the follow-up of the treated neck, but also that USgFNAC is superior to CT in detecting recurrent disease [39–41]. It is generally recommended that the post-treatment baseline CT/MRI should be performed three to six months after treatment. In general, the same imaging modality as the pre-treatment study should be used as subsequent imaging [42, 43]. This first baseline imaging scan demonstrates treatment-caused changes in the tissue, which render accurate interpretation between treatment changes and residual disease difficult [44–46]. A part of the routine follow-up of HNSCC is imaging the chest to detect lung metastasis and second primaries in the lung [47]. CT is showed to be superior as a screening tool to detect lung malignancies in comparison to a normal chest radiograph. Therefore chest CT seems to be necessary for the follow-up of high risk patients [48–50]. In the era of metabolic imaging, again, in high risk patients the chest will be examined as part of a ‘one stop shop strategy’ in the context of 18F-FDG-PET/CT.
After (chemo-) radiation tumour recurrence appears as a tissue mass at the primary site on CT or MRI. However, distinguishing between persistent viable tumour tissue and posttherapeutic changes can be difficult [51]. MRI is recommended for patients with base of tongue, sinonasal, skull base and nasopharyngeal tumours and with suspicion of perineural or intracranial spreading [52]. Diffusion-weighted MRI (DW-MRI) was shown to be even superior to anatomical imaging [53]. 18F-FDG-PET/CT has also found widespread acceptance for restaging after radiotherapy and chemo-radiotherapy [54, 55] It was seen that the effectiveness of 18F-FDG-PET/CT in detecting recurrence or relapse leads to a specificity of 94%, the positive predictive value was 75%, the negative predictive value 95% and the sensitivity was greater for scans performed after 10 weeks of treatment [56–58]. The accuracy of 18F-FDG-PET/CT for distant metastasis in patients with laryngeal cancer is almost 100% [59]. 18F-FDG-PET alone shows a high ratio of false positive results in patients with suspected recurrent disease. The combination of 18F-FDG-PET with a ceCT part reduces these false positive rates by over 50% compared to CT alone [60]. The high negative predictive value suggests that salvage surgery can be avoided in many cases [60–62].
In the case of a combined treatment for advanced lesions a repeated 18F-FDG-PET/CT scan is indicated to evaluate treatment response and further follow-up. Since metabolic imaging was indicated at the time of diagnosis to exclude distant disease or second primaries, it is advantageous to repeat the same scan post treatment for distinctive comparison. There are no guidelines for optimal timing of the post-treatment 18F-FDG-PET/CT. Overall, recent studies show a tendency towards a greater sensitivity for 18F-FDG PET/CT performed 10 weeks or more after treatment. Therefore, it shouldn’t be performed earlier than two to three months after treatment [56, 63–66]. There is still a debate about the benefit of ongoing surveillance scans. In a previous publication the authors were able to show significance in outcome between patients with or without distant disease. However, the time of diagnosis did not play any significant role [29]. Head and neck cancer patients with a negative first post-treatment scan (e.g., after three months) appear to derive limited benefit from subsequent 18F-FDG PET/CT surveillance [67].
Since there are some difficulties in the interpretation of these scans due to scarring and inflammation after surgery as well as irradiation of the tissue repeated scans may be indicated Furthermore, complex reconstructions may result in diffuse FDG-uptake rendering a definitive diagnosis difficult and repeated biopsies of the suspicious areas may be necessary.
After all there is no consensus of the perfect time for baseline and follow-up imaging. At our institution first imaging will be done 10 weeks after treatment. After that follow-up imaging is based on the previous findings. Since locoregional recurrence is often seen during the first two years after initial treatment the authors feel there is a legitimate reason for another subsequent scan, e.g., after 12, and 24 months. 18F-FDG-PET/CT is shown to be more accurate than conventional follow-up imaging alone regarding the detection rate of recurrences (fig. 2, and 3) [68]. Any additional imaging modalities should be performed on clinical signs. Suspicious lymph nodes can best be evaluated with USgFNAC.
Conclusions
The assessment of the primary tumour with CT or MRI will always be completed with ultrasound-guided FNAC for the assessment of the neck, because it seems to correlate best with the exact histologic staging. In surgical cases a patient with a clinical negative neck should be offered minimally invasive SNB or risk-level-based ENDs. In patients with advanced tumour stages 18F-FDG-PET/CT will be performed as a one stop shop strategy for the exclusion of second primaries and distant metastases. In selected cases (e.g., base of tongue cancer), the locoregional assessment warrants the addition of a MRI. In case of cancer of unknown primary18F-FDG-PET/CT is the most accurate choice of imaging prior to panendoscopy with biopsies.
The timely detection of residual or recurrent head and neck cancer after therapy is important to allow a prompt salvage treatment. Besides clinical examination, post-treatment imaging is crucial for follow-up. A baseline post-treatment imaging study should be performed 10 weeks after therapy. As shown in studies above, 18F-FDG-PET/CT shows an advantage in detection of locoregional persistence, recurrence and distant disease. The positive predictive value of the 18F-FDG-PET/CT is somewhat suboptimal at the primary site and the neck. However, its negative predictive value remains extraordinary high, so that a negative finding in post-treatment follow-up imaging by 18F-FDG-PET/CT is highly suggestive of the absence of recurrence or distant disease. In case of suspicious lymph nodes at clinical/radiologic examination USgFNAC should be performed in any case.
References
1 Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108.
2 NICER. National Institute for Cancer Epidemiology and Registration Switzerland. 2010.
3 Sankaranarayanan R, Masuyer E, Swaminathan R, Ferlay J, Whelan S. Head and neck cancer: a global perspective on epidemiology and prognosis. Anticancer Res. 1998;18:4779–86.
4 Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of 25 major cancers in 1990. Int J Cancer. 1999;80:827–41.
5 Schantz SP, Yu GP. Head and neck cancer incidence trends in young Americans, 1973–1997, with a special analysis for tongue cancer. Arch Otolaryngol Head Neck Surg. 2002;128:268–74.
6 Lindel K, Beer KT, Laissue J, Greiner RH, Aebersold DM. Human papillomavirus positive squamous cell carcinoma of the oropharynx: a radiosensitive subgroup of head and neck carcinoma. Cancer. 2001;92:805–13.
7 Portugal LG, Goldenberg JD, Wenig BL, et al. Human papillomavirus expression and p53 gene mutations in squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 1997;123:1230–4.
8 Syrjänen K, Syrjänen S, Lamberg M, Pyrhönen S, Nuutinen J. Morphological and immunohistochemical evidence suggesting human papillomavirus (HPV) involvement in oral squamous cell carcinogenesis. Int J Oral Surg. 1983;12:418–24.
9 Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007;121:1813–20.
10 Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–9.
11 Dayyani F, Etzel CJ, Liu M, Ho CH, Lippman SM, Tsao AS. Meta-analysis of the impact of human papillomavirus (HPV) on cancer risk and overall survival in head and neck squamous cell carcinomas (HNSCC). Head Neck Oncol. 2010;2:15.
12 Robinson M, Sloan P, Shaw R. Refining the diagnosis of oropharyngeal squamous cell carcinoma using human papillomavirus testing. Oral Oncol. 2010;46:492–6.
13 Seiwert TY, Cohen EE. State-of-the-art management of locally advanced head and neck cancer. Br J Cancer. 2005;92:1341–8.
14 Kim SY, Kim JS, Yi JS, et al. Evaluation of 18F-FDG PET/CT and CT/MRI with histopathologic correlation in patients undergoing salvage surgery for head and neck squamous cell carcinoma. Ann Surg Oncol. 2011;18:2579–84.
15 Vidiri A, Guerrisi A, Pellini R, et al. Multi-detector row computed tomography (MDCT) and magnetic resonance imaging (MRI) in the evaluation of the mandibular invasion by squamous cell carcinomas (SCC) of the oral cavity. Correlation with pathological data. J Exp Clin Cancer Res. 2010;29:73.
16 Bolzoni A, Cappiello J, Piazza C, et al. Diagnostic accuracy of magnetic resonance imaging in the assessment of mandibular involvement in oral-oropharyngeal squamous cell carcinoma: a prospective study. Arch Otolaryngol Head Neck Surg. 2004;130:837–43.
17 Ahmad A, Branstetter BF. CT versus MR: still a tough decision. Otolaryngol Clin North Am. 2008;41:1–22, v.
18 Haerle SK, Strobel K, Ahmad N, Soltermann A, Schmid DT, Stoeckli SJ. Contrast-enhanced 18F-FDG-PET/CT for the assessment of necrotic lymph node metastases. Head Neck. 2011;33:324–9.
19 Stokkel MP, Terhaard CH, Hordijk GJ, van Rijk PP. The detection of unknown primary tumors in patients with cervical metastases by dual-head positron emission tomography. Oral Oncol. 1999;35:390–4.
20 Regelink G, Brouwer J, de Bree R, et al. Detection of unknown primary tumours and distant metastases in patients with cervical metastases: value of FDG-PET versus conventional modalities. Eur J Nucl Med Mol Imaging. 2002;29:1024–30.
21 Rudmik L, Lau HY, Matthews TW, et al. Clinical utility of PET/CT in the evaluation of head and neck squamous cell carcinoma with an unknown primary: a prospective clinical trial. Head Neck. 2011;33:935–40.
22 Layland MK, Sessions DG, Lenox J. The influence of lymph node metastasis in the treatment of squamous cell carcinoma of the oral cavity, oropharynx, larynx, and hypopharynx: N0 versus N+. Laryngoscope. 2005;115:629–39.
23 Grandi C, Alloisio M, Moglia D, et al. Prognostic significance of lymphatic spread in head and neck carcinomas: therapeutic implications. Head Neck Surg. 1985;8:67–73.
24 Stoeckli SJ, Haerle SK, Strobel K, Haile SR, Hany TF, Schuknecht B. Initial staging of the neck in head and neck squamous cell carcinoma: a comparison of CT, PET/CT, and ultrasound-guided fine-needle aspiration cytology. Head Neck. 2012;34:469–76.
25 Knappe M, Louw M, Gregor RT. Ultrasonography-guided fine-needle aspiration for the assessment of cervical metastases. Arch Otolaryngol Head Neck Surg. 2000;126:1091–6.
26 de Bondt RB, Nelemans PJ, Hofman PA, et al. Detection of lymph node metastases in head and neck cancer: a meta-analysis comparing US, USgFNAC, CT and MR imaging. Eur J Radio.l 2007;64:266–72.
27 Stoeckli SJ. Sentinel node biopsy for oral and oropharyngeal squamous cell carcinoma of the head and neck. Laryngoscope. 2007;117:1539–51.
28 Robbins KT, Medina JE, Wolfe GT, Levine PA, Sessions RB, Pruet CW. Standardizing neck dissection terminology. Official report of the Academy's Committee for Head and Neck Surgery and Oncology. Arch Otolaryngol Head Neck Surg. 1991;117:601–5.
29 Haerle SK, Schmid DT, Ahmad N, Hany TF, Stoeckli SJ. The value of (18)F-FDG PET/CT for the detection of distant metastases in high-risk patients with head and neck squamous cell carcinoma. Oral Oncol. 2011;47:653–9.
30 Byers RM. Modified neck dissection. A study of 967 cases from 1970 to 1980. Am J Surg. 1985;150:414–21.
31 El-Bassiouni M, Ciernik IF, Davis JB, et al. [18FDG] PET-CT-based intensity-modulated radiotherapy treatment planning of head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;69:286–93.
32 Kim JW, Roh JL, Kim JS, et al. (18)F-FDG PET/CT surveillance at 3–6 and 12 months for detection of recurrence and second primary cancer in patients with head and neck squamous cell carcinoma. Br J Cancer. 2013;109:2973–9.
33 Strobel K, Haerle SK, Stoeckli SJ, et al. Head and neck squamous cell carcinoma (HNSCC)--detection of synchronous primaries with (18)F-FDG-PET/CT. Eur J Nucl Med Mol Imaging. 2009;36:919–27.
34 Virgo KS, Paniello RC, Johnson FE. Costs of posttreatment surveillance for patients with upper aerodigestive tract cancer. Arch Otolaryngol Head Neck Surg. 1998;124:564–72.
35 Cooney TR, Poulsen MG. Is routine follow-up useful after combined-modality therapy for advanced head and neck cancer? Arch Otolaryngol Head Neck Surg. 1999;125:379–82.
36 de Visscher AV, Manni JJ. Routine long-term follow-up in patients treated with curative intent for squamous cell carcinoma of the larynx, pharynx, and oral cavity. Does it make sense? Arch Otolaryngol Head Neck Surg. 1994;120:934–9.
37 Lester SE, Wight RG. 'When will I see you again?' Using local recurrence data to develop a regimen for routine surveillance in post-treatment head and neck cancer patients. Clin Otolaryngol. 2009;34:546–51.
38 Boysen M, Lövdal O, Tausjö J, Winther F. The value of follow-up in patients treated for squamous cell carcinoma of the head and neck. Eur J Cancer. 1992;28:426–30.
39 Westhofen M. Ultrasound B-scans in the follow-up of head and neck tumors. Head Neck Surg 1987;9:272–8.
40 Ahuja A, Leung SF, Ying M, Metreweli C. Echography of metastatic nodes treated by radiotherapy. J Laryngol Otol. 1999;113:993–8.
41 Steinkamp HJ, Mäurer J, Cornehl M, Knöbber D, Hettwer H, Felix R. Recurrent cervical lymphadenopathy: differential diagnosis with color-duplex sonography. Eur Arch Otorhinolaryngol. 1994;251:404–9.
42 Pfister DG, Ang KK, Brizel DM, et al. Head and neck cancers. J Natl Compr Canc Netw. 2011;9:596–650.
43 Daisne JF, Duprez T, Weynand B, et al. Tumor volume in pharyngolaryngeal squamous cell carcinoma: comparison at CT, MR imaging, and FDG PET and validation with surgical specimen. Radiology. 2004;233:93–100.
44 Mukherji SK, Mancuso AA, Kotzur IM, et al. Radiologic appearance of the irradiated larynx. Part II. Primary site response. Radiology. 1994;193:149–54.
45 Schwartz DL, Barker J, Chansky K, et al. Postradiotherapy surveillance practice for head and neck squamous cell carcinoma--too much for too little? Head Neck. 2003;25:990–9.
46 Hermans R, Pameijer FA, Mancuso AA, Parsons JT, Mendenhall WM. Laryngeal or hypopharyngeal squamous cell carcinoma: can follow-up CT after definitive radiation therapy be used to detect local failure earlier than clinical examination alone? Radiology. 2000;214:683–7.
47 O'Meara WP, Thiringer JK, Johnstone PA. Follow-up of head and neck cancer patients post-radiotherapy. Radiother Oncol. 2003;66:323–6.
48 Warner GC, Cox GJ. Evaluation of chest radiography versus chest computed tomography in screening for pulmonary malignancy in advanced head and neck cancer. J Otolaryngol. 2003;32:107–9.
49 Hsu YB, Chu PY, Liu JC, et al. Role of chest computed tomography in head and neck cancer. Arch Otolaryngol Head Neck Surg. 2008;134:1050–4.
50 Mercader VP, Gatenby RA, Mohr RM, Fisher MS, Caroline DF. CT surveillance of the thorax in patients with squamous cell carcinoma of the head and neck: a preliminary experience. J Comput Assist Tomogr. 1997;21:412–7.
51 Bahadur S, Amatya RC, Kacker SK. The enigma of post-radiation oedema and residual or recurrent carcinoma of the larynx and pyriform fossa. J Laryngol Otol .1985;99:763–5.
52 Manikantan K, Khode S, Dwivedi RC, et al. Making sense of post-treatment surveillance in head and neck cancer: when and what of follow-up. Cancer Treat Rev. 2009;35:744–53.
53 Wang J, Takashima S, Takayama F, et al. Head and neck lesions: characterization with diffusion-weighted echo-planar MR imaging. Radiology. 2001;220:621–30.
54 Lowe VJ, Boyd JH, Dunphy FR, et al. Surveillance for recurrent head and neck cancer using positron emission tomography. J Clin Oncol. 2000;18:651–8.
55 Goerres GW, Schmid DT, Bandhauer F, et al. Positron emission tomography in the early follow-up of advanced head and neck cancer. Arch Otolaryngol Head Neck Surg. 2004;130:105–9; discussion 20–1.
56 Isles MG, McConkey C, Mehanna HM. A systematic review and meta-analysis of the role of positron emission tomography in the follow up of head and neck squamous cell carcinoma following radiotherapy or chemoradiotherapy. Clin Otolaryngol. 2008;33:210–22.
57 Gupta T, Master Z, Kannan S, et al. Diagnostic performance of post-treatment FDG PET or FDG PET/CT imaging in head and neck cancer: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2011;38:2083–95.
58 Gupta T, Jain S, Agarwal JP, et al. Diagnostic performance of response assessment FDG-PET/CT in patients with head and neck squamous cell carcinoma treated with high-precision definitive (chemo)radiation. Radiother Oncol. 2010;97:194–9.
59 Gordin A, Daitzchman M, Doweck I, et al. Fluorodeoxyglucose-positron emission tomography/computed tomography imaging in patients with carcinoma of the larynx: diagnostic accuracy and impact on clinical management. Laryngoscope. 2006;116:273–8.
60 Ong SC, Schöder H, Lee NY, et al. Clinical utility of 18F-FDG PET/CT in assessing the neck after concurrent chemoradiotherapy for Locoregional advanced head and neck cancer. J Nucl Med. 2008;49:532–40.
61 Porceddu SV, Pryor DI, Burmeister E, et al. Results of a prospective study of positron emission tomography-directed management of residual nodal abnormalities in node-positive head and neck cancer after definitive radiotherapy with or without systemic therapy. Head Neck. 2011;33:1675–82.
62 Yao M, Smith RB, Graham MM, et al. The role of FDG PET in management of neck metastasis from head-and-neck cancer after definitive radiation treatment. Int J Radiat Oncol Biol Phys. 2005;63:991–9.
63 Greven KM, Williams DW, Keyes JW, et al. Positron emission tomography of patients with head and neck carcinoma before and after high dose irradiation. Cancer. 1994;74:1355–9.
64 Lee JC, Kim JS, Lee JH, et al. F-18 FDG-PET as a routine surveillance tool for the detection of recurrent head and neck squamous cell carcinoma. Oral Oncol. 2007;43:686–92.
65 Lonneux M, Lawson G, Ide C, Bausart R, Remacle M, Pauwels S. Positron emission tomography with fluorodeoxyglucose for suspected head and neck tumor recurrence in the symptomatic patient. Laryngoscope. 2000;110:1493–7.
66 Ryan WR, Fee WE, Le QT, Pinto HA. Positron-emission tomography for surveillance of head and neck cancer. Laryngoscope. 2005;115:645–50.
67 Ho AS, Tsao GJ, Chen FW, et al. Impact of positron emission tomography/computed tomography surveillance at 12 and 24 months for detecting head and neck cancer recurrence. Cancer. 2013;119:1349–56.
68 Abgral R, Querellou S, Potard G, et al. Does 18F-FDG PET/CT improve the detection of posttreatment recurrence of head and neck squamous cell carcinoma in patients negative for disease on clinical follow-up? J Nucl Med. 2009;50:24–9.


