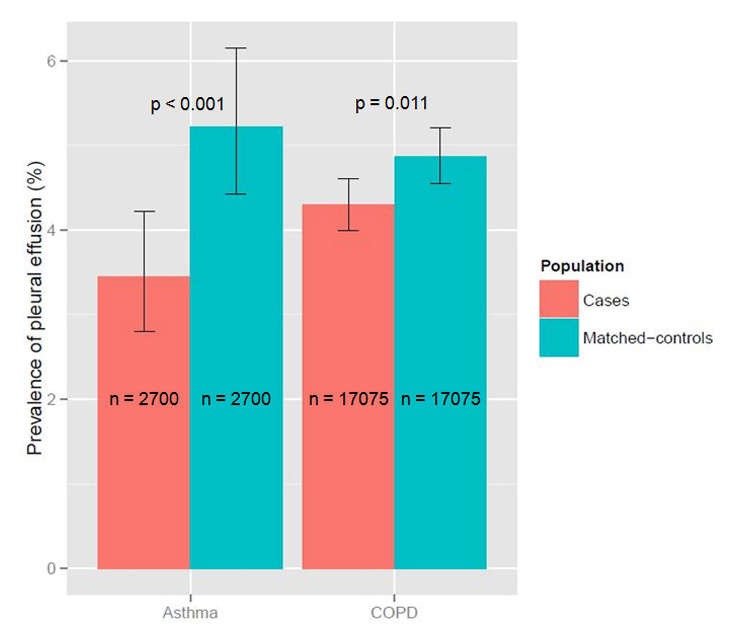
Figure 1
Prevalence of pleural effusion in hospitalised COPD and asthma patients compared to their matching controls without lung disease.
DOI: https://doi.org/10.4414/smw.2014.14013
A case control study
Abbreviations
AIP Acute interstitial pneumonia
ARDS Acute respiratory distress syndrome
CAP Community acquired pneumonia
COP Cryptogenic organising pneumonia
DIP Desquamative interstitial pneumonia
HIV Human immunodeficiency virus
ICD International classification of diseases
ICS Inhaled corticosteroids
ICU Intensive care unit
ILD Interstitial lung disease
IPF Idiopathic pulmonary fibrosis
LIP Lymphoid interstitial pneumonia
LOS Length of hospital stay
NS Not significant
NSIP Non-specific interstitial pneumonia
RB-ILD Respiratory bronchiolitis interstitial lung disease
Community-acquired pneumonia (CAP) is a frequent disease, causing more than one million hospitalisations annually in the US [1]. It is associated with high morbidity and mortality, being the ninth leading cause of death [2]. Several host conditions predispose to the development of CAP, including acute and chronic lung diseases such as toxic inhalations, pulmonary oedema, bronchial obstruction, cystic fibrosis, chronic obstructive pulmonary disease (COPD) and lung cancer, amongst many other risk factors [3].
The impact of pre-existing chronic lung diseases on disease-related complications and outcome in CAP is controversial and not well established. Some studies found COPD to be a protective factor against the development of complications of CAP [4, 5], whereas others describe COPD as a risk factor for higher CAP severity [6].
Inhaled corticosteroids (ICS) are a main pillar of the treatment of asthma and COPD. Their use in the treatment of COPD has been associated with an increased risk of pneumonia [7–11]. In a recent study, prior treatment with ICS for different chronic pulmonary diseases was associated with a lower incidence of parapneumonic effusion in CAP patients [12].
We aimed to investigate the impact of COPD, asthma and interstitial lung disease (ILD) on the incidence of complications (lung abscess, parapneumonic pleural effusion, empyema, acute respiratory distress syndrome [ARDS]) and outcome (in-hospital mortality, length of hospital stay [LOS]) in hospitalised adult CAP-patients in a retrospective case-control study.
For our analysis, we used a nationwide database including all hospitalisations in Switzerland from 2002 to 2010, provided by the Swiss Federal Office for Statistics, Neuchâtel, Switzerland. Due to the design of the database, individual level data for medications including ICS, the level of asthma control, COPD stage and smoking status were not available.
Inclusion criteria were hospitalisation due to CAP (ICD-10-codes J12.x, J13, J14, J15.x, J16.x, J17.x and J18.x) and age >17 years.
Exclusion criteria were HIV-infection, cancer and chronic lung diseases except COPD, asthma and ILD.
All diagnoses were based on the ICD-10 codes used in the database.
For this study, we analysed three pairs of comparisons:
1. Patients with CAP and a diagnosis of COPD (as the single chronic lung disease, ICD-10-codes J43.x and J44.x) versus patients with CAP, but without any chronic lung disease.
2. Patients with CAP and a diagnosis of asthma (as the single chronic lung disease, ICD-10-codes J45.x and J46) versus patients with CAP, but without any chronic lung disease.
3. Patients with CAP and a diagnosis of ILD (as the single chronic lung disease, ICD-10-code J84.x) versus patients with CAP, but without any chronic lung disease.
In order to control for a possible detection bias and performance bias in patients who are followed closely and frequently by specialists for chronic diseases for which patients might be encouraged to seek early medical attention if they develop dyspnoea or cough, we performed an additional comparison: we compared patients with heart failure and CAP but no underlying chronic lung disease with their matched controls as an internal plausibility control.
As a second internal plausibility control, we calculated the Charlson comorbidity index for all different groups to compare overall morbidity and to exclude a higher overall disease burden in the control groups, which could lead to results in favour of the disease groups.
ILD included idiopathic pulmonary fibrosis (IPF), acute interstitial pneumonia (AIP), desquamative interstitial pneumonia (DIP), nonspecific interstitial pneumonia (NSIP), respiratory bronchiolitis interstitial lung disease (RB-ILD), cryptogenic organising pneumonia (COP) and lymphoid interstitial pneumonia (LIP) (summarised by ICD-10-code J84.1), alveolar proteinosis (J84.0) and interstitial pulmonary diseases without further specification (J84.8 and J84.9).
We performed a retrospective, matched case control study. The groups were matched according to age, gender and month of hospitalisation to exclude seasonal effects. Matching for all relevant comorbidities was not feasible. We had initially tried to do so, but this would have reduced the sample size dramatically and would have excluded a large proportion of patients due to missing matched control patients, thereby limiting generalisability.
Endpoints were the incidence of complications of CAP (lung abscess [J85.x], parapneumonic pleural effusion [J90, J91], empyema [J86.x], ARDS [J80]), in-hospital mortality and LOS.
Descriptive statistics are reported as median and inter-quartile-range (IQR). Logistic regression with generalised estimating equations (using working independence correlation structure) was used to test the association between binary endpoints (presence/absence of complications, in-hospital mortality) within the two groups of our case-control design while adjusting for covariates (age and gender) and accounting for within-patient variability. P-values from the Wald-test statistic are reported. Similarly, Gaussian linear models with generalised estimating equations were used to test the association between continuous variables (length of stay) in our case control design, adjusting for covariates (age, gender) and accounting for within patient variability. Non-parametric two-sample Wilcoxon Rank Sum test was used in procedures involving continuous endpoints where the normality assumption was violated. All analyses were done using the R statistical software (v. 2.15.2).
According to the inclusion and exclusion criteria, the search of the database identified a total of 112,103 eligible patients hospitalised due to CAP in Switzerland between 2002 and 2010.

Figure 1
Prevalence of pleural effusion in hospitalised COPD and asthma patients compared to their matching controls without lung disease.
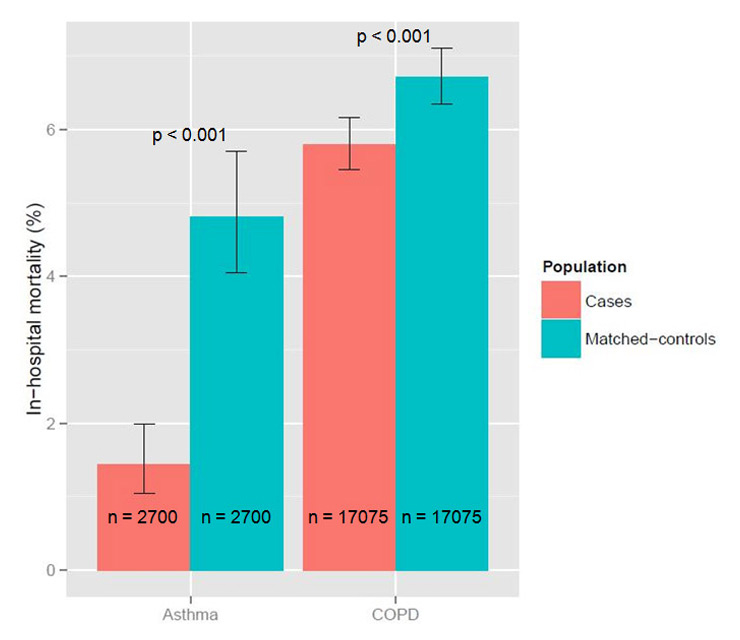
Figure 2
In-hospital mortality of hospitalised COPD and asthma patients compared to their matching controls without lung disease.
Of these, 90,998 (81.2%) had no pre-existing chronic lung disease, 21,105 (18.8%) had a history of COPD, asthma, ILD or a combination of these diseases.
17,075 patients (15.2%) had a diagnosis of COPD as single chronic lung disease, 2,700 patients (2.4%) had a diagnosis of asthma as single chronic lung disease and 916 patients (0.8%) had a diagnosis of ILD as single chronic lung disease.
The remaining 414 patients (0.4%) had a combination of these chronic lung diseases and were therefore excluded.
We compared 17,075 COPD patients with their matching controls. The median age was 75 years (IQR 67–82), 67.4% were males. At least one of the above mentioned complications occurred in 5.0% of COPD patients with CAP in comparison to 5.6% of the controls (p = 0.019). The most frequent complication was pleural effusion, which was less frequent in COPD patients (4.3% vs 4.9%, p = 0.011, fig. 1). The frequency of the other complications did not differ between COPD patients and controls. In-hospital mortality was slightly lower in COPD patients (5.8% vs 6.7%, p <0.001, fig. 2). LOS was longer in COPD patients (nine vs eight days, p <0.001).
We compared 2,700 asthma patients with their matching controls. The median age was 68 years (IQR 47–79), 37.1% were males. At least one of the above mentioned complications occurred in 4.2% of asthma patients with CAP in comparison to 6.3% of the controls (p <0.001). The most frequent complication was again pleural effusion, which was less frequent in asthma patients (3.4% vs 5.2%, p <0.001, fig. 1). The frequency of the other complications did not differ between asthma patients and controls. In-hospital mortality was markedly lower in asthma patients (1.4% vs 4.8%, p <0.001, fig. 2). LOS did not differ between the groups.
We compared 916 ILD patients with their matching controls. The median age was 78 years (IQR 69–83), 61.0% were males. At least one of the above mentioned complications occurred in 5.4% of ILD patients with CAP in comparison to 6.1% of the controls (p = 0.482). The most frequent complication was again pleural effusion, with no significant difference between the groups, which was also found for the other complications. In-hospital mortality was significantly higher in ILD patients (16.3% vs 6.8%, p <0.001), as was LOS (ten vs nine days, p <0.001).
We compared 7084 heart failure patients (and no additional chronic lung disease) with their matching controls. The median age was 83 years (IQR 77–88), 50.5% were males. At least one of the above mentioned complications occurred in 9.5% of heart failure patients with CAP in comparison to 4.9% of the controls (p <0.001). The most frequent complication was again pleural effusion, which was more frequent in heart failure patients (8.8% vs 4.2%, p <0.001). The frequency of the other complications did not differ between heart failure patients and controls. In-hospital mortality was markedly higher in heart failure patients (12.5% vs 9.4%, p <0.001), LOS was only slightly higher in this group (10 vs 9 days, p <0.001).
All results are summarised in table 1.
The Charlson comorbidity index was highest in heart failure patients, followed by COPD, ILD and asthma patients. In all groups, values were significantly higher as compared to the control patients (table 2).
| Table 1: Outcome results of hospitalised CAP patients. Comparison of COPD, asthma, ILD and heart failure patients with their corresponding matched control groups. | |||
| COPD | Control | p | |
| N | 17,075 | 17,075 | – |
| Age in years (IQR) | 75 (67–82) | 75 (67–82) | – |
| Male gender (%) | 67.4 | 67.4 | – |
| Any complication (%) | 5.0 | 5.6 | 0.019 |
| Lung abscess (%) | 0.1 | 0.1 | 0.100 |
| Pleural effusion (%) | 4.3 | 4.9 | 0.011 |
| Empyema (%) | 0.5 | 0.5 | 0.817 |
| ARDS (%) | 0.2 | 0.3 | 0.150 |
| In-hospital mortality (%) | 5.8 | 6.7 | <0.001 |
| LOS (IQR) | 9 (6–14) | 8 (5–13) | <0.001 |
| Asthma | Control | p | |
| N | 2700 | 2700 | – |
| Age in years (IQR) | 68 (47–79) | 68 (47–79) | – |
| Male gender (%) | 37.1 | 37.1 | – |
| Any complication (%) | 4.2 | 6.3 | <0.001 |
| Lung abscess (%) | 0.2 | 0.0 | 0.214 |
| Pleural effusion (%) | 3.4 | 5.2 | 0.001 |
| Empyema (%) | 0.5 | 0.9 | 0.141 |
| ARDS (%) | 0.3 | 0.3 | 0.620 |
| In-hospital mortality (%) | 1.4 | 4.8 | <0.001 |
| LOS (IQR) | 7 (4–11) | 7 (4–12) | 0.83 |
| ILD | Control | p | |
| N | 916 | 916 | – |
| Age in years (IQR) | 78 (69–83) | 78 (69–83) | – |
| Male gender (%) | 61.0 | 61.0 | – |
| Any complication (%) | 5.4 | 6.1 | 0.482 |
| Lung abscess (%) | 0.0 | 0.0 | – |
| Pleural effusion (%) | 4.2 | 5.1 | 0.318 |
| Empyema (%) | 0.2 | 0.7 | 0.180 |
| ARDS (%) | 1.0 | 0.6 | 0.287 |
| In-hospital mortality (%) | 16.3 | 6.8 | <0.001 |
| LOS (IQR) | 10 (6–16) | 9 (5–13) | <0.001 |
| Heart failure | Control | p | |
| N | 7084 | 7084 | – |
| Age in years (IQR) | 83 (77–88) | 83 (77–88) | – |
| Male gender (%) | 50.5 | 50.5 | – |
| Any complication (%) | 9.5 | 4.9 | <0.001 |
| Lung abscess (%) | 0.0 | 0.1 | 0.272 |
| Pleural effusion (%) | 8.8 | 4.2 | <0.001 |
| Empyema (%) | 0.4 | 0.5 | 0.705 |
| ARDS (%) | 0.4 | 0.2 | 0.104 |
| In-hospital mortality (%) | 12.5 | 9.4 | <0.001 |
| LOS (IQR) | 10 (6–16) | 9 (5–13) | <0.001 |
| Table 2: Charlson comorbidity indices for the different patient groups. | |||
| Case | Control | p | |
| COPD | 2.1 | 1.0 | <0.001 |
| Asthma | 1.7 | 0.7 | <0.001 |
| ILD | 1.8 | 1.0 | <0.001 |
| Heart failure | 2.4 | 0.9 | <0.001 |
We found significantly less disease-related complications of CAP in hospitalised COPD and asthma patients compared with their controls, mainly related to a lower incidence of pleural effusion. In-hospital mortality was also lower in the COPD and – much more pronounced – asthma cohorts.
Chalmers et al. identified a history of COPD as a protective factor against the development of complicated parapneumonic effusion and empyema in patients hospitalised due to CAP (adjusted odds ratio 0.18) [4]. Cillóniz et al. also found COPD to be a protective factor against pulmonary complications of pneumococcal CAP (odds ratio 0.38) [5]. These findings are in line with our results.
A recent study found that prior treatment with ICS in patients with different chronic respiratory disorders who developed CAP was associated with a lower incidence of parapneumonic pleural effusion (5% vs 12%) [12]. This is also in line with our results for COPD and asthma patients, who are often treated with ICS. Of note, our database did not provide information about the medical therapy, so that a direct association with ICS use could not be tested in our study.
A recent meta-analysis aimed to investigate the mortality risk from CAP in COPD patients and revealed heterogeneous results without clear evidence for a higher mortality of COPD patients or a lower mortality for patients with prior ICS use [13]. In our cohort, mortality was slightly lower in COPD patients compared to their matching controls.
Other recent studies suggested a causal association between the use of ICS and the risk of developing CAP in patients with COPD, but without an association with increased mortality [14, 15].
Taken together our results and the previously mentioned findings suggest that COPD is not associated with a higher in-hospital mortality in patients hospitalised due to CAP, and it could possibly be a protective factor against complications of CAP.
As far as asthma is concerned, CAP patients with a history of asthma in our cohort had a markedly lower risk of parapneumonic pleural effusion (3.4% vs 5.2%) and in-hospital mortality (1.4% vs 4.8%) as compared to their matching controls without asthma. In the literature, there is very limited information on the effect of asthma in patients with CAP. A Japanese study compared asthmatic CAP patients under ICS therapy with asthmatic CAP patients not under ICS therapy and found no differences regarding outcome [16]. The reason for the markedly lower CAP-mortality in our cohort of patients with asthma is not obvious. One hypothesis could be the anti-inflammatory effect of ICS, which are frequently given in asthmatics. ICS could possibly mitigate the degree of inflammation in the lung tissue and thereby improve outcome in CAP.
When hypothesizing about other possible explanations for a better outcome of COPD and asthma patients hospitalised for CAP, one could imagine a higher degree of cautiousness among general practitioners while treating these patients, with a consecutively lower threshold to hospitalise, when CAP is suspected. Additionally, patients may seek care at an earlier time because they are trained to assess themselves or because their cardiopulmonary reserve is lower. This could lead to an earlier administration of adequate CAP therapy. The same assumption could be made for the physicians in the hospital, who might provide more attention to patients with underlying chronic lung diseases and thereby recognize clinical deterioration earlier, thus improving outcome.
Possibly, the different infective agent spectrum between patients with or without chronic lung disease may also contribute to a different severity of CAP complications. A rising topic regarding this issue is the lung microbiome, which seems to differ between healthy and diseased lungs with implications for immune regulation [17]. A bidirectional interaction between the host immune defences and the constitution of the lung microbiome has been proposed [18]. The microbial diversity seems to be dependent on age, disease severity and medication use [19].
Another hypothesis could be a different immune response due to colonisation in patients with chronic lung diseases. A recent study detected different early inflammatory patterns between CAP patients with and without associated COPD [20]. Arguably, colonisation might lead to a preformed humoral and cellular immune response, which could provide earlier and more profound protection and improve outcome. This association is suggested in studies, which found a lower mortality from bacteraemia in patients who are colonised with Staphylococcus aureus[21]. Alternatively, colonisation might also lead to immune tolerance and less detrimental inflammatory responses, again with beneficial outcome [22], which also could contribute to a better microbial control.
For ILD patients, we did not find a difference in the frequency of the pre-specified CAP complications; as a possible explanation, one could again hypothesize that due to the fact that ICS is not a standard therapy in this patient group, the formerly mentioned possible protective anti-inflammatory effect is missing. We found a markedly higher mortality in comparison to the control group (16.3% vs 6.8%), which can be expected due to the more or less severe limitation of baseline lung function, especially of the lung parenchyma, in comparison to the control group without chronic lung disease. Subsequently, relatively minor infections can lead to a rapid worsening of the pre-existing unstable gas exchange balance in ILD patients. Of course, the probability of respiratory failure with concomitant need for ventilation and/or ICU admission is considerably higher in this patient group.
As an internal plausibility control of our results, we additionally analysed heart failure patients hospitalised for CAP with matching controls without heart failure. We regarded heart failure patients similar to patients with severe chronic lung diseases, e.g., in terms of overall morbidity and limitation.
Interestingly, the higher mortality of patients with heart failure suggests that the beneficial effect of asthma and COPD on pneumonia complications was unlikely to be due to earlier attention to symptoms or a lower cardiopulmonary response but rather due to an alternative effect. Of course, this is only indirect evidence for the plausibility of the results. Alternatively, the higher mortality from pneumonia in patients with heart failure might be explained by erroneously suspecting a cardiac origin of symptoms instead of a respiratory infection and possibly later initiation of appropriate therapy. The markedly higher in-hospital mortality in the heart failure control group in comparison to the other control groups is most likely due to the higher average age in this cohort (83 years vs 75, 68 and 78 years for COPD, asthma and ILD, respectively).
The fact that the Charlson comorbidity index was also markedly higher in COPD and asthma patients compared with their controls (table 2) additionally strengthens the results, because according to this one would expect higher complication and in-hospital mortality rates for COPD and asthma patients and not vice versa.
One limitation of our study is its retrospective design and the fact that the database provided by the Swiss Federal Office for Statistics did not contain certain clinical parameters, which also would have been of interest, e.g. ICU admission and the need for ventilator support. Further, mistakes in ICD-10-coding by the treating physicians cannot be excluded. Adjustment for disease severity was not possible due to the design of the database, so that a certain degree of selection bias cannot be excluded. Another limitation is the lack of information regarding ICS use in the database, so that we can only speculate about a possible influence of ICS use on our results. Further, the level of asthma control, COPD stage and smoking state were not available.
The main strength of our study is the comprehensive coverage of the database covering all hospitals in Switzerland with a consecutively high number of patients. This fact should also contribute to balancing mistakes in ICD-10-coding between case and control groups and thereby diminish the relevance of such possible mistakes. Furthermore, due to the high number of patients, we were able to perform a well-balanced matching of case and control groups.
In summary, bearing in mind the above mentioned limitations, our results indicate a lower incidence of composite complications of CAP in COPD patients and an even more pronounced reduction in asthma patients compared to matched control groups without any chronic lung disease, mainly due to a lower incidence of parapneumonic pleural effusion. This could not be shown for ILD patients, where the incidence of composite CAP complications did not differ between case and control group. We could show slightly lower in-hospital mortality in COPD patients and a markedly lower in-hospital mortality in asthma patients hospitalised due to CAP, a finding, which has not been described until now. In contrast and as expected, ILD patients with CAP had markedly higher in-hospital mortality than matched controls without chronic lung disease.
Our results are novel and provocative and should be viewed as hypothesis generating, with different possible explanations for our findings, including possible influence of ICS therapy, a possibly higher awareness of general practitioners and hospital physicians while treating patients with chronic lung diseases, the different microbial spectrum or a differently regulated immune response.
We found less complications and a lower in-hospital mortality of CAP in COPD and asthma patients compared with their controls.
1 http://www.cdc.gov/nchs/data/nhds/2average/2010ave2_firstlist.pdf
2 Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America, American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl2):S27.
3 Almirall J, Bolíbar I, Balanzó X, González CA. Risk factors for community-acquired pneumonia in adults: a population-based case-control study. EurRespir J. 1999;13(2):349.
4 Chalmers JD, Singanayagam A, Murray MP, Scally C, Fawzi A, Hill AT. Risk factors for complicated parapneumonic effusion and empyema on presentation to hospital with community-acquired pneumonia. Thorax. 2009;64(7):592–7.
5 Cillóniz C, Ewig S, Polverino E, Muñoz-Almagro C, Marco F, Gabarrús A, et al. Pulmonary complications of pneumococcal community-acquired pneumonia: incidence, predictors, and outcomes. Clin Microbiol Infect. 2012;18(11):1134–42.
6 Ishiguro T, Takayanagi N, Yamaguchi S, Yamakawa H, Nakamoto K, Takaku Y, et al. Etiology and factors contributing to the severity and mortality of community-acquired pneumonia. Intern Med. 2013;52(3):317–24.
7 Eurich DT, Lee C, Marrie TJ, Majumdar SR. Inhaled corticosteroids and risk of recurrent pneumonia: a population-based, nested case-control study. Clin Infect Dis. 2013;57(8):1138–44.
8 Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–89.
9 Calverley PM, Stockley RA, Seemungal TA, Hagan G, Willits LR, Riley JH, et al. Reported pneumonia in patients with COPD: findings from the INSPIRE study. Chest. 2011;139(3):505–12.
10 Singh S, Amin AV, Loke YK. Long-term use of inhaled corticosteroids and the risk of pneumonia in chronic obstructive pulmonary disease: a meta-analysis. Arch Intern Med. 2009;169(3):219–29.
11 Drummond MB, Dasenbrook EC, Pitz MW, Murphy DJ, Fan E. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300(20):2407–16.
12 Sellares J, López-Giraldo A, Lucena C, Cilloniz C, Amaro R, Polverino E, et al. Influence of Previous Use of Inhaled Corticoids on the Development of Pleural Effusion in Community-Acquired Pneumonia. Am J Respir Crit Care Med. 2013;187(11):1241–8.
13 Loke YK, Kwok CS, Wong JM, Sankaran P, Myint PK. Chronic obstructive pulmonary disease and mortality from pneumonia: meta-analysis. Int J Clin Pract. 2013;67(5):477–87.
14 Marzoratti L, Iannella HA, Waterer GW. Inhaled corticosteroids and the increased risk of pneumonia. Ther Adv Respir Dis. 2013;7(4):225–34.
15 Yawn BP, Li Y, Tian H, Zhang J, Arcona S, Kahler KH. Inhaled corticosteroid use in patients with chronic obstructive pulmonary disease and the risk of pneumonia: a retrospective claims data analysis. Int J COPD. 2013;8:295–304.
16 To M, To Y, Yamada H, Ogawa C, Otomo M, Suzuki N, et al. Influence of inhaled corticosteroids on community-acquired pneumonia in patients with bronchial asthma. Intern Med. 2004;43(8):674–8.
17 Sze MA, Hogg JC, Sin DD. Bacterial microbiome of lungs in COPD. Int J Chron Obstruct Pulmon Dis. 2014;21(9):229–38.
18 Segal LN, Rom WN, Weiden MD. Lung Microbiome for Clinicians. New Discoveries about Bugs in Healthy and Diseased Lungs. Ann Am Thorac Soc. 2014;11(1):108–16.
19 Martinez FJ, Erb-Downward JR, Huffnagle GB. Significance of the microbiome in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2013;Suppl:170–9.
20 Crisafulli E, Menéndez R, Huerta A, Martinez R, Montull B, Clini E, et al. Systemic inflammatory pattern of patients with community-acquired pneumonia with and without COPD. Chest. 2013;143(4):1009–17.
21 Wertheim HF, Vos MC, Ott A, van Belkum A, Voss A, Kluytmans JA, et al. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet. 2004;364(9435):703–5.
22 Derrien M, Van Baarlen P, Hooiveld G, Norin E, Müller M, de Vos WM. Modulation of Mucosal Immune Response, Tolerance, and Proliferation in Mice Colonized by the Mucin-Degrader Akkermansia muciniphila. Front Microbiol 2011;2:166.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article were reported.
Authors' contribution: Frank Dusemund and Joannis Chronis are equally contributing first authors