Cell therapies for tendons: Old cell choice for modern innovation
DOI: https://doi.org/10.4414/smw.2014.13989
Lee
Laurent-Applegate, Anthony
Grognuz, Nathalie
Hirt-Burri, Ilias
Petrou, Wassim
Raffoul
Summary
Although tissue engineering and cell therapies are becoming realistic approaches for medical therapeutics, it is likely that musculoskeletal applications will be among the first to benefit on a large scale. Cell sources for tissue engineering and cell therapies for tendon pathologies are reviewed with an emphasis on small defect tendon injuries as seen in the hand which could adapt well to injectable cell administration. Specifically, cell sources including tenocytes, tendon sheath fibroblasts, bone marrow or adipose-derived stem cells, amniotic cells, placenta cells and platelet-derivatives have been proposed to enhance tendon regeneration. The associated advantages and disadvantages for these different strategies will be discussed and evolving regulatory requirements for cellular therapies will also be addressed. Human progenitor tenocytes, along with their clinical cell banking potential, will be presented as an alternative cell source solution. Similar cell banking techniques have already been described with other progenitor cell types in the 1950’s for vaccine production, and these “old” cell types incite potentially interesting therapeutic options that could be improved with modern innovation for tendon regeneration and repair.
Introduction
Tendon disorders are frequent and create functional and productivity problems in sports and in the workplace. Incomplete healing of tendon injuries not only leads to disability, but can also be associated with debilitating pain. Accidents with acute tendon laceration of the hand are particularly common. Approximately 1,775 new cases are reported in Switzerland each year with a mean insurance cost of 23,843 CHF [1]. The Swiss hand surgeon, Claude Verdan, was the first to introduce the classification of flexor tendon injuries, separating them into five anatomical zones [2]. There are also associated pathologies of tendons and ligaments that can frequently occur with hand overuse, degenerative diseases and aging. These can promote joint instability and arthritis and eventually may require surgical intervention. Following the pioneering endeavors of tendon surgery, modern therapeutic strategies with primary suture and precocious-controlled motion have been developed with good but not always ideal results.

Figure 1
Tendon structure, histology and cell culture.
Ultrastructure of tendon showing longitudinal fibres and histological sections stained with haematoxylin and eosin depicting extracellular matrix colored in pink (~80% composition) and cellular components mainly with tenocytes stained in blue (~20% composition)(horse tendon for illustration). Primary cultures from tissue can be developed by culturing cells in minimal medium (DMEM + 10% FBS + 1% glutamine) for approximately 1 week in a monolayer culture to provide “fibroblast-like” pure tenocyte populations (human progenitor tenocytes).

Figure 2
Tendon pathologies and therapeutic strategy.
Tendon pathologies can be either acute or degenerative. Degenerative therapies could be treated with biological cellular therapies with injectable forms to delay degenerative responses and more invasive surgery. Acute injuries, depending on their severity, could be treated with either non-invasive to invasive technique with cells in viscous biogels to complex neo-tissue constructs.

Figure 3
Progenitor human tenocyte cell bank production, capacity and usage for off-the-shelf therapeutic options for tendinopathies.
From one single tendon tissue (2 mm3, organ donation of foetal Achilles tendon tissue at 14 weeks gestation), clinical cell banks can be produced including parental primary cultures, Master and Working Cell Banks that contain 200 vials each of progenitor tenocytes at low passages (P. 3–6). Expansion of cells produces high quantities of cells that can be stocked in vapor phase of liquid nitrogen (–165 °C) providing cell stocks for hundreds of thousands of patients. Cells can be administered in hydrogels or on other delivery systems for testing in animal models and pre-clinical patient treatments in the process for therapeutic agent development.
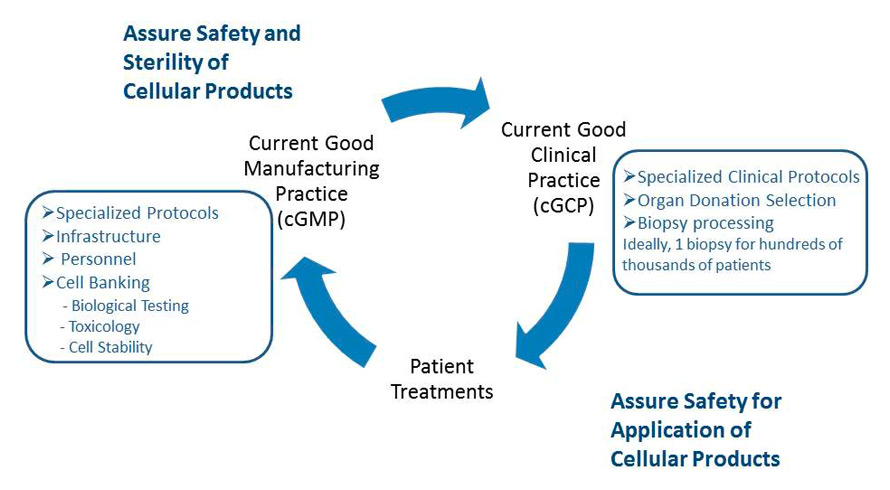
Figure 4
Good Clinical Practice and Good Manufacturing Practice associated with cellular therapeutics.
Current Good Clinical Practices (cGCP) are necessary to begin the process of cellular therapies and tissue engineering with detailed protocols for organ donation and biopsy processing. Tissue and cellular handling then needs to abide by Current Good Manufacturing Processes (cGMP) which include specific protocols for infrastructure, equipment and personnel. cGCP is then needed for appropriate administration of cellular therapies. Thus, the entire process requires high collaboration between biologists, technicians, nurses and surgeons.
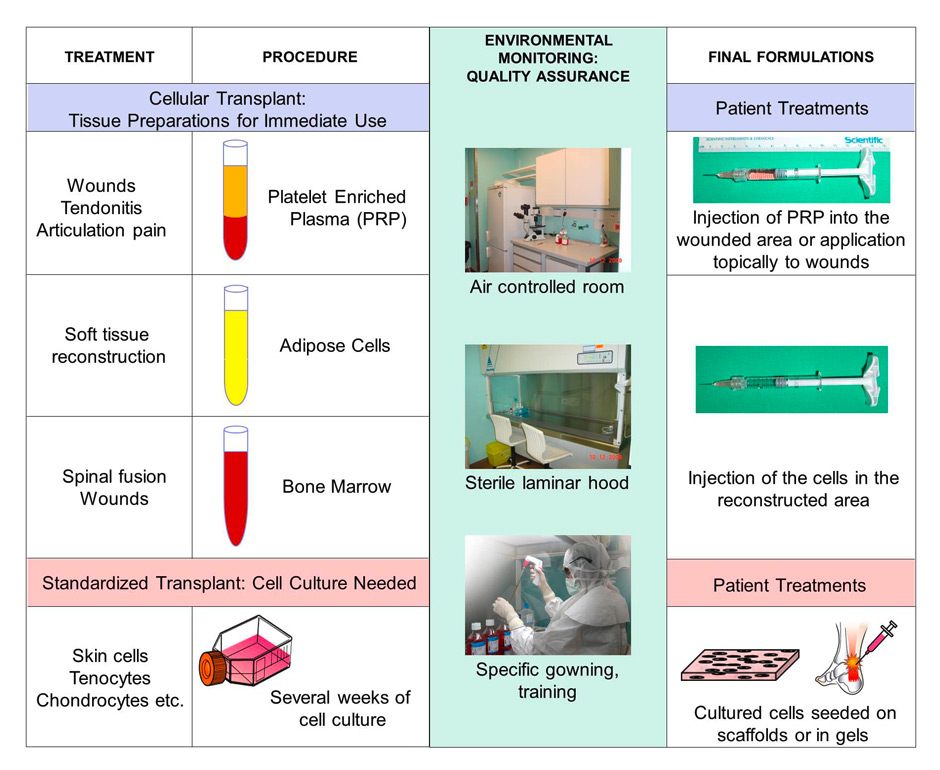
Figure 5
Regulatory requirements for cellular therapy products.
Regulatory requirements have become more extensive for cellular therapies and tend towards conservative drug development in their production, pre-clinical testing and clinical trials. Blood products also have to be produced under cGMP and only in institutions with a license for manipulation. Organs, cells and tissues (PRP, adipose, bone marrow, skin, cartilage, tendon etc.) need to be processed with environmental monitoring and full quality assurance with cGMP processing through to the final formulation and delivery to the patient.
On the pathophysiological level, intrinsic tenocyte regenerative response, adequate nutrition and prevention of adhesions are some of the key elements that have to be considered in order to achieve an optimal functional result after tendon injury. Despite early surgical treatment and mobilisation, adhesions are frequent and secondary tendon rupture is still a major concern. Joint instability is usually the consequence of trauma to bone and degeneration of cartilage. Tendon transfers are used to stabilise the joint, but treatment failures are frequent where there is tendon degeneration. It has been proposed that cell stimulation could prevent surgical failure. Tendon regeneration is a time consuming procedure because of slow metabolism in adult tenocytes. Immediately following an injury an inflammatory phase takes place, during which chemo-attractive substances are released to recruit and stimulate tenocytes. After a few days and during the first few weeks, the second step of healing is controlled by tenocytes and macrophages and there is a high deposition of matrix, mostly composed of type 3 collagen. After one or two months, a third phase appears with the extracellular matrix (ECM) continuing to be produced but with a higher amount of type 1 collagen. The tissue is reorganised and becomes more and more aligned. This process takes as long as one year or more, with the cell density and activity diminishing with time [3, 4].
The architecture and orientation of the collagen fibres of the tendon are of paramount importance, providing an exquisite anatomical structure assuring elasticity, mobility and tensile strength and therefore becoming a functional tissue with unique biomechanical qualities (fig. 1A & B). The role of the tendon is to provide correct transmission of forces between muscle and bone and to protect tension and compression of structures upon forces. This conjunctive tissue can come in various forms and lengths [3, 5, 6]. Interestingly, the composition of tendon is mostly ECM at 80% and cells at approximately 20% [7] with 90–95% of the cells being tenocytes [3, 5]. A minor portion of chondrocytes, synovial, vascular and tendon stem cells can also be detected. The longitudinal cells present in histological sections (fig. 1B) represent tenocytes and are distributed fairly uniformly throughout the ECM and when cultured from primary tissue form “fibroblastic-like” cells in 2–D culture (fig. 1C). The ECM is mainly composed of collagen fibres representing 65–80% of tendon dry matter with Type I and III being the most frequent (95% Type I) [6]. Elastic fibres (1–2%), proteoglycans along with glycoproteins (less tan 1%) and inorganic molecules (less than 0.2%) are the remaining elements of the ECM [5].
In the case of injury, the healed tendon cannot restore its exact natural structure, resulting in the formation of scar tissue which weakens its overall functional abilities [8]. In clinical practice it is frequently observed that patient recovery is long and full strength mobilisation takes almost 3 months after the time of the injury to return. Tendon injuries can be classified as acute or degenerative conditions (fig. 2). Acute injuries associated with partial ruptures or fissures could be treated first with injectable functional cell populations before turning towards more invasive tissue replacement. Tendinopathies with degenerative conditions could be stabilised with injectable biological cell therapy solutions in order to delay any surgical procedures. With tissue defects, the length of time before primary repair, the failure of the primary closure and enhanced chances of re-rupture are common problems encountered. Under these circumstances, the use of tendon grafts or transfers is sometimes necessary (fig. 2). However, these procedures are associated with donor site morbidity (if autologous graft), increased operating time, risk of infection and higher cost.
These drawbacks have promoted translational research in the search for solutions of off-the-shelf tendon engineered constructs and cellular therapies that could be available whenever and wherever needed. The optimal result of new therapeutic strategies would be the achievement of a scar-free regenerated tissue. Cadaveric de-cellularised tendons are routinely used and new tissue engineering strategies would be important for clinical use. As for any tissue engineering therapeutic strategy, the cell source and type is crucial. The implicated cells should be easy to collect, easily and rapidly cultured and expanded, and should possess maximum stability of the desired phenotype which would assure patient safety upon implantation. In addition, they should also possess a high tendon formation potential without triggering an immunological reaction of the recipient patient.
In this short review on innovation we will present the existing cell sources used in cellular therapies of tendon with their advantages and drawbacks, and we will present the potential use of human allogenic progenitor tenocytes for the repair and enhancement of tissue regeneration of the tendon.
Embryonic cell sources for tendon tissue engineering applications
Embryonic stem cells (ESCs) have been proposed as a cell source for tendon repair and regeneration. These are undifferentiated cells which are able to create all cell types and the original tissue source is the early age embryo (around five days following ovum fertilisation). The derived cells are “totipotent” until eight cell divisions occur. Then, approximately 2 weeks post-fertilisation, the cells become pluripotent and can no longer develop into another embryo, but they can develop into every cell type with proper stimulation (i.e. growth factors). All of these stages of development up to 8 weeks post-fertilisation are considered to be an embryo.
A tendon model derived from ESCs was developed successfully and grafted in a rat. Maturation and differentiation of the cells into a tenocyte-type was achieved and observed 30 days after implantation. Using the same cells in a fibrin gel to treat rat patellar tendons led to better structural and functional results than the controls with fibrin gel only. The cells survived for 4 weeks and activated the intrinsic tendon regeneration procedure with no presence of ectopic tissue or teratomas. However, ESCs have the potential to differentiate into many phenotypes and the presence of factors like GDF5 and BMP2 in the wound could potentially lead to the formation of bone or cartilage tissues [9].
In a large animal model with induced horse flexor tendonitis (collagenase gel – physical defect), the injection of ESCs to the injured tendon led to an important clinical amelioration. The double-blinded experiment using MRI as well as ultrasound analysis showed improved structural changes in the animals treated with ESCs over placebo [10] but could not define the specific mechanism of amelioration through exogenous cell transplant, local cytokine or immunological modulation or simply by the stimulation of environmental endogenous horse cells.
The use of ESCs remains fairly complicated on the technical front with high concerns in terms of consistency and security of the cells as they could dedifferentiate when introduced into an in vivo environment. Cultures of these stem cell types are technically very demanding since the amount of tissue to begin the culture is very small (<100 cells) and up-scaling the stem cells in an undifferentiated state requires many growth factor supplements. In addition, in many countries ethical issues for the use of embryonic stem cells have been associated with specific directives and licenses to allow cell manipulation and research. More sophisticated techniques of cell encapsulation and cellular cloning could provide strategies to improve the delivery of the correct cell population and therefore provide an allogenic (cells not from the same patient) cell source ready-for-use, which would address safety issues for the patient.
Adult cell sources for tendon tissue engineering applications
Various adult cell sources are under current research and development to achieve tendon regeneration. The tissue engineering strategies can thereby be described depending on the differentiation level of the cells used and on the original tissue source. In addition to the above described embryonic and allogenic cell source, autologous cells have also been proposed and various adult stem cell populations have been envisioned for tendon repair. Cell sources implemented include: i) isolated, cultured “mesenchymal cells” (MSCs) from bone marrow (BM-MSCs), adipose tissue (AD-MSCs); ii) fully differentiated cell lineages such as tenocytes, and; iii) blood-derivatives such as platelets or plasma-enriched platelet preparations (PRP).
Fresh blood or bone marrow have been extensively used for over 40 years, since the beginning of the tissue engineering era. Although cells from fresh bone marrow or blood are accessible without much effort, allotransplantation remains difficult as these transplantations could lead to an acute graft-versus host disease [11, 12]. This is why modern cell-based therapeutic techniques focus on the use of specific “purified” and culture expanded lineages. Interestingly, Osiris Therapeutics demonstrated in 1997 that BM-MSCs isolated by density gradient, purified to eliminate non-MSC cell sources and expanded in cell culture, could be used as a secure allogenic cell source for different clinical affections [13]. The first clinical trials using BM-MSCs allogenic stocked cells for burns and wounds will take place at the University of Miami as they were awarded a Department of Defense and Armed Forces Institute of Regenerative Medicine Grant (DOD-AFIRM) for tissue repair (http://med.miami.edu/news/miller-school-physician-scientists-receive-3–million-defense-grant-to-treat/). Combining stem cells with autografting techniques used in burn wounds may help in diffusion of the necessary nutrients, growth factors and oxygen for successful transplantation. Likewise, adipose cells derived from the stromal fraction have been shown to improve diffusion and help overall tissue homeostasis in a mouse model to date [14]. Additionally, other stem cell sources such as tonsil mesenchymal cells can aid in the preparation of skin engineered grafts [15] and full lymphatic vascular systems can be linked to skin grafts with the development of lymphatic capillaries constructed in vitro [16]. Especially for burn patients these techniques need to be continually improved and optimised to allow shorter production and earlier delivery times to the patient [17].
Both adipose-derived and bone-marrow-derived MSCs have been used for tendon repair and regeneration potential in animal models including rats, horses, and sheep [18–21]. Only slight improvements have been reported in these models and the regeneration quality cannot be compared to that seen intrinsically in foetal tendon [10, 22, 23]. These cell types have also been used on acellularised allogenic tendons or on various scaffolds and tested in animal models. The optimal goal is to produce a bioengineered construct with high force before failure, adequate tensile stiffness and absence of calcifications [24–27]. So far in human trials, autotransplantation of mononuclear stem cells extracted from the iliac crest has given encouraging results in terms of safety and ability to enhance intrinsic tendon regeneration [28].
Each cell type possesses different advantages and disadvantages. MSCs isolated from different tissue could present immunological advantages for autologous use. As only one out of every 100,000 cells derived from bone marrow is a stem cell, a technically demanding procedure of isolation and expansion is needed. AD-MSCs are more readily available (>100–fold compared to BM-MSC) and have better growth capacity in vitro. Although the use of MSCs has shown tendon regenerative properties, the formation of calcifications in almost all the experimental models prevents their establishment as a successful bioengineered therapeutic agent for tendons. Recently discovered tendon stem cells [29] could potentially allow regeneration with less calcification. Unfortunately it is difficult to harvest these cells in an autologous tendon.
It is possible that differentiated cells will be more competent in the in vivo environment due to their higher production of specific components of the extracellular matrix and lower de-differentiation potential. Cell choices from local tendon environment such as tenocytes, and tendon sheath fibroblasts were proposed [27] and comparisons between adipose-derived MSC and bone-marrow derived MSC to these two differentiated cell types were assessed for their qualities both in vitro and in vivo. As all cell types were viable and seemed to deposit matrix, differentiated cell sources might avoid calcification formation and prove more useful. However, adult tendon cells present the same issue as tendon stem cells and an autologous source does not yet seem technically imaginable.
Another recent popular cell source for tendon regeneration is platelets. Platelet rich plasma (PRP) can be used alone or with biocompatible scaffolds, providing better structural results resulting in increased functional outcomes [30, 31]. Until now, PRP is used as an autotransplantation as there is always a small but existing risk of immunological reactions in the case of allotransplantation [32]. On the one hand there is still strong controversy regarding the real positive effect that this therapeutic agent can obtain [33] but on the contrary the isolation and preparation can be done rapidly and inexpensively [34].
Progenitor cell sources in tissue engineering applications
Placenta, umbilical cord or even amniotic liquid contain various types of foetal progenitor cells that have been employed in bioengineering [35–37]. Besides these above mentioned tissues, most foetal cell research is based on specific material derived at the latter end of the first trimester (11 to 14 weeks of gestation). At this stage, cell lineages with tissue specific cells can be established. The tissue can be considered an organ donation when the mother-donor is contacted only after her decision to proceed with a voluntary pregnancy interruption, when she (and her partner) gives an informed consent and when there is no payment [38]. This procedure is authorised by legislation in most countries.
The use of foetal tissues or cells in research began in the laboratories of immunologists and neurologists. The polio vaccine, which led to a Nobel Prize for Medicine in 1954 to American immunologists, was developed using cultures of human foetal cells which are still used in contemporary vaccine development today. Transplantation of foetal neural cells has been used to treat conditions such as Huntington’s [39, 40] or Parkinson’s disease [41]. Similarly, foetal transplants have been used for spinal cord affections or injuries by providing encouraging results in motor function recovery and also by offering a safe transplantation procedure for patients [42–45]. Moreover, human foetal liver cells have been used for more than 25 years to treat severe immunodeficiency, haematological disorders and congenital disorders of metabolism [46].
Recently, liver failures and diabetes have been targeted by foetal cell therapy strategies [47]. Better understanding of developmental embryology has substantially helped technological progress. Studies have also revealed important inductive signals and transcription factors that can play crucial roles in the differentiation of hepatocytes and b-cells from various stem and progenitor cell types [48]. It should be noted that human foetal liver cells were successfully isolated to treat end-stage liver disease. In a case report it was shown that the patient model for end-stage liver disease (MELD) score improved significantly within the first 18 months of follow-up [49]. In another study including 25 patients, clinical and biochemical parameters improved, patients did not present with hepatic encephalopathy and their mean MELD score decreased during 6 months of observation [50].
In recent years, three-dimensional biological bandages, developed from human foetal skin progenitor cells have been used to treat burns in children and also for chronic wounds [51, 52]. With this particular technique, cells from one dedicated cell bank can be expanded to produce over 35 x 109 tissue engineering skin constructs (9 x12 cm) providing an off-the shelf cell-based therapy [53]. Following this approach, human foetal bone cells [54] and chondro-progenitor cells [55] have been used as potential regenerative agents for human skeletal tissue, and depending on delivery systems, the cells can be used either in injectable techniques for difficult to treat areas or on scaffolds for cavity filling [56]. Surprisingly, tendon tissue engineering using foetal progenitor cells has barely begun to be investigated. Lately, our group has studied the characteristics of human progenitor tenocytes for a better comprehension of their biology in vitro (http://www.unil.ch/webdav/site/fbm/shared/recherche/FBM_Day_2012/FBMDAY_ABSTRACTBOOK_2012_FINAL.pdf, abstract on p.93) and to evaluate their potential use for tendon tissue engineering and for the production of a biocompatible neo-tendon. A short description follows below.
Potential of human progenitor tenocytes for tendon tissue engineering applications
Up to now, it has been shown that the determining factor in cell therapies is the cell choice and technical specifications that are related to their collection, culture, expansion, storage and stability. In addition, therapeutically, the cells should have high tissue regenerative properties, produce low or no immunological induced reactions, and have no pro-inflammatory issues. In Figure 3, a clinical cell bank of human progenitor tenocytes is depicted that can be developed in a very short time period from one single organ donation of achilles heel tendon (~2 mm3). These cells have been developed in a registered transplantation programme in Switzerland since 2007 (08.2007, protocol #62/07: Development of foetal cell banks for tissue engineering) [34]. The primary culture or parental cell bank can be produced in less than 14 days using a simple medium as nutrient (Dulbecco’s Modified Eagles Medium [DMEM] supplemented with 10% foetal bovine serum and 1% glutamine) (fig. 3A). Master and working cell bank vials (MCB & WCB) of 1–1010 cells can be stored at –165 °C in the vapor phase of liquid nitrogen for at least 5 years with no incidence on stability (fig. 3B). Other progenitor cell types have been shown to remain stable for 20 years to date (i.e. skin). From the original 2 mm3 of tissue, it would be possible to develop around 200 vials of a MCB and an equivalent quantity of WCB vials. The overall potential of one, unique organ donation can thus be illustrated in that at least 35 x 109 treatments could be produced from the clinical cell bank. The cells produced could be delivered either in hydrogels to allow minimal invasive options or within a scaffold to provide possibilities for larger defect repair and regeneration (fig. 3C).
Evolving regulatory requirements for cellular therapies
The main reason why cellular therapies have not been implemented more rapidly in the clinical setting is because of the fluctuating regulatory requirements that have surrounded cellular products over the last years. The EU regulation on advanced therapy medicinal products (ATMP) was adopted in all European member states in 2008 and Switzerland modified its Transplantation Act in July of 2007 (Federal Act on the Transplantation of Organs, Tissues and Cells of 8 October 2004, RS 810.21). Since these dates, all cellular products must be in compliance with Current Good Clinical Practice (cGCP) guidelines for their procurement and collection, and with Current Good Manufacturing Procedures (cGMP) for the production and manufacturing of cells and tissues. These new regulations impose strict criteria for the production of cellular products and the environment for which they are produced (fig. 4 & 5). The necessary requirements can be assimilated to the industry that produces vaccines for world-wide use. As a result, cellular products and therapies used in early clinical trials require hospital settings that have appropriate licensed clean-rooms for stocking and manufacturing of cells; a major task that takes time and extremely high budgets. Most of the major hospitals in Switzerland have invested in facilities or are investing in them to assure cellular therapeutic options. These types of clean rooms and quality assurance (QA) systems associated with them are now necessary even for PRP preparations following a recent decision by SwissMedic (August, 2013: http://www.swissmedic.ch/zulassungen/00196/01956/index.html?lang=fr). All preparations of blood and serum derivatives for the treatments of tendinopathies are obliged to be considered as medicinal products under the interpretation of the Therapeutic Products Act (Federal Act on Medicinal Products and Medical Devices of 15 December 2000, TPA, RS 812.21). Consequently, preparations can only be prepared in appropriate laboratories that have a license for manufacturing and fabrication must respect “GMP on a small industrial scale”, which are edited by SwissMedic in Pharmacopoea Helvetica (11.1, chapter 20.1 and 21.1). Clinical indications that are regularly treated with these PRP and associated preparations include achilles tendinopathy, patellar tendinopathy (‘jumper’s knee’), rotator cuff disorders, calcaneal and plantar fasciitis in the foot, muscle strains, ligament sprains, articular cartilage injury and even for preparation before hair transplants. In the field of hand surgery, the conditions including lateral epicondylitis (‘tennis elbow’), medial epicondylitis (golfer's elbow), osteoarthritis, acute flexor/extensor tendon rupture/laceration or even hand rejuvenation techniques will now need to follow these new strict regulations. Consequently, any product destined to be transferred to patients, which is composed of organs, cells and living tissues from autologous, allogenic or xenogeneic sources (PRP, MSCs, skin progenitor cells, tenocytes) will need to follow environmental monitoring and different degrees of GMP manufacturing. Manufacturing will include all manipulations ranging from simple tissue and cell separation techniques (i.e. centrifugations) to the more complex manipulations of cell culturing and extensive cell separation (fig. 5). With the advancement of technology and the ever increasing interest in tendon regeneration research, tendon pathologies could be included in more complex cell-based therapeutic strategies in the near future.
Benefits for patient care with these new innovative therapies offer promise in repairing, replacing or restoring the functionality of damaged tissues and in the management of pain. In order to provide potential cell-based therapies to increasing patient numbers, it will be necessary to optimise the cell choice for isolation, proliferation and stability to assure the highest patient security. In addition, all of these new innovative therapies will evolve in relation to regulatory requirements and more importantly adapted technical specifications (fig. 4). Biologists and technicians, in their roles as “laboratory surgeons”, will need to work even more closely with clinicians to adapt innovative cell therapeutics to the patient.
Acknowledgements: We would like to thank the Foundation S.A.N.T.E and Foundation Family Sandoz for financing our Progenitor Transplantation Program. We also would like to thank the Orthopaedic Hospital Foundation for support for the doctoral thesis of one of the students (AG).
References
1 Service de centralization des statistiques de l’assurance accident LAA. http://www.unfallstatistik.ch
2 Verdan CE. Half a century of flexor-tendon surgery. Current status and changing philosophies. J Bone Joint Surg Am. 1972;54(3):472–91.
3 Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am. 2005;87(1):187–202.
4 Voleti PB, Buckley MR, Soslowsky LJ. Tendon healing: repair and regeneration. Annual Rev Biomed Eng. 2012;14:47–71.
5 Kannus P. Structure of the tendon connective tissue. Scand J Med Sci Sports. 2000;10(6):312–20.
6 O’Brien M. Structure and metabolism of tendons. Scand J Med Sci Sports. 1997;7(2):55–61.
7 Tai CC, Williams A. Ligaments and tendons, in Basic Orthopaedic Sciences, Ed. Ramachandram, CRC Press 2006, chapter 8, pp. 71–78.
8 James R, Kesturu G, Balian G, Chhabra AB. Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg Am. 2008;33(1):102–12
9 Chen X, Song XH, Yin Z, Zou X-Y, Wang L-L, Hu H, et al. Stepwise differentiation of human embryonic stem cells promotes tendon regeneration by secreting fetal tendon matrix and differentiation factors. Stem Cells. 2009;27(6):1276–1287.
10 Watts AE, Yeager AE, Kopyov OV, Nixon AJ. Fetal derived embryonic-like stem cells improve healing in a large animal flexor tendonitis model. Stem Cell Res Ther. 2011;2(1):4.
11 Ferrara JL, Cooke KR, Teshima T. The pathophysiology of acute graft-versus-host disease. Int J Hematol. 2003;78(3):181–7.
12 Petersdorf EW, Shuler KB, Longton GM, Spies T, Hansen JA. Population study of allelic diversity in the human MHC class I-related MIC-A gene. Immunogenetics. 1999;49(7–8):605–12.
13 Mack GS. Osiris seals billion-dollar deal with Genzyme for cell therapy. Nature Biotech. 2009;27(2):106–7.
14 Klar AS, Güven S, Biedermann T, Luginbühl J, Böttcher-Haberzeth S, Meuli-Simmen C, Meuli M, Martin I, Scherberich A, Reichmann E. Tissue-engineered dermo-epidermal skin grafts prevascularized with adipose-derived cells. Biomaterials. 2014;35(19):5065–78.
15 Böttcher-Haberzeth S, Biedermann T, Klar AS, Pontiggia L, Rac J, Nadal D, et al. Tissue engineering of skin: human tonsil-derived mesenchymal cells can function as dermal fibroblasts. Pediatr Surg Int. 2014;30(2):213–22.
16 Daniela Marino, Joachim Luginbühl, Simonetta Scola, Martin Meuli, Ernst Reichmann. Bioengineering Dermo-Epidermal Skin Grafts with Blood and Lymphatic Capillaries. Sci Transl Med. 2014;6(221): 221ra14 DOI: 10.1126/scitranslmed.3006894.
17 Pontiggia L, Klar AS, Böttcher-Haberzeth S, Biedermann T, Meuli M, Reichmann E. Optimizing in vitro culture conditions leads to a significantly shorter production time of human dermo-epidermal skin substitutes. Pediatr Surg Int. 2013;29(3):249–56.
18 Ju YJ, Muneta T, Yoshimura H, Koga H, Sekiya I. Synovial mesenchymal stem cells accelerate early remodeling of tendon-bone healing. Cell Tissue Res. 2008;332(3):469–78.
19 Lacitignola L, Crovace A, Rossi G, Francioso E. Cell therapy for tendinitis, experimental and clinical report. Vet Res Commun. 2008;32:s33–38.
20 Nixon AJ, Dahlgren LA, Haupt JL, Yeager AE, Ward DL. Effect of adipose-derived nucleated cell fractions on tendon repair in horses with collagenase-induced tendinitis. Am J Vet Res. 2008;69(7):928–37.
21 Smith RK. Mesenchymal stem cell therapy for equine tendinopathy. Disabil Rehabil. 2008;30(20–22 ):1752–8.
22 Favata M, Beredjiklian PK, Zgonis MH, Beason DP, Crombleholme TM, Jawad AF, Soslowsky LJ. Regenerative properties of fetal sheep tendon are not adversely affected by transplantation into an adult environment. J Orthop Res. 2006;24(11):2124–32.
23 Beredjiklian PK, Favata M, Cartmell JS, Flanagan CL, Crombleholme TM, Soslowsky LJ. Regenerative versus reparative healing in tendon: a study of biomechanical and histological properties in fetal sheep. Annals of Biom Eng. 2003;31(10):1143–52.
24 Kryger GS, Chong AKS, Costa M, Pham H, Bates SJ, Chang J. A comparison of tenocytes and mesenchymal stem cells for use in flexor tendon tissue engineering. J Hand Surg. 2007;32(5):597–605.
25 Ouyang HW,Goh JCH, Thambyah A, Teoh SH, Lee EH. Knitted poly-lactide-co-glycolide scaffold loaded with bone marrow stromal cells in repair and regeneration of rabbit achilles tendon. Tissue Eng. 2003;9(3):431–9.
26 Chong AKS, Ang AD, Goh JCH, Hui JHP, Lim AYT, Lee EH, et al. Bone marrow derived mesenchymal stem cells influence early tendon-healing in a rabbit Achilles tendon model. J Bone Joint Surgery A. 2007;89(1):74–81.
27 Gulotta LV, Kovacevic D, Packer JD, Deng XH, Rodeo SA. Bone marrow-derived mesenchymal stem cells transduced with scleraxis improve rotator cuff healing in a rat model. Am J Sports Med. 2011;39(6):1282–9.
28 Ellera Gomes JL, Canquerini da Silva R, Silla LMR, Abreu MR, Pellanda R. Conventional rotator cuff repair complemented by the aid of mononuclear autologous stem cells. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):373–7.
29 Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nature Med. 2007;13(10):1219–27.
30 Fernández-Sarmiento JA, Domínguez JM, Granados MM, Morgaz J, Navarrete R, Carrillo JM, et al. Histological study of the influence of plasma rich in growth factors (PRGF) on the healing of divided Achilles tendons in sheep. J Bone Joint Surg Am. 2013;95(3):246–55.
31 Sato D, Takahara M, Narita A, Yamakawa J, Hashimoto J, Ishikawa H, Ogino T. Effect of platelet-rich plasma with fibrin matrix on healing of intrasynovial flexor tendons. J Hand Surg Am. 2012;37(7):1356–63.
32 Cid J, Harm SK, Yazer MH. Platelet transfusion – the art and science of compromise. Transfus Med Hemother. 2013;40(3):160–71.
33 Schepull T, Kvist J, Norrman H, Trinks M, Berlin G, Aspenberg P. Autologous platelets have no effect on the healing of human achilles tendon ruptures: a randomized single-blind study. Am J Sports Med. 2011;39(1):38–47.
34 Akhundov K, Pietramaggiori G, Waselle L, Darwiche S, Guerid S, Scaletta C, et al. Development of a Cost-Effective Method for Platelet Rich Plasma (PRP) Preparation for Topical Wound Healing. Ann Burns Fire Disasters. 2012;25(4):207–13.
35 Kaviani A, Guleserian K, Perry TE, Russel W, Jennings RW, Moritz M, et al. Fetal tissue engineering from amniotic fluid. J Am Coll Surg. 2003;196(4):592–7.
36 Kadner A, Hoerstrup SP, Tracy J, Breymann C, Maurus CF, Melnitchouk S, et al. Human umbilical cord cells: A new cell source for cardiovascular tissue engineering. Ann Thorac Surg. 2002;74:S1422–8.
37 Kaviani A, Perry TE, Barnes CM, Oh JT, Ziegler MM, Fishman SJ, et al. The placenta as a cell source in fetal tissue engineering. J Pediatr Surg. 2002;37:995–9.
38 Applegate LA, Weber D, Simon J-P, Scaletta C, Hirt-Burri N, de Buys Roessingh A, Raffoul W. Chapter 7 – Organ donation and whole-cell bioprocessing in the Swiss Fetal Progenitor Cell Transplantation Platform. Ed. RF Saidi, Organ Donation and Organ Donors. Nova Science Publishers; 2013. p.125–147.
39 Rosser AE, Bachoud-Lévi A-C. Clinical trials of neural transplantation in Huntington’s disease. Prog Brain Res. 2012;200:345–71.
40 Schackel S, Pauly M-C, Piroth T, Nikkhah G, Döbrössy MD. Donor age dependent graft development and recovery in a rat model of Huntington’s disease: Histological and behavioral analysis. Behav Brain Res. 2013;256:56–63.
41 Lindvall, O. Developing dopaminergic cell therapy for Parkinson’s disease – give up or move forward? Mov. Disord. 2013;28(3):268–73.
42 Wirth ED 3rd, Reier PJ, Fessler RG, Thompson FJ, Uthman B, Behrman A, et al. Feasibility and safety of neural tissue transplantation in patients with syringomyelia. J Neurotrauma. 2001;18(9):911–29.
43 Akesson E, Piao JH, Samuelsson EB, Holmberg L, Kjaeldgaard A, Falci S, et al. Long-term culture and neuronal survival after intraspinal transplantation of human spinal cord-derived neurospheres. Physiol Behav. 2007;92(1–2):60–6.
44 Iwai H, Nori S, Nishimura S, Yasuda A, Takano M, Tsuji O, et al. Transplantation of neural stem/progenitor cells at different locations in mice with spinal cord injury. Cell Transplant. 2013 Aug 30. doi: 10.3727/096368913X670967. (Epub ahead of print)
45 Mothe, AJ, Tator CH. Review of transplantation of neural stem/progenitor cells for spinal cord injury. Int J Dev Neurosci. 2013;31(7):701–13.
46 Touraine JL, Raudrant D, Golfier F, Rebaud A, Sembeil R, Roncarolo MG, et al. Reappraisal of in utero stem cell transplantation based on long-term results. Fetal Diagn Ther. 2004;19(4):305–12.
47 Montanucci P, Pennoni I, Pescara T, Basta G, Calafiore R. Treatment of diabetes mellitus with microencapsulated fetal human liver (FH-B-TPN) engineered cells. Biomaterials. 2013;34(16):4002–12.
48 Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322(5907):1490–4.
49 Gridelli B, Vizzini G, Pietrosi G, Luca A, Spada M, Gruttadauria S, et al. Efficient human fetal liver cell isolation protocol based on vascular perfusion for liver cell–based therapy and case report on cell transplantation. Liver Transpl. 2012;18(2):226–37.
50 Khan AA, Shaik MV, Parveen N, Rajendraprasad A, Aleem MA, Habeeb MA, et al. Human fetal liver-derived stem cell transplantation as supportive modality in the management of end-stage decompensated liver cirrhosis. Cell Transplant. 2010;19(4):409–18.
51 Hohlfeld J, de Buys Roessingh A, Hirt-Burri N, Chaubert P, Gerber S, Scaletta C, et al. Tissue engineered fetal skin constructs for paediatric burns. Lancet. 2005;366:840–2.
52 Ramelet A-A, Hirt-Burri N, Raffoul W, Scaletta C, Pioletti D, Offord E, et al. Chronic wound healing by fetal cell therapy may be explained by differential gene profiling observed in fetal versus old skin cells. Exp Gerontol. 2009;44:208–18.
53 Applegate LA, Weber D, Simon J-P, Scaletta C, de Buys Roessingh A, Raffoul W. Organ donation and whole-cell bioprocessing in the swiss fetal progenitor cell transplantation program. In: Organ donation and Organ donors: Issues, challenges and perspectives. Nova Science Publishers, Ed. Saidi RF. pp 125–147, 2013.
54 Pioletti DP, Montjovent MO, Zambelli PY, Applegate L. Bone tissue engineering using foetal cell therapy. Swiss Med Wkly. 2006;136(35–36):557–60.
55 Darwiche S, Scaletta C, Raffoul W, Pioletti DP, Applegate LA. Epiphyseal Chondroprogenitors Provide a Stable Cell Source for Cartilage Cell Therapy. Cell Medicine. 2012;4:23–32.
56 Tenorio DM, Scaletta C, Jaccoud S, Hirt-Burri N, Pioletti DP, Jaques B, Applegate LA. Human fetal bone cells in delivery systems for bone engineering. J Tissue Eng Regen Med. 2011;5(10):806–14.




