Parenteral nutrition in the intensive care unit: cautious use improves outcome
DOI: https://doi.org/10.4414/smw.2014.13997
Ronan
Thibault, Claudia Paula
Heidegger, Mette M
Berger, Claude
Pichard
Summary
Critical illness is characterised by nutritional and metabolic disorders, resulting in increased muscle catabolism, fat-free mass loss, and hyperglycaemia. The objective of the nutritional support is to limit fat-free mass loss, which has negative consequences on clinical outcome and recovery. Early enteral nutrition is recommended by current guidelines as the first choice feeding route in ICU patients. However, enteral nutrition alone is frequently associated with insufficient coverage of the energy requirements, and subsequently energy deficit is correlated to worsened clinical outcome. Controlled trials have demonstrated that, in case of failure or contraindications to full enteral nutrition, parenteral nutrition administration on top of insufficient enteral nutrition within the first four days after admission could improve the clinical outcome, and may attenuate fat-free mass loss. Parenteral nutrition is cautious if all-in-one solutions are used, glycaemia controlled, and overnutrition avoided. Conversely, the systematic use of parenteral nutrition in the ICU patients without clear indication is not recommended during the first 48 hours. Specific methods, such as thigh ultra-sound imaging, 3rd lumbar vertebra-targeted computerised tomography and bioimpedance electrical analysis, may be helpful in the future to monitor fat-free mass during the ICU stay. Clinical studies are warranted to demonstrate whether an optimal nutritional management during the ICU stay promotes muscle mass and function, the recovery after critical illness and reduces the overall costs.
Introduction
Patients with critical illness, admitted to the intensive care unit (ICU) are characterized by a systemic inflammatory response (SIRS), which triggers metabolic and nutritional disorders: increased muscle catabolism, lipolysis, insulinoresistance, and hyperglycaemia. These metabolic changes aim at increasing the synthesis of inflammatory proteins by the liver, and at furnishing glucose as an energy source to vital organs (e.g., heart, kidney, etc.). During the ICU stay, fat-free mass loss is about 400 g/day. In that context, delivering the adequate energy and protein provision may limit the negative consequences of hypercatabolism and limit fat-free mass loss (fig. 1). The protection of fat-free mass is a challenge in ICU patients. As a matter of fact, the medical environment and the patients in the ICU are changing: the improvements of medical technology (e.g., better mechanical ventilation, infection control and haemodynamic management), ageing, obesity, increased prevalence of chronic diseases (cancer, degenerative neurological diseases, organ insufficiencies, etc.), and sedentary contribute to it. Patients with pre-existing undernutrition and/or lean tissues depletion (e.g., sarcopenic obesity, advanced age, severe chronic diseases) are becoming prevalent. As these conditions are incompatible with stress-induced catabolism and rapid healing and recovery the prevention of their onset or worsening is warranted. Therefore, the nutritional support should be considered as a mean of optimization of fat-free mass and energy balance during the ICU stay.
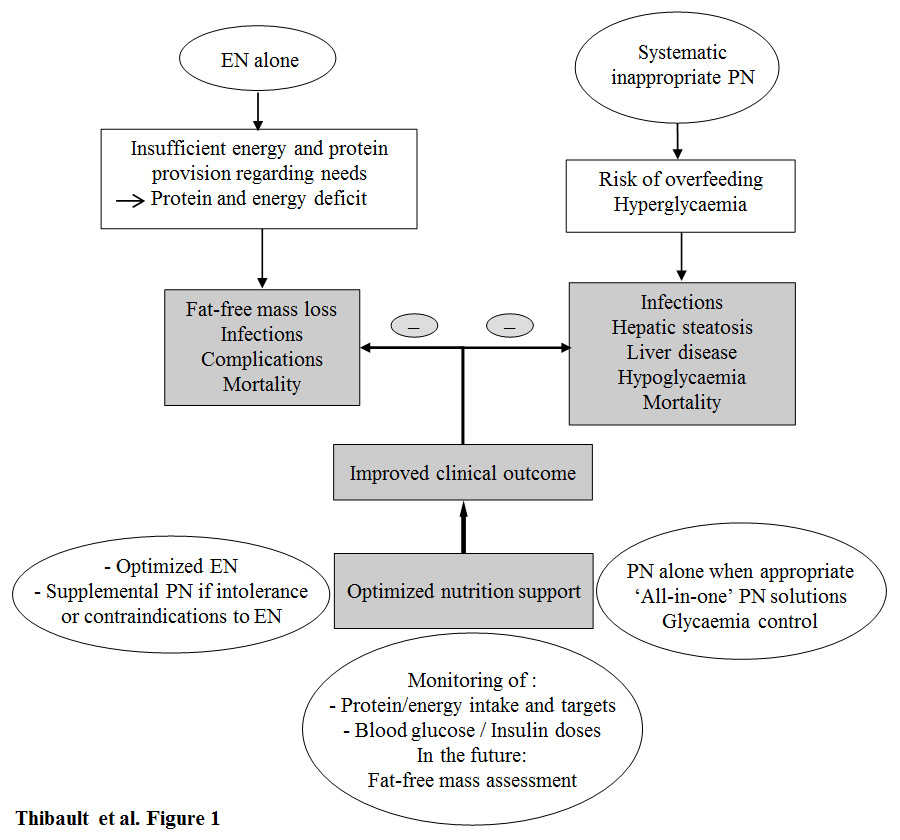
Figure 1
Improved clinical outcome with optimised nutrition support and monitoring in the intensive care unit. Enteral nutrition (EN) alone is often associated with an insufficient energy provision, leading to energy deficit; the latter is associated with fat-free mass loss, increased risk of infections and complications, and increased mortality. Systematic parenteral nutrition (PN) without appropriate indication is associated with increased risks of overfeeding, hyperglycaemia, and promotes infections, hepatic steatosis, liver disease, hypoglycaemia (as a result of high insulin doses), and mortality. Optimal nutrition support improves the clinical outcome. It includes the adequate choice of nutritional support: i) EN in first line, then together with supplemental parenteral nutrition in case of failure or contraindication to optimised EN; ii) parenteral nutrition alone when appropriate with respect to the indications, the preferred use of ‘all-in-one’ solutions, and the glycaemic control; iii) the nutritional and metabolic monitoring: adequation of protein/energy provision towards target, blood glucose and insulin doses according to on-going protocols. The assessment of fat-free mass may become the key part of the nutritional management of ICU patients, but validation studies are needed.
Early enteral nutrition is recommended by current guidelines as the first choice feeding route in ICU patients [1]. However, enteral nutrition alone is often unable to fully cover the nutritional needs of patients [2–3]. The resulting cumulated energy deficit is associated with a worse clinical outcome [4–7] (fig. 1). Prospective randomised studies indicate that the administration of parenteral nutrition to supplement insufficient enteral nutrition within the first four days post-admission in the patients with failure or contraindications to enteral nutrition could improve the clinical outcome [8–9]. The use of parenteral nutrition is safe if overfeeding and hyperglycaemia are avoided. The optimal use of parenteral nutrition in the ICU should be based on the assertion that limiting the energy deficit and preventing overfeeding would improve the clinical outcome, shorten the recovery period, and reduce overall costs.
The burden of energy deficit
Numerous studies have shown that enteral nutrition is insufficient to cover energy requirements [2–3]. Analyses conducted in U.S. [10], Canadian [11], and Swiss [2] hospitals found that a maximum of 52% to 70% of prescribed calories were actually delivered through enteral nutrition in the ICU patients. This is the consequence of frequent enteral nutrition interruption because of radiologic or endoscopic investigations, surgery and technical problems regarding nutrition pumps or feeding tubes, gastrointestinal intolerance [12], and irrelevant medical prescriptions or inadequate routine nursing procedures such as repeated gastric residues measurements. The implementation of feeding protocols based on current guidelines was supposed to improve energy delivery [13], but has failed constantly to reach the energy target [10, 11]. The use of prokinetics, such as erythromycin [14], and the suppression of gastric residues measurements [15] might contribute to improve energy provision. Optimisation of enteral nutrition in the ICU remains a great challenge even when a well-trained and experienced nutrition team is available [2]. The resulting energy deficit has been associated with increased mortality, and increased infection rate [4–7], impaired wound healing [5], adult respiratory distress syndrome, renal failure, need for surgery, and pressure sores [5]. Energy deficit also leads to fat-free mass loss, undernutrition [16], and its related complications, which, in turn, increases global health care costs [17]. The additional risk of underfeeding during the ICU stay is further amplified since patients are frequently undernourished prior to their ICU admission [18–19]. Whereas some degrees of deficit are probably acceptable during the first ICU days (around –50 kcal/kcal/kg [4, 5]), limiting cumulated energy deficit is the key challenge to prevent the worsening of undernutrition and improve the clinical outcome [20]. When enteral nutrition is insufficient to cover the energy target, parenteral nutrition could be a reliable means to match energy requirements with delivery, and avoid further energy deficit [8].
Supplemental parenteral nutrition and improved clinical outcome
Supplemental parenteral nutrition to improve energy provision and nutritional status
The combination of parenteral nutrition with enteral nutrition increases calorie delivery in comparison with enteral nutrition alone (28 ± 5 vs 20 ± 5 kcal/kg/day, p<0.0001) [8], suggesting that the combination of enteral nutrition and parenteral nutrition allows the achievement of the energy target sooner during critical illness. In a study of 49 undernourished ICU patients with mechanical ventilation, an increase in serum transthyretin and the Maastricht index, an assessment tool of nutritional status that incorporates serum transthyretin and albumin, lymphocyte count, and percentage of ideal weight, was observed after two weeks of supplemental parenteral nutrition, whereas no improvement was observed in patients treated with enteral nutrition or parenteral nutrition alone [21]. This effect could be related to the optimisation of energy delivery.
Evidence from recent prospective randomised controlled trials (table 1)
Two prospective, controlled, randomised trials have provided evidence of the clinical benefit of parenteral nutrition in ICU patients [8–9]. The Swiss SPN trial [8] was conducted in 305 mixed medical-surgical ICU patients (7% with cardiac arrest, 5% with myocardial infarction) with insufficient enteral nutrition or failure to optimised enteral nutrition. These represented 12% of the admitted patients during the study period. The delivery of the 100% of energy target from day 4 to day 8 by enteral nutrition and supplemental parenteral nutrition in ICU patients receiving less than 60% of their energy needs during the first three days of their ICU stay reduced the number of patients with nosocomial infections, as well as the duration of mechanical ventilation [8]. In the Early Parenteral Nutrition Trial, Doig et al showed, in 1372 mixed medical-surgical ICU patients (20% with cardiovascular diseases) with relative contra-indications to enteral nutrition, no deleterious effect of early parenteral nutrition provided within the 24 hours following admission in comparison with standard care at the physician’s discretion [9]. In the interventional group receiving parenteral nutrition, patients received parenteral nutrition during a short time period: a mean of 6.0 days (95% confidence interval (CI), 5.6–6.4). Early parenteral nutrition even showed clinical benefits on the duration of invasive ventilation, 60–day quality of life and body composition [9]. This approach was cost-effective [22]. Improved quality of life and body composition associated with early parenteral nutrition suggest a positive impact of optimal nutrition on post-ICU rehabilitation. This reinforces the hypothesis that timely individualised nutritional support is likely to limit fat-free mass loss and to shorten the recovery phase in ICU patients with preexisting undernutrition at admission.
|
Table 1:Prospective randomised controlled trials evaluating the clinical outcome of supplemental parenteral nutrition (PN) in intensive care units (ICU) patients. EN, enteral nutrition; GI, gastrointestinal; PN, parenteral nutrition. |
|
References
|
Patients (n)
|
ICU patientstypes
|
Study groups
|
Main results
|
Practical messages
|
| TICACOS
[50] |
112 |
Mechanically ventilated
Surgical: 45%
Medical: 55% |
EN with an
energy target determined by:
indirect calorimetry (study group), or
according to 25 kcal/kg/day (control group). EN supplemented with PN when required |
Trend towards improved hospital
mortality in the study group, but increased length of ventilation
and ICU stay |
Nutritional intervention based on indirect calorimetry may be beneficial |
| EPaNIC
[28] |
4640 |
Surgical: 89%, 60% of cardiac surgery
Medical: 11% |
Glucose load followed by PN initiation on day 3
vs PN initiation on day 8 |
PN initiation on day 8: faster recovery and fewer complications |
Avoid PN in the first 48 hours of ICU stay, if not indicated |
| SPN study
[8] |
305 |
Patients receiving <60% of energy target by day 3
Surgical: 46%
Medical: 54% |
Supplemental PN from day 4 to day 8 to reach 100% of measured energy target vs standard EN |
Supplemental PN: reduction in nosocomial infections, antibiotic use, and duration of mechanical ventilation |
– Accept low energy EN the first 4 days
– Consider PN if insufficient EN by day 4 |
| Early PN trial
[9] |
1372 |
Surgical: 63%
Medical: 37% |
Standard nutrition vs PN within 24 hours if contraindication to EN |
Early PN: fewer days of invasive ventilation, but similar 60–day mortality and ICU-hospital lengths of stay |
Supplemental parenteral nutrition to reduce enteral nutrition-associated symptoms
Another approach for promoting supplemental parenteral nutrition in the ICU is to prevent enteral nutrition-related side effects. For instance, we have recently showed in a prospective observational study that diarrhoea was reported at least one day in 14% of patients during their ICU stay [23]. There was a clear relationship between enteral nutrition providing ≤60% of energy target and diarrhoea onset. This suggests that in some patients, the combination of enteral nutrition and parenteral nutrition may be helpful, reducing the burden and the cost of managing diarrhoea (manpower, investigations, treatment).
Summary
In patients with failure, intolerance, or relative contra-indications to enteral nutrition, supplemental parenteral nutrition initiated between 24 and 72 hours after admission could prevent the worsening of energy deficit, and improve the clinical outcome of ICU patients. Furthermore, supplemental parenteral nutrition could reduce the incidence of enteral nutrition-related side effects, and their related costs. When parenteral nutrition is administered, the respect of good clinical practices is mandatory. Calories delivery should be adjusted to energy deficit and monitored daily. In fact the prevention of overfeeding and its related complications is as important as the limitation of energy deficit.
Inappropriate use of parenteral nutrition and risk of complications
Parenteral nutrition has been repeatedly associated with overfeeding, especially in the 1980s and 1990s, at a period when it was thought that the more calories was administered, the better the outcome would be. In 2014, an inappropriate use of parenteral nutrition remains associated with an increased risk of infections [25–28], and liver metabolic complications [29].
Parenteral nutrition and the risk of overfeeding
It has been extensively demonstrated that parenteral nutrition can induce metabolic disorders, known as “overfeeding”, such as hyperglycaemia, hypertriglyceridemia, liver steatosis, endocrine dysfunction, impairment of immunity, infections, and increased mortality [27]. Parenteral nutrition related infectious complications have been linked to hyperglycaemia, which was not considered a serious issue before 2001 [27, 30]. Large randomised, controlled, prospective studies have shown that an optimised glycaemic control with the aim to obtain a glycaemia less than 10 mmol/l and avoiding hypoglycaemia reduces mortality and morbidity [31–32]. Overfeeding has no beneficial impact on the nutritional status, and is deleterious. Hart et al showed that feeding burned patients at more than their energy expenditure leads to fat rather fat-free mass accretion [33]. In summary, overfeeding whatever the route is associated with increased complications that increase mortality, and is not associated to an improvement of nutritional status and body composition. Therefore, the prevention of overfeeding is deeply warranted in the ICU.
Parenteral nutrition and infections
Multicentre studies of ICU patients indicate that the use of parenteral nutrition or its duration is associated with an increased risk of Candida colonisation or candidemia [34–35]. However, other independent risk factors of Candida infections have been identified: sepsis, multifocal colonisation, and surgery [35]. An Italian multicentre randomised study conducted in 33 general ICUs (326 patients) found that, in the subgroup of patients without septic shock (n = 142), the administration of parenteral nutrition was associated with more episodes of severe sepsis or septic shock than on patients with enteral feeding [36]. In another study performed in 415 patients with severe sepsis or septic shock from 454 ICUs from 310 hospitals, the use of parenteral nutrition was independently associated with an increased risk of death, after adjustment for patient morbidity [37]. In trauma patients with a quite good enteral nutrition tolerance, it was shown that early parenteral nutrition was associated with an increased infectious morbidity [38]. However, a randomised controlled study performed in patients with brain trauma found no differences in terms of duration of mechanical ventilation, survival, or long term sequelea between patients treated with enteral nutrition or parenteral nutrition [39]. The prospective randomised controlled EPaNIC trial [28] was conducted on 4,640 patients, mainly with surgical patients and short-stayers, showed the deleterious effects of the early systematic initiation of parenteral nutrition at day 3 post-admission, preceded by high iv 20% glucose load (day 1:400 kcal, day 2:800 kcal) during the first two days after admission. These deleterious effects were further evidenced by fewer patients being discharged alive during the first 8 days of hospital stay; additionally, there was an increased rate of new infections, longer ICU stay and mechanical ventilation, when compared to a late initiation of parenteral nutrition, at day 8, i.e., seven days after underfeeding in the ICU [28]. In that context, late parenteral nutrition was more cost-effective than early parenteral nutrition [40]. In the late parenteral nutrition group, only 25% of patients actually received parenteral nutrition [28]. Strict glycaemic control, shown to be deleterious [31], was applied. In the EPaNIC trial, 89% of patients were surgical including 60% from cardiac surgery, a very rare indication of parenteral nutrition. An experimental study performed in burned rabbits suggests that the complications of early parenteral nutrition described by Casaer et al [28] could be secondary to autophagia deficit in liver and skeletal muscle [41]. The EPaNIC trial allows for the conclusion that overfeeding is deleterious at the early phase of critical illness; and that early parenteral nutrition, i.e., in the 48 hours, must be avoided in surgical patients, specifically if they have undergone cardiac surgery. Indeed the parenteral nutrition group required more insulin in relation to unverified energy targets and high initial glucose load: insulin is known to be an inhibitor of autophagy in diabetes [42]. The later findings of the Leuven group [41] indeed emphasize the importance of a precise energy target – only possible with indirect calorimetry: as a matter of fact, in the SPN trial where targets were largely guided by indirect calorimetry, the insulin requirements had not increased.
Parenteral nutrition and liver dysfunction
The use of parenteral nutrition in the ICU remains associated with liver dysfunction in ICU patients. In a multicentre study which included 3,409 patients from 40 Spanish ICUs, Grau et al found that among the 725 patients on artificial nutrition (total parenteral nutrition, n = 303, enteral nutrition, n = 422), a liver dysfunction was observed in 23% of patients (30% in total parenteral nutrition, 18% in enteral nutrition) [29]. In that study, three profiles of liver tests were considered as liver dysfunction: (a) cholestasis: increased alkaline phosphatase, gamma-GT, or bilirubin blood concentrations; (b) liver necrosis: increased transaminases or Quick time; (c) mixed pattern - both associated. Importantly there was no difference between the patients on enteral nutrition or parenteral nutrition during the first 11 days, the liver dysfunction was related to non-nutritional causes. In multivariate analysis, the factors associated with liver dysfunction were total parenteral nutrition, sepsis, early enteral nutrition or parenteral nutrition, and energy target >25 kcal/kg/day. It is noteworthy that patients with liver dysfunction had received mostly parenteral nutrition (30% vs 18% for enteral nutrition, p <0.001), and for a longer duration than enteral nutrition (13 days [interquartiles 8–25] vs 8 days [interquartiles 4–16], p <0.001). These findings clearly indicate that parenteral nutrition, initiated early, with an energy target >25 kcal/kg/day, and lasting more than 10 days, is associated with liver dysfunction, probably in relation with overfeeding. Indeed it is much easier to overfeed with parenteral nutrition than with enteral nutrition.
Summary
Parenteral nutrition per se does not affect mortality contrary to former beliefs, except in patients with severe septic shock. Nevertheless, exclusive parenteral nutrition is associated with an increased risk of hyperglycaemia, infections and liver dysfunction, in relation to overfeeding. These risks are increased by the early initiation and the duration of parenteral nutrition, the presence of sepsis, recent surgery and multifocal colonization, and by energy provision >25 kcal/kg/day. However, the respect of good clinical practices and academic societies’ recommendations minimises the risk of parenteral nutrition-related complications, and allows a safe use of parenteral nutrition.
The optimal parenteral nutrition in clinical practice
Feeding of the patients according to energy requirements
Indirect calorimetry is recommended to optimise the energy delivery for real needs. Future developments of this technique of energy expenditure measurement are awaited [43]. In the absence or unavailability of indirect calorimetry, the European Society for Clinical Nutrition and Metabolism (ESPEN) recommends avoiding delivering ≥25 kcal/kg actual body weight (BW)/day during the acute phase, and ≥30 kcal/kg actual BW/day during the post-acute phase [1, 44]. Importantly, energy from non-nutritional sources should be included in the calculations [45]. Severely malnourished patients on parenteral nutrition should initially receive 10 kcal/kg actual BW/day, then the target should progressively be increased to reach 25–30 kcal/kg actual BW/day over 3–4 days [1]. In obese or overweight patients, the energy requirements could be estimated as 15 kcal/kg actual BW/day, or 20 kcal/kg ideal BW/day [1, 44].
Limitation of energy deficit and prevention of overfeeding and hyperglycaemia
The systematic use of parenteral nutrition must be avoided. In selected patients, i.e., with an appropriate indication, ‘all-in-one’ parenteral nutrition can be administered successfully and safely, if it is used by a trained team [27], if energy delivery is adapted to the energy target, if a glycaemic control is obtained [31], and if parenteral nutrition is limited through the time [8, 29]. The prevention of overfeeding-related complications is facilitated by the fact that the industrial parenteral nutrition solutions have considerably evolved during the last two decades. They contain protein, carbohydrate, lipid, and electrolytes, supplemented with trace elements and vitamins. They allow a lower and constant load of glucose and lipids, reducing the risk of hyperglycaemia, hypertriglyceridemia, and liver fat overload. This risk has also been reduced by the increased use of emulsions containing both long and medium chain triglycerides. In addition, parenteral nutrition should be administered continuously over 24h.
Glucose delivery should not exceed 6 g/kg/day, at a rate below 5 mg/kg/min, and lipid supply must not exceed 23 mg/kg/min or 60% of the total energy input. However, there is no consensus regarding the ideal quantity of lipids. An initial supply of 0.5 to 1 g/kg/day of long chain triglycerides seem to be best; this can be increased up to a maximum of 2 g/kg/day if triglyceridemia and serum lactescence are regularly monitored [27]. “Hidden” fat from the sedative propofol should be included in the calculations [45]. The immunosuppressive effect of standard lipid emulsions remains controversial. Within the context of severe trauma/sepsis, lipid supply is limited to about 30%–40% of non-protein calorie input.
Indications of parenteral nutrition in ICU patients
The current indications of parenteral nutrition in ICU patients are shown in table 2. In most situations, a minimal enteral nutrition (e.g., 250 ml/day) may contribute to maintaining the integrity of the intestinal epithelial barrier. The use of enteral nutrition also optimises the glycaemic control, compared to parenteral nutrition, by reducing the risk of hyperglycaemia and decreasing insulin needs [46]. A sequential approach should be considered and parenteral nutrition should be gradually weaned over time when enteral nutrition reaches the energy target, to avoid overfeeding and infectious complications of parenteral nutrition.
|
Table 2:Indications of exclusive or supplemental parenteral nutrition (PN) in the intensive care unit (ICU) patients. |
| General indications |
Haemodynamically stabilised patient |
| Post-acute phase (≥4 days post-admission): in case of EN failure or insufficiency = full oral nutrition or EN not reached within 3 days |
| Contra-indication to EN (within 48 hours post-admission): gastrointestinal occlusion, mesenteric ischemia, active gastrointestinal bleeding |
| Acute phase (48 hours following ICU admission): avoid systematic exclusive or supplemental PN |
| Most common situations associated with PN use in the ICU |
Gastrointestinal occlusion (functional or mechanical) |
| Mesenteric ischemia |
| Active gastrointestinal bleeding |
| Intestinal insufficiency
– Short bowel syndrome: postsurgical remnant small bowel from duodeno-jejunal angulus to the most distal part of small bowel <1.5 m
– Radiation enteritis
– Proximal (duodenum, jejunum) high output fistulae: >2 liters / 24 h)
– Inflammatory bowel diseases in acute phase
– Splanchnic ischemia |
Monitoring of parenteral nutrition in the ICU
Table 3 proposes different strategies that could be useful for the monitoring of parenteral nutrition during the different phases of critical illness. Overfeeding-related metabolic complications of parenteral nutrition must be tracked. The monitoring of nutritional and metabolic care in the ICU has three main goals: first, the control of the amount of delivered macronutrients (glucose, protein, fat) and micro-nutrients (vitamins and trace-elements); second, the assessment of the adequation between energy needs and delivery; finally, the glycaemic control (fig. 1). The monitoring should be assisted by computerised systems that contribute to optimizing energy delivery and glycaemic control [47], thus improving adherence to guidelines and clinical outcome. Such a goal could be achieved if computerised monitoring is integrated into a global educational and interdisciplinary program of nutritional care [48]. The presence of an ICU-dedicated dietician further improves energy delivery in the ICU [48].
Fat-free mass loss is a consequence of stress, physical immobilisation and energy deficit. Limited actions can be taken for the first two factors, but energy deficit can be prevented, thus having a positive impact on clinical outcome and post-ICU recovery. In the future, body composition evaluation could be integrated into clinical practice, for an early and optimised nutritional management (table 3). Clinical studies are ongoing (Phase angle project, NCT #01907347) to assess whether specific methods, such as bioelectrical impedance analysis and 3rd lumbar vertebra-targeted computerised tomography, could help to assess and monitor fat-free mass during the ICU stay. The use of ultra-sound imaging of the thigh seems promising, hardly invasive and with limited costs [49]. Also, the assessment of muscle strength could be valuable, since fat-free mass loss during the ICU stay has an impact on muscular force, functional capacity, and therefore quality of life, during the months following ICU discharge. Whether an optimal nutritional management during the whole ICU stay could enhance the recovery after critical illness and improve post-ICU muscle mass and function remains to be demonstrated.
|
Table 3: Monitoring of PN during the different phases of critical illness. |
|
Criteria
|
Objective
|
Evaluation methods
|
Period of ICU stay
|
| Adequation between nutritional provision and target |
Energy target |
Prevention of energy deficit |
Measurement of energy expenditure by indirect calorimetry |
If available, at postacute and rehabilitation phases |
| Predictive formulas |
At any time, during the 48h post-admission, and at postacute and rehabilitation phases |
| Protein target |
Prevention of energy and protein deficit |
Predictive formulas |
At any time, during the 48h post-admission, and at postacute and rehabilitation phases |
| Macro-nutrients provision: total energy, cumulated energy deficit, provision in protein, carbohydrate, fat |
Prevention of energy and protein deficit, and overfeeding |
Monitoring sheet
Computerised software |
Several times daily to tailor nutrition support according to delivery and target |
| Micro-nutrients provision: vitamins and trace-elements |
Prevention of micronutrient deficiency and optimization of macronutrient metabolism |
Monitoring sheet
Computerised software |
Daily |
| Glycaemic control |
Glycaemia |
Prevention of overfeeding and hypoglycaemia |
Venous, arterial, or capillary blood collection |
Several times daily at the acute phase, then daily adaptation |
| Insulin doses |
Prevention of overfeeding and hypoglycaemia |
Monitoring sheet
Computerized software
Dynamic therapeutical algorithm |
Several times daily at the acute phase, then daily adaptation |
| Biological monitoring |
Blood sodium, potassium, phosphates, magnesium, urea, creatinin |
Prevention of refeeding syndrome |
Venous blood collection |
At the initiation of nutritional support, then daily if abnormalities then at least several times a week a nutrition |
| Liver tests |
Prevention of overfeeding and PN-related liver disease |
Venous blood collection |
| Albumin / Transthyretin |
Follow up of nutritional status in the absence of inflammation |
Venous blood collection |
Weekly during the post-acute and the rehabilitation phases
Not at the acute phase because of inflammation |
| Body composition assessment |
Weight, weight loss, body mass index |
Evaluation of nutritional status |
Weight: weighing bed or chair-weigh scale |
Post-acute and rehabilitation phases
Not at the acute phase because of hydration variations |
| Height: heel-knee distance |
Post-acute and rehabilitation phases |
| Fat-free mass, fat mass, total ± intra- and extra-cellular water |
Evaluation of body composition including fat-free mass loss (nutritional status) |
Bioimpedance analysis |
Absence of fluid retention
Post-acute and rehabilitation phases |
| Phase angle |
Evaluation of clinical prognosis? (under evaluation) |
Bioimpedance analysis |
At any time?
(under evaluation) |
| Skeletal muscular mass index |
Evaluation of body composition including fat-free mass loss (nutritional status) |
Third lumbar vertebrae-targeted computerised tomography |
At each abdominal routine scan?
(under evaluation) |
| Muscular strength |
Evaluation of muscular function |
Dynamometer |
Post-ICU rehabilitation phase |
| Quality of life |
Evaluation of overall health and muscular function |
Specific questionnaires |
Post-ICU rehabilitation phase |
Conclusion
In the ICU, optimal nutrition support should prevent both energy deficit and overfeeding, thereby improving the clinical outcome. Parenteral nutrition should be limited to enteral nutrition contraindications or failure. Parenteral nutrition is a safe therapy for ICU patients as long as overfeeding and hyperglycaemia are avoided. Inadequate use of parenteral nutrition is associated with an increased infection rate and liver dysfunction. The prescription of parenteral nutrition to supplement insufficient enteral nutrition (i.e., the “SPN concept”) should be initiated 24–72 hours after ICU admission, since it results in improving clinical outcome and cost-savings. The safe use of parenteral nutrition is of great interest, since it could preserve fat-free mass in patients presenting more and more to a certain extent with clinical situations of ageing, sarcopenic obesity, chronic diseases and pre-existing undernutrition.
References
1 Kreymann KG, Berger MM, Deutz NE, Hiesmayr M, Jolliet P, Kazandjiev G, et al. ESPEN Guidelines on Enteral Nutrition: Intensive care. Clin Nutr. 2006;25(2):210–23.
2 Genton L, Dupertuis YM, Romand JA, Simonet ML, Jolliet P, Huber O, et al. Higher calorie prescription improves nutrient delivery during the first 5 days of enteral nutrition. Clin Nutr. 2004;23(3):307–15.
3 De Jonghe B, Appere-De-Vechi C, Fournier M, Tran B, Merrer J, Melchior JC, et al. A prospective survey of nutritional support practices in intensive care unit patients: what is prescribed? What is delivered? Crit Care Med. 2001;29(1):8–12.
4 Villet S, Chiolero RL, Bollmann MD, Revelly JP, Cayeux RNM, Delarue J, et al. Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clin Nutr. 2005;24(4):502–9.
5 Dvir D, Cohen J, Singer P. Computerized energy balance and complications in critically ill patients: an observational study. Clin Nutr. 2006;25(1):37–44.
6 Faisy C, Candela Llerena M, Savalle M, Mainardi JL, Fagon JY. Early ICU energy deficit is a risk factor for Staphylococcus aureus ventilator-associated pneumonia. Chest. 2011;140(5):1254–60.
7 Alberda C, Gramlich L, Jones N, Jeejeebhoy K, Day A, Dhaliwal R, et al. The relationship between nutritional intake and clinical outcomes in critically ill patients: results of an international multicenter observational study. Intens Care Med. 2009;35(10):1728–37.
8 Heidegger CP, Berger MM, Graf S, Zingg W, Darmon P, Costanza MC, et al. Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: a randomised controlled clinical trial. Lancet. 2013;381(9864):385–93.
9 Doig GS, Simpson F, Sweetman EA, Finfer SR, Cooper DJ, Heighes PT, et al. Early parenteral nutrition in critically ill patients with short-term relative contraindications to early enteral nutrition: a randomized controlled trial. JAMA. 2013;309(20):2130–8.
10 Spain DA, Mcclave SA, Sexton LK, Adams JL, Blanford BS, Sullins ME, et al. Infusion protocol improves delivery of enteral tube feeding in the critical care unit. JPEN J Parenter Enteral Nutr. 1999;23(5):288–92.
11 Sigalet DL, Mackenzie SL, Hameed SM. Enteral nutrition and mucosal immunity: implications for feeding strategies in surgery and trauma. Can J Surg. 2004;47(2):109–16.
12 Mackenzie SL, Zygun DA, Whitmore BL, Doig CJ, Hameed SM. Implementation of a nutrition support protocol increases the proportion of mechanically ventilated patients reaching enteral nutrition targets in the adult intensive care unit. JPEN J Parenter Enteral Nutr. 2005;29(2):74–80.
13 Martin CM, Doig GS, Heyland DK, Morrison T, Sibbald WJ. Multicentre, cluster-randomized clinical trial of algorithms for critical-care enteral and parenteral therapy (ACCEPT). Can Med Assoc J. 2004;170(2):197–204.
14 Reignier J, Bensaid S, Perrin-Gachadoat D, Burdin M, Boiteau R, Tenaillon A. Erythromycin and early enteral nutrition in mechanically ventilated patients. Crit Care Med. 2002;30(6):1237–41.
15 Reignier J, Mercier E, Le Gouge A, Boulain T, Desachy A, Bellec F, et al. Effect of not monitoring residual gastric volume on risk of ventilator-associated pneumonia in adults receiving mechanical ventilation and early enteral feeding: a randomized controlled trial. JAMA. 2013;309(3):249–56.
16 Reid CL, Campbell IT, Little RA. Muscle wasting and energy balance in critical illness. Clin Nutr. 2004;23(2):273–80.
17 Amaral TF, Matos LC, Tavares MM, Subtil A, Martins R, Nazare M, et al. The economic impact of disease-related malnutrition at hospital admission. Clin Nutr. 2007;26(6):778–84.
18 Pichard C, Kyle UG, Morabia A, Perrier A, Vermeulen B, Unger P. Nutritional assessment: lean body mass depletion at hospital admission is associated with an increased length of stay. Am J Clin Nutr. 2004;79(4):613–8.
19 Pirlich M, Schutz T, Norman K, Gastell S, Lubke HJ, Bischoff SC, et al. The German hospital malnutrition study. Clin Nutr. 2006;25(4):563–72.
20 Elke G, Wang M, Weiler N, Day AG, Heyland DK. Close to recommended caloric and protein intake by enteral nutrition is associated with better clinical outcome of critically ill septic patients: secondary analysis of a large international nutrition database. Crit Care. 2014;18(1):R29.
21 Huang YC, Yen CE, Cheng CH, Jih KS, Kan MN. Nutritional status of mechanically ventilated critically ill patients: comparison of different types of nutritional support. Clin Nutr. 2000;19(2):101–7.
22 Doig GS, Simpson F. Early parenteral nutrition in critically ill patients with short-term relative contraindications to early enteral nutrition: a full economic analysis of a multicenter randomized controlled trial based on US costs. Clinicoecon Outcomes Res. 2013;5(369–79.
23 Thibault R, Graf S, Clerc A, Delieuvin N, Heidegger CP, Pichard C. Diarrhoea in the ICU: respective contribution of feeding and antibiotics. Crit Care. 2013;17(4):R153.
24 Peter JV, Moran JL, Phillips-Hughes J. A metaanalysis of treatment outcomes of early enteral versus early parenteral nutrition in hospitalized patients. Crit Care Med. 2005;33(1):213–20.
25 Simpson F, Doig GS. Parenteral vs. enteral nutrition in the critically ill patient: a meta-analysis of trials using the intention to treat principle. Intens Care Med. 2005;31(1):12–23.
26 Sena MJ, Utter GH, Cuschieri J, Maier RV, Tompkins RG, Harbrecht BG, et al. Early supplemental parenteral nutrition is associated with increased infectious complications in critically ill trauma patients. J Am Coll Surg. 2008;207(4):459–67.
27 Ziegler TR. Parenteral nutrition in the critically ill patient. N Engl J Med. 2009;361(11):1088–97.
28 Casaer MP, Mesotten D, Hermans G, Wouters PJ, Schetz M, Meyfroidt G, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011;365(6):506–17.
29 Grau T, Bonet A, Rubio M, Mateo D, Farre M, Acosta JA, et al. Liver dysfunction associated with artificial nutrition in critically ill patients. Crit Care. 2007;11(1):R10.
30 Marik PE, Pinsky M. Death by parenteral nutrition. Intens Care Med. 2003;29(6):867–9.
31 Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–97.
32 Van Den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–61.
33 Hart DW, Wolf SE, Herndon DN, Chinkes DL, Lal SO, Obeng MK, et al. Energy expenditure and caloric balance after burn: increased feeding leads to fat rather than lean mass accretion. Ann Surg. 2002;235(1):152–61.
34 Chow JK, Golan Y, Ruthazer R, Karchmer AW, Carmeli Y, Lichtenberg DA, et al. Risk factors for albicans and non-albicans candidemia in the intensive care unit. Crit Care Med. 2008;36(7):1993–8.
35 Leon C, Alvarez-Lerma F, Ruiz-Santana S, Leon MA, Nolla J, Jorda R, et al. Fungal colonization and/or infection in non-neutropenic critically ill patients: results of the EPCAN observational study. Eur J Clin Microbiol Infect Dis. 2009;28(3):233–42.
36 Radrizzani D, Bertolini G, Facchini R, Simini B, Bruzzone P, Zanforlin G, et al. Early enteral immunonutrition vs parenteral nutrition in critically ill patients without severe sepsis: a randomized clinical trial. Intens Care Med. 2006;32(8):1191–8.
37 Elke G, Schadler D, Engel C, Bogatsch H, Frerichs I, Ragaller M, et al. Current practice in nutritional support and its association with mortality in septic patients – results from a national, prospective, multicenter study. Crit Care Med. 2008;36(6):1762–7.
38 Dissanaike S, Pham T, Shalhub S, Warner K, Hennessy L, Moore EE, et al. Effect of immediate enteral feeding on trauma patients with an open abdomen: protection from nosocomial infections. J Am Coll Surg. 2008;207(5):690–7.
39 Koretz RL, Avenell A, Lipman TO, Braunschweig CL, Milne AC. Does enteral nutrition affect clinical outcome? A systematic review of the randomized trials. Am J Gastroenterol. 2007;102(2):412–29; quiz 68.
40 Vanderheyden S, Casaer MP, Kesteloot K, Simoens S, De Rijdt T, Peers G, et al. Early versus late parenteral nutrition in ICU patients: cost analysis of the EPaNIC trial. Crit Care. 2012;16(3):R96.
41 Derde S, Vanhorebeek I, Guiza F, Derese I, Gunst J, Fahrenkrog B, et al. Early parenteral nutrition evokes a phenotype of autophagy deficiency in liver and skeletal muscle of critically ill rabbits. Endocrinology. 2012;153(5):2267–76.
42 Strappazzon F, Campello S, Cecconi F. Non-apoptotic roles for death-related molecules: when mitochondria chose cell fate. Exp Cell Res. 2012;318(11):1309–15.
43 Guttormsen AB, Pichard C. Determining energy requirements in the ICU. Curr Opin Clin Nutr Metab Care. 2014;17(2):171–6.
44 Singer P, Berger MM, Van Den Berghe G, Biolo G, Calder P, Forbes A, et al. ESPEN Guidelines on Parenteral Nutrition: intensive care. Clin Nutr. 2009;28(4):387–400.
45 Devaud JC, Berger MM, Pannatier A, Marques-Vidal P, Tappy L, Rodondi N, et al. Hypertriglyceridemia: a potential side effect of propofol sedation in critical illness. Intens Care Med. 2012;38(12):1990–8.
46 Petrov MS, Zagainov VE. Influence of enteral versus parenteral nutrition on blood glucose control in acute pancreatitis: a systematic review. Clin Nutr. 2007;26(5):514–23.
47 Kipnis E, Ramsingh D, Bhargava M, Dincer E, Cannesson M, Broccard A, et al. Monitoring in the intensive care. Crit Care Res Pract. 2012;2012(473507.
48 Soguel L, Revelly JP, Schaller MD, Longchamp C, Berger MM. Energy deficit and length of hospital stay can be reduced by a two-step quality improvement of nutrition therapy: the intensive care unit dietitian can make the difference. Crit Care Med. 2012;40(2):412–9.
49 Puthucheary ZA, Rawal J, Mcphail M, Connolly B, Ratnayake G, Chan P, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310(15):1591–600.
50 Singer P, Anbar R, Cohen J, Shapiro H, Shalita-Chesner M, Lev S, et al. The tight calorie control study (TICACOS): a prospective, randomized, controlled pilot study of nutritional support in critically ill patients. Intens Care Med. 2011;37(4):601–9.
