Vitamin D status among children and adolescents on anticonvulsant drugs in Southern Switzerland
DOI: https://doi.org/10.4414/smw.2014.13996
Vera
Ramelli, Gian Paolo
Ramelli, Sebastiano A. G.
Lava, Giordano M.
Siegenthaler, Marco
Cantù, Mario G.
Bianchetti, Alessandro
Ceschi
Summary
INTRODUCTION: It is recognised that vitamin D status is often inadequate (<50 nmol/l) in epileptic children, mainly because some anticonvulsant drugs induce the enzymes responsible for its metabolism. The purpose of the present study was to address vitamin D status among children and adolescents treated with anticonvulsant drugs and control subjects who reside in southern Switzerland, a high solar radiation region.
METHODS: Between January and May 2013, total serum 25-hydroxyvitamin D was assessed by liquid chromatography-tandem mass spectrometry in 58 children and adolescents with epilepsy and 29 controls residing in southern Switzerland. Dark-skinned individuals, females wearing dress styles covering practically the whole body and subjects with body mass index ≥85th percentile for age and sex were excluded.
RESULTS: Concentration of serum 25-hydroxyvitamin D was similar in epilepsy patients (48 [37–62] nmol/l; median and interquartile range) and controls (53 [47–64] nmol/l). An inadequate serum 25-hydroxyvitamin D concentration was common both among patients (55%) and control subjects (34%). Serum 25-hydroxyvitamin D was significantly lower among patients treated with anticonvulsant drugs that induce the metabolism of vitamin D (30 [21–51] nmol/l) than among the remaining patients (51 [40–65] nmol/l) and controls.
CONCLUSIONS: The present study indicates a relevant tendency towards inadequate vitamin D status among children with and without anticonvulsant drug management who reside in southern Switzerland. This tendency is more prominent in patients treated with anticonvulsant drugs that induce the metabolism of 25-hydroxyvitamin D.
Introduction
In childhood, vitamin D is crucial for bone growth and mineralisation because it promotes the assimilation of nutritional calcium and phosphate. New research indicates that the benefits of vitamin D extend to the musculoskeletal system as a whole [1–5]. Furthermore, vitamin D might also play a role in susceptibility to diabetes, cancer, infections and cardiovascular, neurologic or psychiatric diseases. Finally, it might modulate the inflammatory pathways [1–5]. As a consequence, the 25-hydroxyvitamin D blood concentration of ≥25 nmol/l that was thought to be indicative of “health” in the early 1970s is currently considered inadequate [5].
It has been recognised since the 1970s that vitamin D status is often poor in epileptic children, mainly because some anticonvulsant drugs such as carbamazepine, oxcarbazepine, phenobarbital and phenytoin induce the enzymes responsible for the metabolism of 25-hydroxyvitamin D, resulting in its increased conversion into inactive metabolites [6–10]. In addition, sun exposure might often be low in epileptic children concurrently affected with developmental delay or cerebral palsy. Nonetheless, epileptic patients are only rarely supplemented with this vitamin [7, 10].
The purpose of the present study was to address vitamin D status among children and adolescents treated with anticonvulsant drugs residing in southern Switzerland, a high solar radiation region [5]. Since recent data suggest that, although overt vitamin D deficiency is no longer common, lesser degrees of hypovitaminosis D are widespread, a control group was included.
Subjects and methods
All epileptic patients 2 to 20 years of age on anticonvulsant drug therapy, who received no vitamin D supplementation and were on regular follow-up at the Department of Pediatrics, Ente Ospedaliero Cantonale Ticinese, were eligible for enrollment in the trial. They had to meet all of the following criteria to qualify for participation: anticonvulsant drug treatment for ≥12 months, stable drug management and absence of seizure activity for ≥4 months, as well as absence of liver or kidney disease. Eligible for the study as controls were also non-pharmacologically treated children with functional voiding disorders, idiopathic childhood nephrotic syndrome cured for ≥2 years, attention deficit hyperactivity disorder, or admitted for minor surgical procedures ( e.g., inguinal hernia repair, phimosis surgery or hydrocelectomy) in a ratio of 1:2 cases to epilepsy patients.
Vitamin D status was assessed exclusively in epilepsy patients and controls for whom a blood collection was ordered for medical reasons independent of the study. Dark-skinned individuals, female adolescents wearing dress styles covering practically the whole body and subjects with body mass index ≥85th percentile for age and sex were excluded both from the study group and the control group [5].
The epileptic disease was categorised as “associated” in patients who also had developmental delay or cerebral palsy and as “isolated” in those without these difficulties. Liquid chromatography-tandem mass spectrometry, which quantitatively measures both 25-hydroxyvitamin D2and 25-hydroxyvitamin D3, was applied for the determination of total 25-hydroxyvitamin D concentration, as previously reported [11]. Between-run coefficients of variation were 4.6% at a 25-hydroxyvitamin D3 concentration of 69 nmol/l and 4.4% at a 25-hydroxyvitamin D3 concentration of 145 nmol/l, respectively [11]. A total 25-hydroxyvitamin D level <50 nmol/l was considered inadequately low [1, 5].
The study design had been approved by the local Ethics Committee and written informed consent was obtained. The D’Agostino-Pearson omnibus test for normality demonstrated that most numerical data are not sampled from a Gaussian distribution. Numerical data are presented as median and interquartile range, categorical data as relative frequency. Statistical evaluations included the two-tailed Kruskal-Wallis test with the Dunn’s post hoc test, regressions (with the rank correlation coefficient), and the Fisher exact test [12]. P <0.05 was accepted to indicate statistical significance [12].
Results
Between January and May 2013, 58 Swiss-Italian patients with epilepsy, 33 affected by “isolated” and 25 affected by “associated” epilepsy, and a group of 29 controls entered the study (table 1). The 58 patients were on treatment with one (N = 43), two (N = 13) or three (N = 2) anticonvulsant drugs. Age and body weight were significantly (Kruskall-Wallis test; P <0.01) lower in controls than in patients. On the contrary, concentration of serum 25-hydroxyvitamin D was similar in the study group and in controls. 25-hydroxyvitamin D2level was always ≤6 nmol/l both in patients and controls. Patients with “isolated” and patients with “associated” epilepsy significantly differed with respect to age and weight but not with respect to serum 25-hydroxyvitamin D concentration. An inadequate serum 25-hydroxyvitamin D concentration was common among control subjects (34%), patients with “associated” (52%) and patients with “isolated” (58%) epilepsy (without any significant difference between the groups; Fisher exact test). Serum 25-hydroxyvitamin D was similar (Kruskal-Wallis test) in female and male patients (47 [39–60] versus 48 [31–64] nmol/l), in female and male controls (55 [48–70] versus 53 [45–59] nmol/l) and age-independent (regression analysis) both in controls and epilepsy patients. Serum 25-hydroxyvitamin D was similar (Kruskal-Wallis test) in 16 control subjects admitted for minor surgical procedures (53 [47–66] nmol/l) and in the remaining 13 control subjects (53 [45–66] nmol/l).
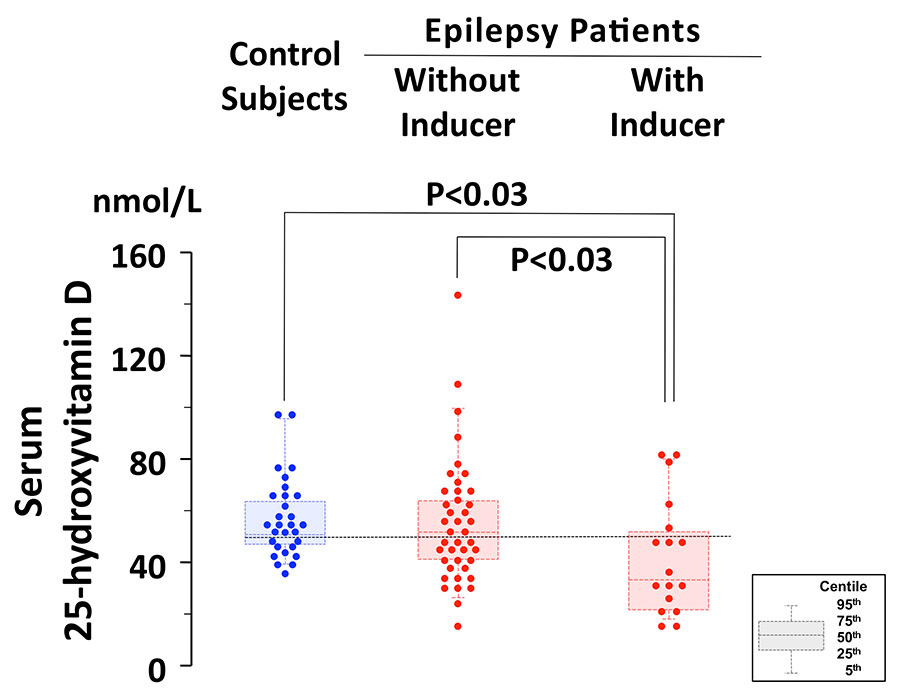
Figure 1
25-hydroxyvitamin D concentration in 29 controls, in 41 epilepsy patients without drugs that induce the metabolism of vitamin D and in 17 patients treated with at least one drug that induces vitamin D metabolism (carbamazepine, oxcarbazepine, phenobarbital or phenytoin). The results are given both as individual values as well as "box and whiskers plot" (the bottom and top of the box are the 25th and 75th centile, the middle of the box is the 50thcentile, i.e., the median, the ends of the whiskers represent the 5th and the 95th centile). The horizontal dotted line denotes the adequacy threshold level of 50 nmol/l.
Serum 25-hydroxyvitamin D concentration was significantly lower (P <0.03; Kruskal-Wallis test) in 17 patients on treatment with at least one drug that induces the metabolism of vitamin D (carbamazepine, oxcarbazepine, phenobarbital or phenytoin) as compared with the remaining 41 patients and the 29 controls (fig. 1 and table 2). Patients managed with drugs that induce the metabolism of vitamin D were significantly older (Kruskal-Wallis test) than the remaining patients (P <0.05) and the control subjects (P <0.01).
|
Table 1: Clinical data and antiepileptic drug management in 58 pediatric patients with epilepsy and 29 control patients. Numerical data are presented as median and interquartile range, categorical data as relative frequency. |
| |
Controlsubjects
|
Epilepsy Patients
|
| |
All
|
“Isolated”
|
“Associated”
|
| N |
29 |
58 |
33 |
25 |
| Gender, male to female |
16:13 |
34:24 |
19:14 |
15:10 |
| Age, years |
4.8 [2.9–6.2] |
12.2* [7.3–17.3] |
11.5** [7.5–14.6] |
15.7**, # [6.1–18.1] |
| Body weight, kg |
16.9 [13.9–21.0] |
52.4* [26.0–68.3] |
45.0** [26.0–61.9] |
55.5**, # [32.0–71.1] |
| Total serum 25-hydroxyvitamin D |
|
|
|
|
| Concentration, nmol/l |
53 [47–64] |
48 [37–62] |
48 [37–66] |
47 [32–61] |
| Concentration <50 nmol/l, N (%) |
10 (34) |
32 (55) |
19 (58) |
13 (52) |
| Drug treatment |
|
|
|
|
| Carabamazepine, N |
– |
10 |
5 |
5 |
| Oxcarbazepine, N |
– |
3 |
|
3 |
| Phenytoin, N |
– |
1 |
|
1 |
| Phenobarbital, N |
– |
3 |
1 |
2 |
| Clobazam, N |
– |
2 |
|
2 |
| Ethosuximide, N |
– |
7 |
6 |
1 |
| Lamotrigine, N |
– |
9 |
5 |
4 |
| Levetiracetam, N |
– |
5 |
2 |
3 |
| Stiripentol, N |
– |
1 |
|
1 |
| Sultiam, N |
– |
4 |
4 |
|
| Topiramate, N |
– |
6 |
2 |
4 |
| Valproic acid, N |
– |
23 |
14 |
9 |
| Vigabatrin, N |
– |
1 |
|
1 |
| * P <0.01 and ** P<0.03 versus control subjects (Kruskal-Wallis test); # P<0.05 versus patients with “isolated” epilepsy (Kruskal-Wallis test). |
|
Table 2: Clinical data in 41 epileptic patients without and 17 with drugs that induce the metabolism of vitamin D (carbamazepine, oxcarbazepine, phenobarbital or phenytoin). Numerical data are presented as median and interquartile range, categorical data as relative frequency. |
| |
Without inducer of vitamin D
metabolism |
With inducer
of vitamin D
metabolism |
Significance |
| N |
41 |
17 |
|
| Gender, male to female |
25:16 |
9:8 |
Not significant#
|
| Age, years |
11.9 [7.2–15.7] |
16.8 [10.1–18.5] |
P <0.05* |
| Body weight, kg |
47.3 [24.3–61.9] |
52.7 [35.0–68.7] |
Not significant* |
| Total serum 25-hydroxyvitamin D |
|
|
|
| Concentration, nmol/l |
51 [40–65] |
30 [21–51] |
P <0.03* |
| Concentration <50 nmol/l, N (%) |
20 (49) |
12 (71) |
Not significant* |
| Epilepsy type |
|
|
|
| “Isolated” |
26 |
7 |
|
| “Associated” |
15 |
10 |
|
|
# Fisher exact test; * Kruskal-Wallis test. |
Discussion
The present investigation was performed among children and adolescents on anticonvulsant drug management and control subjects residing in Southern Switzerland between January and May, i.e., during a season of the year with low solar exposure. It indicates that serum 25-hydroxyvitamin D concentration is inadequate in approximately half of the participants. Unsurprisingly, the tendency to hypovitaminosis D was more pronounced in epileptic patients on drugs that induce vitamin D metabolism. However, the concentration of 25-hydroxyvitamin D was similar in patients with and without developmental delay or cerebral palsy, suggesting a similar solar exposure in these two groups of patients. It is therefore concluded that in the winter half-year, solar radiation is often insufficient to maintain the vitamin D level in our region. In the summer half-year exposure of arms, hands and face 2 to 3 times a week for 10–20 minutes is believed to be adequate to maintain vitamin D level [5].
There is some controversy regarding the best methodology for assessing 25-hydroxyvitamin D concentration in clinical practice. In this study, 25-hydroxyvitamin D was measured using liquid chromatography-tandem mass spectrometric analysis, a robust technology that is more accurate than immunological assays [13, 14].
This study was performed in a high solar radiation region. The substantial advancement over recent preceding work [15] was the inclusion of a control group of healthy children and adolescents, whose 25-hydroxyvitamin D level was similar to that found on anticonvulsant drugs. It has been often stated that vitamin D supplementation is imperative in children and adolescents on long-term antiepileptic drugs [6–10, 15]. That is true, but our results suggest that all children and adolescents residing in southern Switzerland are at risk, at least during winter months, to have an inadequate level of 25-hydroxyvitamin D. Similar observations were recently made among healthy Italian children [16] and among Swiss children with type 1 diabetes mellitus residing in a rather low solar radiation region [17].
Standards for defining vitamin D adequacy in children are not well established. X-ray changes of rickets and reduced bone density have been observed at 25-hydroxyvitamin D levels of <40 nmol/l and alkaline phosphatase levels have been noted to rise at levels <50 nmol/l [1–5]. Hence, for this study we used the adequacy threshold level of ≥50 nmol/l, recommended among others by the Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society and the German Nutrition Society [1]. There is little evidence from studies in children that vitamin D levels ≥75 nmol/l are necessary, as suggested by some authorities, including among others the Canadian Pediatric Society and the Italian Society for Osteoporosis, Mineral Metabolism and Bone Diseases [1–5].
A limitation of our study is that we were not able to assess the 25-hydroxyvitamin D concentration in patients on anticonvulsants and in healthy subjects matched for age and gender in a ratio of 1:1 cases. Nonetheless, in this study 25-hydroxyvitamin D levels were age and gender independent both in epilepsy patients and controls. Furthermore, considering that in general vitamin D levels are lower in adolescence [1–5], the inclusion of a well-matched control group would not have relevantly modified the results. Another limitation is the heterogeneity of the drugs used in our patients and the resulting small case numbers for each drug. Moreover, the distinction in two groups regarding the effect on vitamin D metabolism (inducers versus non-inducers) is to some extent arbitrary as induction of metabolism may occur to different extents.
In conclusion, the present study indicates a relevant tendency towards inadequate vitamin D status among children without and with anticonvulsant drug management residing in southern Switzerland, a high solar radiation region. The tendency, which deserves further confirmation in a larger and better-designed trial, is slightly more pronounced in epileptic patients treated with drugs that induce the metabolism of 25-hydroxyvitamin D. This alarmingly high prevalence of hypovitaminosis D in the general pediatric population during the winter and spring warrants action at a population level rather than at a risk group level [1–5, 18]. The recommendation that Swiss infants receive a supplementation of 400 units of vitamin D3 per day during the first 12 months of life has been recently extended [5]. Nowadays, 600 units of vitamin D3 per day are recommended also for the second and third year of life and 600 units per day exclusively during the winter and spring for older children and adolescents [5]. Based on our data, this supplementation might well be advised also for epileptic children. For children and adolescents managed with drugs that induce vitamin D, year-round vitamin D supplementation might seem appropriate. At our institutions we also advise screening of 25-hydroxyvitamin D level in the latter patients, considering that for every 100 units of added vitamin D3, 25-hydroxyvitamin D concentration is expected to increase by approximately 2–3 nmol/L [1, 5].
References
1 Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M; Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398–417.
2 Wacker M, Holick MF. Vitamin D – effects on skeletal and extraskeletal health and the need for supplementation. Nutrients. 2013;5:111–48.
3 Shaw NJ, Mughal MZ. Vitamin D and child health part 1 (skeletal aspects). Arch Dis Child. 2013;98:363–7.
4 Shaw NJ, Mughal MZ. Vitamin D and child health: part 2 (extraskeletal and other aspects). Arch Dis Child. 2013;98:368–72.
5 Lava SA, Simonetti GD, Bianchetti AA, Ferrarini A, Bianchetti MG. Prevention of vitamin D insufficiency in Switzerland: A never-ending story. Int J Pharm. 2013;457:353–6.
6 Hunter J, Maxwell JD, Stewart DA, Parsons V, Williams R. Altered calcium metabolism in epileptic children on anticonvulsants. BMJ. 1971;263:202–4.
7 Sheth RD. Metabolic concerns associated with antiepileptic medications. Neurology. 2004;63(Suppl 4):S24–9.
8 Mintzer S, Boppana P, Toguri J, DeSantis A. Vitamin D levels and bone turnover in epilepsy patients taking carbamazepine or oxcarbazepine. Epilepsia. 2006;47:510–5.
9 Misra A, Aggarwal A, Singh O, Sharma S. Effect of carbamazepine therapy on vitamin D and parathormone in epileptic children. Pediatr Neurol. 2010;43:320–4.
10 Wirrell E. Vitamin D and bone health in children with epilepsy: fad or fact? Pediatr Neurol. 2010;42:394–5.
11 Cantù M, Keller F. Vitamin D LC-MS/MS: optimization of chromatographic parameters to increased 60% of commercial kit througput. Biochim Clin. 2013;37:S442.
12 Brown GW, Hayden GF. Nonparametric methods. Clinical applications. Clin Pediatr (Phila). 1985;24:490–8.
13 Hollis BW. Editorial: The determination of circulating 25-hydroxyvitamin D: no easy task. J Clin Endocrinol Metab. 2004;89:3149–51.
14 Guessous I, Dudler V, Glatz N, Theler JM, Zoller O, Paccaud F, Burnier M, Bochud M; Swiss Survey on Salt Group. Vitamin D levels and associated factors: a population-based study in Switzerland. Swiss Med Wkly. 2012;142:w13719.
15 Fong CY, Riney CJ. Vitamin D deficiency among children with epilepsy in South Queensland. J Child Neurol. 2014;29:368–73.
16 Vierucci F, Del Pistoia M, Fanos M, Gori M, Carlone G, Erba P, Massimetti G, Federico G, Saggese G. Vitamin D status and predictors of hypovitaminosis D in Italian children and adolescents: a cross-sectional study. Eur J Pediatr. 2013;172:1607–17.
17 Janner M, Ballinari P, Mullis PE, Flück CE. High prevalence of vitamin D deficiency in children and adolescents with type 1 diabetes. Swiss Med Wkly. 2010;140:w13091.
18 Stoll D, Lamy O, Hans D, Zufferey P, So A, Krieg MA, Aubry-Rozier B. Changing the awareness of low vitamin D status in a rheumatology population: a pre/post-study. Swiss Med Wkly. 2013;143:w13891.
