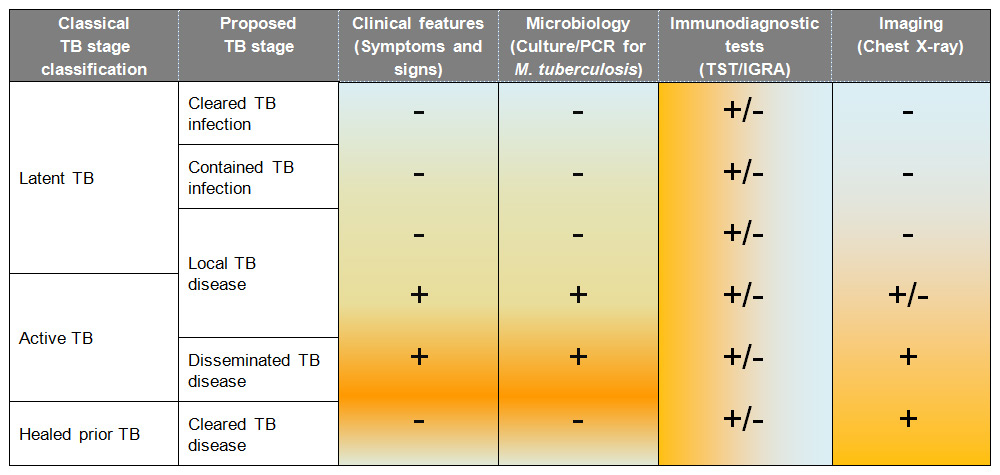
Table 2
Proposed continuum of stages between infection and disease in childhood tuberculosis (adapted from [9]).
DOI: https://doi.org/10.4414/smw.2014.14000
Tuberculosis (TB) is one of the oldest diseases known to mankind. Evidence of TB has been found in lung and bone tissue from Egyptian mummies and Neolithic settlements. Recent work based on phylogenetic analysis of Mycobacterium tuberculosis strains goes further to suggest that M. tuberculosis has been infecting humans for more than 70,000 years [1]. The modern humans who migrated from Africa around 62,000 to 75,000 years ago were responsible for the early worldwide spread of TB. Given the long historical relationship between TB and humans, it may seems surprising that TB still ranks as the second leading cause of death from infection worldwide [2]. However, the long co-evolution of the human immune system with M. tuberculosis may also explain the remarkable ability of this bacterium to evade the immune response.
Before clinical diagnosis of TB started in the 18th century and M. tuberculosis was first described by Robert Koch in 1882, there is limited information on global TB incidence and mortality. However, it is believed that the incidence of TB increased with the global population and crowding during the Middle Ages to become a leading cause of death in the 18th and 19th century in Europe. In the 20th century, TB rates in industrialised countries dramatically declined only to resurge in the 1980s as a result of the human immunodeficiency virus (HIV) epidemic and a lack of interest in the medical community [3]. This eventually led to the declaration of TB as a ‘global emergency’ by the World Health Organization (WHO) in 1993. Renewed interest in TB resulted in the WHO publishing the first data on global TB incidence and mortality in 1997. However, it took another 15 years until data specifically relating to childhood TB was included in this report [2]. The absence of paediatric TB data in previous reports was predominantly the result of the WHO’s definition of a TB case, which required a positive sputum smear as diagnostic criterion. As children often have a lower bacillary load, the majority of paediatric patients with TB disease are sputum smear negative and many are also culture negative (see below). Despite a number of limitations, the inclusion of data on childhood TB in the most recent two editions of the WHO TB report is a major improvement and provides a first estimate of the burden of TB in children worldwide.
The most recent WHO TB report, published in autumn 2013, states that of the 8.6 million new cases in 2012, an estimated 510,000 to 550,000 cases were in children under 15 years of age [4]. Despite being a curable disease, around 74,000 children died from TB in 2012. Compared to the first report on childhood TB published a year earlier, estimates of new cases in children have increased by 40,000 cases and those of children dying from TB by 10,000 per year [2, 4]. This is in marked contrast to the decline in new TB cases and TB mortality in the adult population and most likely reflects improved case finding and reporting rather than a true increase in disease burden in children.
The European Centre for Prevention and Diseases Control (ECDC) published their first paediatric TB report in 2011. In a 10–year-period, a total of 39’695 cases of paediatric TB were notified in Europe, which accounted for 4.3% of all notified TB cases [5]. As with the data published by the WHO, it is widely acknowledged that the estimates of the burden of TB disease in children is likely to be a substantial underestimate. The burden of TB disease in children is difficult to determine for a number of reasons:
‒TB disease in children frequently presents with non-specific clinical features.
‒A higher proportion of paediatric cases are extra-pulmonary forms of TB resulting in a different clinical presentation.
‒Children with pulmonary TB are more likely to have paucibacillary disease which commonly results in a negative sputum smear microscopy and culture.
‒Obtaining a sample for microbiological diagnosis is often difficult in children, as younger children are not able to produce sputum.
‒For culture-negative children with TB there is no universally applicable and validated clinical diagnostic algorithm.
‒Smear or culture negative cases are not reported in many countries due to the lack to national surveillance systems.
In Switzerland, TB became a mandatory notifiable disease in 1988. The definition used in this country includes confirmed cases (M. tuberculosis detected by culture or nucleic acid amplification testing) as well as non-confirmed cases, which are defined as patients treated for TB disease with at least three anti-tuberculous drugs. This kind of reporting system is more suitable for the evaluation of TB in children. Based on these data collected by the Federal Office of Public Health, a recent study investigated the epidemiology of childhood TB in Switzerland between 1996 and 2011 [6]. The study showed that over the last 16 years, 20 to 30 cases of paediatric TB have been notified per year and incidence rates have remained at a consistently low level of 1.4 /100,000 children. This low rate results in reduced opportunities for clinicians to gain experience in childhood TB, and consequently diminished knowledge of diagnosis and prevention [7].
| Table 1: Differences between tuberculosis in children and adults. | ||
| Feature | Children | Adults |
| Contact history | Contact history positive (parents, care-givers, teachers) in approximately 50% of cases | Contact history frequently negative |
| Most important risk factors for TB disease | Age <2 (5) years, HIV infection, malnutrition | HIV infection, immunosuppressive treatment, prior episode of TB, chronic renal insufficiency, diabetes mellitus, smoking, alcohol abuse |
| Social risk factors | Same bed, bedroom or household as a smear positive adult case, living in refugee camps, orphan | Homeless, iv-drug abuse, prisoner |
| Clinical clues | Weight loss or failure to thrive | Unwanted weight loss |
| Chronic non-remitting cough (>2–3 weeks) | Chronic non-remitting cough (>2–3 weeks) | |
| Wheezing | ||
| Chronic fever | Chronic fever | |
| Risk of progression from TB infection to disease | <1 year of age: up to 50% annual risk <5 years of age: 6–24% annual risk 6–15 years of age: 6–12% annual risk | HIV negative: 8–10% lifelong risk (5% in the first 2 years) HIV-positive: 10% annual risk |
| Frequency of extra-pulmonary TB | 30% | 15% |
| Most common radiologic findings | Hilar or mediastinal lymphadenopathy, Ghon focus, disseminated (miliary disease) | Cavitary disease, Ghon focus, Pleural effusion |
| Microbiological confirmation | 1–15% smear positive 10–50% culture positive | 50% smear positive 90% culture positive |
| Immuno-diagnostic testing | TST can be false positive, particularly after BCG immunisation | TST can be false positive after exposure to non-tuberculous mycobacteria |
| IGRA may be false negative particularly in young children | IGRA has replaced TST testing in most resource-rich countries | |
| Treatment | Weight-adapted dosing (dose per kg higher than in adults) Young children require special formulations (eg syrup) as unable to take tablets | Fixed dosing |
| Hepatotoxicity less likely than in adults | Hepatotoxicity increases with age and in cases with underlying liver disease | |
| Pyridoxine only in malnourished children | Routine pyridoxine | |
| Risk of transmission | Low in children <5 years because of absent force of cough and paucibacillary disease | Increased in sputum positive individuals |
| Protection afforded by BCG immunisation | 77% for disseminated forms of TB 50% for pulmonary TB | Highly variable: 0–80% for all forms of TB |
As childhood TB has been a neglected disease, knowledge about TB has largely been based on studies in adults. It is therefore not surprising that clinicians and scientists are commonly unaware of the considerable differences between TB in children and adults. The spectrum of disease, risk factors, clinical features and diagnostic results differ considerably according to age. The following table summarises what we consider to be the most important differences between TB in children and in adults (table 1).
For many years TB has been catergorised into two distinct stages: “latent” and “active”. The understanding was that in “latent TB”, M. tuberculosisbacilliwere dormant or non-replicating. However, in recent years, evidence has emerged that in both stages there are non-replicating and replicating M. tuberculosis bacilli but that in TB infection (“latent TB”) the number of replicating M. tuberculosis bacilli is low and in TB disease (“active TB”) it is high [8]. To highlight this change in understanding, we prefer to use the terms TB infection and disease rather “latent” and “active TB”. It is therefore now hypothesised that in TB infection, non-replicating M. tuberculosis bacilli sporadically return to a replicating stage [8]. This would explain why isoniazid, which is used as treatment of TB infection but which is only active against replicating M. tuberculosis, is effective in reducing the risk of TB disease. It has therefore been suggested by a number of experts that TB is better envisaged as a continuum between infection and disease rather than there being distinct binary stages [9–11]. A proposed novel classification of this continuum of different stages is illustrated in table 2. Although currently this classification does not impact clinical management, in the future it may be possible to determine whether M. tuberculosis infection has been cleared in individuals currently classified as “latent TB” for example and therefore suggest different management.

Table 2
Proposed continuum of stages between infection and disease in childhood tuberculosis (adapted from [9]).
Confirmation of TB in both children and adults involves detection of M. tuberculosisby culture or by nucleic acid amplification testing (NAAT). For individuals with TB who have negative culture and/or NAAT, there is currently no accurate diagnostic test for TB. This scenario is typically more common in children. In these situations, the diagnosis of TB disease is usually based on a combination of clinical, radiological and immunodiagnostic criteria.
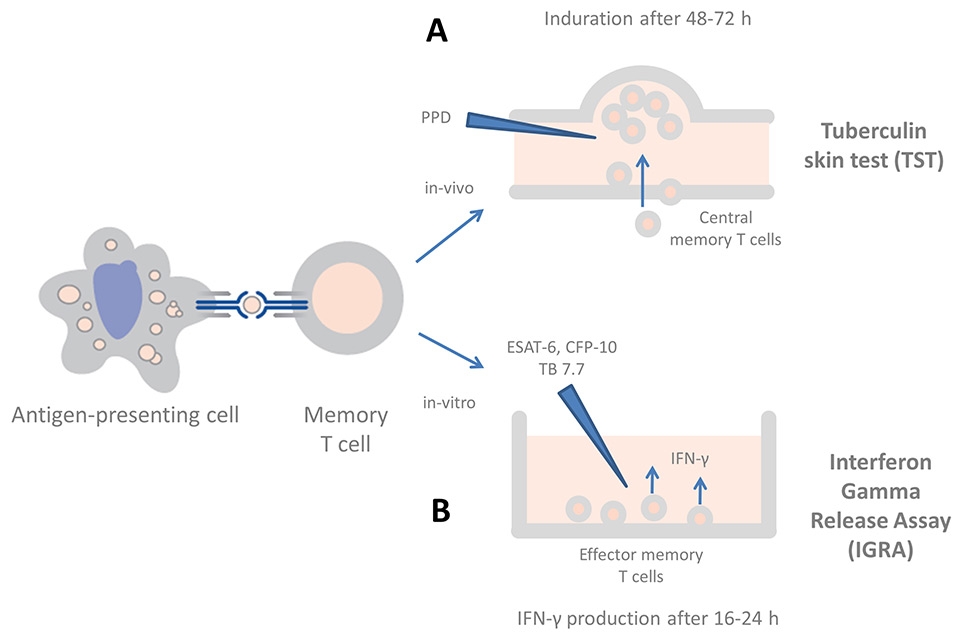
Figure 1
The current immunodiagnostic tests in clinical use for the diagnosis of tuberculosis (adapted from: Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. Lancet. 2000;356(9235):1099–104, with permission from Elsevier). Exposure to M. tuberculosis leads to uptake by antigen-presenting cells and primes antigen-specific T cells in the local lymph node. (A) In the TST, after re-stimulation with PPD, mycobacterial-specific central memory T cells infiltrate the skin at the injection site and cause a palpable induration. (B)In IGRAs, blood is stimulated in vitro with ESAT-6, CFP-10 and (in the QFT-GIT only) TB 7.7, resulting in IFN-γ production by circulating effector memory T cells, which is measured by ELISA or ELISPOT.
The symptoms classically associated with TB diseases are cough, fever and weight loss. A landmark study in children with TB disease, in a high prevalence setting in South Africa, combining the classic symptoms of persisting cough for more than two weeks, failure to thrive and prolonged fatigue had an overall sensitivity of 63% [12]. Particularly worrying, however, is the finding that below the age of three years, and in children infected with HIV, the sensitivity declined to 52–56% [12]. Several other approaches using clinical scores have reported similar results. A recent evaluation including nine different scores developed to improve and standardise diagnosis of TB showed variable results with between 7% and 89% of children identified by different scoring systems [13]. Importantly, scores relying on clinical and radiological features only had the lowest sensitivity.
Culture confirmation of presumed pulmonary TB disease is usually done using a sputum sample. Younger children, particularly those under the age of five years, are unable to expectorate sputum and therefore alternative samples are commonly used including gastric aspirates, induced sputum, nasopharyngeal aspirates, string test samples or bronchoalveolar lavage (BAL) fluid.
These sample collection techniques need specifically-trained staff and facilities, and some are associated with a high degree of invasiveness and discomfort for the child. Regardless of the sample technique, microbiological confirmation of TB disease in children is generally limited and at best reaches 50% [6, 14]. In adults, improved confirmation rates in pulmonary TB disease have recently been achieved using NAAT from sputum. Based on this, the WHO has advocated universal use of Xpert MTB/RIF (Cepheid, CA, USA) for the diagnosis of TB. The clear advantage of this assay is that it detects significantly more cases than sputum smear microscopy and, importantly, results are available within one day, including detection of rifampicin resistance. However, studies in children show that Xpert MTB/RIF is unfortunately unable to detect a significant proportion of children with TB disease who are culture negative. This new technique therefore does not improve the overall detection rate and will not increase the proportion of cases of confirmed TB disease in children [15].
The immunodiagnosis of TB infection and disease relies on the detection of a cell mediated immune response (CMI). The tuberculin skin test (TST), which was developed more than a century ago, is still the standard test in children. A recent study shows that the skin induration in the TST results from a cellular infiltrate of central memory T cells (CD4+/CD45RO+) (fig. 1) [16]. There are numerous disadvantages of TST including low specificity, particularly in BCG-immunised children, sub-optimal sensitivity, the need for a technically-demanding and difficult-to-standardise intradermal injection, the requirement for the patient to return for the reading of the test and an unsophisticated error-prone reading technique using a ruler to measure the palpable induration.
To overcome the shortcomings of the TST, in vitro cytokine-based immunodiagnostic tests were developed in the 1990s. These tests measure the concentration of IFN-γ in stimulated blood in response to mycobacterial antigens (fig. 1) [17, 18]. Two commercial kits are licensed for use: the QuantiFERON-TB Gold In-Tube test (QFT-GIT, Cellestis/Qiagen) and the T-SPOT.TBtest (Oxford Immunotech, Oxford, UK). Rather than purified protein derivative (PPD), both tests use more specific TB antigens. Sensitivity and specificity is similar for both IGRAs, with the QFT being more frequently used in resource-rich countries [19]. The QFT offers additional advantages over the T-SPOT.TBtest including no requirement for peripheral blood mononuclear cell (PBMC) separation and importantly the potential to measure additional cytokines from the same supernatant. It is now clear, that many other expressed cytokines might be suitable biomarkers for TB infection and disease (reviewed in [20, 21]). Interferon gamma-inducible protein (IP)-10 has been most studied. It is produced by antigen-presenting cells in response to IFN-γ, has similar kinetics to IFN-γ but is expressed in higher concentrations. In studies in children, the addition of IP-10 has been shown to increase the sensitivity of commercial IGRAs [22–24]. In one study, although children under the age of five years had reduced IFN-γ responses, IP-10 responses were not affected by age [22]. Several other cytokines, including interleukin (IL)-2, IL-1 receptor antagonist (IL-1 ra) and tumour-necrosis factor (TNF)-α are currently being evaluated as potential immunodiagnostic markers.
IGRAs were developed, optimised and standardised for adults. They are now well-established tests for the immunodiagnosis of TB in adults and have replaced the TST in many resource-rich countries [25]. Evidence for the performance of IGRAs in children is limited but recent meta-analyses have failed to show improved sensitivity compared to TST and in resource-limited, high burden TB-countries sensitivity was lower for IGRAs than for TSTs [19, 26]. Therefore the majority of national guidelines – including Switzerland – and the two supranational guidelines (WHO and ECDC) recommend using a TST as the immunodiagnostic test of choice for TB in children [27–30]. The underlying reason for the lower performance of IGRAs in children is uncertain but the age of the child, the immune status, nutrition and co-infections all likely play an important role [31–33]
More accurate diagnostic tests for TB in children are therefore urgently needed. There is growing recognition that there are fundamental differences in children and adults in relation to the underlying immunology and consequent diagnosis of TB. The recently published roadmap for childhood TB by the WHO highlights that the evaluation of new diagnostics is a research priority with particular emphasis on assays that are suitable for paediatric samples and that have the ability to identify disease progression in children [34].
The Bacille Calmette-Guérin (BCG) vaccine is one of the oldest vaccines still in use today. Although it is no longer used or licensed in many Western European countries, more than 100 million children, or approximately 90% of all children born worldwide each year, receive a BCG immunisation. A common misconception about BCG is that the vaccine has little or no protective efficacy. The vaccine is most effective for the prevention of severe forms of TB that particularly affect infants and young children, including TB meningitis and miliary TB. In the 1980s and 1990s, many studies in different settings repeatedly confirmed the protective efficacy of BCG for TB meningitis (67% to 79%) and for miliary TB (58% to 87%) (reviewed in [35]). However, BCG has limited efficacy for the prevention of pulmonary TB disease in children and limited to no efficacy for the prevention of adult pulmonary TB disease. It is unclear whether this is the result of waning immunity of the vaccine’s protective efficacy or if BCG is less effective in preventing pulmonary forms of TB disease. In addition, in children infected with HIV, BCG immunisation does not induce protective immunity and potentially leads to severe adverse effects, including disseminated BCG disease [36]. HIV infection or immunosupression is therefore a contraindication to BCG immunisation. In particular, WHO now recommends that BCG immunisation is delayed in infants born to HIV-infected mothers until these infants are confirmed to be HIV negative.
Another common misconception is that BCG is one single product and that children immunised worldwide all receive the same vaccine. For historical reasons, however, a number of different BCG-vaccine strains are produced and licensed worldwide today [37, 38]. Two recent studies provide evidence that the specific BCG vaccine strain influences both the immune response and protection against TB [39, 40]. Knowledge of the BCG vaccine strain used for immunisation is also important in the treatment of potential BCG adverse effects. All BCG vaccine strains are resistant to pyrazinamide. In addition, at least two currently-used strains also exhibit low level resistance to isoniazid [41]. Given the fact that BCG vaccine strain influence immune response, protection and anti-mycobacterial resistance, it seems surprising that in a recent European survey in two TB specialist networks, one third of participants did not know which BCG vaccine strain was used in their country [42].
In addition to protection against TB, BCG has a number of other beneficial heterologous (‘non-specific’) effects on the immune system [43, 44]. The immunomodulatory and anti-tumour effect of BCG is used in the treatment of bladder cancer [45]. In addition, BCG influences vaccine response to routine immunisations [46, 47]. Moreover, there are a large number of reports showing that BCG is associated with reduced all-cause mortality, independent of TB, in resource limited settings [48, 49].
| Table 3: Novel tuberculosis vaccines. | |||
| Vaccine type | Intended use | Intended endpoint | Example vaccines in phase 2–3 clinical trials |
| Prime | Replace BCG in infants, before the individual’s immune system has been exposed to M. tuberculosis or other mycobacteria | Prevent infection and disease | H56/AERAS-456 +IC31 H1+IC31 VPM 1002 |
| Boost | Given as a booster vaccine either after BCG immunisation at birth as a ‘primer’ or after exposure to non-tuberculous mycobacteria later in life | Prevent infection, disease and/or reactivation | Crucell Ad35/AERAS-402 H1+IC31 H4/AERAS-404 +IC31 H56/AERAS-456 +IC31 M72+AS01 |
| Therapeutic | Adjunctive treatment for individuals with TB disease in addition to standard anti-tuberculous medication | Shorten treatment duration, prevention of transmission | M. vaccae RUTI |
After the first use of BCG in humans in 1921 in France, despite limited efficacy data, the vaccine was distributed worldwide. Encouraging studies in the 1950s and 60s led to the inclusion of BCG in the expanded immunisation programme (EPI) by WHO in 1974. Subsequent studies from high-prevalence TB countries suggesting variable, sometimes absent, protection by BCG prompted the search for improved TB vaccines. A number of other factors paved the way for the development of novel vaccines against TB including the improved understanding of the complex mechanisms underlying the adaptive immune response against TB in the early 1990s, the analysis of the complete genome sequence of M. tuberculosisin 1998 [50] and the comparative analysis of the genome of several BCG vaccine strains in 1999 [51]. This led to the first novel TB vaccine candidates being tested in animal models and, over the last two decades, 14 TB vaccine candidates have advanced to clinical trials. There are now three different types of novel TB vaccines: priming vaccines, boosting vaccines and therapeutic vaccines (table 3).
The most advanced of these vaccines is a boosting vaccine called MVA85A that uses a modified vaccinia virus expressing M. tuberculosis-85A antigen. In a recently completed, phase 2b, randomised placebo-controlled trial (RCT), MVA85A was given as a booster vaccine at the age of 4 to 6 months following BCG immunisation at birth [52]. In the clinical follow-up of the nearly 2800 HIV-negative infant participants over two years, there was no evidence that MVA85 offered additional protection over BCG immunisation. While the results are disappointing, the completion of this trial is a milestone in the history of TB vaccines. It is the first large-scale RCT investigating the efficacy of a novel TB vaccine in infants. The capacity building that allowed such a trial to be completed efficiently and according to good clinical practice standards in a resource limited, high TB-prevalence country should not be underestimated.
The results of this trial have also stimulated the discussion about current knowledge gaps which will have a profound influence on future research into novel TB vaccines [53, 54]. Firstly, it has become very clear that the mouse model – which was the most commonly-used animal model for preclinical testing of TB vaccine candidates – has several shortcomings and may fail to predict efficacy of a TB vaccine candidate in humans. There is therefore now debate whether other animal models, including non-human primate and bovine models, need to be included in future preclinical trials of novel TB vaccine candidates [53]. Secondly, unlike in other infectious diseases, there is currently no marker or set of markers which clearly predict protection against TB. Correlates of protection against TB have been a research priority for many years, yet many attempts to define accurate biomarkers, though increasing our knowledge about the complex immune response to TB, have failed to produce a clear answer [55–57]. Whilst the MVA85 vaccine induced A85–specific T cells that produced several cytokines four weeks after immunisation, this immune response did not correspond to protective efficacy. Many factors could have contributed to this including measurement of the wrong immunological outcome (ie other cells or cell-products are responsible for protection) or the in-vitro stimulation with A85 does not predict protection against M. tuberculosis. Lastly, the diagnosis of TB, particularly confirmation of disease by novel accurate immunodiagnostic tests, would considerably improve the diagnostic certainty in the follow-up of all children included in future TB vaccine trials.
In summary, a number of candidates are currently being evaluated as novel vaccines against TB representing a major achievement in this research field. However, the development of novel TB vaccines and clinical trials are significantly hampered by the absence of biomarkers that correlate with protection and the lack of an ideal animal model. It is therefore important to improve the diagnostic accuracy of all tests used to diagnose TB disease in children included in TB vaccine trials. In the light of these challenges, it is likely that BCG will remain the only licensed TB vaccine for at least the next 10 years.
A number of major advances have been made in the understanding of the epidemiology, diagnosis and protection of childhood TB in the last few years. This has led to childhood TB being lifted out of the shadow of adult TB. The increased public attention, commitment of clinicians and researchers and increased funding for treatment and research at both local and global levels is invaluable. Addressing research priorities, including the lack of accurate diagnostic tests that can detect all TB cases in children and improved TB vaccines, is now the responsibility of the current generation of clinicians and researchers alike.
1 Comas I, Coscolla M, Luo T, Borrell S, Holt KE, Kato-Maeda M, et al. Out-of-Africa migration and Neolithic coexpansion of Mycobacterium tuberculosis with modern humans. Nat Genet. 2013;45(10):1176–82.
2 World Health Organization. TB report. 2012 [cited 2013 14 Aug]; Available from: http://www.who.int/tb/publications/global_report/en/.
3 Reichman LB. The U-shaped curve of concern. Am Rev Respir Dis. 1991;144(4):741–2.
4 World Health Organization, Global Tuberculosis Report 2012, 2013.
5 Sandgren A, Hollo V, Quinten C, Manissero D. Childhood tuberculosis in the European Union/European Economic Area, 2000 to 2009. Euro Surveill. 2011;16(12).
6 Oesch Nemeth, G., Nemeth J, Altpeter E, Ritz N. Epidemiology of childhood tuberculosis in Switzerland between 1996 and 2011. Eur J of Pediatr. 2013; in press.
7 Nendaz M, Perrier A. Diagnostic errors and flaws in clinical reasoning: mechanisms and prevention in practice. Swiss Med Wkly. 2012;142:w13706.
8 Gengenbacher M, Kaufmann SH. Mycobacterium tuberculosis: success through dormancy. FEMS Microbiol Rev. 2012;36(3):514–32.
9 Lawn SD, Wood R, Wilkinson RJ. Changing concepts of “latent tuberculosis infection” in patients living with HIV infection. Clin Dev Immunol. 2011. 2011.
10 Young DB, Gideon HP, Wilkinson RJ. Eliminating latent tuberculosis. Trends Microbiol. 2009;17(5):183–8.
11 Barry CE, 3rd, Boshoff HI, Dartois V, Dick T, Ehrt S, Flynn J, et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol. 2009;7(12):845–55.
12 Marais BJ, Gie RP, Hesseling AC, Schaaf HS, Lombard C, Enarson DA, et al. A refined symptom-based approach to diagnose pulmonary tuberculosis in children. Pediatrics. 2006;118(5):e1350–9.
13 Hatherill M, Hanslo M, Hawkridge T, Little F, Workman L, Mahomed H, et al. Structured approaches for the screening and diagnosis of childhood tuberculosis in a high prevalence region of South Africa. Bull World Health Organ. 2010;88(4):312–20.
14 Nejat S, Buxbaum C, Eriksson M, Pergert M, Bennet R. Pediatric tuberculosis in Stockholm: a mirror to the world. Pediatr Infect Dis J. 2012;31(3):224–7.
15 Dodd LE, Wilkinson RJ. Diagnosis of paediatric tuberculosis: the culture conundrum. Lancet Infect Dis. 2013;13(1):3–4.
16 Sarrazin H, Wilkinson KA, Andersson J, Rangaka MX, Radler L, van Veen K, et al. Association between tuberculin skin test reactivity, the memory CD4 cell subset, and circulating FoxP3–expressing cells in HIV-infected persons. J Infect Dis. 2009;199(5):702–10.
17 Ritz N, Connell TG, Paxton GA, Buttery JP, Curtis N, Ranganathan SC. A three-way comparison of tuberculin skin testing, QuantiFERON-TB gold and T-SPOT.TB in children. PLoS ONE. 2008;3(7):e2624.
18 Connell T, Tebruegge M, Ritz N, Curtis N. Interferon-gamma release assays for the diagnosis of tuberculosis. Pediatr Infect Dis J. 2009;28(8):758–9.
19 Sun L, Xiao J, Miao Q, Feng WX, Wu XR, Yin QQ, et al. Interferon gamma release assay in diagnosis of pediatric tuberculosis: a meta-analysis. FEMS Immunol Med Microbiol. 2011;63(2):165–73.
20 Walzl G, Ronacher K, Hanekom W, Scriba TJ, Zumla A. Immunological biomarkers of tuberculosis. Nat Rev Immunol. 2011;11(5):343–54.
21 Chegou NN, Heyckendorf J, Walzl G, Lange C, Ruhwald M. Beyond the IFN-gamma horizon: Biomarkers for immunodiagnosis of infection with M. tuberculosis. Eur Respir J. 2013.
22 Lighter J, Rigaud M, Huie M, Peng CH, Pollack H. Chemokine IP-10: an adjunct marker for latent tuberculosis infection in children. Int J Tuberc Lung Dis. 2009;13(6):731–6.
23 Whittaker E, Gordon A, Kampmann B. Is IP-10 a better biomarker for active and latent tuberculosis in children than IFNgamma? PLoS One, 2008. 3(12): p. e3901.
24 Ruhwald M, Petersen J, Kofoed K, Nakaoka H, Cuevas LE, Lawson L, et al. Improving T-cell assays for the diagnosis of latent TB infection: potential of a diagnostic test based on IP-10. PLoS One. 2008;3(8):e2858.
25 Diel R, Loddenkemper R, Nienhaus A. Evidence-based comparison of commercial interferon-gamma release assays for detecting active TB: a metaanalysis. Chest. 2010;137(4):952–68.
26 Machingaidze S, Wiysonge CS, Gonzalez-Angulo Y, Hatherill M, Moyo S, Hanekom W, et al. The utility of an interferon gamma release assay for diagnosis of latent tuberculosis infection and disease in children: a systematic review and meta-analysis. Pediatr Infect Dis J. 2011;30(8):694–700.
27 Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K. Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection – United States, 2010. MMWR Recomm Rep. 2010;59(RR-5):1–25.
28 Lungenliga Schweiz, Handbuch Tuberkulose, 2011, Lungenliga Schweiz und Bundesamt für Gesundheit.
29 National Institute for Health and Clinical Excellence Tuberculosis:Clinical diagnosis and management of tuberculosis, and measures for its prevention and control. 2011.
30 Denkinger CM, Dheda K, Pai M. Guidelines on interferon-gamma release assays for tuberculosis infection: concordance, discordance or confusion? Clin Microbiol Infect. 2011;17(6):806–14.
31 Connell TG, Tebruegge M, Ritz N, Bryant PA, Leslie D, Curtis N. Indeterminate interferon-gamma release assay results in children. Pediatr Infect Dis J. 2010;29(3):285–6.
32 Thomas TA, Mondal D, Noor Z, Liu L, Alam M, Haque R, et al. Malnutrition and helminth infection affect performance of an interferon gamma-release assay. Pediatrics. 2010;126(6):e1522–9.
33 Debord C, De Lauzanne A, Gourgouillon N, Guerin-El Khourouj V, Pedron B, Gaudelus J, et al. Interferon-gamma release assay performance for diagnosing tuberculosis disease in 0– to 5–year-old children. Pediatr Infect Dis J. 2011;30(11):995–7.
34 World Health Organization Roadmap for childhood tuberculosis towards zero deaths. 2013.
35 Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367(9517):1173–80.
36 Hesseling AC, Marais BJ, Gie RP, Schaaf HS, Fine PE, Godfrey-Faussett P, et al. The risk of disseminated Bacille Calmette-Guerin (BCG) disease in HIV-infected children. Vaccine. 2007;25(1):14–8.
37 Ritz N, Curtis N. Mapping the global use of different BCG vaccine strains. Tuberculosis (Edinb). 2009;89(4):248–51.
38 Ritz N, Hanekom WA, Robins-Browne R, Britton WJ, Curtis N. Influence of BCG vaccine strain on the immune response and protection against tuberculosis. FEMS Microbiol Rev. 2008;32(5):821–41.
39 Ritz N, Dutta B, Donath S, Casalaz D, Connell TG, Tebruegge M, et al. The influence of bacille Calmette-Guerin vaccine strain on the immune response against tuberculosis: a randomized trial. Am J Respir Crit Care Med. 2012;185(2):213–22.
40 Favorov M, Ali M, Tursunbayeva A, Aitmagambetova I, Kilgore P, Ismailov S, et al. Comparative tuberculosis (TB) prevention effectiveness in children of Bacillus Calmette-Guerin (BCG) vaccines from different sources, Kazakhstan. PLoS ONE. 2012;7(3):e32567.
41 Ritz N, Tebruegge M, Connell TG, Sievers A, Robins-Browne R, Curtis N. Susceptibility of Mycobacterium bovis BCG vaccine strains to antituberculous antibiotics. Antimicrob Agents Chemother. 2009;53(1):316–8.
42 Dierig A, Tebruegge M, Krivec U, Heininger U, Ritz N. Current status of Bacille Calmette-Guérin (BCG) immunisation in Europe – a ptbnet survey, 2014: Abstract submitted: Annual meeting of the European Society for Paediatric Infectious Diseases.
43 Flanagan KL, van Crevel R, Curtis N, Shann F, Levy O. Heterologous (“nonspecific”) and sex-differential effects of vaccines: epidemiology, clinical trials, and emerging immunologic mechanisms. Clin Infect Dis. 2013;57(2):283–9.
44 Ritz N, CasalazD, Hanekom WA, Britton WJ, Dutta B, Donath S, et al. Reply: Bacille Calmette-Guerin vaccine: innate immunity and nonspecific effects. Am J Respir Crit Care Med. 2013;187(7):779–80.
45 Gan C, Mostafid H, Khan MS, Lewis DJ. BCG immunotherapy for bladder cancer – the effects of substrain differences. Nat Rev Urol. 2013;10(10):580–8.
46 Ritz N, Mui M, Balloch A, Curtis N. Non-specific effect of Bacille Calmette-Guerin vaccine on the immune response to routine immunisations. Vaccine. 2013;31(30):3098–103.
47 Ota, M.O., J. Vekemans, S.E. Schlegel-Haueter, K. Fielding, M. Sanneh, M. Kidd, et al. Influence of Mycobacterium bovis bacillus Calmette-Guerin on antibody and cytokine responses to human neonatal vaccination. J Immunol. 2002;168(2):919–25.
48 Roth A, Garly ML, Jensen H, Nielsen J, Aaby P. Bacillus Calmette-Guerin vaccination and infant mortality. Expert Rev Vaccines. 2006;5(2):277–93.
49 Shann F. The non-specific effects of vaccines. Arch Dis Child. 2010;95(9):662–7.
50 Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393(6685):537–44.
51 Behr MA, Wilson MA, Gill WP, Salamon H, Schoolnik GK, Rane S, et al. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science. 1999;284(5419):1520–3.
52 Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet. 2013.
53 Lalvani A, Sridhar S, von Reyn CF. Tuberculosis vaccines: time to reset the paradigm? Thorax. 2013;68(12):1092–4.
54 Bishai W, Sullivan Z, Bloom BR, Andersen P. Bettering BCG: a tough task for a TB vaccine? Nat Med. 2013;19(4):410–1.
55 Kagina BM, Abel B, Scriba TJ, Hughes EJ, Keyser A, Soares A, et al. Specific T Cell Frequency and Cytokine Expression Profile do not Correlate with Protection against Tuberculosis, Following BCG Vaccination of Newborns. Am J Respir Crit Care Med. 2010;182(8):1073–9.
56 Ritz N, Strach M, Yau C, Dutta B, Tebruegge M, Connell TG, et al. A comparative analysis of polyfunctional T cells and secreted cytokines induced by Bacille Calmette-Guerin immunisation in children and adults. PLoS ONE. 2012;7(7):e37535.
57 Zufferey C, Germano S, Dutta B, Curtis N, Ritz N. The Contribution of Non-Conventional T Cells and NK Cells in the Mycobacterial-Specific IFNgamma Response in Bacille Calmette-Guerin (BCG)-Immunized Infants. PLoS ONE. 2013;8(10):e77334.
58 Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. Lancet. 2000;356(9235):1099–104.
Funding / potential competing interests: NR is supported by a career advancement grant from the University of Basel and the Rozalia foundation. No other potential conflict of interest relevant to this article was reported.