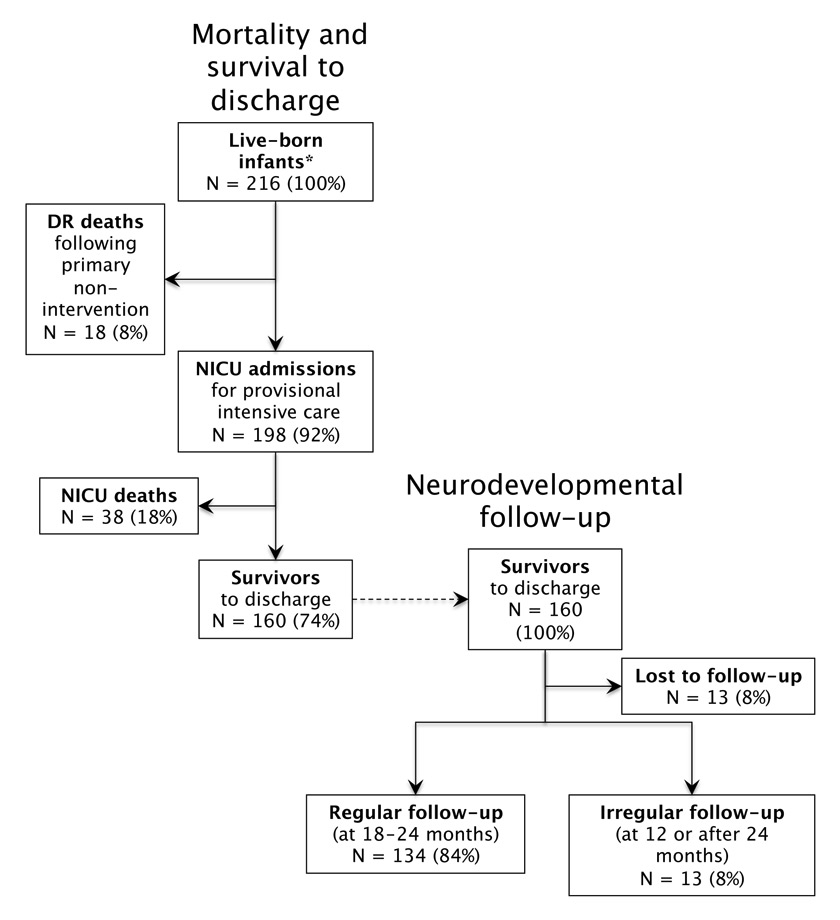
Figure 1
ELGAN patient population cared for at the Children’s Hospital of Lucerne between 2000 and 2009: DR and NICU mortality, survival and neuro-developmental follow-up rates.
* After exclusion of one ELGAN with trisomy 21.
DOI: https://doi.org/10.4414/smw.2014.14014
A Swiss single centre experience over 10 years
Abbreviations
ANC: Antenatal corticosteroid
BPD: Bronchopulmonary dysplasia
BSID-II: Bayley Scales of Infant Development, second edition
cPVL : Cystic periventricular leukomalacia
CTC: Centre-to-centre;
DR: delivery room
DQ: Developmental quotients
ELGANs: Extremely low gestational age newborns
EXPRESS: Extremely preterm infants in Sweden study
GMDS: Griffiths Mental Developmental Scales
MDI: Mental Developmental Index
MNDS: Swiss Minimal Neonatal Data Set
NDI: Neuro-developmental impairment;
NEC: Necrotising enterocolitis
NICHD NRN: National Institute of Child Health and Development Neonatal Research Network
NICU: Neonatal intensive care unit
PDI: Psychomotor Developmental Index
PIVH: Periventricular/intraventricular haemorrhage
ROP: Retinopathy of prematurity
SD: Standard deviation
VON: Vermont-Oxford Network
Major advances in perinatal care, such as antenatal corticosteroid (ANC) administration, surfactant replacement therapy and improved techniques for mechanical respiratory support, have led to significantly improved survival rates of extremely low gestational age neonates (ELGANs; gestational age (GA) <28 weeks) over the last three decades [1, 2]. On the other hand, the incidence of major neonatal morbidities in this population has remained unchanged [1, 3]. Concerns have been raised as to whether the increased survival rates of ELGANs would be associated with increased rates of neuro-developmental impairment (NDI) [4, 5] or not [6–10].
These uncertainties have fuelled ethical concerns regarding the best approach to ELGANs, particularly at the limit of viability (i.e., at <25 weeks of gestation). Consequently, ethical decision-making in neonatal intensive care varies widely across Europe [11]. The Swiss Society of Neonatology published its own recommendations for the care of infants born at the limit of viability in 2002 and a revised version in 2011 [12, 13]. Following the 2002 publication, survival rates of extremely preterm infants with a gestational age between 22 and 25 completed weeks increased from 31% to 40% in Switzerland without affecting the incidence of short-term morbidity. Interestingly, considerable centre-to-centre (CTC) outcome differences were noted and appeared to be unaffected by the publication of national recommendations [14]. These observations were confirmed in a ten-year-study of very low gestational age neonates (gestational age <32 weeks); in addition, risk-factor adjusted CTC differences in survival rates extended beyond the borderline viable infant population [15]. Whether these differences can be explained by unmeasured patient-level factors or whether they result from variations in the effectiveness of the care provided remains a matter of debate.
At the Children's Hospital of Lucerne, a pro-active approach to the care of ELGANs has been practiced for more than 15 years, and has been associated with significantly higher survival rates compared to the national average [15]. Generally, provisional intensive care is instituted without a priori restriction of any therapeutic options. This is followed by frequent reassessments of the potential burden and benefit for the patient. As long as the benefit appears to outweigh the burden, life-sustaining therapies are continued. On the other hand, once prognosis has become grim, redirection of care is considered and palliative care becomes a priority.
This retrospective cohort study was carried out to determine the impact of the centre's high survival rates of ELGANs on the rates of both severe neonatal morbidity and neuro-developmental impairment (NDI) at the age of 18 to 24 months. The results were compared with published data from contemporary national and international cohorts.
We assessed all infants with a gestational age between 23 0/7 and 27 6/7 weeks born alive between January 1st, 2000 and December 31st, 2009 who were admitted to the neonatal intensive care unit (NICU) of the Children's Hospital of Lucerne in Switzerland. Infants who died in the delivery room (DR) were also included regardless of whether resuscitative efforts were initiated or not. Infants transferred to our centre from other level III NICUs and ELGANs with major congenital anomalies (such as genetic disorders or malformations of major organ systems) were excluded. The following perinatal data were recorded: gestational age, birth weight, ANC administration (none, incomplete: 1 dose, complete: 2 doses), singleton/multiple births, and sex. Gestational age was determined as the best obstetric estimate based on ultrasound and/or date of the last menstrual period. Gestational age was defined according to the International Classification of Disease as the postmenstrual age in weeks and days [16]. The time period between 24 weeks and 0 days and 24 weeks and 6 days, for example, is termed 24 completed weeks of gestation; the foetus has completed 24 weeks and is in the 25th week of gestation.
Survival rates and severe neonatal morbidities were assessed for all live-born infants as well as for those infants admitted to the NICU. The following neonatal morbidities were included: Periventricular/intraventricular haemorrhage (PIVH) was graded according to Papile et al. [17] and cystic periventricular leukomalacia (cPVL) was defined as proposed by de Vries et al. [18]. Severe brain injury was defined as the presence of either PIVH grade III or IV and/or cPVL. Severe retinopathy of prematurity (ROP) was defined as ≥ stage 3 disease using the International Committee for the Classification of Retinopathy of Prematurity [19]. Necrotising enterocolitis (NEC) was diagnosed in the presence of intestinal pneumatosis or portal venous gas and/or pneumoperitoneum (Bell's stage ≥2) [20]. Diagnosis of moderate or severe bronchopulmonary dysplasia (BPD) was based on the National Institute of Health consensus definition as a requirement for supplemental oxygen and/or mechanical respiratory support at 36 weeks postmenstrual age [21].
Comprehensive neuro-developmental assessment was performed at 18 to 24 months' corrected age. Neurological evaluation included assessment of vision impairment (blindness in one or both eyes), hearing deficit (need for corrective hearing aids in one or both ears) and abnormal muscle tone (hypotonia, hypertonia). Cerebral palsy (quadriplegia, hemiplegia, diplegia) was defined as a non-transient disorder of movement or posture, or both, causing activity limitations [22]. Developmental evaluation was performed using either the Bayley Scales of Infant Development (second edition [BSID-II], German version [23]), or the Griffiths Mental Developmental Scales (GMDS, German version [24]). Both tests are most reliable when performed at or around 24 months corrected age. The BSID-II included determination of the Mental Developmental Index (MDI) and the Psychomotor Developmental Index (PDI). MDI and PDI scores of 100 ± 15 represent the mean ± 1 SD in the general population. The GMDS consists of five subscales: locomotion, personal-social, hearing-speech, eye-hand and performance. This test is designed to yield both global (sum of five subscales) and subscale developmental quotients (DQ) with a mean DQ ± 1 SD score for the general population of 100 ± 15 [24].
Severe NDI was defined as cerebral palsy resulting in a severely impaired mobility (PDI <55), severe cognitive impairment (MDI <55), GMDS DQ <55, bilateral blindness or deafness. Moderate NDI was defined as a PDI and/or an MDI between 55 and 69, a GMDS DQ between 55 and 69, or bilateral visual or hearing impairment. Mild NDI was defined as a PDI and/or an MDI between 70 and 84, a GMDS DQ between 70 and 84 and/or minor sensory impairments such as unilateral visual or hearing impairment. Finally, no NDI was defined as normal mobility (PDI >84), normal cognitive development (MDI >84), a GMDS DQ >84 and the absence of any visual or hearing impairment.
Gestational age-specific mortality and morbidity rates were calculated. Descriptive statistics were performed to compare baseline characteristics; data are presented as median and range for not normally distributed data and mean and standard deviation for normally distributed data, respectively. All statistical analyses were performed with the statistical software Stata (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP).
Over the entire 10-year study period, a total of 216 ELGANs were born alive at the perinatal centre of Lucerne (fig. 1). The median gestational age was 26 0/7 weeks (range 23 2/7–27 6/7 weeks), and the median birth weight was 780 g (range 390–1380 g). Of all 216 live-born infants, 113 (52%) were male, and 54 (25%) were multiple birth infants (52 twins, 2 triplets). Overall, 165 (76%) infants had been exposed to a complete or an incomplete course of ANC. In contrast, only 4 of the 18 infants (22%) with primary non-intervention and palliative care were given any corticosteroids prior to birth. Gestational age-specific characteristics at birth are shown in table 1.

Figure 1
ELGAN patient population cared for at the Children’s Hospital of Lucerne between 2000 and 2009: DR and NICU mortality, survival and neuro-developmental follow-up rates.
* After exclusion of one ELGAN with trisomy 21.
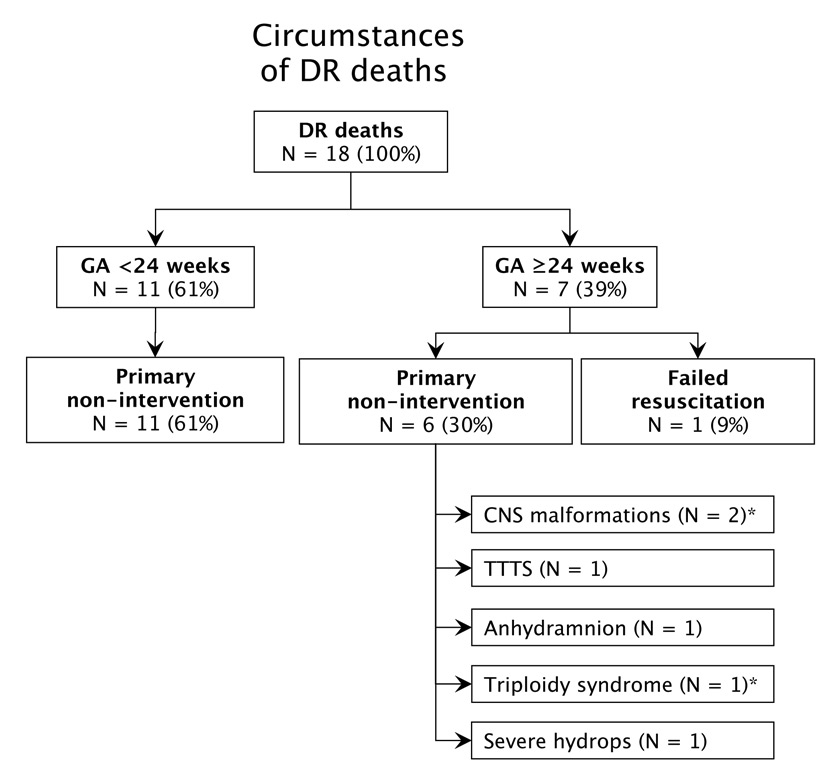
Figure 2
Circumstances of death in ELGANs who died in the DR.
CNS = central nervous system; TTTS = twin-to-twin transfusion syndrome. * Late termination of pregnancy.
The survival rate of all live-born infants (i.e., including 18 infants who died in the DR) was 74% (160/216), ranging from 22% at 23 weeks to 92% at 27 weeks (table 2). The majority of infants who died in the DR (fig. 2) had a gestational age <24 weeks (n = 11) and no life-sustaining therapies were initiated at the request of parents. Among those who were born with a gestational age ≥24 weeks (n = 7), 6 patients died following primary non-intervention, and 1 patient died following failed DR resuscitation. The survival rate of infants admitted to the NICU (i.e., excluding infants who died in the DR) was 81%, increasing with advancing gestational age from 57% at 23 weeks to 93% at 27 weeks (table 2). Among the 160 survivors, 25% (n = 40) sustained at least one major neonatal complication: 10% had severe brain injury, 16% had moderate or severe BPD, 1% had ROP ≥ stage 3, and 2% had NEC. Gestational age-specific rates of severe neonatal morbidity are summarised in table 2.
Neuro-developmental outcome data were available for 92% (147/160) of all surviving infants, including 13 infants with irregular follow-up (i.e., only with 12 months or after 24 months). No follow-up information was available for 13 (8%) of the survivors (fig. 1). The median BSID-II MDI was 95 (range 55–122), and the median BSID-II PDI was 92 (range 54–130). The median GMDS was 97 (range 49–116). Favourable outcome, defined as no or mild NDI, was found in 88% (129/147) of survivors assessed. Moderate NDI was present in 10%, and severe NDI was seen in 2% of the children. Survival with no or mild NDI was less frequent among ELGANs with a gestational age of 23 and 24 weeks (75 and 64%, respectively) compared with more mature infants born at 25, 26 and 27 weeks (93, 89, and 96%, respectively) (table 2).
| Table 1: Gestational age-specific characteristics of the ELGAN patient population. | ||||||
| Gestational age, weeks | ||||||
| 23 | 24 | 25 | 26 | 27 | Total <28 weeks | |
| Liveborn infants | N = 18 | N = 40 | N = 48 | N = 51 | N = 59 | N = 216 |
| Median birth weight, g (range) | 600(390–780) | 672 (400–880) | 750 (460–970) | 840 (500–1250) | 1030 (630–1380) | 780 (390–1380) |
| Male sex | 56%(n = 10) | 50% (n = 20) | 42% (n = 20) | 63% (n = 32) | 53% (n = 31) | 52% (n = 113) |
| Multiple birth infants | 17% (n = 3) | 10% (n = 4) | 27% (n = 13) | 20% (n = 10) | 39% (n = 23) | 25% (n = 53) |
| Antenatal corticosteroids (ANC) | ||||||
| – Complete course | 17% (n = 3) | 68% (n = 27) | 65% (n = 31) | 63% (n = 32) | 66% (n = 39) | 61% (n = 132) |
| – Incomplete course | 28% (n = 5) | 8% (n = 3) | 15% (n = 7) | 22% (n = 11) | 12% (n = 7) | 15% (n = 33) |
| – None | 56% (n = 10) | 25% (n = 10) | 21% (n = 10) | 16% (n = 8) | 22% (n = 13) | 24% (n = 51) |
| Table 2:Gestational age-specific survival, morbidity rates and rates of neuro-developmental impairment among ELGAN patient population cared for at the Children‘s Hospital of Lucerne between 2000 and 2009. PIVH = periventricular/intraventricular haemorrhage; cPVL = cystic periventricular leukomalacia; BPD = bronchopulmonary dysplasia; ROP= retinopathy of prematurity; NEC = necrotising enterocolitis). | ||||||
| Gestational age, weeks | ||||||
| 23 | 24 | 25 | 26 | 27 | Total <28 weeks | |
| Liveborn infants | N = 18 | N = 40 | N = 48 | N = 51 | N = 59 | N = 216 |
| Overall survival | 22% (n = 4) | 55% (n = 22) | 69% (n = 33) | 92% (n = 47) | 92% (n = 54) | 74% (n = 160) |
| Admitted to NICU | N = 7 | N = 36 | N = 46 | N = 51 | N = 58 | N = 198 |
| NICU survival | 57% (n = 4) | 61% (n = 22) | 72% (n = 33) | 92% (n = 47) | 93% (n = 54) | 81% (n = 160) |
| Morbidity among survivors | N = 4 | N = 22 | N = 33 | N = 47 | N = 54 | N = 160 |
| PIVH ≥3 | 25% (n = 1) | 9% (n = 2) | 12% (n = 4) | 6% (n = 3) | 4% (n = 2) | 8% (n = 12) |
| cPVL | 0% (n = 0) | 5% (n = 1) | 3% (n = 1) | 4% (n = 2) | 2% (n = 1) | 3% (n = 5) |
| BPD, moderate or severe | 25% (n = 1) | 32% (n = 7) | 24% (n = 8) | 13% (n = 6) | 7% (n = 4) | 16% (n = 26) |
| ROP stage ≥3 | 0% (n = 0) | 5% (n = 1) | 0% (n = 0) | 2% (n = 1) | 0% (n = 0) | 1% (n = 2) |
| NEC stage ≥2 | 0% (n = 0) | 5% (n = 1) | 0% (n = 0) | 4% (n = 2) | 0% (n = 0) | 2% (n = 3) |
| None | 25% (n = 1) | 59% (n = 13) | 67% (n = 22) | 79% (n = 37) | 89% (n = 48) | 76% (n = 121) |
| Neuro-developmental outcome | ||||||
| Survivors assessed, % (n/N) | 100% (4/4) | 100% (22/22) | 88% (29/33) | 94% (44/47) | 89% (48/54) | 92% (147/160) |
| No impairment, % (n/N) | 75% (3/4) | 55% (12/22) | 79% (23/29) | 70% (31/44) | 71% (34/48) | 70% (103/147) |
| Mild impairment, % (n/N) | 0% (0/4) | 9% (2/22) | 14% (4/29) | 19% (8/44) | 25% (12/48) | 18% (26/147) |
| Moderate impairment, % (n/N) | 25% (1/4) | 32% (7/22) | 3% (1/29) | 9% (4/44) | 4% (2/48) | 10% (15/147) |
| Severe impairment, % (n/N) | 0% (0) | 4% (1/22) | 3% (1/29) | 2% (1/44) | 0% (0/48) | 2% (3/147) |
In this single centre cohort of 216 ELGANs, we observed an overall survival rate of 74%. Despite the fact that 25% of the 160 survivors sustained at least one severe neonatal complication, 88% had a favourable outcome when examined at 18–24 months (i.e., no or mild NDI); in contrast, moderate and severe NDI were only observed in a minority of patients (10% and 2%, respectively).
Survival rates of ELGANs increase with advancing gestational age and, in addition, are strongly influenced by patient-level factors (birth weight, sex, single or multiple birth) and interventions (ANC, mode of delivery) that are known prior to delivery [25–27]. Several studies from the USA, Canada, Australia, and Switzerland have shown that substantial CTC outcome differences persist even after adjusting for these patient-level factors and perinatal interventions [15, 25, 28–30]. This may be due to unmeasured inherent risk factors of the patient population or, alternatively, due to differences in the treatment approach (i.e., provisional intensive care vs. primary non-intervention) or the effectiveness of the care provided.
This study reports survival rates, incidence of severe neonatal morbidity and neuro-developmental impairment at 18–24 months of age following a pro-active treatment approach to ELGANs at the Children's Hospital of Lucerne, Switzerland. Given the wide range of national and international CTC outcome differences [15, 25, 28–30], centre-specific outcome information is important when counselling parents at risk of imminent extremely preterm delivery.
Compared with published data from the Swiss Minimal Neonatal Data Set (MNDS) [14, 15, 31], overall and most gestational age-specific survival rates at the Children's Hospital of Lucerne are higher. For example, Schlapbach et al. reported survival rates of 30, 57 and 76% at 24, 25, and 26 weeks' gestation, respectively, for a Swiss national cohort born between 2000 and 2008 [31]; the corresponding survival rates for our centre are 55, 69 and 92% (table 2).
The survival rates at the limit of viability in our hospital are comparable to those reported from the National Institute of Child Health and Development Neonatal Research Network (NICHD NRN) [25, 28] and the Vermont-Oxford Network (VON) [32] in the United States: these large networks reported survival rates of 55%-63% and 72–76% at 24 and 25 gestational weeks, respectively [25, 28, 32]. Finally, the EXPRESS study group from Sweden has reported excellent survival rates following a pro-active approach to extremely preterm infants born in Sweden between 2004 and 2007 [3, 33]. The survival rates of 67 and 81% at 24 and 25, respectively, exceed those observed in our study or those reported from the NICHD NRN and VON in the United States.
Despite the fact that survival rates of ELGANs cared for at our perinatal centre are above the national average [15] the incidence of severe neonatal morbidities is not higher (i.e., major brain injury, NEC, moderate/severe BPD, severe ROP).This suggests that higher survival rates are not necessarily associated with increased rates of short-term complications (i.e., severe neonatal morbidity). Life sustaining therapies were not initiated in patients considered to have only minimal chances of survival and to be at the highest risk of severe morbidity; these patients (n = 18) died in the delivery room (fig. 1, 2). With this individualised approach, increased mortality rates are accepted in order to avoid an excessive burden of futile therapy or severe short- and long-term morbidities. This type of selection bias is likely to occur in other studies of infants born at the limit of viability. When compared with the results from the EXPRESS study group from Sweden [3, 33], rates of severe brain injury and NEC were comparable among surviving ELGANs; however, rates of moderate or severe BPD or severe ROP were lower in our cohort.
When compared with the results from the Swiss MNDS cohort, higher survival rates of ELGANs with a gestational age of 24 to 26 completed weeks (73 and 58% in our and the MNDS cohorts, respectively) were not associated with increased rates of NDI. In fact, we even found lower rates of impaired neuro-developmental outcome among survivors (16 and 39% for our and the MNDS cohorts, respectively) [31]. Our results are comparable to those reported by the EXPRESS study group [34].
The results of our study suggest that a pro-active treatment approach can increase the chances for survival without increasing the rates of severe neonatal morbidities and severe NDI. Active perinatal management starts before delivery, is of paramount importance in the delivery room and continues potentially for weeks in the NICU. Provisional intensive care without a priori restriction of any effective therapeutic options offers the opportunity to assess a preterm infant's individual response to life-sustaining therapies. It thus has the potential to more accurately predict an individual's chances for a favourable outcome. In contrast, primary non-intervention will always result in death of the ELGAN (self-fulfilling prophecy).
In our centre, a number of potentially beneficial therapeutic strategies are routinely used for ELGANs. These include – but are not restricted to – the use of lung-protective ventilation protocols [35], close monitoring of oxygen saturation targets [36], PIVH prophylaxis with indomethacin [37] (while avoiding the simultaneous use of corticosteroids [38]), initiation of gut protection on the first day of life [39], exclusive use of breast milk or donor breast milk for enteral nutrition [40, 41], and strict guidelines regarding the use of central lines. In addition, we have unrestricted access to subspecialties relevant for the care of ELGANs (e.g., cardiology, paediatric anaesthesia, paediatric surgery, radiology, paediatric neurology, etc.).
Redirection of care is only considered when complications occur that have a high likelihood of causing severe long-term morbidity with significant impact on quality of life. In contrast, severe complications that are potentially reversible are treated as vigorously as they would in more mature infants. Finally, all involved subspecialists participate in redirection of care decisions, help to fully inform parents and allow shared decision-making.
In Switzerland, recommendations for perinatal care at the limit of viability between 22 and 26 completed weeks of gestation were first published in 2002 and revised in 2011 [13]. While the new recommendations still leave room for interpretation, they are more specific regarding the suggested treatment approaches. Whether this will lead to more uniform outcomes across the Swiss centres in the next 10 years remains to be seen.
There are several limitations to our study. Even though we summarised data from a recent 10-year-period, the number of patients included in each gestational age category is small. Limited sample size also prohibited analyses of temporal trends. No follow-up data was available for 8% of survivors. This rate compares favourably to other national and international studies [31, 32, 34]. Finally, assessment of neuro-development among survivors was not uniform since both BSID-II and GMDS were used, possibly limiting the comparability with other studies.
A pro-active treatment approach to ELGANs at our centre was associated with higher overall survival rates compared with those reported from a contemporary Swiss cohort. Importantly, the higher survival rates were not associated with increased rates of severe neonatal morbidity or neuro-developmental impairment at 18 to 24 months of age. Finally, our results compared favourably with those reported from large international networks (NICHD-NRN, VON, EXPRESS study group) with comparable rates of survival, severe neonatal morbidity and neuro-developmental impairment.
1 Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196:147.e1–8.
2 Håkansson S, Farooqi A, Holmgren PA, Serenius F, Högberg U. Proactive management promotes outcome in extremely preterm infants: a population-based comparison of two perinatal management strategies. Pediatrics 2004;114:58–64.
3 EXPRESS Group. Incidence of and risk factors for neonatal morbidity after active perinatal care: extremely preterm infants study in Sweden (EXPRESS). Acta Paediatr. 2010;99:978–92.
4 Lorenz JM, Paneth N, Jetton JR, Ouden den L, Tyson JE. Comparison of management strategies for extreme prematurity in New Jersey and the Netherlands: outcomes and resource expenditure. Pediatrics. 2001;108:1269–74.
5 Wilson-Costello D, Friedman H, Minich N, Fanaroff AA, Hack M. Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990s. Pediatrics. 2005;115:997–1003.
6 Lefebvre F, Glorieux J, St-Laurent-Gagnon T. Neonatal survival and disability rate at age 18 months for infants born between 23 and 28 weeks of gestation. Am J Obstet Gynecol. 1996;174:833–8.
7 O'Shea TM, Klinepeter KL, Goldstein DJ, Jackson BW, Dillard RG. Survival and developmental disability in infants with birth weights of 501 to 800 grams, born between 1979 and 1994. Pediatrics. 1997;100:982–6.
8 Jacobs SE, O'Brien K, Inwood S, Kelly EN, Whyte HE. Outcome of infants 23–26 weeks' gestation pre and post surfactant. Acta Paediatr. 2000;89:959–65.
9 The Victorian Infant Collaborative Study Group. Improved outcome into the 1990s for infants weighing 500–999 g at birth. The Victorian Infant Collaborative Study Group. Arch Dis Child Fetal Neonatal Ed. 1997;77:F91–4.
10 Hack M, Fanaroff AA. Outcomes of children of extremely low birthweight and gestational age in the 1990s. Semin Neonatol. 2000;5:89–106.
11 Cuttini M, Nadai M, Kaminski M, Hansen G, de Leeuw R, Lenoir S, et al. End-of-life decisions in neonatal intensive care: physicians' self-reported practices in seven European countries. EURONIC Study Group. Lancet. 2000;355:2112–8.
12 Berger TM, Fauchère JC, Holzgreve W, Kind C, Largo R, Moessinger A, et al. Empfehlungen zur Betreuung von Frühgeborenen an der Grenze der Lebensfähigkeit (Gestationsalter 22–26 SSW). Schweizer Ärztezeitung. 2002;83:1589–95. German.
13 Berger TM, Bernet V, Alama El S, Fauchère JC, Hösli I, Irion O, et al. Perinatal care at the limit of viability between 22 and 26 completed weeks of gestation in Switzerland. Swiss Med Wkly. 2011;141:w13280
14 Fischer N, Steurer MA, Adams M, Berger TM, Swiss Neonatal Network. Survival rates of extremely preterm infants (gestational age <26 weeks) in Switzerland: impact of the Swiss guidelines for the care of infants born at the limit of viability. Arch Dis Child Fetal Neonatal Ed. 2009;94:F407–13.
15 Berger TM, Steurer MA, Woerner A, Meyer-Schiffer P, Adams M, Swiss Neonatal Network. Trends and centre-to-centre variability in survival rates of very preterm infants (<32 weeks) over a 10–year-period in Switzerland. Arch Dis Child Fetal Neonatal Ed. 2012;97:F323–8.
16 International Classification of Diseases (ICD) 10th revision. World Health Organization [Homepage on the Internet]. 2013 ed. Available from: http://www.who.int/classifications/icd/en/
17 Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–34.
18 de Vries LS, Eken P, Dubowitz LM. The spectrum of leukomalacia using cranial ultrasound. Behav Brain Res. 1992;49:1–6.
19 International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005;123:991–9.
20 Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187:1–7.
21 Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–9.
22 Bax M, Goldstein M, Rosenbaum P, Leviton A, Paneth N, Dan B, et al. Proposed definition and classification of cerebral palsy. Dev Med Child Neurol. 2005;47:571–6.
23 Bayley N. Bayley scales of infant development. 2nd edition. San Antonio (TX). The Psychological Corporation; 1993. German version, G. Reuner, J. Rosenkranz, J. Pietz, R. Horn, Pearsson 2008.
24 Griffiths R. The abilities of babies: a study in mental measurment. Association for Research in Infants and Child Development. Amersham, UK; 1976. German version, I. Brandt, E.J. Sticker, 2nd revised and enlarged edition, Beltz Test GmbH 2001.
25 Tyson JE, Parikh NA, Langer J, Green C, Higgins RD, National Institute of Child Health and Human Development Neonatal Research Network. Intensive care for extreme prematurity – moving beyond gestational age. N Engl J Med. 2008;358:1672–81.
26 Cole TJ, Hey E, Richmond S. The PREM score: a graphical tool for predicting survival in very preterm births. Arch Dis Child Fetal Neonatal Ed. 2010;95:F14–9.
27 Medlock S, Ravelli ACJ, Tamminga P, Mol BWM, Abu-Hanna A. Prediction of mortality in very premature infants: a systematic review of prediction models. PLoS ONE. 2011;6:e23441.
28 Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–56.
29 Ge WJ, Mirea L, Yang J, Bassil KL, Lee SK, Shah PS, et al. Prediction of neonatal outcomes in extremely preterm neonates. Pediatrics. 2013;132:e876–85.
30 Boland RA, Davis PG, Dawson JA, Doyle LW, The Victorian Infant Collaborative Study Group. Predicting death or major neurodevelopmental disability in extremely preterm infants born in Australia. Arch Dis Child Fetal Neonatal Ed. 2013;98:F201–4.
31 Schlapbach LJ, Adams M, Proietti E, Aebischer M, Grunt S, Borradori-Tolsa C, et al. Outcome at two years of age in a Swiss national cohort of extremely preterm infants born between 2000 and 2008. BMC Pediatr. 2012;12:198.
32 Mercier CE, Dunn MS, Ferrelli KR, Howard DB, Soll RF, Vermont Oxford Network ELBW Infant Follow-Up Study Group. Neurodevelopmental outcome of extremely low birth weight infants from the Vermont Oxford network: 1998–2003. Neonatology. 2010;97:329–38.
33 EXPRESS Group, Fellman V, Hellström-Westas L, Norman M, Westgren M, Källén K, et al. One-year survival of extremely preterm infants after active perinatal care in Sweden. JAMA. 2009;301:2225–33.
34 Serenius F, Källén K, Blennow M, Ewald U, Fellman V, Holmström G, et al. Neurodevelopmental outcome in extremely preterm infants at 2.5 years after active perinatal care in Sweden. JAMA. 2013;309:1810–20.
35 Berger TM, Fontana M, Stocker M. The journey towards lung protective respiratory support in preterm neonates. Neonatology. 2013;104:265–74.
36 Sola A, Golombek S, Bueno MTM, Lemus-Varela L, Zuluaga C, Domínguez F, et al. Safe oxygen saturation targeting and monitoring in preterm infants. Can we avoid hypoxia and hyperoxia? Acta Paediatr. 2014; [Epub ahead of print]
37 Fowlie PW, Davis PG, McGuire W. Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Fowlie PW, editor. Cochrane Database Syst Rev. Chichester, UK: John Wiley & Sons, Ltd; 2010;7:CD000174.
38 Gordon PV. Understanding intestinal vulnerability to perforation in the extremely low birth weight infant. Pediatr Res. 2009;65:138–44.
39 Ramani M, Ambalavanan N. Feeding practices and necrotizing enterocolitis. Clin Perinatol. 2013;40:1–10.
40 Quigley M, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Quigley M, editor. Cochrane Database Syst Rev. Chichester, UK: John Wiley & Sons, Ltd. 2014;4:CD002971.
41 Sisk PM, Lovelady CA, Dillard RG, Gruber KJ, O'Shea TM. Early human milk feeding is associated with a lower risk of necrotizing enterocolitis in very low birth weight infants. J Perinatol. 2007;27:428–33.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article were reported.